Abstract
Background
COVID-19 is associated with androgenetic alopecia (AGA), telogen effluvium (TE), and alopecia areata (AA). No studies have analyzed the aggregate data to date.
Objective
We conducted a systematic review to characterize the types, incidence, timing, and clinical outcomes of COVID-19–associated alopecia.
Methods
We searched PubMed/MEDLINE, Scopus, and Embase for articles published between November 2019 and August 2021 using the key words “alopecia” or “hair” and COVID-19–related search terms, identifying 41 original articles describing patients with alopecia and COVID-19.
Results
The current review included 1826 patients with alopecia and COVID-19 (mean age, 54.5 years; 54.3% male). The most common types of alopecia identified were AGA (30.7%, 86.4% male), TE (19.8%, 19.3% male), and AA (7.8%, 40.0% male). AGA preceded COVID-19 symptoms. TE was usually newly triggered by COVID-19 (93.6%). AA usually occurred in patients with preexisting disease (95.1%).
Limitations
Definitions of COVID-19 onset varied. Studies differed in methodology and were susceptible to reporting and sampling bias. Studies with large sample sizes may exert a disproportionate influence on data.
Conclusion
AGA may be a risk factor for severe COVID-19, whereas TE presents as a sequela of COVID-19. AA generally occurs as a relapse in patients with preexisting alopecia.
Key words: alopecia areata, alopecia, anagen effluvium, androgenetic alopecia, coronavirus disease 2019, COVID-19, hair loss, SARS-CoV-2, telogen effluvium
Abbreviations used: AA, alopecia areata; ADT, androgen-deprivation therapy; AE, anagen effluvium; AGA, androgenetic alopecia; PA, pressure-induced alopecia; TE, telogen effluvium
Capsule Summary.
-
•
Telogen effluvium and alopecia areata may be associated with COVID-19, while adrogenetic alopecia may be associated with severe infection.
-
•
Patients with COVID-19 and androgenetic alopecia may benefit from antiandrogen therapy, though further research is needed.
Introduction
SARS-CoV-2, the causative agent of the COVID-19 pandemic, has given rise to a global health emergency. Although dermatologic signs1 of COVID-19 have been described, considerably more attention has been directed toward skin-related, rather than hair-related, manifestations.2,3
Recent observational reports have documented associations between COVID-19 and various types of alopecia, including androgenetic alopecia (AGA), alopecia areata (AA), telogen effluvium (TE), anagen effluvium (AE), and pressure-induced alopecia (PA). Mechanisms of these associations are not entirely clear but are believed to be multifactorial; hair loss, like other cutaneous manifestations of COVID-19, may be related to various virus-induced or delayed immunologic responses to infection.2,3
Given the growing number of reports documenting associations between COVID-19 and certain types of alopecia, we sought to summarize these findings in a systematic review and meta-analysis. Recently published review articles have summarized findings from reports documenting associations between COVID-19 and AGA; however, no reviews have pooled data from all types of COVID-19–associated alopecia.4, 5, 6 To our knowledge, this article is the first comprehensive review to include all published studies describing hair-related manifestations of COVID-19. In this report, we have summarized the demographic information of affected patients and the types, incidence, timing, and clinical outcomes of types of alopecia associated with COVID-19.
Methods
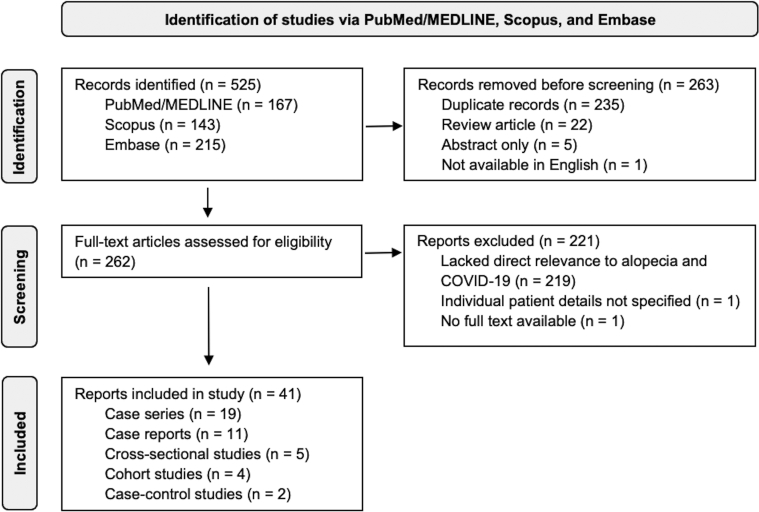
A flowchart summarizing the steps for study identification according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines is shown in Figure 1. We searched PubMed/MEDLINE, Scopus, and Embase for articles available in English from November 1, 2019, through August 31, 2021, using the key words “alopecia” or “hair” and COVID-19–related search terms adapted from the Medical Library Association Clinical Librarians Caucus’ COVID-19 hedge (search terms will be available upon request), yielding 525 articles. Broad search terms were intentionally used to minimize the chance of excluding relevant studies. A reviewer (BN) screened the articles on the basis of titles and abstracts to remove the duplicate, abstract-only, non-English, and review articles, yielding 262 articles. Articles were further excluded if they had no full text available, did not specify individual patient details, or lacked direct relevance to alopecia and COVID-19. After these exclusions, 41 reports (19 case series, 11 case reports, 5 cross-sectional studies, 4 cohort studies, and 2 case-control studies) were included in this review. When available, information collected from each article included the country of the patient population, type of study, the incidence of alopecia, mean age of patients, sex of patients, and survival rate. Data were further characterized on the basis of whether patients had new-onset alopecia or exacerbation of a preexisting alopecia diagnosis.
Fig 1.
Flowchart of study identification via PubMed/MEDLINE, Scopus, and Embase according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Results
From the 41 articles included in this review, we identified 1826 patients with alopecia and COVID-19.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47 Identifying information of each study (article title, author, country, and study type) and patient information (age, sex, alopecia incidence, alopecia type, and survival rate) are shown in Table I. When reported, the mean age of patients was 54.5 years (range, 7-100 years), with a slight male predominance (54.3%). Of the 1826 patients, age and sex were not reported in 1056 (57.8%) and 709 (38.8%) patients, respectively. A total of 17 distinct countries were represented in the patient populations of the included studies, with no clear geographic patterns apparent.
Table I.
Characteristics and findings of 41 original articles reporting alopecia in 1826 patients with COVID-19
| Title | Author; country of patient population | Type of study, number of alopecia cases (n)/number of COVID-19–positive patients, age (y), (sex, male:female) | Type of alopecia | Clinical outcomes |
|---|---|---|---|---|
| Time of onset and duration of post-COVID-19 acute telogen effluvium | Abrantes; United States, Brazil, Spain7 | Case series: n = 30/30 Mean age: 40.5 (9 M: 21 F) |
PA: 1 TE: 29 |
30 (100%) survival |
| Rapidly progressive alopecia areata totalis in a COVID-19 patient, unresponsive to tofacitinib | Berbert; Brazil8 | Case report: n = 1/1 Age: 24 (0 M: 1 F) |
AE: 1 | 1 (100%) survival |
| Alopecia areata in a COVID-19 patient: a case report | Capalbo; Italy9 | Case report: n = 1/1 Age: 38 (1 M: 0 F) |
AA: 1 | 1 (100%) survival |
| Clinical characteristics and outcomes of adult patients admitted with COVID-19 in East London: a retrospective cohort analysis | Cheng; United Kingdom10 | Cohort study: n = 9/139 Age: NR (Sex NR) |
Unclassified: 9 | 9 (100%) survival |
| COVID-19: association with rapidly progressive forms of alopecia areata | Di Landro; Italy11 | Case series: n = 39/39 Mean age: 64.6 (9 M: 30 F) |
TE: 39 | 39 (100%) survival |
| The impact of individual lifestyle and status on the acquisition of COVID-19: a case-control study | Flvenson; United States12 | Case series, n = 1/1 Age: 56 (0 M: 1 F) |
AA (universalis): 1 | 1 (100%) survival |
| A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain—a potential clue to the role of androgens in COVID-19 severity | Gao; China13 | Case-control study, n = 30/105 Mean age: 55 (Sex NR) |
Unclassified: 30 | 30 (100%) survival |
| Different hair loss patterns in two pediatric patients with COVID-19-associated multisystem inflammatory syndrome in children | Goren; Spain14 | Case series, n = 41/41 Mean age: 58 (41 M: 0 F) |
AGA: 41 | NR |
| Pathobiology questions raised by telogen effluvium and trichodynia in COVID-19 patients | Hayran; Turkey15 | Case series: n = 2/2 Mean age: 11.5 (2 M: 0 F) |
AA: 1 TE: 1 |
2 (100%) survival |
| Male balding is a major risk factor for severe COVID-19 | Lee; United Kingdom16 | Case-control study, n = 274/336 Mean age: 59.0 (274 M: 0 F) |
AGA: 274 | NR |
| A case of acute telogen effluvium after SARS-CoV-2 infection | Lv; China17 | Case report: n = 1/1 Age: 38 (0 M: 1 F) |
TE: 1 | 1 (100%) survival |
| Acral rash in a child with COVID-19 | Mazzotta; Italy18 | Case report: n = 1/1 Age: 9 (1 M: 0 F) |
AA (universalis): 1 | 1 (100%) survival |
| Telogen effluvium: a sequela of COVID-19 | Mieczkowska; United States19 | Case series, n = 10/10 Mean age: 52.4 (0 M: 10 F) |
TE: 10 | NR |
| Prolonged and late-onset symptoms of coronavirus disease 2019 | Miyazato; Japan20 | Case series, n = 14/58 Mean age: NR (9 M: 5 F) |
Unclassified: 14 | NR |
| SARS-CoV-2-induced telogen effluvium: a multicentric study | Moreno-Arrones; Spain21 | Case series, n = 191/191 Mean age: 47.4 (41 M: 150 F) |
TE: 191 | NR |
| Alopecia and grey hair are associated with COVID-19 severity | Müller Ramos; Brazil22 | Cross-sectional survey, n = 513/43,595 Mean age: NR (Sex NR) |
Unclassified: 513 | NR |
| Telogen effluvium associated with COVID-19 infection | Olds; United States23 | Case series, n = 10/10 Mean age: 48.5 (1 M: 9 F) |
TE: 10 | 10 (100%) survival |
| Clinical characteristics, mortality and short term follow up of patients admitted with COVID-19 in a North East London NHS Trust: a retrospective analysis | Patel; United Kingdom24 | Cohort study: n = 5/109 Mean age: NR (Sex NR) |
Unclassified: 5 | NR |
| Pressure-induced alopecia due to proning in COVID-19 | Perry; United States25 | Case report: n = 1/1 Age: 49 (1 M: 0 F) |
PA: 1 | 1 (100%) survival |
| Comparing outcomes of hospitalized patients with moderate and severe COVID-19 following treatment with hydroxychloroquine plus atazanavir/ritonavir | Rahmani; Iran26 | Cohort study, n = 3/213 Mean age: 60 (Sex NR) |
Unclassified: 3 | NR |
| Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence | Rinaldi; Italy27 | Cross-sectional study: n = 133/133 Mean age: 36.1 (Sex NR) |
AA: 133 | NR |
| Telogen effluvium related to post severe Sars-Cov-2 infection: clinical aspects and our management experience | Rizzetto; Italy28 | Case series, n = 3/3 Mean age: 64.6 (0 M: 3 F) |
TE: 3 | 3 (100%) survival |
| Telogen effluvium after SARS-CoV-2 Infection: a series of cases and possible pathogenetic mechanisms | Rossi; Italy29 | Case series: n = 14/14 Mean age: 47.6 (3 M: 11 F) |
TE: 14 | 14 (100%) survival |
| New onset of alopecia areata in a patient with SARS-CoV-2 infection: possible pathogenetic correlations? | Rossi; Italy30 | Case report: n = 1/1 Age: 29 (0 M: 1 F) |
AA (totalis): 1 | 1 (100%) survival |
| Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients | Rudnicka; Poland31 | Case series: n = 10/32 Mean age: 33.6 (Sex NR) |
TE: 10 | 10 (100%) survival |
| Telogen effluvium: long term COVID-19 symptom | Saeed; Pakistan32 | Case series: n = 3/3 Mean age: NR (0 M: 3 F) |
TE: 3 | 3 (100%) survival |
| Alopecia and severity of COVID-19: a cross-sectional study in Peru | Salazar Arenas; Peru33 | Cross-sectional study: n = 45/98 Mean age: 62 (45 M: 0 F) |
AGA: 45 | 35 (77.8%) survival |
| Hair loss as a late complication of multisystem inflammatory syndrome in children | Savaş Şen; Turkey34 | Case report: n = 1/1 Age: 7 (0 M: 1 F) |
TE: 1 | 1 (100%) survival |
| Alopecia areata in a patient with SARS-Cov-2 infection | Sgubbi; Italy35 | Case report: n = 1/1 Age: 54 (0 M: 1 F) |
AA: 1 | 1 (100%) survival |
| COVID-19 related anagen effluvium | Shanshai; Iraq36 | Case report, n = 1/1 Age: 35 (0 M: 1 F) |
AE: 1 | 1 (100%) survival |
| COVID-19 infection is a major cause of acute telogen effluvium | Sharquie; Iraq37 | Cross-sectional study: n = 39/39 Mean age: 41.3 (3 M: 36 F) |
TE: 39 | 39 (100%) survival |
| Mild COVID-19 in ANCA-associated vasculitis treated with rituximab | Suárez-Diáz; Netherlands38 | Case report: n = 1/1 Age: 64 (0 M: 1 F) |
AA: 1 | 1 (100%) survival |
| Clinical course of alopecia after COVID-19 | Suzuki; Japan39 | Case report: n = 1/1 Age: 49 (1 M: 0 F) |
TE (unconfirmed): 1 | 1 (100%) survival |
| The development of dermatologic diseases in patients recovered from COVID-19 | Temiz; Turkey40 | Case series: n = 8/33 Mean age: 37.2 (2 M: 6 F) |
AA: 2 TE: 6 |
8 (100%) survival |
| Patient recovery from COVID-19 infections: follow-up of hair, nail, and cutaneous manifestations | Thuangtong; Thailand41 | Case series, n = 22/93 Mean age: 43.5 (8 M: 14 F) |
Unclassified: 22 | NR |
| Androgenetic alopecia in women and men is not related to COVID-19 infection severity: a prospective cohort study of hospitalized COVID-19 patients | Torabi; Iran42 | Cohort study: n = 77/116 Mean age of cohort: 60.4 (45 M: 32 F) |
AGA: 77 | NR |
| Skin signs resembling vascular acrosyndromes during the COVID-19 outbreak in Italy | Tosti; Italy43 | Case series: n = 1/4 Age: 16 (0 M: 1 F) |
AA (universalis): 1 | 1 (100%) survival |
| What can the hair tell us about COVID-19? | Trüeb; Brazil, Switzerland44 | Case series, n = 10/10 Mean age: 55.5 (3 M: 7 F) |
AGA: 6 TE: 4 |
10 (100%) survival |
| Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the "Gabrin sign" | Wambier; Spain45 | Case series, n = 118/175 Mean age: 66.7 (96 M: 22 F) |
AGA: 118 | NR |
| COVID-19 dermatological manifestations: results from the Mexican Academy of Dermatology COVID-19 registry | Welsh; Mexico46 | Cross-sectional study: n = 6/164 Mean age: NR (Sex NR) |
Unclassified: 6 | NR |
| Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study | Xiong; China47 | Case series, n = 154/538 Mean age: 52 (12 M: 142 F) |
Unclassified: 154 | 154 (100%) survival |
AA, Alopecia areata; AE, anagen effluvium; AGA, androgenic alopecia; F, female; M, male; NR, not reported; PA, pressure-induced alopecia; TE, telogen effluvium.
Table II summarizes the patient demographic and survival data stratified by type of alopecia. When classified, the 3 most common types of alopecia identified were AGA (n = 561/1826, 30.7%), TE (n = 362/1826, 19.8%), and AA (n = 143/1826, 7.8%). The 2 less common types of alopecia identified were AE (n = 2/1826, 0.1%) and PA (n = 2/1826, 0.1%). The type of alopecia in the remaining patients (n = 756/1826, 41.4%) was not specified. The mean age of patients with AGA was the highest (mean, 61.1 years; range, 18-100 years), followed by TE (mean, 48.0 years; range, 15-88 years) and PA (mean, 43.0 years; range, 37-49 years). The mean age of patients with AE was the lowest (mean, 29.5 years; range, 24-35 years), followed by AA (mean, 36.1 years; range, 7-64 years). Male predominance was observed in AGA (n = 504/561, 89.8%), whereas female predominance was observed in TE (n = 284/352, 80.7%) and AA (n = 6/10, 60%). The sample size of patients with AE (n = 2) and PA (n = 2) was too small to draw any meaningful conclusions about sexual preference. When reported, the survival rates for all types of alopecia were 100%, except AGA, which had a 79.2% (n = 38/48) survival rate; survival outcomes were not reported for most patients (n = 1402/1826, 76.8%).
Table II.
Summary of types of alopecia associated with COVID-19 in 1826 patients and survival data
| Characteristics | AA | AE | AGA | PA | TE | Unclassified |
|---|---|---|---|---|---|---|
| Number of patients (n = 1826) | 143 (7.8%) | 2 (0.1%) | 561 (30.7%) | 2 (0.1%) | 362 (19.8%) | 756 (41.4%) |
| Mean age (y), number of patients with reported age, age range | 36.1 (n = 10) | 29.5 (n = 2) | 61.1 (n = 484) | 43.0 (n = 2) | 48.0 (n = 352) | 51.5 (n = 206) |
| Range: 7-64 | Range: 24-35 | Range: 18-100 | Range: 37-49 | Range: 15-88 | Range: NR | |
| Sex | 4 M, 6 F 133 NR |
0 M, 2 F | 504 M, 57 F | 2 M, 0 F | 68 M, 284 F 10 NR |
29 M, 161 F 566 NR |
| Country of patient | Italy Netherlands Turkey United States |
Brazil Iraq |
Brazil Iran Peru Spain Switzerland United Kingdom |
Brazil United States |
Brazil China Iraq Italy Pakistan Poland Spain Switzerland Turkey United States |
Brazil China Iran Japan Mexico Thailand United Kingdom |
| Survival | 10/10 (100%) 133 NR |
2/2 (100%) 0 NR |
38/48 (79.2%) 513 NR |
Unknown 2 NR |
171/171 (100%) 191 NR |
193/193 (100%) 563 NR |
AA, Alopecia areata; AE, anagen effluvium; AGA, androgenic alopecia; F, female; M, male; NR, not reported; PA, pressure-induced alopecia; TE, telogen effluvium.
Table III summarizes composite data, stratified by alopecia type, to show whether alopecia symptoms in patients with COVID-19 represented worsening of a preexisting alopecia diagnosis or a newly triggered event. When reported, all patients with COVID-19 who experienced symptoms of AGA had a preexisting diagnosis of AGA (n = 287/287, 100%). Similarly, most patients with COVID-19 who experienced symptoms of AA also had a preexisting diagnosis of AA (n = 136/143, 95.1%); of these 136 patients, 58 (42.6%) patients experienced a new AA flareup worsened by COVID-19, whereas 78 (57.4%) patients had no new AA symptoms. In contrast, patients with COVID-19 presenting with TE generally had no preexisting diagnoses of alopecia (n = 339/362, 93.6%), although a small percentage of these patients had a preexisting diagnosis of AA (n = 10/362, 2.8%) or AGA (n = 13/362, 3.6%). Thus, unlike AGA and AA, TE symptoms in patients with COVID-19 all represented the first-time occurrences of disease rather than worsening of a previous alopecia diagnosis. Notably, the onset of TE symptoms generally lagged that of COVID-19 symptoms, with a mean duration to symptom onset of 56.5 days. When reported, TE resolved in 100% (86/86) of the patients within approximately 1 to 6 months of hair loss onset. Treatment was not required in most cases, although minoxidil, finasteride, and topical clobetasol were used in some cases.
Table III.
Prevalence of the types of classified alopecia in 1070 patients with COVID-19 and the number of patients with preexisting alopecia versus new-onset alopecia
| Types of classified alopecia | Preexisting alopecia diagnosis |
No preexisting alopecia diagnosis |
Not reported | |
|---|---|---|---|---|
| No new symptoms triggered by COVID-19 | New alopecia flareup worsened by COVID-19 | New-onset alopecia triggered by COVID-19 | ||
| AA (n = 143, 13.4%) | 78 (54.5%) | 58 (40.6%) | 7 (4.9%) | 0 |
| AE (n = 2, 0.2%) | 0 | 1 (50%) | 1 (50%) | 0 |
| AGA (n = 561, 52.4%) | 287 (51.2%)∗ | 0 | 274 (48.8%) | |
| PA (n = 2, 0.2%) | 0 | 1 (50%) | 1 (50%) | 0 |
| TE (n = 362, 33.8%) | 0 | 23 (6.4%) (n = 10 had prior AA) (n = 13 had prior AGA) |
339 (93.6%) | 0 |
AA, Alopecia areata; AE, anagen effluvium; AGA, androgenic alopecia; PA, pressure-induced alopecia; TE, telogen effluvium.
Not reported whether alopecia symptoms worsened (or were stable) after COVID-19.
Table IV summarizes data from studies that report the prevalence of AGA in patients with severe COVID-19 requiring hospitalization. Among the studies that specify this information, the prevalence of AGA in these patients ranged from 70.7% to 91.4% (mean, 75.5%; n = 449/595) in men and from 41.5% to 56.1% (mean, 49.1%; n = 54/110) in women.
Table IV.
Prevalence of androgenetic alopecia among men and women with severe COVID-19
| Study | Prevalence of AGA in patients with severe COVID-19 |
|---|---|
| Men | |
| Goren et al,14 2020 | 70.7% (29/41) |
| Lee et al,16 2020 | 73.5% (247/336) |
| Salazar et al,33 2021 | 91.4% (32/35) |
| Torabi et al,42 2021 | 73.8% (45/61) |
| Wambier et al,45 2020 | 78.7% (96/122) |
| Total | 75.5% (449/595) |
| Women | |
| Torabi et al,42 2021 | 56.1% (32/57) |
| Wambier et al,45 2020 | 41.5% (22/53) |
| Total | 49.1% (54/110) |
AGA, Androgenic alopecia.
Discussion
Overview
Understanding the hair-related manifestations of COVID-19 is clinically important for both patients and providers. Many distinct types of alopecia appear to be associated with COVID-19, although the mechanisms for these associations likely differ among these types. Findings from our review strengthen the current understanding of the relationship between the types of alopecia associated with COVID-19. Although we identified 5 distinct types of alopecia associated with COVID-19, we lacked a sufficient sample size of patients with PA (n = 2, 0.1%) and AE (n = 2, 0.1%) to draw any meaningful conclusions. Thus, we discuss implications for only the 3 most common types of alopecia identified: AGA, TE, and AA.
AGA: Risk factor for COVID-19
AGA is reported as a risk factor for, rather than sequela of, COVID-19. In our study, patients with COVID-19 with symptoms of AGA all had a preexisting diagnosis of AGA, although it was not reported whether AGA had worsened or remained stable after COVID-19. An increased prevalence of AGA in patients with severe COVID-19 has been reported by 5 of 6 studies.14,16,33,42,45 A report found that 71% of patients (n = 41) hospitalized with COVID-19–related pneumonia in Spain had AGA,14 compared with an expected prevalence of 31% to 53% in otherwise healthy Spanish Caucasian men.48,49 A follow-up study by the same authors expanded on this data set to include an additional 175 patients who were hospitalized with COVID-19 and reported a similar prevalence of AGA of 67% (72% in men and 49% in women).45 A separate report by Torabi et al42 on patients hospitalized with severe COVID-19 in Iran also found a comparable 73.7% prevalence of AGA in men and 56.1% in women; interestingly, the authors of this study found no association between AGA and COVID-19 severity,42 whereas 3 separate reports found that AGA was associated with increased COVID-19 severity.16,33,45 After pooling data from these reports, we found that the prevalence of AGA among men with severe COVID-19 requiring hospitalization (n = 449/595, 75.5%) exceeded the expected AGA prevalence of approximately 31% to 53% in men without COVID-19.48,50 In women, the prevalence of AGA among patients with severe COVID-19 (n = 54/110, 49.1%) also exceeded the expected AGA prevalence of approximately 6% to 38% in women without COVID-19.51 Although a causal relationship cannot be established from these studies, these composite data suggest that AGA may be a risk factor for severe COVID-19.
The mechanism of this association is believed to be related to androgen-mediated upregulation of the transmembrane serine protease 2, facilitating entry of SARS-CoV-2 into cells through the angiotensin-converting enzyme 2 receptor.52,53 We identified 2 studies that reported that androgen-deprivation therapies (ADTs) could be protective against COVID-19. One prospective cohort study of 77 men hospitalized with COVID-19 found that patients who had received ADT for at least 6 months prior to hospitalization were less likely to be admitted to the intensive care unit (n = 1/12, 8%) compared with those who had not received ADT (n = 38/65, 58%) (P = .0015).54 In a large population-based study (n = 4532) of patients with prostate cancer diagnosed with COVID-19 in Italy, patients who received ADT had a significantly lower risk of COVID-19 than those who did not receive ADT (odds ratio, 4.05; 95% confidence interval, 1.55-10.59).55 Two additional studies found that treatment with an androgen receptor antagonist, proxalutamide, decreases both time to clinical remission and hospitalization from COVID-19. In a double-blinded, randomized controlled trial of 236 patients with COVID-19, proxalutamide treatment significantly reduced the time of clinical infection (4.2 ± 5.4 days vs placebo of 21.8 ± 13.0 days) (P < .001) by accelerating viral clearance of COVID-19.56 In a similar double-blinded, randomized controlled trial of 268 men with COVID-19, patients treated with proxalutamide had a 91% reduction in 30-day hospitalization rate compared with those receiving placebo (2.2% vs 26%; risk ratio = 0.09; 95% confidence interval, 0.03-0.27).57 Collectively, findings from these studies suggest that the association between AGA and COVID-19 may be mediated by androgens, and antiandrogen therapy may be beneficial for patients with AGA and COVID-19.
TE: Triggered by COVID-19
TE is a sequela of COVID-19 that is most likely triggered by cytokine storm. Unlike patients with AGA, who all had a preexisting diagnosis of AGA, no patients with TE had a preexisting diagnosis of TE prior to COVID-19. In our review, the mean duration to the onset of TE symptoms was 56.5 days after COVID-19, which is slightly less than the duration of 2 to 3 months reported for TE caused by other factors.58,59
The mechanism of the association between COVID-19 and TE is believed to be related to the upregulation of proinflammatory cytokines, including interleukin 1b, interleukin 6, tumor necrosis factor-α, and interferon gamma, that may induce catagen development and subsequent TE.11,60, 61, 62, 63 Although the large sex bias for TE seen in our review (80.7% female) may be due to sex differences in immune responses, females may be more likely to notice hair thinning than males (and, therefore, more likely to seek treatment) or be more susceptible to TE because of postpartum hormonal changes.37,58,64
AA: Worsened by COVID-19
AA typically occurred as a relapse of a preexisting diagnosis of AA, rather than new-onset symptoms triggered by COVID-19. A recent cohort study of 7958 COVID-19–positive individuals with no prior history of AA found that only 0.2% (18/7958) of the patients developed a new diagnosis of AA from COVID-19.65 Of the 143 patients with COVID-19 in our review, 95.1% (n = 136/143) had a preexisting diagnosis of AA, compared with only 4.9% (n = 7/143) without a preexisting diagnosis. Most patients with AA and COVID-19 included in our review were derived from a cross-sectional study (n = 133) in Italy that found an AA relapse rate of 42.5% after COVID-19, compared with a relapse rate of 12.5% in patients without COVID-19, suggesting that AA may be worsened by COVID-19.27 Relapses of AA have been associated with several other viruses, including Epstein-Barr virus, cytomegalovirus, and hepatitis B vaccination.66
The mechanism of AA is believed to be an autoimmune reaction related to loss of immune privilege of hair follicles in the anagen stage.67 Viral infections can cause buildup of reactive oxygen species; this oxidative stress, in turn, can upregulate the expression of major histocompatibility complex class I ligands in hair root sheaths, leading to increased T-cell activation.30,67 T cells release interferon gamma and tumor necrosis factor-α around hair follicles, causing autoimmune-induced hair loss.67
Limitations
There are several limitations of our study, including reporting and sampling bias. Findings from our review relied heavily on case reports and series with small sample sizes, from which generalized conclusions may be difficult to draw. Although several articles had significantly larger sample sizes (n > 100), these frequently lacked individual patient details, such as patient age, sex, and/or the type of alopecia. These few studies may also exert a disproportionate influence on our data compared with case reports and series with fewer patients, as approximately 75% of patients included in the review come from only approximately 15% of articles. Moreover, some cross-sectional studies presented patient data from self-reported questionnaires, which may be susceptible to both reporting and sampling bias. Some of our statistics may also be overestimated for various reasons. For example, some studies on AGA were conducted at male-only hospitals, which could overestimate the true proportion of males with AGA. Survival rates may similarly be overestimated in patients with TE, given that the onset of TE symptoms generally only occurs in the weeks to months after COVID-19. Many studies that reported hair outcomes of TE or AA associated with COVID-19 did not describe the severity of the symptoms, and, therefore, comparisons to hair outcomes in TE or AA without COVID-19 could not be made. Definitions of COVID-19 onset also varied across studies. Our meta-analysis is based only on aggregate data from each study and only includes a descriptive analysis. Despite these limitations, our review provides meaningful and clinically relevant information to providers, as it is, to our knowledge, the first to summarize findings from all published articles that describe hair manifestations of COVID-19.
Conclusion
AGA appears to be a risk factor for severe COVID-19, whereas TE presents as a new-onset sequela of COVID-19. AA generally occurs as a relapse in patients with preexisting AA, although, rarely, COVID-19 may also trigger new-onset AA. In this review, we have summarized findings from studies published to date describing any type of alopecia in patients with COVID-19. Although we have also included information about the current understanding of the relationships between COVID-19 and these various forms of alopecia, further studies are needed to elucidate mechanisms underlying these associations.
Conflicts of interest
Dr Tosti is a consultant for DS Laboratories, Monat Global, Almirall, Thirty Madison, Eli Lilly, Bristol Myers Squibb, P&G, Pfizer, and Myovant. Author Nguyen has no conflicts of interest to declare.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Fahmy D.H., El-Amawy H.S., El-Samongy M.A., et al. COVID-19 and dermatology: a comprehensive guide for dermatologists. J Eur Acad Dermatol Venereol. 2020;34(7):1388–1394. doi: 10.1111/jdv.16545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wollina U., Karadag A.S., Rowland-Payne C., Chiriac A., Lotti T. Cutaneous signs in COVID-19 patients: a review. Dermatol Ther. 2020;33(5) doi: 10.1111/dth.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seirafianpour F., Sodagar S., Pour Mohammad A., et al. Cutaneous manifestations and considerations in COVID-19 pandemic: a systematic review. Dermatol Ther. 2020;33(6) doi: 10.1111/dth.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moravvej H., Pourani M.R., Baghani M., Abdollahimajd F. Androgenetic alopecia and COVID-19: a review of the hypothetical role of androgens. Dermatol Ther. 2021;34(4) doi: 10.1111/dth.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed M.S., Moulin T.C., Schioth H.B. Sex differences in COVID-19: the role of androgens in disease severity and progression. Endocrine. 2021;71(1):3–8. doi: 10.1007/s12020-020-02536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moradi F., Enjezab B., Ghadiri-Anari A. The role of androgens in COVID-19. Diabetes Metab Syndr. 2020;14(6):2003–2006. doi: 10.1016/j.dsx.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrantes T.F., Artounian K.A., Falsey R., et al. Time of onset and duration of post-COVID-19 acute telogen effluvium. J Am Acad Dermatol. 2021;85(4):975–976. doi: 10.1016/j.jaad.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berbert Ferreira S., Gavazzoni Dias M.F.R., Berbert Ferreira R., Neves Neto A.C., Trueb R.M., Lupi O. Rapidly progressive alopecia areata totalis in a COVID-19 patient, unresponsive to tofacitinib. J Eur Acad Dermatol Venereol. 2021;35(7):e411–e412. doi: 10.1111/jdv.17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capalbo A., Giordano D., Gagliostro N., et al. Alopecia areata in a COVID-19 patient: a case report. Dermatol Ther. 2021;34(2) doi: 10.1111/dth.14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng D., Calderwood C., Skyllberg E., Ainley A. Clinical characteristics and outcomes of adult patients admitted with COVID-19 in East London: a retrospective cohort analysis. BMJ Open Respir Res. 2021;8(1) doi: 10.1136/bmjresp-2020-000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Landro A., Naldi L., Glaser E., Paus R., Tosti A. Pathobiology questions raised by telogen effluvium and trichodynia in COVID-19 patients. Exp Dermatol. 2021;30(7):999–1000. doi: 10.1111/exd.14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FIvenson D. COVID-19: association with rapidly progressive forms of alopecia areata. Int J Dermatol. 2021;60(1):127. doi: 10.1111/ijd.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao C., Zhao Z., Li F., et al. The impact of individual lifestyle and status on the acquisition of COVID-19: a case-control study. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0241540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goren A., Vano-Galvan S., Wambier C.G., et al. A preliminary observation: male pattern hair loss among hospitalized COVID-19 patients in Spain—a potential clue to the role of androgens in COVID-19 severity. J Cosmet Dermatol. 2020;19(7):1545–1547. doi: 10.1111/jocd.13443. [DOI] [PubMed] [Google Scholar]
- 15.Hayran Y., Yorulmaz A., Gur G., Aktas A. Different hair loss patterns in two pediatric patients with COVID-19-associated multisystem inflammatory syndrome in children. Dermatol Ther. 2021;34(2) doi: 10.1111/dth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee J., Yousaf A., Fang W., Kolodney M.S. Male balding is a major risk factor for severe COVID-19. J Am Acad Dermatol. 2020;83(5):e353–e354. doi: 10.1016/j.jaad.2020.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv S., Wang L., Zou X., et al. A case of acute telogen effluvium after SARS-CoV-2 infection. Clin Cosmet Investig Dermatol. 2021;14:385–387. doi: 10.2147/CCID.S307982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzotta F., Troccoli T., Caselli D., Bonifazi E. Acral rash in a child with COVID-19. Eur J Pediatr Dermatol. 2020;30:79–82. [Google Scholar]
- 19.Mieczkowska K., Deutsch A., Borok J., et al. Telogen effluvium: a sequela of COVID-19. Int J Dermatol. 2021;60(1):122–124. doi: 10.1111/ijd.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazato Y., Morioka S., Tsuzuki S., et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020;7(11):ofaa507. doi: 10.1093/ofid/ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno-Arrones O.M., Lobato-Berezo A., Gomez-Zubiaur A., et al. SARS-CoV-2-induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol. 2021;35(3):e181–e183. doi: 10.1111/jdv.17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller Ramos P., Ianhez M., Amante Miot H. Alopecia and grey hair are associated with COVID-19 severity. Exp Dermatol. 2020;29(12):1250–1252. doi: 10.1111/exd.14220. [DOI] [PubMed] [Google Scholar]
- 23.Olds H., Liu J., Luk K., Lim H.W., Ozog D., Rambhatla P.V. Telogen effluvium associated with COVID-19 infection. Dermatol Ther. 2021;34(2) doi: 10.1111/dth.14761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel G., Dadey E., Gosal E., et al. Clinical characteristics, mortality and short term follow up of patients admitted with COVID-19 in a North East London NHS Trust: a retrospective analysis. Thorax. 2021;76(suppl 1):A5. [Google Scholar]
- 25.Perry T., II, Rosen H., Pettit C., Trinidad J.C. Pressure-induced alopecia due to proning in COVID-19. Dermatol Ther. 2021;34(2) doi: 10.1111/dth.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahmani H., Davoudi-Monfared E., Nourian A., et al. Comparing outcomes of hospitalized patients with moderate and severe COVID-19 following treatment with hydroxychloroquine plus atazanavir/ritonavir. Daru. 2020;28(2):625–634. doi: 10.1007/s40199-020-00369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rinaldi F., Trink A., Giuliani G., Pinto D. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb) 2021;11(2):339–345. doi: 10.1007/s13555-021-00498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizzetto G., Diotallevi F., Campanati A., et al. Telogen effluvium related to post severe SARS-CoV-2 infection: clinical aspects and our management experience. Dermatol Ther. 2021;34(1) doi: 10.1111/dth.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi A., Magri F., Sernicola A., et al. Telogen effluvium after SARS-CoV-2 infection: a series of cases and possible pathogenetic mechanisms. Skin Appendage Disord. 2021;21:1–5. doi: 10.1159/000517223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi A., Magri F., Michelini S., et al. New onset of alopecia areata in a patient with SARS-CoV-2 infection: possible pathogenetic correlations? J Cosmet Dermatol. 2021;20(7):2004–2005. doi: 10.1111/jocd.14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudnicka L., Rakowska A., Waskiel-Burnat A., Kurzeja M., Olszewska M. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85(3):723–725. doi: 10.1016/j.jaad.2021.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saeed W., Hussain I., Altaf F. Telogen effluvium: long term Covid-19 symptom. J Pak Assoc Dermatol. 2020;30(4):700–703. [Google Scholar]
- 33.Salazar Arenas M.A., Munoz Del Carpio-Toia A., Aybar Galdos J., Rodriguez-Morales A.J. Alopecia and severity of COVID-19: a cross-sectional study in Peru. Infez Med. 2021;29(1):37–45. [PubMed] [Google Scholar]
- 34.Savas Sen Z., Polat M., Oz F.N., Tanir G. Hair loss as a late complication of multisystem inflammatory syndrome in children. Pediatr Infect Dis J. 2021;40(6):e251–e252. doi: 10.1097/INF.0000000000003115. [DOI] [PubMed] [Google Scholar]
- 35.Sgubbi P., Savoia F., Calderoni O., Longo R., Stinchi C., Tabanelli M. Alopecia areata in a patient with SARS-CoV-2 infection. Dermatol Ther. 2020;33(6) doi: 10.1111/dth.14295. [DOI] [PubMed] [Google Scholar]
- 36.Shanshal M. COVID-19 related anagen effluvium. J Dermatolog Treat. 2020:1–2. doi: 10.1080/09546634.2020.1792400. [DOI] [PubMed] [Google Scholar]
- 37.Sharquie K.E., Jabbar R.I. COVID-19 infection is a major cause of acute telogen effluvium. Ir J Med Sci. 2021:1–5. doi: 10.1007/s11845-021-02754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suarez-Diaz S., Moran-Castano C., Coto-Hernandez R., Mozo-Avellaneda L., Suarez-Cuervo C., Caminal-Montero L. Mild COVID-19 in ANCA-associated vasculitis treated with rituximab. Ann Rheum Dis. 2020;80(6):e99. doi: 10.1136/annrheumdis-2020-218246. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T., Kutsuna S., Saito S., et al. Clinical course of alopecia after COVID-19. Int J Infect Dis. 2021;107:255–256. doi: 10.1016/j.ijid.2021.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Temiz S.A., Kutlu O. The development of dermatologic diseases in patients recovered from COVID-19. Dermatol Ther. 2021;34(2) doi: 10.1111/dth.14791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thuangtong R., Angkasekwinai N., Leeyaphan C., et al. Patient recovery from COVID-19 infections: follow-up of hair, nail, and cutaneous manifestations. Biomed Res Int. 2021;2021:5595016. doi: 10.1155/2021/5595016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torabi S., Mozdourian M., Rezazadeh R., Payandeh A., Badiee S., Darchini-Maragheh E. Androgenetic alopecia in women and men is not related to COVID-19 infection severity: a prospective cohort study of hospitalized COVID-19 patients. J Eur Acad Dermatol Venereol. 2021;35(9):e553–e556. doi: 10.1111/jdv.17353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tosti G., Barisani A., Queirolo P., et al. Skin signs resembling vascular acrosyndromes during the COVID-19 outbreak in Italy. Clin Exp Dermatol. 2020;45(6):757–758. doi: 10.1111/ced.14267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trueb R.M., Dutra Rezende H., Gavazzoni Dias M.F.R. What can the hair tell us about COVID-19? Exp Dermatol. 2021;30(2):288–290. doi: 10.1111/exd.14259. [DOI] [PubMed] [Google Scholar]
- 45.Wambier C.G., Vano-Galvan S., McCoy J., et al. Androgenetic alopecia present in the majority of patients hospitalized with COVID-19: the “Gabrin sign”. J Am Acad Dermatol. 2020;83(2):680–682. doi: 10.1016/j.jaad.2020.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh E.C., Alfaro Sanchez A.B., Ortega Gutierrez G.L., et al. COVID-19 dermatological manifestations: results from the Mexican Academy of Dermatology COVID-19 registry. Int J Dermatol. 2021;60(7):879–881. doi: 10.1111/ijd.15544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong Q., Xu M., Li J., et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect. 2021;27(1):89–95. doi: 10.1016/j.cmi.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Severi G., Sinclair R., Hopper J.L., et al. Androgenetic alopecia in men aged 40-69 years: prevalence and risk factors. Br J Dermatol. 2003;149(6):1207–1213. doi: 10.1111/j.1365-2133.2003.05565.x. [DOI] [PubMed] [Google Scholar]
- 49.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamilton J.B. Patterned loss of hair in man; types and incidence. Ann NY Acad Sci. 1951;53(3):708–728. doi: 10.1111/j.1749-6632.1951.tb31971.x. [DOI] [PubMed] [Google Scholar]
- 51.Birch M.P., Messenger J.F., Messenger A.G. Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol. 2001;144(2):297–304. doi: 10.1046/j.1365-2133.2001.04018.x. [DOI] [PubMed] [Google Scholar]
- 52.Cattrini C., Bersanelli M., Latocca M.M., Conte B., Vallome G., Boccardo F. Sex hormones and hormone therapy during COVID-19 pandemic: implications for patients with cancer. Cancers (Basel) 2020;12(8):2325. doi: 10.3390/cancers12082325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goren A., Wambier C.G., Herrera S., et al. Anti-androgens may protect against severe COVID-19 outcomes: results from a prospective cohort study of 77 hospitalized men. J Eur Acad Dermatol Venereol. 2021;35(1):e13–e15. doi: 10.1111/jdv.16953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montopoli M., Zumerle S., Vettor R., et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cadegiani F.A., McCoy J., Gustavo Wambier C., et al. Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial. Cureus. 2021;13(2) doi: 10.7759/cureus.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCoy J., Goren A., Cadegiani F.A., et al. Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial. Front Med (Lausanne) 2021;8:668698. doi: 10.3389/fmed.2021.668698. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Malkud S. Telogen effluvium: a review. J Clin Diagn Res. 2015;9(9):WE01–03. doi: 10.7860/JCDR/2015/15219.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sinclair R. Diffuse hair loss. Int J Dermatol. 1999;38(suppl 1):8–18. doi: 10.1046/j.1365-4362.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 60.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the ‘cytokine storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito T., Ito N., Saathoff M., Bettermann A., Takigawa M., Paus R. Interferon-gamma is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br J Dermatol. 2005;152(4):623–631. doi: 10.1111/j.1365-2133.2005.06453.x. [DOI] [PubMed] [Google Scholar]
- 63.Webb B.J., Peltan I.D., Jensen P., et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet Rheumatol. 2020;2(12):e754–e763. doi: 10.1016/S2665-9913(20)30343-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hughes E.C., Saleh D. StatPearls; 2021. Telogen Effluvium. [PubMed] [Google Scholar]
- 65.Kim J., Hong K., Gomez Gomez R.E., Kim S., Chun B.C. Lack of evidence of COVID-19 being a risk factor of alopecia areata: results of a national cohort study in South Korea. Front Med (Lausanne) 2021;8:758069. doi: 10.3389/fmed.2021.758069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richardson C.T., Hayden M.S., Gilmore E.S., Poligone B. Evaluation of the relationship between alopecia areata and viral antigen exposure. Am J Clin Dermatol. 2018;19(1):119–126. doi: 10.1007/s40257-017-0312-y. [DOI] [PubMed] [Google Scholar]
- 67.Rajabi F., Drake L.A., Senna M.M., Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. 2018;179(5):1033–1048. doi: 10.1111/bjd.16808. [DOI] [PubMed] [Google Scholar]