Abstract
Purpose
Macrophages play crucial roles as early responders to bacterial pathogens and promote/ or impede chronic inflammation in various tissues. Periodontal macrophage-induced pyroptosis results in physiological and pathological inflammatory responses. The transcription factor Dec2 is involved in regulating immune function and inflammatory processes. To characterize the potential unknown role of Dec2 in the innate immune system, we sought to elucidate the mechanism that may alleviate macrophage pyroptosis in periodontal inflammation.
Methods
Porphyromonas gingivalis lipopolysaccharide (LPS) was used to induce pyroptosis in RAW 264.7 macrophages. Subsequently, we established an LPS-stimulated Dec2 overexpression cellular model in macrophages. Human chronic periodontitis tissues were employed to evaluate potential changes in inflammatory marker expression and pyroptosis. Finally, the effects of Dec2 deficiency on inflammation and pyroptosis were characterized in a P. gingivalis-treated experimental periodontitis Dec2-knockout mouse model.
Results
Macrophages treated with LPS revealed significantly increased messenger RNA expression levels of Dec2 and interleukin (IL)-1β. Dec2 overexpression reduced IL-1β expression in macrophages treated with LPS. Overexpression of Dec2 also repressed the cleavage of gasdermin D (GSDMD), and the expression of caspase-11 was concurrently reduced in macrophages treated with LPS. Human chronic periodontitis tissues showed significantly higher gingival inflammation and pyroptosis-related protein expression than non-periodontitis tissues. In vivo, P. gingivalis-challenged mice exhibited a significant augmentation of F4/80, tumor necrosis factor-α, and IL-1β. Dec2 deficiency markedly induced GSDMD expression in the periodontal ligament of P. gingivalis-challenged mice.
Conclusions
Our findings indicate that Dec2 deficiency exacerbated P. gingivalis LPS-induced periodontal inflammation and GSDMD-mediated pyroptosis. Collectively, our results present novel insights into the molecular functions of macrophage pyroptosis and document an unforeseen role of Dec2 in pyroptosis.
Keywords: Dec2, Macrophage, Periodontal inflammation, Porphyromonas gingivalis, Pyroptosis
Graphical Abstract

INTRODUCTION
Inflammation is a complex biological phenomenon facilitated by innate and adaptive immune responses against damaged cells and pathogens that is strictly regulated by macrophages, which cause activation of the inflammatory network [1,2]. During periodontal inflammation, pathogens interfere with immune cells to induce inflammatory surveillance with constant alterations in gene expression. The unrestricted production of inflammatory molecules provokes consequential damage to tissues, leading to detrimental effects on the local environment. Macrophages are heterogeneous immune cells with various functions that can aggravate or ameliorate the severity of diseases [3,4]. Macrophages are important innate immune cells that act as sentinels in tissues against lipopolysaccharide (LPS)-induced periodontal inflammation [5]. Macrophage activation leads to the secretion of pro-inflammatory cytokines, such as interleukin (IL)-1β, during early stages of infection [6,7]. In the late stages of inflammation, macrophages undergo pyroptosis, an inflammatory form of programmed cell death [8,9]. LPS is a canonical inducer of the process of pyroptosis [10]. In this context, the interplay between pyroptosis and periodontal inflammation has drawn profound interest.
Pyroptosis enables the elimination of intracellular pathogens [11,12]. Pyroptosis is activated by harmful microorganisms [13] and is dependent on inflammatory caspase [14]. Bacterial and viral infections are the primary causes of intensified macrophage pyroptosis [14]. In recent years, increasing evidence has emerged that macrophage pyroptosis contributes to uncontrolled inflammation in various tissues. As a keystone pathogen in periodontal inflammation, Porphyromonas gingivalis [15] regulates innate immunity [16] and may boost macrophage pyroptosis and the secretion of cytokines to mediate inflammation. The release of mediators of inflammation promotes the migration of polymorphonuclear neutrophils (PMNs) to sites of inflammation in the periodontal ligament. Active caspase-1 and caspase-11 are involved in pyroptosis and cleave the cytosolic protein gasdermin D (GSDMD) [14,17]. LPS can activate caspase-11 and stimulate the secretion of IL-1β/IL-18, which subsequently triggers pyroptosis [18].
IL-1β has long been known as a critical pyrogen, and enhanced expression of IL-1β has been found in the gingival tissues of patients with periodontitis [19]. In addition, P. gingivalis induces the secretion of IL-1β and subsequent pyroptosis [20]. P. gingivalis can persist within macrophages [21,22]. Accordingly, we speculated that P. gingivalis might induce periodontal inflammation through GSDMD-mediated pyroptosis. Thus, this study used a mouse model of P. gingivalis-challenged periodontal inflammation and cell culture experiments with RAW 264.7 macrophages to imitate the inflammatory response of macrophages in periodontal pyroptosis.
Differentiated embryo chondrocyte 2 (Dec2/Sharp-1/Bhlhe41) is a transcription factor that is involved in immune function and the regulation of inflammatory processes. Dec2 has been shown to regulate Th2 cell differentiation and induce Th2-type cytokine stimulation [23]. These inter-regulatory mechanisms of immune cells, pro-inflammatory cytokines, and pyroptosis can have detrimental effects on periodontal homeostasis and can lead to chronic periodontitis [24]. We recently reported the effects of Dec2 on the activation of inflammasomes and the pyroptosis of gingival and periodontal ligament fibroblasts [25]. However, the definitive role of Dec2 in fundamental processes involved in the activation of periodontal macrophage pyroptosis has not been determined.
Therefore, the aim of this study was to determine whether there is a relationship between Dec2 and LPS-induced inflammatory macrophages that governs GSDMD-dependent pyroptosis. Using an in vivo Dec2-knockout (Dec2KO) mouse model of periodontal inflammation, we demonstrated that Dec2 might possess bona fide periodontal homeostasis activity, as Dec2-deficient cells exhibited higher levels of inflammatory parameters.
MATERIALS AND METHODS
Cellular model
Cell culture
The cell line of RAW 264.7 macrophages was purchased from the ATCC (Manassas, VA, USA). The cells were cultured in Dulbecco's modified Eagle medium (Wako, Tokyo, Japan) with 10% fetal bovine serum, 100 U/mL penicillin and 100 mg/mL streptomycin under 5% CO2 at 37°C in a humidified environment. The cells were incubated for 24 hours in the presence or absence of 10 µg/mL P. gingivalis LPS (LPS-PG Ultrapure, InvivoGen, San Diego, CA, USA) to induce an inflammatory response.
Overexpression of Dec2
RAW 264.7 macrophages were seeded at 1.0–2.0 × 105 in 6-well plates and cultured in antibiotic-free normal growth medium. An empty vector or a Dec2 overexpression vector was transfected to the cells using Lipofectamine3000 (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription-polymerase chain reaction (RT-PCR) and Western blot assays were used to verify the transfection efficacy.
RT-PCR
Total RNA was extracted using QIAZOL (Qiagen KK, Tokyo, Japan) according to the manufacturer's instructions. A high-capacity complementary DNA (cDNA) archive kit (Applied Biosystems, Foster City, CA, USA) was used for cDNA transcription. TaqMan probes of the target genes, including Dec2 (Mm00470512_m1), IL-1β (Mm00434228_m1), and beta-actin (ACTB) (Mm02619580_g1), were used for RT-PCR.
Western blots
Whole-cell lysates were collected using radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Santa Cruz, CA, USA). In this process, 20 μg of protein was separated on precast gels (10% or 15%, Wako, Osaka, Japan) and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% skim milk followed by overnight incubation at 4°C with antibodies to caspase-11 (1:500, Abcam, Tokyo, Japan), GSDMD (1:500, Abcam) and glyceraldehyde-3-phosphate dehydrogenase (1:1,000, Cell Signaling Technology, Danvers, MA, USA). Antibodies bound to protein bands were visualized using an ECL Plus Western Blotting Detection System (GE Healthcare, Tokyo, Japan).
Human gingival tissue model
Gingival tissues were obtained from chronic periodontitis patients and prepared as previously described [26]. The review board of Dalian Stomatological Hospital Committee of Ethics on Human Experiments approved this study (DLKQLL2020025; 05/15/2020). All subjects (9 males, 8 females) signed an informed consent document. The specimens were subjected to antigen retrieval (citrate buffer pH 6.0, Abcam) and peroxidase blocking (Wako, Tokyo, Japan) followed by overnight incubation at 4°C with antibodies to Dec2 (1:100, Novus Biologicals, LLC, Centennial, CO, USA), CD68 (Nichirei, Tokyo, Japan), GSDMD (1:100, Abcam), F4/80 (1:50, Abcam), PMNs (1:50, Abcam), tumor necrosis factor-α (TNF-α; 1:75, Abcam) and IL-1β (1:100, Abcam). The same 3,3′-diaminobenzidine exposure conditions were performed on each specimen after incubation with the secondary antibodies (MAX-PO, Nichirei Biosciences Inc., Tokyo, Japan). Specimens of the upper jaws were stained with tartrate-resistant acid phosphatase (TRAP; Wako, Tokyo, Japan) according to the manufacturer's protocol.
Mouse periodontitis model
Dec2KO (12-week-old, n=12) mice were purchased from Ingenious Targeting Laboratory, Inc. (Stony Brook, NY, USA) [25]. C57BL/6 (WT, 12-week-old, n=12) (CLEA Japan, Inc., Tokyo, Japan) and Dec2KO mice were used as periodontitis models. The experimental periodontitis model was established according to our previous study [27]. The mice were divided into a control group and a P. gingivalis-treated group and were sacrificed after 30 days. All animal experiments were conducted under the approval of the Animal Ethics Committee of Kanagawa Dental University (approval No. 13-044).
Statistical analysis
SPSS version 16.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The independent 2-tailed Student t-test or analysis of variance was performed to assess the results, which were regarded as significantly different if there was a P value less than 0.05.
RESULTS
The expression of Dec2 in P. gingivalis LPS-treated macrophages
RAW 264.7 macrophages were treated with 10 μg/mL P. gingivalis LPS for 24 hours, which led to a significant increase in the messenger RNA expression level of IL-1β, as shown by RT-PCR (Figure 1A, P<0.05). Interestingly, the expression of the transcription factor Dec2 was also significantly elevated (Figure 1B, P<0.05).
Figure 1. Reverse transcription-polymerase chain reaction analysis showing that the transcription factor Dec2 is elevated following treatment with P. gingivalis LPS for 24 hours. (A) LPS treatment significantly induced mRNA expression of IL-1β in RAW 264.7 macrophages. (B) The mRNA expression level of Dec2 was also significantly elevated. The data shown represent means±standard deviation. All results are representative of at least 3 independent experiments.
IL: interleukin, LPS: lipopolysaccharide, mRNA: messenger RNA.
a)P<0.05.
The role of Dec2 in P. gingivalis LPS-induced pyroptosis
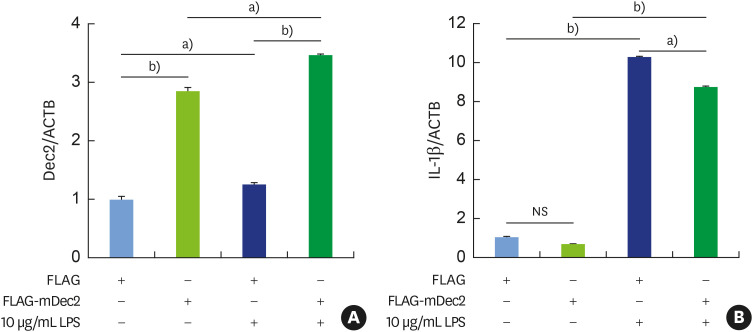
To understand the regulatory role of Dec2 in inflammation mediated by treatment with P. gingivalis LPS, cell pyroptosis was assessed. The overexpression of Dec2 in RAW 264.7 macrophages was confirmed by RT-PCR (Figure 2A, P<0.05, P<0.01), followed by the suppression of IL-1β expression, after treatment with 10 μg/mL P. gingivalis LPS (Figure 2B, P<0.05, P<0.01). Further, treatment with 10 μg/mL P. gingivalis LPS led to a significant induction of GSDMD (Figure 3A and B, P<0.05) and cleaved caspase-11 (Figure 3A and B, P<0.05). The overexpression of Dec2 alleviated the expression of cell pyroptosis markers (Figure 3A and B, P<0.05) and controlled cell pyroptosis under inflammatory conditions. These results revealed the inhibitory effect of Dec2 on LPS-induced cell pyroptosis.
Figure 2. Reverse transcription-polymerase chain reaction analysis showing that Dec2 controls P. gingivalis LPS-induced pyroptosis. (A) The messenger RNA level of Dec2 was highly upregulated after transfection with the Dec2 expression vector. (B) IL-1β expression was suppressed by the overexpression of Dec2 after treatment with 10 μg/mL P. gingivalis LPS. FLAG denotes an empty vector. The data shown represent means±standard deviation. All results are representative of at least 3 independent experiments.
IL: interleukin, LPS: lipopolysaccharide, NS: non-significant.
a)P<0.05, b)P<0.01.
Figure 3. Western blot analysis showing that Dec2 reduces the P. gingivalis LPS-induced pyroptosis. (A, B) Treatment with 10 μg/mL P. gingivalis LPS led to significant increases of GSDMD and pro- and cleaved caspase-11. The overexpression of Dec2 alleviated the expression of cell pyroptosis markers and controlled cell pyroptosis under inflammatory conditions. FLAG denotes an empty vector. The data shown represent means±standard deviation. All results are representative of at least 3 independent experiments.
GSDMD: gasdermin D, LPS: lipopolysaccharide, NS: non-significant.
a)P<0.05.
The expression of Dec2 in human gingival tissue with chronic periodontitis
The expression level of the Dec2 protein was significantly higher in chronic periodontitis tissues than in non-periodontitis healthy tissues. A consistent pattern of significantly higher expression levels of Dec2 (Figure 4, P<0.001), GSDMD (Figure 4, P<0.001), CD68 (Figure 4, P<0.001), PMNs (Figure 5, P<0.001), TNF-α (Figure 5, P<0.001), and IL-1β (Figure 5, P<0.001) was also found in the periodontitis tissues.
Figure 4. Immunohistochemistry showing that Dec2 is upregulated in chronic periodontitis tissues. The expression of Dec2 was higher in periodontitis tissue than in non-periodontitis tissue. The expression of Dec2, GSDMD, and CD68 was also consistently induced in periodontitis tissues. The data shown represent means±standard deviation. All results are representative of at least 3 independent experiments.
H&E: hematoxylin and eosin, GSDMD: gasdermin D.
a)P<0.001.
Figure 5. Immunohistochemistry showing that inflammation is induced in chronic periodontitis tissue. The expression levels of PMNs, TNF-α, and IL-1β were significantly elevated in chronic periodontitis tissue. The data shown represent means±standard deviation. All results are representative of at least 3 independent experiments.
PMN: polymorphonuclear neutrophil, TNF-α: tumor necrosis factor-α, IL-1β: interleukin-1β.
a)P<0.001.
The infiltration of inflammatory cells in a Dec2 deficient mouse periodontitis model
To verify the inflammation-inhibiting role of Dec2, a periodontitis model was used. The expression of GSDMD (Figure 6, P<0.05, P<0.01) was significantly upregulated in Dec2KO mice compared to WT mice, and consistently, the infiltration of inflammatory cells (Figure 6, P<0.05, P<0.01, P<0.001) was higher in Dec2KO mice than in WT mice, as well as inflammatory markers, including IL-1β (Figure 6, P<0.05, P<0.01, P<0.001) and TNF-α (Figure 6, P<0.01). Notably, we also detected a higher number of TRAP-positive cells in Dec2KO mice than in WT mice (Figure 6, P<0.01, P<0.001).
Figure 6. Immunohistochemistry showing that a deficiency of Dec2 facilitates the infiltration of inflammatory cells and osteoclast differentiation in periodontitis models. The expression of GSDMD was upregulated in Dec2KO mice compared with WT mice, and consistently, the infiltration of inflammatory cells was increased more in Dec2KO mice compared with WT mice, as well as the inflammatory markers, which included IL-1β and TNF-α. Notably, a higher number of TRAP-positive osteoclasts was detected in Dec2KO mice than in WT mice; black arrows indicate TRAP-positive cells. The data shown represent means±standard deviation. All results are representative of at least 3 independent experiments.
H&E: hematoxylin and eosin, GSDMD: gasdermin D, PMN: polymorphonuclear neutrophil, Dec2KO: Dec2-knockout, WT: wild-type, TNF-α: tumor necrosis factor-α, IL-1β: interleukin-1β, TRAP: tartrate-resistant acid phosphatase, NS: not significant.
a)P<0.05, b)P<0.01, c)P<0.001.
DISCUSSION
This study demonstrated that Dec2 overexpression inhibits pyroptosis in LPS-treated macrophages. We observed that overexpression of Dec2 inhibited the expression of GSDMD, IL-1β, and pro- and cleaved caspase-11; these findings demonstrate that Dec2 blocks inflammation-triggered pyroptosis. Both caspase-1 and caspase-11 play roles in the innate host defense mechanism against inflammation since caspase-11 predominantly contributes to the response to bacterial infections [28]. Several studies have reported that pyroptosis, a novel inflammatory form of programmed cell death, involves the caspase-1 and/or caspase-11 dependent cleavage of IL-1β [29]. Our results suggest that LPS aids the inflammatory response in macrophages and that Dec2 attenuates LPS-induced pyroptosis in vitro.
We used RAW 264.7 macrophages as our in vitro model to gain insights into the cellular effects of Dec2 in the LPS-induced pyroptosis of macrophages. Under normal conditions, there is a low abundance of resting macrophages, which have a slow metabolism and long-term survival [30]. During periodontal inflammation, the gingiva-resident macrophages recruit monocytes/macrophages from periodontal tissues to the inflammation area to maintain tissue homeostasis. Hence, it is important to use RAW 264.7 macrophages to study the inflammatory response in vitro. We explored the inflammatory activation of macrophages in Dec2KO mice, where they affect the tissue environment concomitantly with pyroptosis.
Pyroptosis, a recently identified type of programmed and pro-inflammatory cell death, allows the removal of intracellular bacterial pathogens to overcome the microbial load [31]. Pyroptosis is initiated by inflammasomes and is executed by GSDMD [32], through a process in which cleaved GSDMD induces pyroptosis by inflammatory caspases-1 and -11 [33]. Caspase-1 is activated upon inflammasome activation, while caspase-11 activation responds to bacterial LPS, and subsequently provokes pyroptosis [34]. GSDMD deletion has consistently alleviated inflammation-driven diseases in various mouse models [35,36]. An enhanced accumulation of pyroptotic cells and increased GSDMD expression were observed following treatment with LPS [37]. In this study, Dec2-overexpressing macrophages treated with LPS had substantially reduced expression levels of the GSDMD protein in comparison to untreated cells. The Dec2KO mouse model reversed this tendency, suggesting the presence of macrophage pyroptosis in periodontal inflammation and showing that Dec2 plays a crucial role in promoting GSDMD-activated macrophage pyroptosis.
Bacterial pathogens quietly evade the host immune responses due to their long-term self-renewal capacity [38]. Treatment with P. gingivalis LPS stimulates inflammasome activation and the consequent GSDMD-triggered macrophage pyroptosis. LPS treatment activates a variety of pathways leading to a dramatic inflammatory response [39]. The transcription level of the pro-inflammatory cytokine IL-1β significantly increased after LPS treatment of macrophages in our study.
Several danger signals stimulate expression of the salient pro-inflammatory mediator IL-1β in various diseases [40]. The levels of IL-1β are higher in patients with periodontitis than in healthy subjects [20]. The representative periodontal pathogenic bacterium P. gingivalis induces periodontal pyroptosis [25]. Our in vivo data suggest that treatment with P. gingivalis LPS substantially enhances periodontitis in the context of induced expression of cleaved caspase-11 and IL-1β. The same trend was evident in our in vitro study, in which GSDMD and cleaved caspase-11 were upregulated following LPS treatment. We demonstrated that Dec2 activation by LPS ameliorated both GSDMD-dependent pyroptosis as well as mature IL-1β levels in macrophages. Our findings illustrate the critical role of macrophage responses to P. gingivalis LPS in periodontal pyroptosis and demonstrate the unique role of P. gingivalis LPS in host-pathogen crosstalk as a regulator of pyroptosis.
In conclusion, these findings show that Dec2 overexpression reduces levels of IL-1β and sequentially regulates caspase-11 and GSDMD, thereby inhibiting pyroptosis. The upregulation of GSDMD is caused, at least in part, by the Dec2 deficiency in LPS-stimulated RAW 264.7 macrophages; this finding provides important mechanistic details regarding the involvement of Dec2 in the context of inflammation and GSDMD activation in macrophages. Finally, by generating Dec2KO mice and analyzing their responses to P. gingivalis-induced periodontal inflammation, we demonstrated that Dec2 deficiency enhances the activation of GSDMD and promotes pyroptosis in vivo. Our results advance our scientific understanding of periodontal pathogenesis and should lead to the design of innovative approaches for this debilitating condition.
ACKNOWLEDGEMENTS
We would like to thank Professor Y. Kato for providing Dec2KO mice; Professor N. Hamada, Dr. T. Toyama, and Dr. T. Sato for the P. gingivalis treatment; and the staff of the animal facility for care of the mice. We thank Dr. Y. Fujita for technical assistance.
Footnotes
Funding: This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a Nihon University Multidisciplinary Research Grant for 2018.
- Conceptualization: Xiaoyan Li, Ujjal K. Bhawal.
- Formal analysis: Dawei He, Xiaoyan Li, Fengzhu Zhang, Ujjal K. Bhawal.
- Investigation: Dawei He, Xiaoyan Li, Fengzhu Zhang, Chen Wang, Ujjal K. Bhawal.
- Methodology: Dawei He, Xiaoyan Li, Fengzhu Zhang, Ujjal K. Bhawal.
- Project administration: Yi Liu, Ujjal K. Bhawal, Jiang Sun.
- Writing - original draft: Xiaoyan Li, Ujjal K. Bhawal.
- Writing - review & editing: Xiaoyan Li, Fengzhu Zhang, Yi Liu, Ujjal K. Bhawal, Jiang Sun.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Dia VP, Bradwell J, Pangloli P. Sorghum phenolics inhibits inflammasomes in lipopolysaccharide (LPS)-primed and adenosine triphosphate (ATP)-activated macrophages. Plant Foods Hum Nutr. 2019;74:307–315. doi: 10.1007/s11130-019-00736-8. [DOI] [PubMed] [Google Scholar]
- 2.Han JW, Shim DW, Shim EJ, Kim MK, Shin YK, Kwak SB, et al. Syneilesis palmata (Thunb.) Maxim. extract attenuates inflammatory responses via the regulation of TRIF-dependent signaling and inflammasome activation. J Ethnopharmacol. 2015;166:1–4. doi: 10.1016/j.jep.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 3.D'Alessio FR, Craig JM, Singer BD, Files DC, Mock JR, Garibaldi BT, et al. Enhanced resolution of experimental ARDS through IL-4-mediated lung macrophage reprogramming. Am J Physiol Lung Cell Mol Physiol. 2016;310:L733–L746. doi: 10.1152/ajplung.00419.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Z, Zhou Q, Gu C, Li D, Zhu L. Depletion of circulating monocytes suppresses IL-17 and HMGB1 expression in mice with LPS-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2017;312:L231–L242. doi: 10.1152/ajplung.00389.2016. [DOI] [PubMed] [Google Scholar]
- 5.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Sahoo M, Lantier L, Warawa J, Cordero H, Deobald K, et al. Caspase-11-dependent pyroptosis of lung epithelial cells protects from melioidosis while caspase-1 mediates macrophage pyroptosis and production of IL-18. PLoS Pathog. 2018;14:e1007105. doi: 10.1371/journal.ppat.1007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascarenhas DPA, Zamboni DS. Inflammasome activation in legionella-infected macrophages. Methods Mol Biol. 2019;1921:305–319. doi: 10.1007/978-1-4939-9048-1_20. [DOI] [PubMed] [Google Scholar]
- 8.Riestra AM, Valderrama JA, Patras KA, Booth SD, Quek XY, Tsai CM, et al. Trichomonas vaginalis induces NLRP3 inflammasome activation and pyroptotic cell death in human macrophages. J Innate Immun. 2019;11:86–98. doi: 10.1159/000493585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Meara TR, Cowen LE. Insights into the host-pathogen interaction: C. albicans manipulation of macrophage pyroptosis. Microb Cell. 2018;5:566–568. doi: 10.15698/mic2018.12.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Murakami T, Suzuki K, Tamura H, Kuwahara-Arai K, Iba T, et al. Antimicrobial cathelicidin peptide LL-37 inhibits the LPS/ATP-induced pyroptosis of macrophages by dual mechanism. PLoS One. 2014;9:e85765. doi: 10.1371/journal.pone.0085765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aachoui Y, Sagulenko V, Miao EA, Stacey KJ. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16:319–326. doi: 10.1016/j.mib.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila . Proc Natl Acad Sci U S A. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–725. doi: 10.1038/nrmicro2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida K, Okamura H, Hiroshima Y, Abe K, Kido JI, Shinohara Y, et al. PKR induces the expression of NLRP3 by regulating the NF-κB pathway in Porphyromonas gingivalis-infected osteoblasts. Exp Cell Res. 2017;354:57–64. doi: 10.1016/j.yexcr.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 17.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 19.Huang X, Yang X, Ni J, Xie B, Liu Y, Xuan D, et al. Hyperglucose contributes to periodontitis: involvement of the NLRP3 pathway by engaging the innate immunity of oral gingival epithelium. J Periodontol. 2015;86:327–335. doi: 10.1902/jop.2014.140403. [DOI] [PubMed] [Google Scholar]
- 20.Park E, Na HS, Song YR, Shin SY, Kim YM, Chung J. Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection. Infect Immun. 2014;82:112–123. doi: 10.1128/IAI.00862-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Hajishengallis G. Lipid raft-dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages. Cell Microbiol. 2008;10:2029–2042. doi: 10.1111/j.1462-5822.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slocum C, Coats SR, Hua N, Kramer C, Papadopoulos G, Weinberg EO, et al. Distinct lipid a moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog. 2014;10:e1004215. doi: 10.1371/journal.ppat.1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Li Z, Mao K, Zou J, Wang Y, Tao Z, et al. Dec2 promotes Th2 cell differentiation by enhancing IL-2R signaling. J Immunol. 2009;183:6320–6329. doi: 10.4049/jimmunol.0900975. [DOI] [PubMed] [Google Scholar]
- 24.Lacey CA, Mitchell WJ, Dadelahi AS, Skyberg JA. Caspase-1 and caspase-11 mediate pyroptosis, inflammation, and control of brucella joint infection. Infect Immun. 2018;86:e00361–e00318. doi: 10.1128/IAI.00361-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oka S, Li X, Sato F, Zhang F, Tewari N, Kim IS, et al. A deficiency of Dec2 triggers periodontal inflammation and pyroptosis. J Periodontal Res. 2021;56:492–500. doi: 10.1111/jre.12849. [DOI] [PubMed] [Google Scholar]
- 26.Sun J, Nemoto E, Hong G, Sasaki K. Modulation of stromal cell-derived factor 1 alpha (SDF-1α) and its receptor CXCR4 in Porphyromonas gingivalis-induced periodontal inflammation. BMC Oral Health. 2016;17:26. doi: 10.1186/s12903-016-0250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F, Suzuki M, Kim IS, Kobayashi R, Hamada N, Sato F, et al. Transcription factor DEC1 is required for maximal experimentally induced periodontal inflammation. J Periodontal Res. 2018;53:883–893. doi: 10.1111/jre.12578. [DOI] [PubMed] [Google Scholar]
- 28.Man SM, Karki R, Briard B, Burton A, Gingras S, Pelletier S, et al. Differential roles of caspase-1 and caspase-11 in infection and inflammation. Sci Rep. 2017;7:45126. doi: 10.1038/srep45126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Py BF, Jin M, Desai BN, Penumaka A, Zhu H, Kober M, et al. Caspase-11 controls interleukin-1β release through degradation of TRPC1. Cell Reports. 2014;6:1122–1128. doi: 10.1016/j.celrep.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arora S, Dev K, Agarwal B, Das P, Syed MA. Macrophages: their role, activation and polarization in pulmonary diseases. Immunobiology. 2018;223:383–396. doi: 10.1016/j.imbio.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaale K, Peters KM, Murthy AM, Fritzsche AK, Phan MD, Totsika M, et al. Strain- and host species-specific inflammasome activation, IL-1β release, and cell death in macrophages infected with uropathogenic Escherichia coli . Mucosal Immunol. 2016;9:124–136. doi: 10.1038/mi.2015.44. [DOI] [PubMed] [Google Scholar]
- 32.Kuang S, Zheng J, Yang H, Li S, Duan S, Shen Y, et al. Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc Natl Acad Sci U S A. 2017;114:10642–10647. doi: 10.1073/pnas.1708194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia C, Zhang J, Chen H, Zhuge Y, Chen H, Qian F, et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 2019;10:778. doi: 10.1038/s41419-019-2021-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 35.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 36.Xiao J, Wang C, Yao JC, Alippe Y, Xu C, Kress D, et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 2018;16:e3000047. doi: 10.1371/journal.pbio.3000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Gan L, Xu Y, Luo D, Ren Q, Wu S, et al. Melatonin alleviates inflammasome-induced pyroptosis through inhibiting NF-κB/GSDMD signal in mice adipose tissue. J Pineal Res. 2017;63:e12414. doi: 10.1111/jpi.12414. [DOI] [PubMed] [Google Scholar]
- 38.Skeldon A, Saleh M. The inflammasomes: molecular effectors of host resistance against bacterial, viral, parasitic, and fungal infections. Front Microbiol. 2011;2:15. doi: 10.3389/fmicb.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan Q, Zhang D, Liu C, Zhang C, Yuan D. Chikusetsusaponin V inhibits LPS-activated inflammatory responses via SIRT1/NF-κB signaling pathway in RAW264.7 cells. Inflammation. 2018;41:2149–2159. doi: 10.1007/s10753-018-0858-8. [DOI] [PubMed] [Google Scholar]
- 40.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]