Abstract
Background
Low systemic blood flow (SBF) is common in extremely premature infants in the first day after birth and has been associated with peri / intraventricular haemorrhage (PIVH), necrotising enterocolitis (NEC), mortality and developmental impairment.
Objectives
To determine the effect of specific inotropes on morbidity and mortality in preterm infants with low systemic blood flow
Search methods
Updated searches were made of CENTRAL (The Cochrane Library, Issue 1, 2010), MEDLINE (1966 to May 2010), EMBASE (1980 to May 2010) and CINAHL (1982 to May 2010), supplemented by searches of abstracts of conference proceedings, citations of reviews and expert informants.
Selection criteria
Random and quasi‐random controlled trials of inotropes enrolling preterm infants with low systemic or organ blood flow in the neonatal period.
Data collection and analysis
Independent assessment of trial eligibility, quality and data extraction by each review author.
Main results
No new studies were found in updated search. No studies that compared an inotrope to no treatment in preterm infants with low SBF were found. One study compared dobutamine versus dopamine in preterm infants with low SVC flow. The study was of adequate methodology. No significant difference was reported in mortality to discharge, PIVH, grade 3 or 4 PIVH or NEC. At three years, there was no significant difference in cerebral palsy, deafness, developmental quotient > 2 sd below norm or combined disability. Surviving infants treated with dobutamine had a significantly higher development quotient. There was no significant difference in death or disability at the latest time reported (RR 0.95, 95% CI 0.66, 1.38). For secondary outcomes, there was no significant difference in periventricular leucomalacia, renal impairment, pulmonary haemorrhage, retinopathy of prematurity or CLD at 36 weeks. There was no significant difference in treatment failure. Dobutamine produced a significantly greater increase in SVC flow at the highest dose reached, whereas dopamine produced a significantly greater increase in mean BP.
Authors' conclusions
In preterm infants with low systemic blood flow, there is some evidence that dobutamine is better than dopamine at increasing and maintaining systemic blood flow. The only eligible trial did not demonstrate any consistent differences in clinical outcomes. However, this study was not sufficiently powered to prove or disprove effects on clinical outcomes. It is unclear what is the most effective strategy for improving the cardiovascular status of immature infants in the first day. Further trials are needed to determine effective strategies for preventing and improving low systemic and organ blood flow.
Plain language summary
The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow
Low systemic blood flow is common in extremely premature infants and has been associated with brain and intestinal injury, death and developmental impairment. It is unclear what is the best strategy to prevent or treat this. The usual strategy for supporting the cardiovascular system of the preterm infant is to treat infants with low blood pressure with agents (inotropes) aimed at increasing blood pressure. However, many of infants with low blood flow have normal blood pressure. One trial was found that examined the effect of inotropes in infants with low systemic blood flow. The trial found that many infants failed to respond to the two commonly used inotropes (dobutamine and dopamine) and neither was better at improving outcomes of very preterm babies. Further research is needed to determine the best strategy for preventing or treating low systemic or organ blood flow in these very immature babies.
Background
Description of the condition
Cardiovascular interventions have most frequently been given to preterm infants on the basis of hypotension, poor peripheral tissue perfusion as measured by increased capillary refill time, or evidence of a developing acidosis or renal impairment. Commonly used criteria for hypotension and cardiovascular intervention have included a mean blood pressure (BP) ≤ 30 mm Hg, which was associated with increased mortality and cerebral injury in a cohort study (Miall‐Allen 1987). Other studies have defined hypotension as below the range (usually the 10th centile) seen in population studies of preterm infants. A commonly used bedside approximation of the 10th centile is a mean BP less than the gestational age in weeks or a systolic BP ≤ 40 mmHg (Subhedar 2003). However, there are several substantial limitations to using hypotension as the criteria for cardiovascular support in preterm infants. There is a poor correlation between BP and systemic blood flow (SBF) in the first days after birth (Kluckow 1996; Osborn 2004a). This is because BP is the product of blood flow and systemic vascular resistance. Using hypotension as a criteria for cardiovascular intervention in the first day after birth results in a considerable delay in treatment of many infants, with many infants with low SBF not receiving treatment for many hours or missing treatment altogether (Kluckow 2000). Infants who have persisting inotrope requirements because of hypotension after the first days after birth frequently have normal or high SBF pointing to low systemic vascular resistance as the problem. Finally, studies of cardiovascular support in preterm infants that have used hypotension as the criterion for treatment and correction of hypotension as the goal of treatment are yet to demonstrate any improvements in morbidity or mortality (Subhedar 2003).
Low systemic and/or organ blood flow occurs in a variety of situations in preterm infants including extremely preterm infants in the first day after birth (Kluckow 2000; Osborn 2002a), infants with perinatal asphyxia, severe respiratory problems and pulmonary hypertension (Evans 1996), sepsis or necrotising enterocolitis. Low SBF is common in the first day after birth and is associated with decreasing gestation, mechanical ventilation, a large diameter ductus arteriosus, high pulmonary and systemic vascular resistances (Kluckow 2000; Osborn 2002a) and poor myocardial contractility (Osborn 2002b). Normally, SBF is measured using echocardiographic measurements of ventricular outputs. However, in the first day after birth, large shunts across the ductus arteriosus and foramen ovale are common and result in the ventricular outputs overestimating SBF by up to 100% (Evans 1996). Alternatives include using echocardiography to measure ventricular inputs such as superior vena cava (SVC) flow (Kluckow 2000b) or using techniques such as near infrared spectroscopy (NIRS) or xenon clearance to measure organ blood flows, particularly cerebral blood flow. In analyses adjusted for potential perinatal confounders, low SVC (Kluckow 2000; Osborn 2002a) and low cerebral blood flows (Meek 1999) in the first day after birth have been independently shown to precede intraventricular haemorrhage in preterm infants. Infants with early low SVC flows are also at increased risk of low urine output and hyperkalaemia (Kluckow 2001), mortality (Osborn 2002a) and poor neurodevelopmental outcome (Hunt 2001).
Description of the intervention
Strategies that have been used to provide cardiovascular support to preterm infants have included the use of volume expansion (see Cochrane reviews: Osborn 2004c; Osborn 2001), inotropes, nitric oxide and corticosteroids. Most studies comparing these interventions enrolled infants with hypotension, not with documented low systemic or organ blood flow. This review will focus on the use of inotropes to provide cardiovascular support for infants with demonstrated low systemic and/or organ blood flows. Inotropes used in preterm infants have included dopamine, dobutamine, isoprenaline (isoproterenol), adrenaline (epinephrine), noradrenaline (norepinephrine) and milrinone.
How the intervention might work
In selecting an inotrope for treatment in preterm infants, it is likely to be important to consider the underlying pathophysiology of the cardiovascular compromise as well as the pharmacokinetics and pharmacodynamics of the inotrope (for reviews see Osborn 2004b; Seri 2001). Dopamine at low doses stimulates dopamine and beta‐adrenergic receptors with little effect on vascular resistance and increases in organ blood flows. At high doses (above 10 μg/kg/min), alpha‐adrenergic effects occur resulting in increases in both pulmonary and systemic vascular resistance and BP. Dobutamine produces predominately beta‐adrenergic effects with resulting inotropy and a reduction in pulmonary and systemic vascular resistance. Isoprenaline is almost purely a beta‐agonist and has a pronounced chronotropic effect and produces pulmonary and systemic vasodilatation. There have been concerns regarding its potential to increase myocardial oxygen consumption. Adrenaline has both alpha and beta adrenergic effects, with beta adrenergic effects predominating at lower doses, producing increases in cardiac output, but alpha effects becoming important at high doses resulting in substantial increases in systemic vascular resistance and reduction in cardiac outputs. Pulmonary vascular resistance is increased to a lesser extent. Noradrenaline has both alpha and beta effects with alpha effects predominant and resulting in an increase in vascular resistance. Milrinone, a selective phosphodiesterase inhibitor, is inotropic and a vasodilator (an 'inodilator'). It is used in infants undergoing cardiac surgery to treat of prevent low cardiac output syndrome associated with poor myocardial contractility, a situation similar to the low SBF that occurs in extremely preterm infants in the first day after birth.
Why it is important to do this review
The primary outcomes of this review that might be expected to be improved by the treatment of low SBF with inotropes are those that have been strongly associated with low SBF in the preterm infant, including mortality, late PIVH and subsequent neurodevelopmental disability including motor impairments and cerebral palsy. There is some data to support necrotising enterocolitis being associated with low SBF (Osborn 2002a). Secondary outcomes for which there is evidence of association include renal impairments and hyperkalaemia. There are no data to date associating periventricular white matter injury with low SBF.
Objectives
The objective was to determine the effect of specific inotropes versus no treatment or another inotrope on morbidity and mortality in preterm infants with low SBF. Secondary objectives included:
Determining the evidence for use of a specific inotrope compared to a different inotrope
Determining whether there is a difference in effect from inotropes that lower vascular resistance (e.g. dobutamine, isoprenaline, milrinone) or those that increase vascular resistance (e.g. noradrenaline, higher dose dopamine and adrenaline)
Determine the evidence for use of inotropes according to clinical indication including immature infants in the first day after birth, infants with asphyxia or infants with sepsis
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials. Trials that use crossover design were eligible for inclusion of data for short term outcomes with the original treatment allocation used for data extraction.
Types of participants
Preterm infants (< 37 weeks gestational age) with low SBF or organ blood flow in the neonatal period. Low SBF may be determined on the basis of echocardiographically measured ventricular outputs or surrogates for SBF such as SVC flow. Low organ blood flow may be determined on the basis of techniques including ultrasound Doppler, near infrared spectroscopy or xenon clearance techniques where there is evidence in the literature that the measurement is associated with substantial clinical outcomes and/or actual organ blood flow. The review does not include studies that include surrogates of flow such as blood pressure, ultrasound Doppler measured velocities, pulsatility or resistive indices.
Types of interventions
Inotropes including adrenaline, dobutamine, dopamine, isoprenaline, noradrenaline and milrinone used to treat infants with low SBF or organ blood flow. Eligible comparisons included no treatment or placebo, or another inotrope.
Types of outcome measures
Primary outcomes
Mortality including neonatal or to near term corrected age
Peri / intraventricular haemorrhage (PIVH) including all and late occurring PIVH (late = not present on an initial head ultrasound in the first hours after birth)
Proven necrotising enterocolitis (pneumatosis on x‐ray, or at surgery or postmortem)
Developmental disabilities including motor impairments and cerebral palsy. Emphasis will be placed on assessments that are blinded to intervention, neurological status assessed by physician, assessments that are made later (e.g. three years for assessment of cerebral palsy status) and assessments that use a validated screening tool that has been shown to predict long term neurodevelopment.
Secondary outcomes
Failure to correct low SBF or organ blood flow. Low SBFs included ventricular outputs or SVC flows below the range normally seen in preterm infants (e.g. RVO or LVO < 150 ml/kg/min; SVC flow < 40 ml/kg/min). Data will be recorded where studies report outcomes that are approximate to these criteria
Persistent patent ductus arteriosus (PDA) after study enrolment
Chronic lung disease at near term equivalent age (defined as need for oxygen or ventilatory support)
Impaired renal function (e.g. creatinine ≥ 120 mmol/l or oliguria ≤ 0.5 ml/kg/hr)
Hyperkalaemia (potassium ≥ 6.5 mmol/l)
Pulmonary haemorrhage
Periventricular leucomalacia and / or periventricular white matter abnormalities on ultrasound or MRI
Retinopathy of prematurity, any or severe (≥ stage 3)
Increase in ventricular output when cardiac shunts excluded (either absolute or as a percentage of baseline)
Increase in organ blood flow (either absolute or as a percentage of baseline)
Increase in BP (either absolute or as a percentage of baseline)
Side effects of inotropes such as excessive tachycardia requiring dose reduction (e.g. heart rates > 180 bpm), hypotension requiring treatment (e.g. mean or systolic BP below 10th centile for gestational age) and arrhythmias
Search methods for identification of studies
See: Cochrane Neonatal Group search strategy.
Electronic searches
The standard search strategy of the Cochrane Neonatal Review Group was used. This included electronic searches of the The Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2006), MEDLINE (1966 to April 2006), EMBASE (1980 to April 2006) and CINAHL (1982 to April 2006).The search strategy was adapted for individual databases and included a search on all fields for [infant, newborn OR infant, newborn, diseases OR neonat*] AND [inotrope OR cardiotonic agents OR dopamine OR dobutamine OR epinephrine OR adrenaline OR noradrenaline OR isoprenaline OR isoproterenol OR milrinone OR amrinone OR phosphodiesterase inhibitors] AND [randomized controlled trial OR clinical trial OR random* OR trial* OR comparative study OR controlled study] was conducted. No language restrictions were used.
The search was updated in May 2010 with additional searches of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2010), MEDLINE (1966 to May 2010), PREMEDLINE (to May 2010), EMBASE (1988 to May 2010).
Searching other resources
References in studies identified as potentially relevant and references in previous reviews were searched. Additionally, the published abstracts of conference proceedings including the Perinatal Society of Australia and New Zealand 1998 to 2005 and the Pediatric Academic Societies and Societies for Pediatric Research Annual Meetings 2000 to 2005 were searched. Expert informants were contacted.
The search was updated in May 2010 with additional searches of cross references of all new studies cited, abstracts and conference proceedings (American Pediatric Society/Society for Pediatric Research Annual Meetings 2005 to 2010; Perinatal Society of Australia and New Zealand Annual Meetings 2005 to 2010).
Data collection and analysis
Standard methods of the Cochrane Collaboration and its Neonatal Review Group were used.
Selection of studies
All review authors independently assessed trials for eligibility for inclusion. Differences between reviewer authors were resolved by consensus.
Data extraction and management
Data was extracted from the included study independently by each reviewer author. Revman 4.2 was used for data management in the original review. This has been updated to Revman 5 format.
Assessment of risk of bias in included studies
All three review authors were to independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). There was no disagreement between the review authors. We planned, had there been trials identified for inclusion, to assess the following.
(1) Sequence generation (checking for possible selection bias)
We planned to describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We planned to assess the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We planned to describe for each included study the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We planned to assess the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear.
(3) Blinding (checking for possible performance bias)
We planned to describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We planned to judge studies to be at low risk of bias if they were blinded, or if we judge that the lack of blinding could not have affected the results. We planned to assess blinding separately for different outcomes or classes of outcomes.
We planned to assess the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We planned to describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We planned to state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or can be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertake. We planned to assess methods as:
adequate (less than 20% missing data);
inadequate;
unclear.
(5) Selective reporting bias
We planned to describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We planned to assess the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We planned to describe for each included study any important concerns we have about other possible sources of bias (e.g. early termination of trial due to data‐dependant process, extreme baseline imbalance, etc). We planned to assess whether each study was free of other problems that could put it at risk of bias. We will assess other sources of bias as:
yes;
no;
unclear.
(7) Overall risk of bias
We planned to make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Standard methods of the Neonatal Review Group were used to synthesise data.
Dichotomous data
For dichotomous data, we planned to present results as risk ratios and risk differences (RD) with 95% confidence intervals (CI). If there is a statistically significant reduction in RD then we planned to calculate the number needed to treat and associated 95% CI.
Continuous data
For continuous data, we planned to use the mean difference if outcomes are measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
The unit of randomisation (individual infant) is the unit of analysis.
Dealing with missing data
The primary analysis was planned to be an intention‐to‐treat analysis reporting only available data (excluding cases where data are missing). For included studies, we planned to note levels of attrition. We planned to obtain missing data from the authors when possible. Where data remains missing, we planned to conduct sensitivity analysis to determine if results are sensitive to assumptions regarding losses, using imputation (both best and worst scenarios) method and last observation carried forward to the final assessment (LOCF) for dichotomous and continuous outcome data respectively.
Assessment of heterogeneity
Heterogeneity was looked for both within the forest plots and statistically using the chi‐square test for heterogeneity. Had we identified substantial heterogeneity, we planned to explore it by pre‐specified subgroup analysis. We planned to grade the degree of heterogeneity as 0 to 30% (might not be important), 31% to 50% (moderate heterogeneity); 51% to 75% (substantial heterogeneity); 76% to 100% (considerable heterogeneity).
Assessment of reporting biases
We planned to investigate reporting and publication bias by examining the degree of asymmetry of a funnel plot. Where we suspect reporting bias (see 'Selective reporting bias' above), we will attempt to contact study authors asking them to provide missing outcome data. Where this is not possible, and the missing data are thought to introduce serious bias, we will explore the impact of including such studies in the overall assessment of results by a Sensitivity analysis.
Data synthesis
The fixed effects model was used using RevMan 4.2 for meta‐analysis.
Subgroup analysis and investigation of heterogeneity
Studies enrolling infants to treatment with different inotropes were not be combined in an overall analysis except where they fulfil criteria for analysis of expected effect on vascular resistance. The following comparisons were prespecified: 1. Individual inotrope versus no inotrope (no treatment or placebo) including separate comparisons of:
adrenaline versus no inotrope
dobutamine versus no inotrope
dopamine versus no inotrope
isoprenaline versus no inotrope
milrinone versus no inotrope
noradrenaline versus no inotrope
2. Individual inotrope (as above) versus a different inotrope.
3. The effect of inotrope according to the expected effect on systemic vascular resistance (see background), including separate comparisons of:
inotropes likely to lower vascular resistance at any dose (e.g. dobutamine, isoprenaline, milrinone)
inotropes at doses likely to raise vascular resistance (e.g. noradrenaline at any dose, dopamine ≥ 10 μg/kg/min, adrenaline ≥ 0.375 μg/kg/min)
inotropes likely to lower vascular resistance (e.g. dobutamine, isoprenaline, milrinone at any dose) versus inotropes at doses likely to raise vascular resistance (e.g. noradrenaline at any dose, dopamine ≥10 μg/kg/min, adrenaline ≥ 0.375 μg/kg/min)
Within each comparison, we planned subgroup analyses according to individual inotrope and dose range.
4. The effect of inotrope according to type of infant enrolled in trials including separate comparisons of the following types of infants:
immature infants in the first day after birth
infants with sepsis
infants with perinatal asphyxia (trials that enrol infants on the basis of a low Apgar score or cord arterial blood gas acidosis)
Sensitivity analysis
Sensitivity analysis was prespecified on the basis of methodological quality. Studies of adequate methodology were defined as those with adequate randomisation and allocation concealment and with < 10% losses to follow up analysed on an intention to treat basis.
Results
Description of studies
Results of the search
No studies were found that compared inotropes to no treatment in preterm infants with low SBF. One study (Osborn 2002a) was found that compared volume and dobutamine versus volume and dopamine in preterm infants with low systemic blood flow.
Included studies
In the 2010 update, no additional eligible studies were found. One study (Osborn 2002a) met inclusion criteria that compared different inotropes. See 'Characteristics of included studies' table.
Osborn 2002a enrolled infants < 30 weeks gestation and < 12 hours after birth with low SVC flow (< 41 ml/kg/min). Forty‐two infants with low SVC flow were given volume (normal saline 10 ml/kg) over 20 minutes and then randomly allocated to dobutamine or dopamine. Infusions were commenced at 10 μg/kg/min. If at any time in the first 24 hours the infant failed to maintain SVC flow > 40 ml/kg/min, the dose was increased to 20 μg/kg/min. Infants crossed over to the other inotrope if SVC flow was still not maintained > 40 ml/kg/min. The primary outcome was maintenance of SVC flow > 40 ml/kg/min. SVC flow and RVO were measured echocardiographically 30 minutes after each change of dose of inotrope and 10 and 24 hours after birth. Head ultrasounds were performed at seven and 28 days. Neonatal morbidity and mortality were reported to discharge. Developmental assessment was performed at one and three years corrected age and included paediatrician examination and the Griffiths Mental Development Scales. A Griffiths development quotient at three years < 75 represents a delay > 2 sd below population norms.
Excluded studies
In the 2010 update, an additional three reports of studies that were excluded were found (Pederson 2009; Paradisis 2009; Pellicer 2005). Nineteen reports of excluded studies and reasons for exclusion are reported in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Osborn 2002a reported adequate randomisation, allocation concealment and blinding of intervention with identical syringes prepared in pharmacy allocated according to a computer generated random number sequence. Echocardiographic measures, neonatal outcomes and one and three years corrected age developmental assessments were also blinded by use of identical syringes with treatment allocation concealed until analysis performed. Four additional infants who were allocated treatment on the basis of hypotension were excluded from analysis as they did not meet prespecified enrolment criteria (SVC flow < 41 ml/kg/min). One surviving infant was not examined for retinopathy of prematurity. Five of 18 surviving infants were not assessed at three years of age. Follow up was complete for all other outcomes.
Allocation
Osborn 2002a reported adequate randomisation and allocation concealment with identical syringes prepared in pharmacy allocated according to a computer generated random number sequence.
Blinding
Osborn 2002a reported adequate blinding of intervention using identical syringes prepared in pharmacy. Echocardiographic measures, neonatal outcomes and one and three years corrected age developmental assessments were also blinded by use of identical syringes with treatment allocation concealed until analysis performed.
Incomplete outcome data
Osborn 2002a reported 4 additional infants who were allocated treatment on the basis of hypotension were excluded from analysis as they did not meet prespecified enrolment criteria (SVC flow < 41 ml/kg/min). One surviving infant was not examined for retinopathy of prematurity. Five of 18 surviving infants were not assessed at three years of age. Follow up was complete for all other outcomes.
Selective reporting
Osborn 2002a prespecified primary and secondary outcomes.
Other potential sources of bias
Osborn 2002a had no other apparent sources of bias including no interim analyses.
Effects of interventions
Inotrope versus no treatment: no study found.
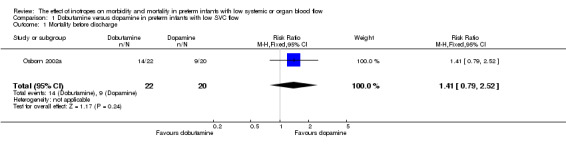
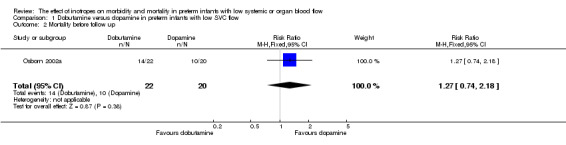
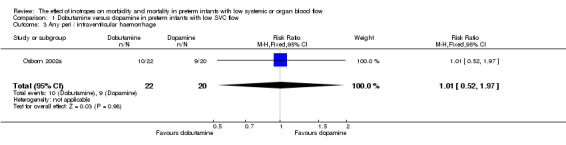
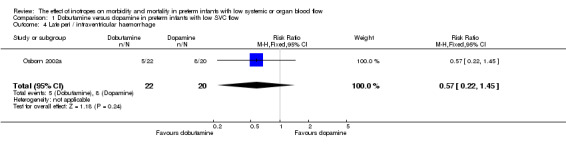
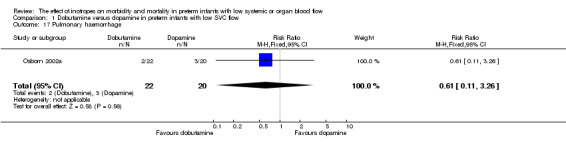
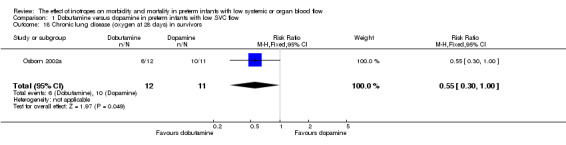
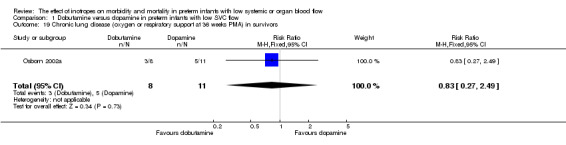
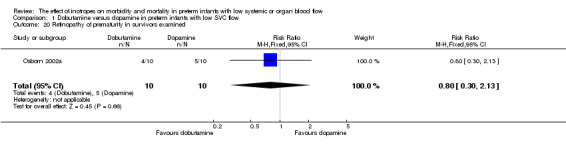
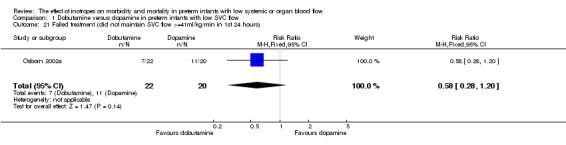
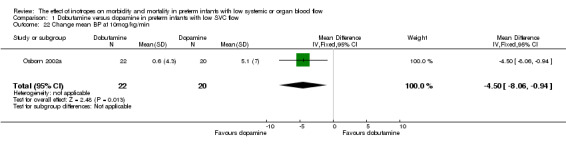
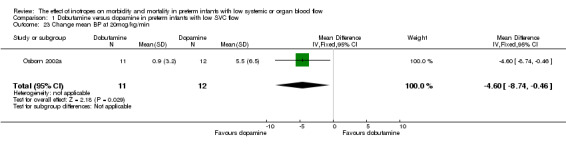
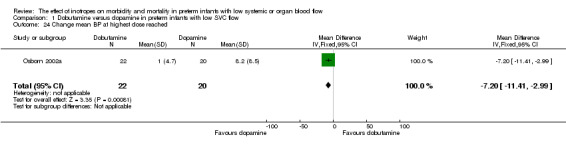
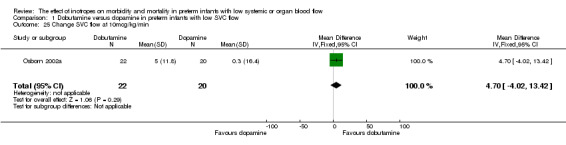
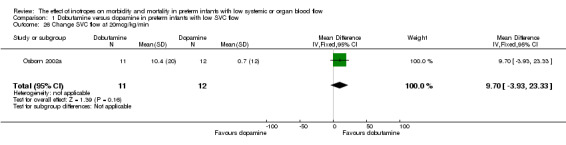
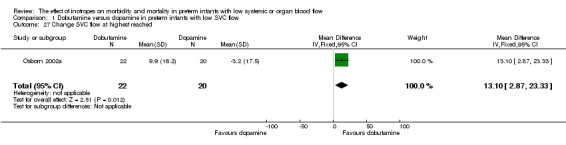
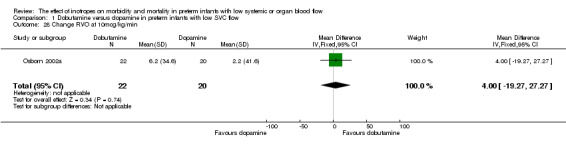
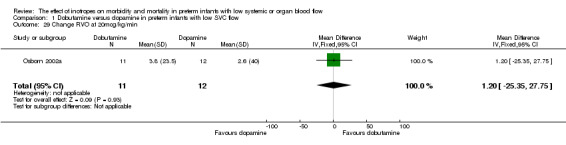
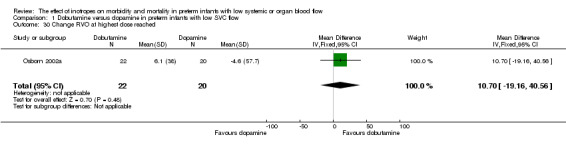
Comparison 01: Dobutamine versus dopamine in preterm infants with low SVC flow: One study eligible study (Osborn 2002a) was included. Primary outcomes: Osborn 2002a reported no significant difference in mortality to discharge (RR 1.41, 95% CI 0.79, 2.52), mortality before follow up (RR 1.27, 95% CI 0.74, 2.18), all PIVH (RR 1.01, 95% 0.52, 1.97), late PIVH (RR 0.57, 95% CI 0.22, 1.45), grade 3 or 4 PIVH (RR 0.39, 95% CI 0.12, 1.31) or necrotising enterocolitis (RR 2.73, 95% CI 0.31, 24.14). At 3 years, there was no significant difference in cerebral palsy (RR 0.16, 95% CI 0.01, 2.64) or deafness (RR 0.16, 95% CI 0.01, 2.64), no blind infant in either group and no difference in Developmental quotient > sd below norm (RR 0.16, 95% CI 0.01, 2.64). There was no significant difference in disability (RR 0.10, 95% CI 0.01, 1.56) defined as a development quotient > sd below norm, cerebral palsy, blind or deaf, or death and disability (RR 0.79, 95% CI 0.57, 1.11). Surviving infants treated with dobutamine had a significantly higher Griffiths quotient (MD 35.00, 95% CI 17.68, 52.32). There was no significant difference in death or disability at the latest time reported (RR 0.95, 95% CI 0.66, 1.38). Secondary outcomes: Osborn 2002a reported no significant difference in periventricular leucomalacia (RR 6.00, 95% CI 0.34, 104.89), renal impairment (RR 0.55, 95% CI 0.15, 2.00), pulmonary haemorrhage (RR 0.61, 95% CI 0.11, 3.26) or retinopathy of prematurity in survivors examined (RR 0.80, 95% CI 0.30, 2.13). There was a reduction of borderline significance in CLD defined as oxygen at 28 days (RR 0.55, 95% CI 0.30, 1.00),but not at 36 weeks (RR 0.83, 95% CI 0.27, 2.49). There was no significant difference in treatment failure (RR 0.58, 95% CI 0.28, 1.20). Dopamine produced a significantly greater increase in mean BP at 10 μg/kg/min (MD ‐4.50 mmHg, 95% CI ‐8.06, ‐0.94), 20 μg/kg/min (MD ‐4.60, 95% CI ‐8.74, ‐0.46) and at the highest dose reached (MD ‐7.20, 95% CI ‐11.41, ‐2.99). There was no significant difference in SVC flow at 10 μg/kg/min (MD 4.70 ml/kg/min, 95% CI ‐4.02, 13.42) or 20 μg/kg/min (MD 9.70, 95% CI ‐3.93, 23.33), but dobutamine produced a significantly greater increase in SVC flow at the highest dose reached (MD 13.10, 95% CI 2.87, 23.33). There was no significant difference in RVO at 10 μg/kg/in (MD 4.00, 95% CI ‐19.27, 27.27) or 20 μg/kg/min (MD 1.20, 95% CI ‐25.35, 27.75) or the highest dose reached (MD 10.70, 95% CI ‐19.16, 40.56).
Subgroup analyses: The only eligible trial compared dobutamine versus dopamine, compared inotropes likely to lower vascular resistance (dobutamine at any dose) versus inotropes at doses likely to raise vascular resistance (dopamine ≥ 10 μg/kg/min) and enrolled immature infants in the first day after birth.
Sensitivity analysis: The only eligible trial meets inclusion criteria for studies of adequate methodology.
Discussion
Summary of main results
The single small study included in this review enrolled preterm infants on the basis of a surrogate for low systemic blood flow (SVC flow) that is not confounded by shunts across the adapting heart, had adequate randomisation and allocation concealment, and blinded treatment and measurement. The study was designed and powered to explore haemodynamic rather than clinical outcomes. However, there are several observations that can be made from this study. Infants treated with dopamine had a significantly greater increase in mean BP at both 10 and 20 μg/kg/min, but little change in SVC flow or RVO. Infants treated with dobutamine had little change in mean BP, but had a significantly greater increase in SVC flow at the highest dose reached with trends to increased SVC flow at both 10 and 20 μg/kg/min. However, treatment failure (defined as failure to increase and maintain normal SVC flows) was substantial on either inotrope, with 40% of the all babies enrolled not improving or maintaining SVC flow within the normal range. No consistent differences were reported in neonatal morbidity (including renal impairment, PIVH, periventricular leucomalacia or necrotising enterocolitis) that we hypothesised may be affected by interventions aimed at improving blood flow. There was no significant difference in mortality or combined death and disability at the latest time measured, although infants treated with dobutamine had significantly higher Griffiths quotients at three years. It is possible that the additional infants who survived in the dopamine group did so with impaired development.
Overall completeness and applicability of evidence
Several methodological issues affect the interpretation of the only study eligible for this review. Most infants enrolled were immature, ventilated preterm infants. However, infants with asphyxia and sepsis were not excluded. The applicability to infants with different reasons for cardiovascular compromise is unclear. All infants were given volume expansion prior to inotrope randomisation. Infants who failed to maintain SVC flow on the highest dose of inotrope crossed over to the other inotrope, so that a substantial proportion of infants were exposed to both inotropes. Cerebral and other organ blood flows were not measured. The dose of inotropes used included relatively high dose dopamine (20 μg/kg/min). This is a dose that some authors may caution using due to the substantial alpha effects that produce substantial increases in vascular resistance. However, it is within the dose range commonly reported in trials (Subhedar 2003). In addition, dopamine is usually titrated to achieve a BP above the minimally accepted pressure on gestation or birth weight criteria, a strategy that was not used in this study.
The inclusion of a single small study enrolling infants in the first day precludes strong conclusions to be made about the use of inotropes in preterm infants. No trial was found that compared inotrope use to another strategy of cardiovascular support in preterm infants with low systemic or organ blood flows. The only comparison able to be made was for dobutamine versus dopamine.
The findings of this review suggest that further research is needed to determine the most effective strategies for providing cardiovascular support for extremely premature infants. Independent research groups considering replicating the trial included in this review should consider using another agent (e.g. low dose adrenaline) in infants who fail to respond to inotrope rather than a blinded crossover to the other agent. The trial should be adequately powered to detect important clinical differences. A trial comparing a strategy of targeting infants with hypotension with dopamine versus targeting infants with low SBF with dobutamine is needed. An alternative strategy that needs evaluation includes one aimed at preventing low SBF. Given that low SBF is associated with high systemic vascular resistance and poor myocardial contractility, researchers conducting trials aimed at preventing low SBF should consider using inotropes that have the potential to lower systemic vascular resistance and improve myocardial contractility, such as dobutamine or milrinone. Future trials should measure the effects of interventions on systemic and organ blood flows using validated measures. Doppler velocity indices of flow are not validated measures of actual organ blood flows. Ventricular outputs have been validated as measures of SBF after the first day, but are confounded by shunts across the adapting heart in the first day. Superior vena cava flow has been validated clinically as a surrogate measure of SBF in the first day. NIRS and xenon measurements are also potential techniques for determining the effect of cardiovascular interventions on organ blood flows in preterm infants.
Quality of the evidence
The single small study included in this review was of adequate methodology and reported outcomes prespecified in the protocol.
Potential biases in the review process
The review has been performed by the research team who conducted the only eligible study.
Agreements and disagreements with other studies or reviews
It is unclear from this study and previous reviews of inotropes for treatment of hypotension (Subhedar 2003) what the optimal strategy is for improving the cardiovascular status of the immature infant. Dopamine has been reported to lead to greater increases in BP in hypotensive infants (Subhedar 2003) and infants with low SBF (Osborn 2002a), but dobutamine increased SBF better than dopamine in infants with low SBF (Osborn 2002a). Neither inotrope has been shown to consistently improve any clinical outcome when given for hypotension or low SBF, and many infants with initial low SBF fail to maintain flows on either inotrope.
Authors' conclusions
Implications for practice.
There were no eligible studies that compared use of an inotrope to no treatment in preterm infants with low SBF. In preterm infants with low SBF, there is some evidence that dobutamine is better than dopamine at increasing and maintaining SBF. The only eligible trial did not demonstrate any consistent differences in clinical outcomes. However, this study was not sufficiently powered to prove or disprove effects on clinical outcomes. It is unclear what is the most effective strategy for improving the cardiovascular status of immature infants in the first day.
Implications for research.
Further trials are needed to determine effective strategies for preventing and improving low systemic and organ blood flows in the first day after birth in extremely immature infants. A trial comparing a strategy of treating hypotension with dopamine versus treating low SBF with dobutamine in preterm infants in the first day is warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 20 May 2010 | New search has been performed | This updates the review "The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow" published in The Cochrane Library (Osborn 2007). No new trials identified in updated search. |
History
Protocol first published: Issue 1, 2005 Review first published: Issue 1, 2007
| Date | Event | Description |
|---|---|---|
| 11 September 2008 | Amended | Converted to new review format. |
| 16 October 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Dobutamine versus dopamine in preterm infants with low SVC flow.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality before discharge | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.79, 2.52] |
| 2 Mortality before follow up | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.74, 2.18] |
| 3 Any peri / intraventricular haemorrhage | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.52, 1.97] |
| 4 Late peri / intraventricular haemorrhage | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.22, 1.45] |
| 5 Peri / intraventricular haemorrhage grade 3 or 4 | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.12, 1.31] |
| 6 Necrotising enterocolitis | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.31, 24.14] |
| 7 Cerebral palsy at 3 years in survivors assessed | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.64] |
| 8 Deafness at 3 years in survivors assessed | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.64] |
| 9 Blind at 3 years survivors assessed | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Griffith's general Quotient >2sd below norm at 3 years in survivors assessed | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.64] |
| 11 Disability at 3 years in survivors assessed | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.56] |
| 12 Death or disability at 3 years | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.11] |
| 13 Death or disability at latest follow up | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.38] |
| 14 Griffith's Mental Scales of Development General Quotient at 3 years in survivors assessed | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 35.0 [17.68, 52.32] |
| 15 Periventricular leucomalacia in survivors | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.34, 104.89] |
| 16 Renal impairment (creatinine >=120 mmol/l) | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.15, 2.00] |
| 17 Pulmonary haemorrhage | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.11, 3.26] |
| 18 Chronic lung disease (oxygen at 28 days) in survivors | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.00] |
| 19 Chronic lung disease (oxygen or respiratory support at 36 weeks PMA) in survivors | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.27, 2.49] |
| 20 Retinopathy of prematurity in survivors examined | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.30, 2.13] |
| 21 Failed treatment (did not maintain SVC flow >=41ml/kg/min in 1st 24 hours) | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.28, 1.20] |
| 22 Change mean BP at 10mcg/kg/min | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐4.5 [‐8.06, ‐0.94] |
| 23 Change mean BP at 20mcg/kg/min | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐4.6 [‐8.74, ‐0.46] |
| 24 Change mean BP at highest dose reached | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐7.20 [‐11.41, ‐2.99] |
| 25 Change SVC flow at 10mcg/kg/min | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 4.7 [‐4.02, 13.42] |
| 26 Change SVC flow at 20mcg/kg/min | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 9.70 [‐3.93, 23.33] |
| 27 Change SVC flow at highest reached | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 13.10 [2.87, 23.33] |
| 28 Change RVO at 10mcg/kg/min | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 4.0 [‐19.27, 27.27] |
| 29 Change RVO at 20mcg/kg/min | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐25.35, 27.75] |
| 30 Change RVO at highest dose reached | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | 10.7 [‐19.16, 40.56] |
1.1. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 1 Mortality before discharge.
1.2. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 2 Mortality before follow up.
1.3. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 3 Any peri / intraventricular haemorrhage.
1.4. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 4 Late peri / intraventricular haemorrhage.
1.5. Analysis.
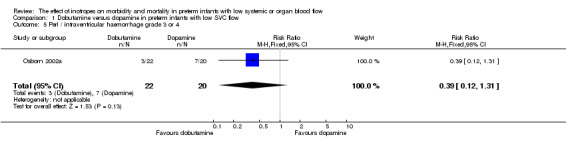
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 5 Peri / intraventricular haemorrhage grade 3 or 4.
1.6. Analysis.
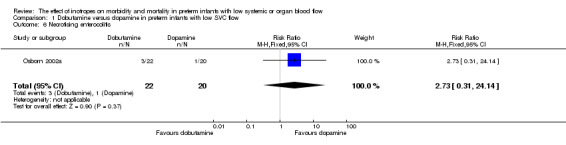
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 6 Necrotising enterocolitis.
1.7. Analysis.
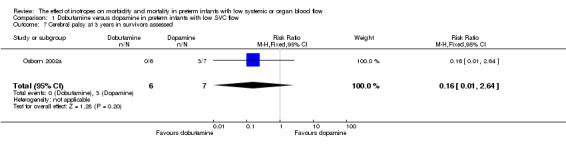
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 7 Cerebral palsy at 3 years in survivors assessed.
1.8. Analysis.
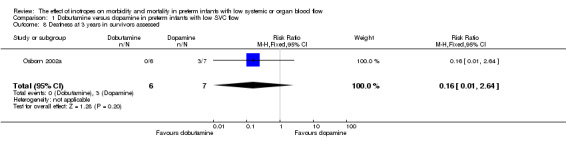
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 8 Deafness at 3 years in survivors assessed.
1.9. Analysis.
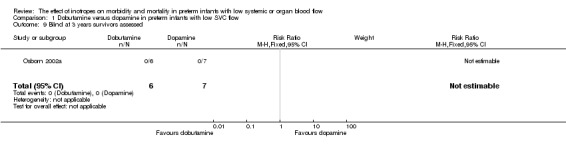
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 9 Blind at 3 years survivors assessed.
1.10. Analysis.
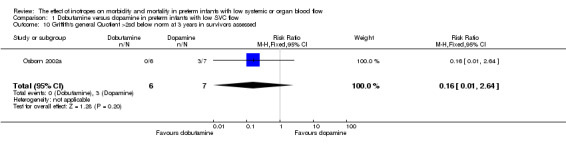
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 10 Griffith's general Quotient >2sd below norm at 3 years in survivors assessed.
1.11. Analysis.
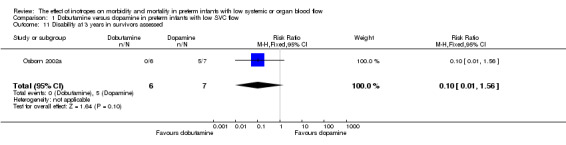
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 11 Disability at 3 years in survivors assessed.
1.12. Analysis.
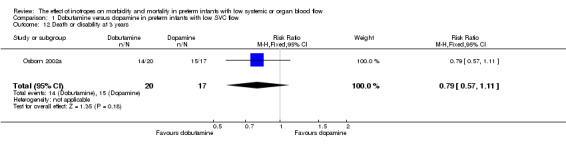
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 12 Death or disability at 3 years.
1.13. Analysis.
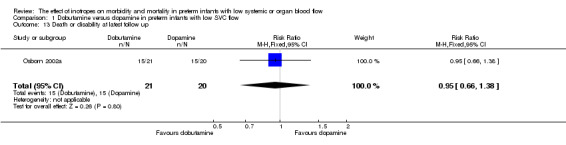
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 13 Death or disability at latest follow up.
1.14. Analysis.
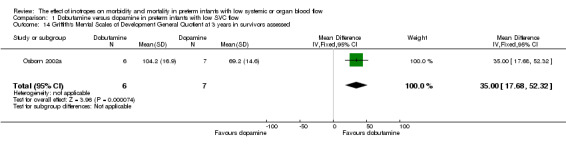
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 14 Griffith's Mental Scales of Development General Quotient at 3 years in survivors assessed.
1.15. Analysis.
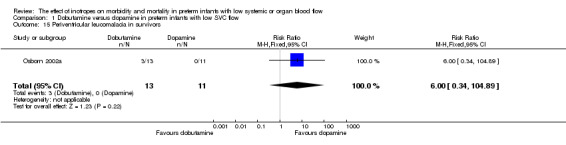
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 15 Periventricular leucomalacia in survivors.
1.16. Analysis.
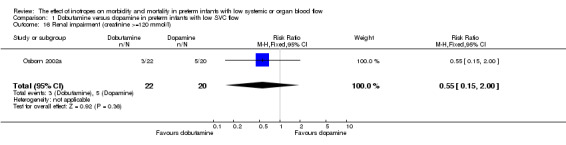
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 16 Renal impairment (creatinine >=120 mmol/l).
1.17. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 17 Pulmonary haemorrhage.
1.18. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 18 Chronic lung disease (oxygen at 28 days) in survivors.
1.19. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 19 Chronic lung disease (oxygen or respiratory support at 36 weeks PMA) in survivors.
1.20. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 20 Retinopathy of prematurity in survivors examined.
1.21. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 21 Failed treatment (did not maintain SVC flow >=41ml/kg/min in 1st 24 hours).
1.22. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 22 Change mean BP at 10mcg/kg/min.
1.23. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 23 Change mean BP at 20mcg/kg/min.
1.24. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 24 Change mean BP at highest dose reached.
1.25. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 25 Change SVC flow at 10mcg/kg/min.
1.26. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 26 Change SVC flow at 20mcg/kg/min.
1.27. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 27 Change SVC flow at highest reached.
1.28. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 28 Change RVO at 10mcg/kg/min.
1.29. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 29 Change RVO at 20mcg/kg/min.
1.30. Analysis.
Comparison 1 Dobutamine versus dopamine in preterm infants with low SVC flow, Outcome 30 Change RVO at highest dose reached.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Osborn 2002a.
| Methods | Randomised controlled trial, two centre. | |
| Participants | Inclusion criteria: <30 weeks gestation, <12 hours age, SVC flow <41 ml/kg/min. Exclusion criteria: structural heart disease, considered by treating physician to be unlikely to survive, previous inotropes. Mean (+/‐sd) gestation (weeks): Dobutamine: 26.0 (2.1); Dopamine: 25.7 (1.5). Mean +/‐sd birth weight (g): Dobutamine: 982 (324); Dopamine: 859 (190). | |
| Interventions | Normal saline 10 ml/kg over 20 minutes, then randomised to: Dobutamine 10 mcg/kg/min titrated up 20mcg/kg/min to maintain SVC flow >41 ml/kg/min (n = 22); or Dopamine 10mcg/kg/min titrated up 20 mcg/kg/min to maintain SVC flow >41 ml/kg/min (n = 20). If failed first inotrope, changed to other syringe. | |
| Outcomes | Primary outcome: change in SVC flow ml/kg/min. Secondary outcomes: late P/IVH, all PIVH, grade 3 or 4 P/IVH, periventricular leucomalacia, mortality to discharge, necrotising enterocolitis, chronic lung disease (oxygen at 36 weeks postmenstrual age), cerebral palsy at 3 years, Griffith's Mental Development Scales at 1 year corrected age and 3 years age, deaf (hearing aids), blind (visual acuity <6/60), disability (deaf, blind, Griffith's quotient <=70, cerebral palsy). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Used computer generated random number sequence to allocate syringes. Stratified by centre. |
| Allocation concealment? | Low risk | |
| Blinding? Cardiovascular measurement | Low risk | Used identical labelled and appearing syringes. |
| Blinding? Neonatal outcomes | Low risk | Used identical labelled and appearing syringes. |
| Blinding? Long term outcomes | Low risk | Used identical labelled and appearing syringes. |
| Blinding? Intervention | Low risk | Used identical labelled and appearing syringes. |
| Incomplete outcome data addressed? All outcomes | Low risk | 4 infants (4/46 = 9%) with hypotension allocated treatment but excluded from analysis as not eligible (did not have low flow). One surviving infant was not examined for ROP. Five of 18 surviving infants were not assessed at 3 years of age. Follow up was complete for all other outcomes. |
| Free of selective reporting? | Low risk | Prespecified primary and secondary outcomes. |
| Free of other bias? | Low risk | No interim analyses. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bourchier 1997 | Enrolled hypotensive preterm infants. Cardiac outputs not reported. |
| Cason 1999 | Enrolled hypotensive infants ≤34 weeks with RDS. |
| DiSessa 1981 | Enrolled asphyxiated term or near term infants with mean BP >50 mmHg. |
| Gill 1993 | Enrolled hypotensive preterm infants. Cardiac outputs not reported. |
| Greenough 1993 | Enrolled hypotensive preterm infants. Cardiac outputs not reported. |
| Hentschel 1995 | Enrolled hypotensive preterm infants. Cardiac outputs not reported. Reported mesenteric artery blood flow velocities. |
| Kawczynski 1996 | Observational study. Enrolled hypotensive preterm infants with oliguria and acidosis. Cardiac outputs not reported. |
| Klarr 1994 | Enrolled preterm infants with hypotension. Cardiac outputs not reported. |
| Lundstrom 2000 | Enrolled normotensive (mean BP 29‐40 mmHg) preterm infants. Reported cardiac outputs. |
| Padbury 1990 | Observational study exploring pharmacokinetics of dopamine in sick neonates. |
| Paradisis 2009 | Enrolled preterm infants with the goal of preventing low systemic blood flow. Incidence of low systemic blood flow developing approximately 20%. |
| Pederson 2009 | Enrolled term infants after elective caesarean section to subcutaneous adrenaline versus placebo for prevention of respiratory distress and hypoglycemia. |
| Pellicer 2005 | Enrolled hypotensive preterm infants in first day. Reported changes and not absolute cerebral blood flow using NIRS. |
| Phillipos 1996 | Enrolled hypotensive preterm infants. |
| Phillipos 2000 | Enrolled hypotensive preterm infants. |
| Rennie 1989 | Observational study in hypotensive preterm infants. |
| Repetto 1999 | Observational study in hypotensive preterm infants. |
| Roze 1993 | Enrolled hypotensive preterm infants. Reported left ventricular outputs. Mean cardiac outputs in normal range. |
| Wong 2009 | Observational study of dopamine in preterm infants with hypotension compared to control infants without hypotension. |
Contributions of authors
DO wrote the protocol and review. All reviewers independently assessed studies for eligibility, assessed study quality and extracted data. All reviewers contributed to the writing of the final version.
DO performed the updated search for the 2010 update and updated the review to Revman 5 format. NE and MP performed searches and checked the review update.
Sources of support
Internal sources
RPA Newborn Care, Royal Prince Alfred Hospital, Australia.
External sources
No sources of support supplied
Declarations of interest
The reviewer authors conducted the only included trial of inotropes in infants with low SBF. No funding was received from pharmaceutical companies.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Osborn 2002a {published and unpublished data}
- Osborn DA. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow ‐ 3 year follow up. Pediatric Research 2005;57:A. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Evans N, Kluckow M. Dopamine but not dobutamine increases left ventricular (LV) stress, but neither improves LV contractility in very preterm infants. Pediatric Research 2002;51:386A. [Google Scholar]
- Osborn DA, Evans N, Kluckow M. Dopamine but not dobutamine increases left ventricular (LV) stress, but neither improves LV contractility in very preterm infants. Proceedings Perinatal Society of Australia and New Zealand 6th Annual Congress. 2002:A145.
- Osborn DA, Evans N, Kluckow M. Left ventricular contractility in extremely premature infants in the first day and response to inotropes. Pediatric Research 2007;61:335‐40. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Evans N, Kluckow M. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow. Journal of Pediatrics 2002;140:183‐91. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Evans N, Kluckow M, Bowen JR, Rieger I. Low superior vena cava flow and effect of inotropes on neurodevelopment to 3 years in preterm infants. Pediatrics 2007;120:372‐80. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Kluckow M, Evans N. Randomized trial of dobutamine and dopamine in preterm infants with low systemic blood flow. Pediatric Research 2000;47:422A. [DOI] [PubMed] [Google Scholar]
- Osborn DA, Kluckow M, Evans N. Randomized trial of dobutamine and dopamine in preterm infants with low systemic blood flow. Proceedings Perinatal Society of Australia and New Zealand 4th Annual Congress. 2000:85.
- Osborn DA, Kluckow M, Evans N. Randomized trial of dobutamine versus dopamine in preterm infants with low systemic blood flow ‐ 3 year follow up. Proceedings Perinatal Society of Australia and New Zealand 9th Annual Congress. 2005:All. [DOI] [PubMed]
References to studies excluded from this review
Bourchier 1997 {published data only}
- Bourchier D, Weston PJ. Randomised trial of dopamine compared with hydrocortisone for the treatment of hypotensive very low birthweight infants. Archives of Disease in Childhood Fetal Neonatal Ed 1997;76:F174‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cason 1999 {published data only}
- Cason DL, Amaker D, Carter D, Sutherland D, Bhatia J. Randomized double‐blind trial of dopamine versus epinephrine for treatment of hypotension in premature infants with respiratory distress syndrome. Journal of Investigative Medicine 1999;47:119A. [Google Scholar]
DiSessa 1981 {published data only}
- DiSessa TG, Leitner M, Ti CC, Gluck L, Coen R, Friedman WF. The cardiovascular effects of dopamine in the severely asphyxiated neonate. Journal of Pediatrics 1981;99:772‐6. [DOI] [PubMed] [Google Scholar]
- Leitner MJ, DiSessa TG, Coen RW, Ti C. Cardiovascular effects of low dose dopamine in the severely asphyxiated infant. Pediatric Research 1980;14:447A. [DOI] [PubMed] [Google Scholar]
Gill 1993 {published data only}
- Gill AB, Weindling AM. Randomised controlled trial of plasma protein fraction versus dopamine in hypotensive very low birthweight infants. Archives of Disease in Childhood 1993;69:284‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill B, Weindling M. Randomised controlled trial to compare plasma protein fraction (PPF) and dopamine in the hypotensive very low birthweight (VLBW) infant. Pediatric Research 1994;35:273 (Abstract A84). [Google Scholar]
Greenough 1993 {published data only}
- Greenough A, Emery EF. Randomized trial comparing dopamine and dobutamine in preterm infants. European Journal of Pediatrics 1993;152:925‐7. [DOI] [PubMed] [Google Scholar]
Hentschel 1995 {published data only}
- Hentschel R, Hensel D, Brune T, Rabe H, Jorch G. Impact on blood pressure and intestinal perfusion of dobutamine or dopamine in hypotensive preterm infants. Biology of the Neonate 1995;68:318‐24. [DOI] [PubMed] [Google Scholar]
Kawczynski 1996 {published data only}
- Kawczynski P, Piotrowski A. Circulatory and diuretic effects of dopexamine infusion in low‐birth‐weight infants with respiratory failure. Intensive Care Medicine 1996;22:65‐70. [DOI] [PubMed] [Google Scholar]
Klarr 1994 {published data only}
- Klarr JM, Faix RG, Pryce CJ, Bhatt‐Mehta V. Randomized, blind trial of dopamine versus dobutamine for treatment of hypotension in preterm infants with respiratory distress syndrome. Journal of Pediatrics 1994;125:117‐22. [DOI] [PubMed] [Google Scholar]
Lundstrom 2000 {published and unpublished data}
- Lundstrom K, Pryds O, Greisen G. The haemodynamic effects of dopamine and volume expansion in sick preterm infants. Early Human Development 2000;57:157‐63. [DOI] [PubMed] [Google Scholar]
- Lundstrom KE. A randomized, controlled study of the influence of dopamine and volume expansion on CBF, LVO and MABP in hypotensive, preterm infants. Proceedings of 14th European Congress of Perinatal Medicine, Helsinki, Finland 1994:500. [Google Scholar]
Padbury 1990 {published data only}
- Padbury JF, Agata Y, Baylen BG, Ludlow JK, Polk D, Habib DM, Martinez AM. Pharmacokinetics of dopamine in critically ill newborn infants. Journal of Pediatrics 1990;117:472‐6. [DOI] [PubMed] [Google Scholar]
Paradisis 2009 {published data only}
- Paradisis M, Evans N, Kluckow M, Osborn D. Randomized trial of milrinone versus placebo for prevention of low systemic blood flow in very preterm infants. Journal of Pediatrics 2009;154:189‐95. [DOI] [PubMed] [Google Scholar]
- Paradisis M, Jiang X, McLachlan AJ, Evans N, Kluckow M, Osborn D. Population pharmacokinetics and dosing regimen design of milrinone in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92:F204‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pederson 2009 {published data only}
- Pedersen P, Avlund OL, Pedersen BL, Pryds O. Intramuscular adrenaline does not reduce the incidence of respiratory distress and hypoglycaemia in neonates delivered by elective caesarean section at term. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94:F164‐7. [DOI] [PubMed] [Google Scholar]
Pellicer 2005 {published and unpublished data}
- Pellicer A, Bravo MC, Madero R, Salas S, Quero J, Cabanas F. Early systemic hypotension and vasopressor support in low birth weight infants: impact on neurodevelopment. Pediatrics 2009;123:1369‐76. [DOI] [PubMed] [Google Scholar]
- Pellicer A, Valverde E, Elorza MD, Madero R, Gaya F, Quero J, Cababnas F. Randomized blinded controlled trial on the effects on brain hemodynamics of dopamine v epinephrine for inotropic support in preterm infants. Pediatric Research 2003;53:Abstract 2364. [Google Scholar]
- Pellicer A, Valverde E, Elorza MD, Madero R, Gaya F, Quero J, et al. Cardiovascular support for low birth weight infants and cerebral hemodynamics: a randomized, blinded, clinical trial. Pediatrics 2005;115:1501‐12. [DOI] [PubMed] [Google Scholar]
- Valverde E, Pellicer A, Madero R, Elorza D, Quero J, Cabañas F. Dopamine versus epinephrine for cardiovascular support in low birth weight infants: analysis of systemic effects and neonatal clinical outcomes. Pediatrics 2006;117:e1213‐22. [DOI] [PubMed] [Google Scholar]
Phillipos 1996 {published data only}
- Phillipos EZ, Barrington KJ, Robertson MA. Dopamine versus epinephrine for inotropic support in the neonate: A randomized double blinded controlled trial. Pediatric Research 1996;39:238A. [Google Scholar]
Phillipos 2000 {published data only}
- Phillipos EZ, Robertson MA. A randomized double blinded controlled trial of dopamine versus epinephrine for inotropic support in premature infants <1750 grams. Pediatric Research 2000;47:425A. [Google Scholar]
Rennie 1989 {published data only}
- Rennie JM. Cerebral blood flow velocity variability after cardiovascular support in premature babies. Archives of Disease in Childhood 1989;64:897‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
Repetto 1999 {published data only}
- Repetto JE, Eyal FG, McHargue L, Alpan G. High dose dopamine for the treatment of neonatal hypotension. Pediatric Research 1999;45:221A. [Google Scholar]
Roze 1993 {published data only}
- Roze JC, Tohier C, Maingueneau C, Lefevre M, Mouzard A. Response to dobutamine and dopamine in the hypotensive very preterm infant. Archives of Disease in Childhood 1993;69:59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2009 {published data only}
- Wong FY, Barfield CP, Horne RS, Walker AM. Dopamine therapy promotes cerebral flow‐metabolism coupling in preterm infants. Intensive Care Medicine 2009;35:1777‐82. [DOI] [PubMed] [Google Scholar]
Additional references
Evans 1996
- Evans N, Kluckow M. Early determinants of right and left ventricular output in ventilated preterm infants. Archives of Disease in Childhood Fetal and Neonatal Edition 1996;74:F88‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Hunt 2001
- Hunt RW, Evans NJ, Rieger I, Kluckow MR. Low superior vena cava flow in the first 24 hours of life and 3 year neurodevelopmental outcome. Pediatric Research 2001;49:336A. [Google Scholar]
Kluckow 1996
- Kluckow M, Evans N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. Journal of Pediatrics 1996;129:506‐12. [DOI] [PubMed] [Google Scholar]
Kluckow 2000
- Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Archives of Disease in Childhood Fetal and Neonatal Edition 2000;82:F188‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kluckow 2000b
- Kluckow M, Evans N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Archives of Disease in Childhood Fetal and Neonatal Edition 2000;82:F182‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kluckow 2001
- Kluckow M, Evans N. Low systemic blood flow and hyperkalemia in preterm infants. Journal of Pediatrics 2001;139:227‐32. [DOI] [PubMed] [Google Scholar]
Lopez 1997
- Lopez SL, Leighton JO, Walther FJ. Supranormal cardiac output in the dopamine‐ and dobutamine‐dependent preterm infant. Pediatric Cardiology 1997;18:292‐6. [DOI] [PubMed] [Google Scholar]
Meek 1999
- Meek JH, Tyszczuk L, Elwell CE, Wyatt JS. Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Archives of Disease in Childhood Fetal and Neonatal Edition 1999;81:F15‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miall‐Allen 1987
- Miall‐Allen VM, Vries LS, Whitelaw AG. Mean arterial blood pressure and neonatal cerebral lesions. Archives of Disease in Childhood 1987;62:1068‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2001
- Osborn DA, Evans N. Early volume expansion versus inotrope for prevention of morbidity and mortality in very preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD002056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2002b
- Osborn D, Evans N, Kluckow M. Left ventricular (LV) contractility and wall stress in very preterm infants in the first day of life. Pediatric Research 2002;51:386A. [DOI] [PubMed] [Google Scholar]
Osborn 2003
- Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late peri/intraventricular hemorrhage in premature infants. Pediatrics 2003;112:33‐9. [DOI] [PubMed] [Google Scholar]
Osborn 2004a
- Osborn DA, Evans N, Kluckow M. Clinical detection of low upper body blood flow in very premature infants using blood pressure, capillary refill time, and central‐peripheral temperature difference. Archives of Disease in Childhood Fetal and Neonatal Edition 2004;89:F168‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2004b
- Osborn D, Evans N, Kluckow M. Diagnosis and treatment of low systemic blood flow in preterm infants. NeoReviews 2004;5:109‐21. [Google Scholar]
Osborn 2004c
- Osborn DA, Evans N. Early volume expansion for prevention of morbidity and mortality in very preterm infants. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD002055.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Padbury 1987
- Padbury JF, Agata Y, Baylen BG, Ludlow JK, Polk DH, Goldblatt E, Pescetti J. Dopamine pharmacokinetics in critically ill newborn infants. Journal of Pediatrics 1987;110:293‐8. [DOI] [PubMed] [Google Scholar]
Seri 1998
- Seri I, Abbasi S, Wood DC, Gerdes JS. Regional hemodynamic effects of dopamine in the sick preterm neonate. Journal of Pediatrics 1998;133:728‐34. [DOI] [PubMed] [Google Scholar]
Seri 2001
- Seri I. Circulatory support of the sick preterm infant. Seminars in Neonatology 2001;6:85‐95. [DOI] [PubMed] [Google Scholar]
Subhedar 2003
- Subhedar NV, Shaw NJ. Dopamine versus dobutamine for hypotensive preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001242] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Osborn 2007
- Osborn DA, Paradisis M, Evans NJ. The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD005090.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


