Abstract
The COVID-19 pandemic has changed routine care practice for older persons, especially in those with frailty living in long term care (LTC) facilities. Due to the high mortality rates of Nursing home (NH) residents during the first wave of the COVID-19 pandemic, priority for COVID-19 vaccinations was given to this vulnerable population. However, the safety and efficacy of such vaccines in older frail elders remains questionable due to the fact that initial randomized clinical trials (RCTs) for such vaccines did not include this population. This type of discrimination in patient participation in RCTs continues and has been recognized in the literature. Nevertheless, in the context of a worldwide emergency, COVID-19 vaccination in older persons living in LTC facilities may provide a solid basis to protect against negative outcomes, such as COVID-19 infection and death. In this report, we present the protocol of the GeroCovid Vax study, an Italian study that began in February 2021 which is aimed at investigating the safety and efficacy of the anti-SARS-CoV-2 vaccinations in older persons living in LTCs. This protocol specially aims to continuously and closely monitor events related to- and following- the anti-SARS-CoV-2 vaccination in elderly living in LTC facilities. In this report, we will provide information related to the study protocol and describe baseline characteristics of the sample.
Keywords: GeroCovid Vax, COVID-19 vaccine, Nursing homes (NH), Long term care (LTC), Frailty syndrome, Elderly, Safety
1. Introduction
The impact of COVID-19 pandemic has held high mortality rates in older Long Term Care (LTC) residents worldwide. In a recent meta-analysis using worldwide data in approximately 50 studies on four continents, age care facility residents showed an attack rate of 45% for SARS-CoV-2 infection, with a death rate of 23% [1] . Italy was one of the first countries to embrace the impact of COVID-19 infection in older persons. In fact, in the first epidemic rate, in March 2020, death rate reached 33.4% in nursing home (NH) residents with symptoms compatible with COVID-19 [2]. Common comorbidities were shown to have a relevant impact on survival of patients with COVID-19 and older adults had the highest case-fatality rate [3], [4]. LTC facilities not only host vulnerable individuals with advanced age and high comorbidity burden, but also represent living environments (group gatherings, more than one person per room, etc.) that can facilitate the spread of COVID-19 infection. Indeed, LTC facilities continuously adapt protective measures against the pandemic war of SARS-CoV-2 (limiting family visits, group gatherings, constant swab testing for infection, use of personal protection equipment). The American Geriatrics Society has indicated four recommendations in regards to elderly living in long term care environments: i) defense against infection, ii) COVID-19 testing and contact tracing, iii) safe transitions of COVID-19 patients, iv) infection control [5]. The European Geriatric Medicine Society (EuGMS) has already launched an interim guidance program aiming to prevent the entrance and spread of COVID-19 into long-term care facilities (LTCFs) [6] . At the moment, there is an urgent priority to assess safety and effectiveness of vaccines especially in comorbid elderly living in LTCF environments.
It is not known to which extent anti-SARS-CoV-2 vaccination protects LTC residents, who are generally frail, older, and have more comorbidities than the general adult population. At the moment, available literature regarding the efficacy and vaccine protection in LTC residents is limited, which may be due to the lack of anti-SARS-CoV-2 vaccine clinical trials in this vulnerable group. Therefore, there is limited knowledge regarding immune response following such vaccines in older frail individuals. A suboptimal response has been suggested in the response to the BNT162b2 mRNA vaccine compared to a control group in the same facility [7]. However, it still remains unclear if there is added immune protection following vaccination in those that had a previous COVID-19 infection.
Currently, the use of second and booster doses remains an important question in the strategy for preventing COVID-19 infection in this population on a worldwide scale. Further investigations are needed to emphasize the absolute necessity of a booster dose as larger sample sizes become available.
Based on this lack of evidence, the Italian Medicines Agency (AIFA) sponsored the GeroCovid Vax study, which is aimed at investigating the safety and effectiveness (including immunity protection duration) of anti-SARS-CoV-2 vaccines, as well as monitoring clinical changes over a 1-year observational period at specific timed intervals in a large population of Italian LTC residents. The study began in February 2021 and in this report, we describe the study protocol and present baseline characteristics of the study sample.
2. Methods
GeroCovid VAX study is promoted by the Italian Society of Gerontology and Geriatrics (SIGG) (Florence, Italy) and the Italian National Institute of Health (Istituto Superiore di Sanità (ISS)) (Rome, Italy) and sponsored by AIFA. GeroCovid Vax study began in February 2021 and is an ongoing multicenter prospective study investigating effects of anti-SARS-CoV-2 vaccine use in elderly residents living in Italian LTC centers, specifically NHs and Assisted living centers [8]. Main study objectives include: i) evaluating efficacy and safety of the vaccine in representative sample of elderly NH residents, and ii) evaluate humoral and cellular immune response in a large subpopulation of study participants. Following the 1st dose of anti-SARS-CoV-2 vaccine, protocol also assesses: i) clinical and functional changes over time following the 1st dose of vaccination, ii) negative clinical outcomes (adverse effects related to vaccination, death, hospitalization, COVID-19 infection). As previously mentioned, the study began in February 2021, and participant enrollment ended on June 30th, 2021. Clinical and immune response, clinical observations and negative health outcomes are still under current collection. Any adverse events from anti-SARS-CoV-2 vaccines are also being registered, as well as any changes in routine blood parameters.
2.1. Study population
The inclusion criteria included: age ≥ 60 years, life expectancy ≥ 3 months, expected Long term care residency ≥ 3 months, undergone at least one dose of any type of anti-SARS-CoV-2 vaccination. Exclusion criteria included: age < 60 years, life expectancy < 3 months, ongoing positive COVID-19 infection, and nursing home discharge planned < 3 months. A signed informed consent form by the participant or his/her proxy was necessary before enrolling in the study, which was approved by the Ethical Committee at the Spallanzani Institute (Rome, Italy (protocol 264_2021).
2.2. Data collection
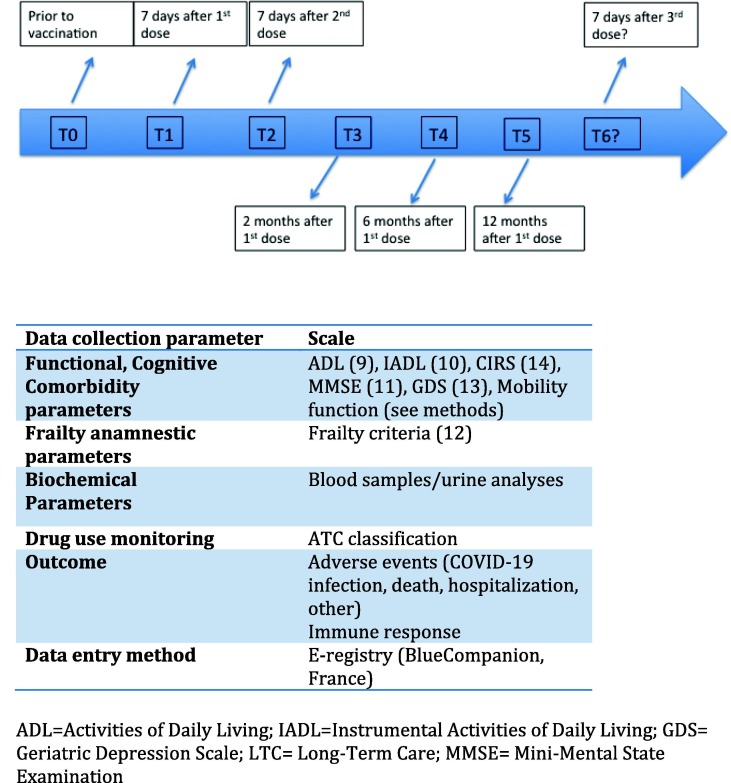
Data collection includes demographic characteristics, lifestyle, mobility function, physical activity, chronic comorbidities, regular pharmacological treatments, nutritional status, frailty status, previous COVID-19 infection, previous anti-influenza, -pneumococcal, and –herpes zoster vaccinations. In particular, data regarding the Activities of Daily Living (ADL) [9], Instrumental Activities of Daily Living (IADL) [10], global cognitive performance with the Mini Mental State Examination (MMSE) [11], Frailty Syndrome (using the Anamnestic modified version) [12], Depression with the Geriatric Depression Scale (GDS) [13] and chronic comorbidities with Cumulative Illness Rating Scale (CIRS) scores [14], as well as routine laboratory analyses are under evaluation (Fig. 2a). We also collected data regarding mobility function (walk independently, walk with a cane, walk with a walker, move around in a wheelchair, is accompanied in a wheelchair, mostly bedridden however sometimes sits in a wheelchair, completely bedridden). Data regarding negative clinical outcomes (adverse effects following vaccination, COVID-19 infection, death and hospitalization) and changes in clinical and biological parameters following the first dose of COVID-19 vaccination are also currently under collection. The GeroCovid VAX study is based on dedicated e-data collection quality (see ahead). Trained physicians or staff use an e-registry platform for data entry completion.
Fig. 2a.
Timeline description for Clinical GeroCovid Vax data collection and parameters at each observation follow up.
2.3. Timeline for data collection
The initial study observation period is planned to last at least 12 months. Data collection includes demographical, clinical, and routine laboratory data if available before and after vaccination doses. The study timeline is specifically aimed at studying all clinical and blood/urine parameters at baseline at timed intervals (Fig. 1 ). In particular, clinical data were collected at baseline (7 days prior to the first dose of vaccine). Follow-up data collections include 7 days following the first dose, 2-, 6- and 12- month intervals following the first dose (Fig. 2a ).
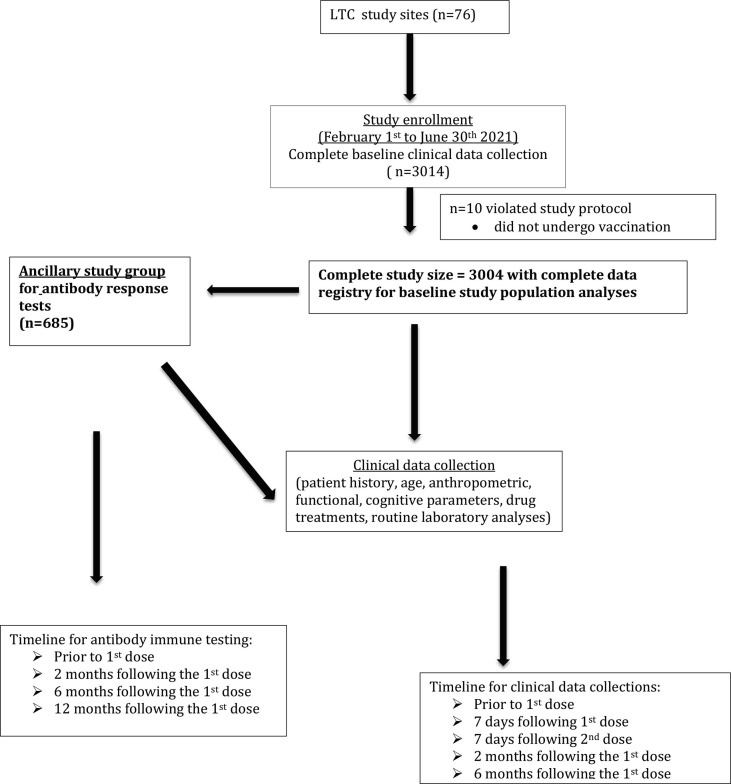
Fig. 1.
Participant recruitment and follow-up flow diagram.
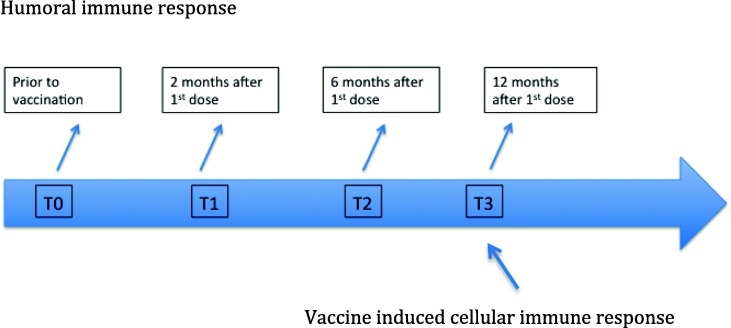
As previously indicated, a subsample group will undergo both humoral and cellular immune response at timed intervals (Fig. 2b ) from the first dose and all sample testing will be performed at a centralized laboratory. In a subsample of LTC residents, in addition to clinical data collections, blood samples are being analyzed for humoral response at baseline, 2-, 6- and 12 months following the first dose (Fig. 2b). All blood samples are properly stored and shipped to a centralized laboratory of the Infective Disease Department at the ISS (Rome, Italy). Anti-S IgG levels will be quantified using a commercial CLIA assay (Liaison SARS-CoV-2 trimeric S IgG assay; Diasorin, Italy). This assay was chosen since it displays a high concordance with neutralizing antibodies titers. In order to check for exposition to SARS-CoV-2, the presence or absence of anti-Nucleocapsid (N) IgG will be checked at each time-point by every peripheral center at time of serum collection. Vaccine-induced cellular immune response will be assessed in a subsample of forty individuals twelve months after the vaccination (T3). The sample population will include 20 individuals who displayed high anti-S IgG levels at T2 and 20 who were anti-S IgG negative/low responders. PBMCs will be purified from peripheral blood, frozen and shipped to the ISS laboratory. A standardized intracellular staining protocol will be used to measure anti-S and anti-N specific cytokines (IFN-g, TNF-a, IL-2) produced by T CD4 and T CD8 cells.
Fig. 2b.
Timeline description.
2.4. E-Registry of clinical data collection
Clinical data collection of GeroCovid VAX uses the same platform as the GeroCovid Observational study provided by the Bluecompanion company (France) and has been previously described in the literature [15]. In particular, the GeroCovid e-Registry was adapted from an existing electronic platform that Bluecompanion developed in 2018 for a project called e-Trajectories. In March 2020, in conjunction with the COVID-19 pandemic, Bluecompanion provided their health data collection system for the GeroCovid initiative. E-Trajectories and GeroCovid adaptation are based on the CleanWeb engine produced by Telemedicine Technologies (Boulogne-Billancourt, France), embedded in a dedicated web platform designed for integrating data from different sources [16]. All data for GeroCovid VAX are recorded on web servers located throughout Italy. ICT operations are compliant with the European General Data Protection Regulation (GDPR) and with the relevant international standards for clinical trials (ISO 9001 certification and FDA CFR 21 part 11). The platform was developed in collaboration with technical-scientific team of Bluecompanion and the GeroCovid coordinators with the goal of capturing the complexity of the geriatric patient. In addition, Bluecompanion continuously provides training sessions for investigators from each investigational site, as well as technical support. Data collection is under constant quality control and investigators are required, when necessary to verify data entry. All individual clinical data are anonymized before data entry.
2.5. Sample size calculation for immune response and statistical analysis
For the humoral response study protocol, we aimed to compare data collected in the present study with those collected in a sample of healthy adults aged < 65 years collected in a national project as an ancillary part of the study. Sample size calculation performed with an α = 0.05% and a β = 0.80 was found to be at least 292 participants in order to prove a significant difference in antibody response of 15% between the two samples. Considering a drop out rated of 55% in the LTC sample, we considered a sample size of at least 649 LTC residents to be enrolled. Such a relevant drop out rate is due to the high 1-year mortality rate observed in LTC residents in Italy [17] and to the fact that LTC residents are often transferred in other settings (hospital or home). Overall, 685 participants were enrolled for this ancillary study.
Descriptive results of variables are presented as means ± standard deviation (SD) or percentages. Analysis of variance (ANOVA) and χ2 test were performed to evaluate differences in clinical characteristics among groups defined according to age (60–75, 76–85, 86 + years). Statistical analyses were performed using SPSS software (SPSS, Inc., Chicago, IL).
3. Results
At the moment, data entry has been collected in 76 LTC centers over Italy and baseline data has been completed in 3014 participants. 10 participants were excluded due to the lack of undergoing the first dose of vaccination. In the present report, descriptive analyses have been performed using available baseline data as of September 12, 2021 and consisted of 3004 participants (Fig. 1); however, data entry related to patients’ enrollment is still undergoing and therefore baseline numbers may change over the next few months. In addition, of the 3004 study participants, 685 have undergone blood tests for immune response analyses (Fig. 1). As of June 30th 2021, 92.8% of the older population living in LTCF has been vaccinated with BNT162b2 and 7.2% with mRNA-1273 (Table 1 ). Clinical characteristics according to age groups are shown in Table 2 . We found that in the younger age groups, there was a significantly higher prevalence of the anti-pneumococcal vaccination compared to the other two age groups. There was not difference in the prevalence of the use of the seasonal flu vaccine across age groups. Chronic comorbidities and frailty syndrome were found to be significantly more prevalent in the oldest age group (Table 2). Potential adverse events related to anti-SARS-CoV-2 vaccines and measured during the study follow-up are listed in Box 1 .
Table 1.
Distribution of Covid vaccines in the Gerocovid Vax Study population (n = 3004).
| N (%) | M/F | Age men yrs (mean (SD)) | Age women yrs (mean (SD)) | |
|---|---|---|---|---|
| BNT162b2 (BioNTech-Pfizer) |
2796 (92.8) | 866/2138 | 79 (9) | 85 (9) |
| mRNA-1273 (Moderna) |
207 (7.2) | 55/152 | 80 (9) | 86 (8) |
| ChAdOx1/nCoV-19 (Vaxrevia) |
1 (0) | 1/0 | 73 | |
| Race | ||||
| White | 2940 (97.9) | 849/2091 | ||
| Black | 18 (0.6) | 1/17 | ||
| Asian | 4 (0.1) | 2/2 | ||
| Other/unknown | 42 (1.4) | 14/28 |
Table 2.
Baseline Clinical Characteristics of GeroCovid Vax study participants according to age group (n = 3004).
| Clinical characteristics | Total |
60–75 yrs |
76–85 yrs |
≥86 yrs |
p |
|---|---|---|---|---|---|
| (n = 3004) | (n = 626) | (n = 1000) | (n = 1378) | ||
| Age, mean (SD) | 83.1 (9.2) | 68.9 (4.6) | 81.3 (2.7) | 91.0 (3.6) | |
| M/F | 866/2138 | 314/312 | 285/715 | 267/1111 | 0.001 |
| Previous COVID-19 infection (n,%) | 833, 27.7 | 153, 24.4 | 271, 27.1 | 409, 29.7 | 0.001 |
| Seasonal Flu vaccine (n,%) | 1900, 63.2 | 403, 64.4 | 619, 61.9 | 878, 63.7 | 0.341 |
| Pneumococcal vaccine (n,%) | 783, 26.1 | 176, 28.1 | 257, 25.7 | 350, 25.4 | 0.010 |
| Number of comorbidities, *% | 0.012 | ||||
| 1–2 | 23.7 | 26.6 | 22.0 | 23.5 | |
| 3–4 | 32.6 | 36.8 | 33.0 | 30.3 | |
| ≥5 | 43.7 | 36.6 | 45.0 | 46.2 | |
| Mobility function* (n, %) | <0.001 | ||||
| Walk | 1140, 41.4 | 329, 55.7 | 399, 42.9 | 412, 33.3 | |
| Does not walk | 1210, 43.9 | 182, 30.8 | 379, 40.8 | 649, 52.5 | |
| Bedridden | 406, 14.7 | 80, 13.5 | 151, 16.3 | 175, 14.2 | |
| Frailty syndrome*, % | 18.80% | 11.80% | 19.50% | 21.70% | <0.001 |
(* = valid percent reported excluding missing variables).
Box 1. Potential Adverse Events after 1st and 2nd dose of anti-SARS-CoV-2 vaccines.
Fever.
Low grade Fever.
Pain and swelling at the injection site.
Headache.
Chills.
Difficulty breathing.
Sneezing.
Cough.
Raynaud syndrome (at room temperature)
Muscle weakness.
Muscles and joints pain.
Site injection itching.
Swollen lymph nodes.
Site injection redness.
Insomnia.
Fast Heartbeat.
Anorexia.
Nausea or Vomiting.
Delirium (hyperkinetic ; hypokinetic)
Increased blood pressure.
Cutaneous rash.
Acute peripheral facial paralysis.
Myelitis transversa.
Anaphylaxis.
Diarrhea.
Weakness/Prostration.
Confusion.
Dizziness.
Guillain-Barrè Syndrome.
Other.
4. Discussion
The GeroCovid Vax study will provide a solid response to the efficacy and safety of the anti-SARS-CoV-2 vaccine implementation in older persons living in LTC facilities. This unique protocol comprises a large representative population of older LTC residents (n = 3004). Baseline data underline over 90% use of the BNT162b2 vaccine compared to the mRNA-1273 or ChAdOx1/nCoV-19 in the study population. In addition, baseline data show that older residents (over 86 years) were more likely to be frail, to have higher number of comorbidities and to have had a previous infection for COVID-19 before vaccination.
At the present, anti-SARS-CoV-2 vaccines remain essential to protecting against COVID-19 infection and mortality. However, older frail NH residents may be less immunogenic to such vaccines and there is a lack of clinical trial data regarding their use in such a vulnerable population. One report tested for antibody levels after the BNT162b2 mRNA vaccine to spike, receptor bindging domain (RBD) and virus neutralization in 149 older NH residents [7]. Overall, they found a significantly lower immune response to the BNT162b2 mRNA vaccine compared to health care workers (control group). Another report tested for both humoral (antibodies against the S1 subunit of spike protein) and cellular (SARS-CoV-2 antigen) immune responses in 64 NH residents with antibodies against SARS-CoV-2 nucleocapsid and 46 residents without antibodies four weeks following the first vaccine dose [18]. This study also indicated a suboptimal response to the BNT162b2 mRNA vaccine in COVID-19 naïve NH residents compared to controls (COVID-19 naïve healthcare workers). Interestingly, their findings indicated a similar response in COVID-19 experienced residents and COVID-19 naïve healthcare workers. Longer prospective studies with large study populations are necessary to reveal if there is only a delay or a quantitative lower immune response in order to adapt vaccination approaches in this frail population. Findings from a retrospective cohort analysis demonstrated that partial vaccination with the BNT162b2 vaccine was associated with a significant reduction in the risk for SARS-CoV-2 infection among NH residents [19]. Another study reported more robust protection after the second dose of the same vaccine in a comparable older adult population [20], thus indicating that complete 2-dose vaccination is an important strategy for preventing COVID-19 in this disproportionately affected population. Further study of this population should continue as larger sample sizes become available.
Correlates of protection against SARS-CoV-2 infection in humans are not yet established, however it has been shown that the cellular immunity persists in recovered individuals but the significance for protection and susceptibility, respectively, remains unclear [21], [22]. Moreover, considering an expected decline in serum antibody levels, the assessment of cellular immunity twelve months after the vaccination will provide important clues on the persistence of vaccine-induced immunity. The design will also allow to test for longitudinal measurement of anti-N IgG, which are not induced by Spike-containing vaccines, to monitor for possible infection during the study period, and thus provide important data on vaccine efficacy in frail elders in LTC settings during the post-vaccine period.
NH residents accounted for a disproportionate share of COVID-19 deaths worldwide since the beginning of the pandemic. Thirty-day mortality rates from COVID 19 in a large study population of NH residents (n = 12,271) significantly declined from 20.9% to 11.2% (March – November 2020) which may be due to improved clinical management within NHs, as well as improved personal protective equipment supplies [23] . Interestingly, a recent Italian case control study compared older NH patients with a previous COVID-19 infection (n = 76) to controls (n = 76) and found an accelerated age-related deterioration of approximately 20% in physical performance and frailty status of previously infected NH residents compared to controls [24]. The recent call for booster doses remains questionable for all age groups. However, government health organizations are rapidly underlining that such dose seems to be essential in maintaining lower COVID-19 infection rates for frail elders.
One important study limitation concerns the potential heterogeneity of e-data registration due to the large number of study sites. However, the study uses a unique electronic platform that is continuously monitored for correct data entry and allows for immediate statistical response to the predictive role of anti-SARS-CoV-2 vaccines on clinical outcomes (including SARS-CoV-2 infection (vaccine failure), adverse events following vaccine doses, hospitalization and death) and changes in biological measures of cellular immunity over time.
In conclusion, the GeroCovid Vax study will provide essential knowledge and practical implications for an optimal use of anti-SARS-CoV-2 vaccines in frail elders, especially those living in LTC environments.
Study Funding
The GeroCovid Vax study was funded by a grant from the Italian Medicines Agency (AIFA).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the members of the Bluecompanion team: Alessandro Loria, Simonetta Demarie, & Patrizia Angelucci and Stefania Del Signore: Bluecompanion Ltd. London UK.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.02.064.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Hashan M.R., Smoll N., King C., Ockenden-Muldoon H., Walker J., Wattiaux A., et al. Epidemiology and clinical features of COVID-19 outbreaks in aged care facilities: A systematic review and meta-analysis. E Clin Med. 2021;33:100771. doi: 10.1016/j.eclinm.2021.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lombardo F.L., Bacigalupo I., Salvi E., Lacorte E., Piscopo P., Mayer F., et al. The Italian national survey on Coronavirus disease 2019 epidemic spread in nursing homes. Int J Geriatr Psychiatry. 2021;36(6):873–882. doi: 10.1002/gps.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbatecola A.M., Antonelli-Incalzi R. Editorial: COVID-19 Spiraling of Frailty in Older Italian Patients. J Nutr Health Aging. 2020;24(5):453–455. doi: 10.1007/s12603-020-1357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.https://www.epicentro.iss.it/coronavirus/sars-cov-2-strutture-socio-assistenziali-sanitarie: accessed 31 July 2021.
- 5.American Geriatrics Society (AGS) Policy Brief: COVID-19 and Assisted Living Facilities. American Geriatrics Society.J Am Geriatr Soc 2020 Jun;68(6):1131–113. [DOI] [PMC free article] [PubMed]
- 6.Blain H., Rolland Y., Schols J.M.G.A., Cherubini A., Miot S., O'Neill D., et al. Interim EuGMS guidance to prepare European Long-Term Care Facilities for COVID-19. Eur Geriatr Med. 2020;11(6):899–913. doi: 10.1007/s41999-020-00405-z. Epub 2020 Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canaday D.H., Carias L., Oyebanji O.A., Keresztesy D., Wilk D., Payne M., et al. Reduced BNT162b2 mRNA vaccine response in SARS-CoV-2-naive nursing home residents. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab447. May 16:ciab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbatecola Angela Marie, Antonelli-Incalzi Raffaele, Malara Alba, et al. Disentangling the impact of COVID-19 Infection on Clinical Outcomes and Preventive Strategies in Older persons: an Italian perspective. Journal of Gerontology and Geriatrics. 2021 doi: 10.36150/2499-6564-N440. In press. [DOI] [Google Scholar]
- 9.Katz S., Ford A.B., Moskowitz R.W., et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 10.Lawton M.P., Brody E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3 Part 1):179–186. [PubMed] [Google Scholar]
- 11.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Pedone C., Costanzo L., Cesari M., Bandinelli S., Ferrucci L., Antonelli Incalzi R. Are Performance Measures Necessary to Predict Loss of Independence in Elderly People? J Gerontol A Biol Sci Med Sci. 2016;71(1):84–89. doi: 10.1093/gerona/glv096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheikh J.I., Yesavage J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 14.Parmelee P.A., Thuras P.D., Katz I.R., Lawton M.P. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130–137. doi: 10.1111/j.1532-5415.1995.tb06377.x. [DOI] [PubMed] [Google Scholar]
- 15.Trevisan C., Del Signore S., Fumagalli S., Gareri P., Malara A., Mossello E., et al. GeroCovid Working Group. Assessing the impact of COVID-19 on the health of geriatric patients: The European GeroCovid Observational Study. Eur J Intern Med. 2021;87:29–35. doi: 10.1016/j.ejim.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.https://www.bluecompanion.eu/ accessed July 1, 2021.
- 17.Onder G., Carpenter I., Finne-Soveri H., Gindin J., Frijters D., Henrard J.C., et al. Assessment of nursing home residents in Europe: the Services and Health for Elderly in Long TERm care (SHELTER) study. BMC Health Serv Res. 2012;12(1) doi: 10.1186/1472-6963-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Praet J.T., Vandecasteele S., De Roo A., De Vriese A.S., Reynders M. Humoral and cellular immunogenicity of the BNT162b2 mRNA Covid-19 Vaccine in nursing home residents. Clin Infect Dis. 2021:ciab300. doi: 10.1093/cid/ciab300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Britton A., Jacobs Slifka K.M., Edens C., Nanduri S.A., Bart S.M., Shang N., et al. Effectiveness of the Pfizer-BioNTech COVID-19 Vaccine Among Residents of Two Skilled Nursing Facilities Experiencing COVID-19 Outbreaks - Connecticut, December 2020-February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(11):396–401. doi: 10.15585/mmwr.mm7011e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breton G., Mendoza P., Hägglöf T., Oliveira T.Y., Schaefer-Babajew D., Gaebler C., et al. Persistent cellular immunity to SARS-CoV-2 infection. J Exp Med. 2021;218(4) doi: 10.1084/jem.20202515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shrotri M., van Schalkwyk M.C.I., Post N., Eddy D., Huntley C., Leeman D., et al. T cell response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE. 2021;16(1):e0245532. doi: 10.1371/journal.pone.0245532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosar C.M., White E.M., Feifer R.A., Blackman C., Gravenstein S., Panagiotou O.A., et al. COVID-19 Mortality Rates Among Nursing Home Residents Declined From March To November 2020. Health Aff (Millwood) 2021;40(4):655–663. doi: 10.1377/hlthaff.2020.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greco G.I., Noale M., Trevisan C., Zatti G., Dalla Pozza M., Lazzarin M., et al. Increase in Frailty in Nursing Home Survivors of Coronavirus Disease 2019: Comparison With Noninfected Residents. J Am Med Dir Assoc. 2021;22(5):943–947.e3. doi: 10.1016/j.jamda.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.