Abstract
Persistent inflammation is a major contributor to healing impairment in diabetic chronic wounds. Paradoxically, diabetic wound environment during the acute phase of healing is completely different in that it exhibits reduced macrophage response due to inadequate expression of CCL2 proinflammatory cytokine. What causes reduction in CCL2 expression in diabetic wound early after injury remains unknown. Here, we report that in contrast to prolonged exposure to high glucose which transforms monocytes proinflammatory, short-term exposure to high glucose causes a rapid monocyte reprograming, manifested by increased expression and secretion of IL-10 which in an autocrine/paracrine fashion, reduces glucose uptake and transforms monocytes into anti-inflammatory phenotype by dampening signaling through toll-like receptors (TLRs). We show that IL-10 expression is significantly increased in diabetic wound during the acute phase of healing, causing significant reductions in TLR signaling and proinflammatory cytokines production, delaying macrophage and leukocyte responses, and underlying healing impairment in diabetic wounds. Importantly, blocking IL-10 signaling during the acute phase of healing improves TLR signaling, increases proinflammatory cytokines production, enhances macrophage and leukocyte responses, and stimulates healing in diabetic wound. We posit that anti-IL-10 strategies have therapeutic potential if added topically after surgical debridement process which resets chronic wounds into acute fresh wounds.
INTRODUCTION
Diabetic foot ulcers are the leading cause of lower extremity amputations in the United States (US), accounting for ~67% of all amputations and are responsible for more hospitalizations than any other complications of diabetes (Brem and Tomic-Canic, 2007, Sen et al., 2009). Persistent non-resolving inflammation, characterized by increased proinflammatory cytokines and leukocytes (e.g., macrophages), is a major contributing factor to impaired healing in diabetic foot ulcers (Bjarnsholt et al., 2008, Blakytny and Jude, 2006). Paradoxically, diabetic wound environment is completely different during the acute phase of healing in that it suffers from inadequate macrophage response, due to dampened expression of CCL2 proinflammatory cytokine (Ishida et al., 2019, Wood et al., 2014). In line with these findings, diabetic monocytes extracted from diabetic patients also exhibit impaired CCL2 expression (Abke et al., 2006). What causes reductions in CCL2 expression and macrophage trafficking in diabetic wound during the acute phase of healing remains unknown, prompting us to revisit this issue.
RESULTS
Proinflammatory cytokine expression is substantially reduced during the acute phase of wound healing early after injury.
CCL2 is one of several chemokines that can recruit monocytes into the wound and promote their differentiation into macrophages (Snyder et al., 2016). The fact that macrophage influx is substantially reduced in diabetic wounds early after injury (Wood et al., 2014) suggested that the expression of other proinflammatory cytokines may be similarly affected in these wounds early after injury. We generated full-thickness excisional wounds in C57B (normal) and db/db (type 2 obese diabetic) mice, as described (Goldufsky et al., 2015, Wood et al., 2014), and assessed them for their IL-1α and TNF-β proinflammatory cytokines - which are expressed early after injury in normal wound (Hubner et al., 1996, Ridiandries et al., 2018) - by ELISA.
Mirroring the macrophage response delay (Wood et al., 2014), IL-1α and TNF-β levels were significantly lower in db/db diabetic wounds early after injury on days 1 and 3 but increased over time reaching their highest levels in day 10 (Figure S1a). Supporting these data, the mRNA levels of IL-1 and TNF, as assessed by RT-PCR (after normalizing the data to 18S to account for reduced macrophage response), were also significantly reduced in day 1 db/db wounds as compared to normal wounds (Figure S1b). Moreover, the expression of CCL2, G-CSF, and GM-CSF - other cytokines that are produced early in wounds (Dipietro et al., 2001, Huang et al., 2017, Mann et al., 2006) - were also significantly reduced in day 1 db/db wounds, as assessed by RT-PCR and Western blotting (Figure S1b-d). Collectively, these data indicated that diabetic wounds suffer from insufficient chemokines during the acute phase of wound healing early after injury. However, it remained unclear why the production of chemokines is dampened in diabetic wounds early after injury.
Toll-like receptor signaling is significantly diminished during the acute phase wound healing in diabetic wound.
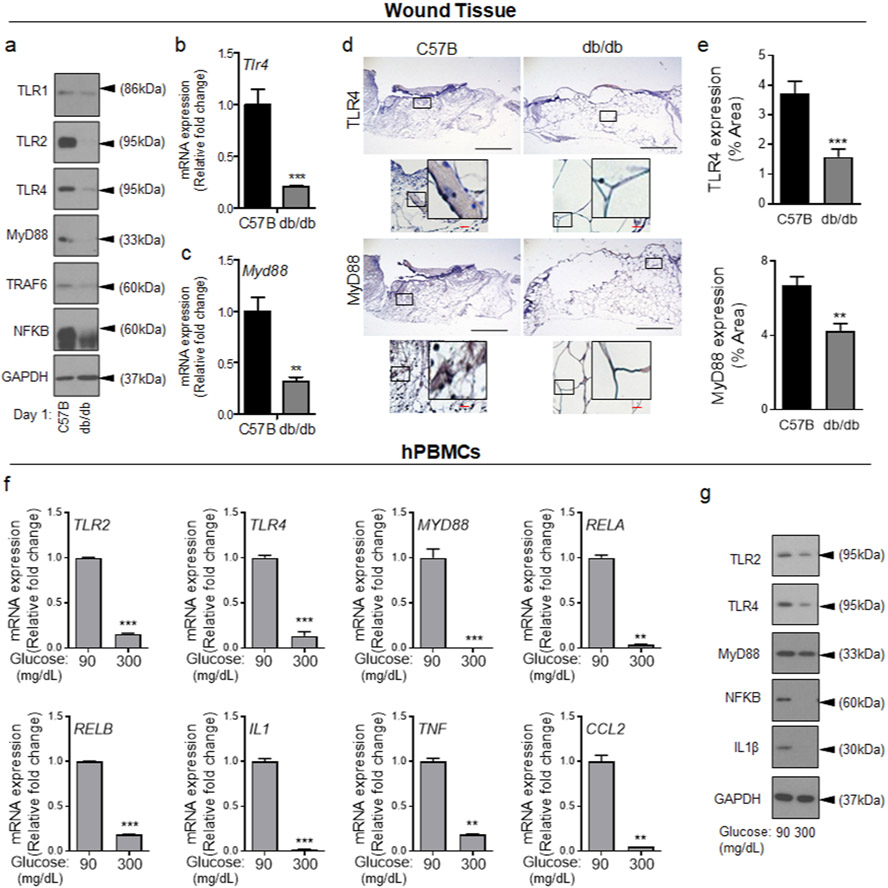
Toll-like receptors (TLRs) are major factories for the production of proinflammatory cytokines, including CCL2, and play critical roles in wound healing (Akhter et al., 2018, Chen and DiPietro, 2017, Kluwe et al., 2009, Lin et al., 2011, Macedo et al., 2007, Sato et al., 2010, Serbina et al., 2012, Suga et al., 2014). Diabetic chronic wounds show elevated TLR signaling (Dasu et al., 2010a), but little is known about TLR signaling in diabetic wound early after injury. Data indicated that the expression of representative TLRs (TLR1, TLR2, and TLR4) and TLR signaling components, (MyD88, TRAF6, and NF-κB (Kawasaki and Kawai, 2014)) were all significantly reduced in day 1 diabetic wound as compared to normal wound (Figure 1a, and Figure S2a). Corroborating these data, mRNA analyses and histological assessments of representative TLR signaling components (TLR4 and MyD88) also showed significant reductions in their expressions in day 1 diabetic wounds (Figure 1b-e). These data indicated that TLR signaling is dampened in diabetic wound early after injury. However, it remained unclear what was causing the reduction in the TLR signaling in diabetic wound early after injury.
Figure 1. Toll-like receptor signaling is diminished during the acute phase wound healing in diabetic wound and in response to short-term exposure to HG.
C57B and db/db wounds were collected 24h after wounding and assessed for the expression of indicated genes by Western blotting (a), by mRNA analysis using RT-PCR (b-c), and by immunohistochemistry (d-e). Representative images are shown in (d) and the corresponding data in (e). (N=5 mice/group, ≥9 random fields/wound/mouse. Black scale bars=500μm; Red scale bars=50μm). (e-f) hPBMCs were exposed to 90mg/dl or 300 mg/dl for 1h and assessed for the expression of indicated genes by mRNA (f), or by Western blotting (g). Data are plotted as mean ± SEM. (Each experiment was repeated at least 2 times for (e) and at least 3 times for (f). *p<0.05, **p<0.01, ***p<0.001).
Short-term exposure to high glucose leads to impaired TLR signaling and reduced chemokine expression in human and murine monocytes.
Circulating monocytes migrate into wound tissues after injury (Schulz et al., 2012). They are generated primarily in the bone marrow and released into the circulation rapidly within 1-3 hours, and injury and/or infection further accelerate their release from bone marrow and their trafficking into injured tissue and/or toward infection (Goto et al., 2003, Zhao et al., 2018). In the absence of injury and/or infection, these classical monocytes circulate in the blood with a lifespan of ~24h (Daley et al., 2010, Patel et al., 2017). Leukocytes (including monocytes/macrophages) extracted from the blood of diabetic patients or diabetic animals have functional impairments, including in their chemotaxis, and these impairments have been attributed to prolonged exposure to HG (Delamaire et al., 1997, Hill et al., 1983). In contrast, leukocytes extracted from bone marrow of diabetic animals appear to be normal (Park et al., 2009, Repine et al., 1980, Scully et al., 2017, Sima et al., 1988), suggesting that bone marrow environment may be protective against the harmful effects of prolonged exposure to HG. Because of chemotaxis impairment in circulating monocytes, we posited that newly released monocytes from bone marrow would likely be the monocytes migrating into the wound early after injury and these monocytes would be exposed to HG for short time (~1-3 hours). Long-term (24-72 hours) exposure to HG transforms monocytes/macrophages proinflammatory (Hotamisligil et al., 1995, Yaghini et al., 2011), as we confirmed (Figure S2b-c), but the impact of short-term acute exposure on inflammatory responses in monocytes has not been assessed.
We purified human Peripheral Blood Monocytes (hPBMCs) from non-diabetic individuals and Bone Marrow Derived Monocytes (mBMDMs) from C57B mice, exposed them to glucose levels in the normal range (90mg/dL) or diabetic range (300mg/dL) for 1h, and assessed the impact of HG on the expression of TLR signaling components and proinflammatory cytokines. Acute (1h) exposure to HG caused significant reductions in the mRNA levels of all indicated genes in hPBMCs and mBMDMs (Figure 1f and Figure S2e). Similarly, the corresponding proteins were also reduced in mBMDMs and hPBMCs, except MyD88 which was significantly reduced in mBMDMs but not in hPBMCs, suggesting that MyD88 protein stability/turnover may be differentially regulated in hPBMCs and mBMDMs (Figure 1g, and Figure S2d, S2f, and S2g). Collectively, these data indicated that, in complete contrast to long-term exposure to HG which transforms monocytes proinflammatory, short-term acute exposure to HG causes reductions in TLR-mediated signaling and proinflammatory cytokines. However, what mediated the reduction in TLR expression and in proinflammatory cytokines production in monocytes after short-term exposure to HG remained unknown.
IL-10 expression is significantly increased during the acute phase of healing in diabetic wounds and in response to short-term exposure to high glucose.
IL-10 is a potent immunosuppressive cytokine that inhibits TLR signaling and proinflammatory cytokines’ production through multiple mechanisms (Curtale et al., 2013, Knödler et al., 2009, Murray, 2005, Wang et al., 1995). Interestingly, IL-10 also inhibits glucose uptake in lipopolysaccharide (LPS)-stimulated macrophages (Ip et al., 2017), linking IL-10 to glucose metabolism. Moreover, long-term (1-5 days) exposure to HG causes mitochondrial damage and culminates in proinflammatory responses (Devi et al., 2013, Park and Park, 2013). We postulated that in diabetic environment where glucose is in excess, IL-10 may be upregulated as a protective measure to prevent excess glucose uptake and to protect monocytes from HG-induced cellular damage.
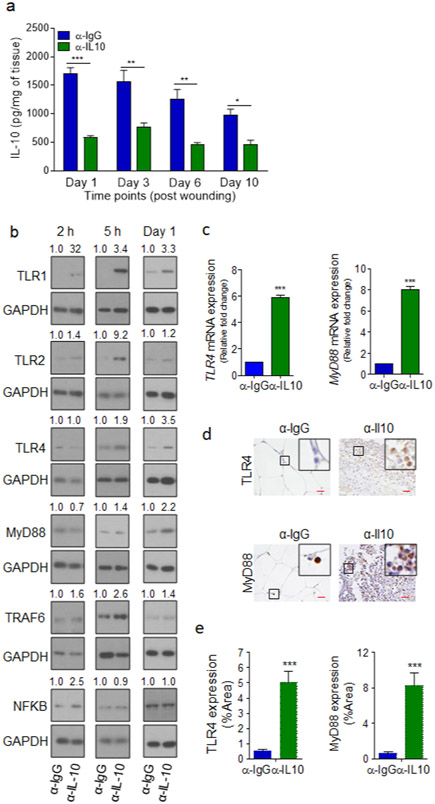
We first measured IL-10 levels in normal and diabetic wounds. IL-10 levels were significantly higher in diabetic wounds early after injury on days 1 and 3, but significantly lower on day 10 (Figure 2a). IL-10 mRNA and protein level assessments corroborated the ELISA data, showing that IL-10 was significantly upregulated in day 1 diabetic wounds (Figure 2b-d). Further corroborating these data, 1h exposure to HG also caused significant increases in IL-10 mRNA and IL-10 protein levels in both cell lysates and culture supernatants of monocytes (Figure 2e-g and Figure S3a-c). Interestingly, IL-10 could not be detected in monocytes or in their culture supernatant after 24h exposure to HG (Figure S2b), suggesting that IL-10 expression is transient and elevated IL-10 in the supernatant of monocytes acutely exposed to HG, is consumed or degraded during prolonged exposure to HG.
Figure 2. IL-10 expression is significantly increased during the acute phase of healing in diabetic wounds and in response to short-term exposure to high glucose.
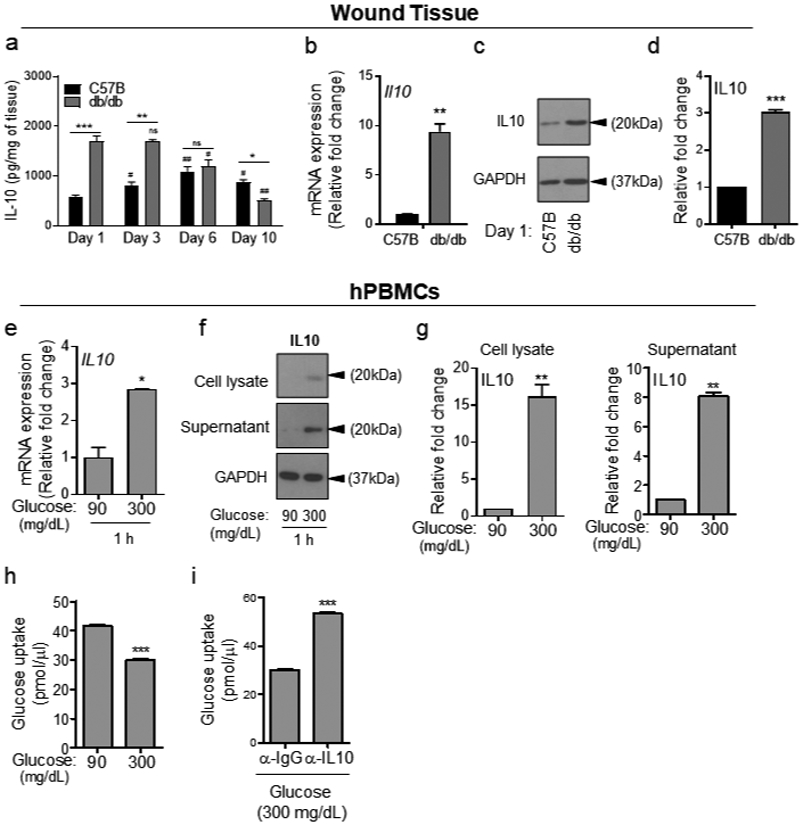
(a) C57B and db/db wounds at indicated times were assessed for IL10 levels by ELISA. (b-d), day 1 wounds were assessed for IL10 by mRNA (b), and by Western blotting (c) and corresponding densitometer of Western blot is shown in (d). (e-h) hPBMCs were exposed to 90 or 300 mg/dl glucose for 1h and analyzed for IL10 expression by mRNA (e), by Western blotting (f-g), or for glucose uptake using 2-NBDG assay in the absence or presence of 5μg/ml anti-IL-10 antibody (α-IL-10) (h-i). Data are plotted as the mean ± SEM. (N≥3 mice/ group (a-d); for (e-h), N≥3 independent times; *p<0.05; **p<0.01; ***p<0.001).
Consistent with our hypothesis, glucose uptake, as determined by 2-NBDG fluorescent d-glucose analog, was significantly reduced in monocytes after 1h exposure to HG (Figure 2h), while blocking IL-10 signaling by anti-IL-10 antibody (α- IL10) increased glucose uptake in monocytes acutely exposed to HG (Figure 2i). Moreover, blocking IL-10 signaling by α- IL10 antibody also significantly increased the expression of representative TLR signaling components, and enhanced proinflammatory cytokines’ production in hPBMCs and mBMDMCs under 1h exposure to HG (Figure 3 and Figure S4), but not in primary epidermal keratinocytes or dermal endothelial cells (Figure S5), which do not express IL-10 (Fiorentino et al., 1989, Li et al., 1999, Moore et al., 2001, Park and Barbul, 2004), indicating that IL-10 autocrine/paracrine signaling is responsible for reducing glucose uptake and dampening TLR signaling in monocytes exposed acutely to HG.
Figure 3. Blocking IL-10 signaling enhances TLR signaling and proinflammatory cytokines’ production in PBMCs in the presence of high glucose.
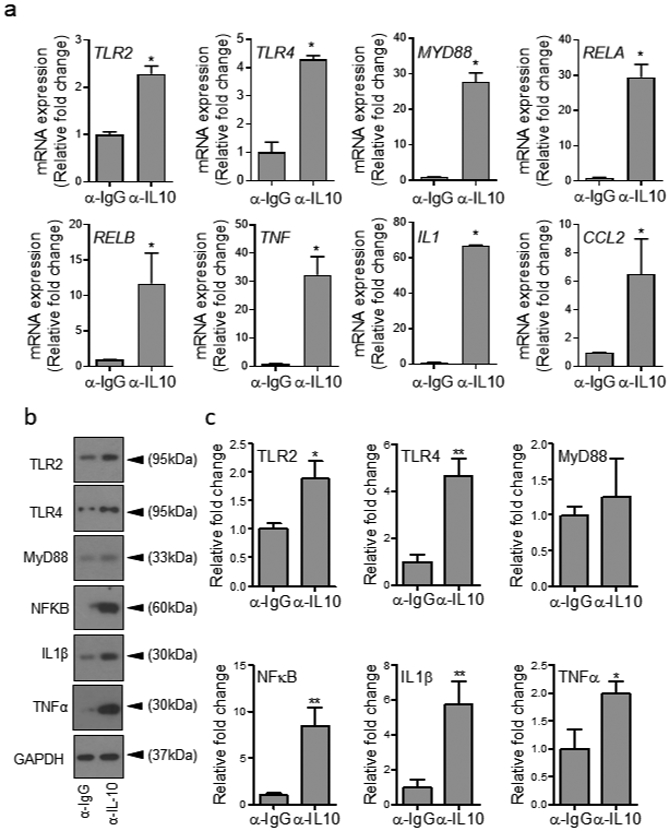
(a-c) hPBMCs were exposed to high glucose (300 mg/dl) for 1h in the presence of either α-IL-10 or α-IgG antibodies at 5μg/ml and assessed for mRNA levels of indicated genes using RT-PCR (a), or by protein analyses using Western blotting (b) and corresponding densitometer tabulated data for Western blots are in (c). All data are plotted as the mean ± SEM. (Each experiment was repeated at least 2 times for (a) and at least 3 times for (b-c); *p<0.05, **p<0.01, ***p<0.001. Pair-wise statistical analyses between groups were performed by unpaired Student’s t-test).
Importantly, blocking IL-10 signaling by α- IL10 antibody significantly reduced IL-10 levels in diabetic wounds (Figure 4a), and increased the expression of indicated TLR components in diabetic wounds early after treatment, albeit with varying kinetics (Figure 4b and Figure S6). We further corroborated these data by assessing the expression of representative TLR signaling components (TLR4 and MyD88) in day 1 wounds treated with α- IL10 by mRNA analysis and by histological analysis (Figure 4c-e).
Figure 4. Blocking IL-10 signaling dampens IL-10 and enhances TLR signaling and proinflammatory cytokines production in diabetic wound.
(a-e) db/db wounds were treated topically with α-IgG or α-IL10 antibodies (10μg/wound), immediately after wounding. Wound tissues were analyzed at indicated timepoints for IL-10 by ELISA (a), for the expression of indicated genes by Western blotting (b), by RT-PCR (c), by IHC (d), and the corresponding data for (d) are plotted as the mean ± SEM in (e). (N=5 mice/group for all experiments. For (d-e) ≥9 random fields/wound/mouse, red scale bars=50μm. Data were then normalized per wound area and plotted as mean ± SEM, *p<0.05, ** p<0.01, ***p<0.001, Statistical analyses between groups were performed by One-way ANOVA, and pair-wise comparisons within groups were performed or by unpaired Student’s t-test).
Blocking IL-10 stimulates healing in diabetic wound.
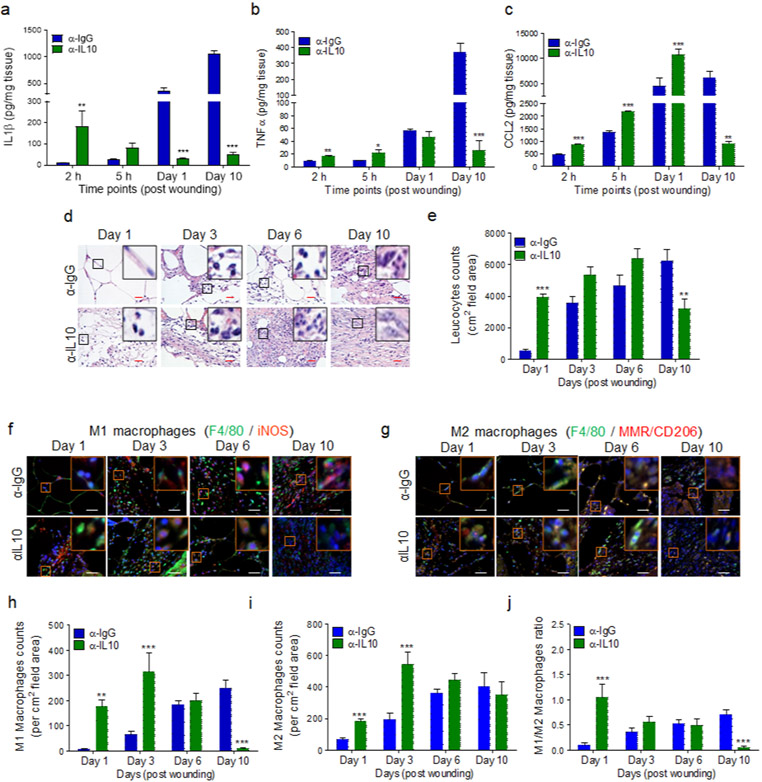
As compared to α-IgG-treated wounds, diabetic wounds treated with α-IL-10 antibody showed significant increases in IL-1β and TNF-α early after treatment within the first 5h, but significantly lower levels in day 10 older wounds (Figure 5a-b). CCL2 expression showed similar pattern, except that it also remained higher in day 1 db/db wounds treated with α-IL-10 but by day 10, CCL2 expression was also significantly diminished in these wounds (Figure 5c). Similarly, leukocyte numbers - as assessed by immunohistochemistry, using Hematoxylin Eosin (H&E) staining (Goldufsky et al., 2015, Wood et al., 2014) - increased significantly early after injury but decreased in day 10 wounds in α-IL10-treated diabetic wounds (Figure 5d-e and Figure S7).
Figure 5. Blocking IL-10 signaling enhances inflammatory responses during the acute phase of wound healing.
db/db wounds were treated with α-IgG or α-IL10 (10μg/wound) after wounding and assessed at indicated timepoints for indicated proinflammatory cytokines (a-c), for leukocytes by H&E staining (d-e), and for M1 and M2 macrophages, by IF microscopy using F4/80/iNOS or F4/80/MMR/CD206 respectively (f-g). The corresponding data of (d, f, & g) are plotted as mean ± SEM (Red scale bars=50μm, White scale bar= 50 μm) in (e, h, & i) respectively. M1/M2 macrophage ratios are shown in (j). (N=5 mice/group; ≥9 random fields/wound/mouse. The number of leukocytes and macrophages were normalized per wound area. *p<0.05, ** p<0.01, ***p<0.001. Statistical analyses between groups were performed by One-way ANOVA, and pair-wise comparisons within groups were performed or by unpaired Student t-test).
Macrophages are generally classified as either classically activated proinflammatory macrophages (M1) or alternatively activated anti-inflammatory and reparative macrophages (M2), although other M2 macrophage subtypes have also been described (Ferrante and Leibovich, 2012, Loegl et al., 2016). In normal healing, M1 macrophages predominate early after injury and contribute to inflammatory responses, whereas M2 macrophages predominate the wound as it transitions into the proliferation phase and play critical roles in new tissue regeneration and remodeling phases (Krzyszczyk et al., 2018, Mirza et al., 2009). We assessed the impact of IL-10 inhibition on the dynamics and distribution of M1 and M2 macrophages by co-staining the wound tissues with monocyte/macrophage marker F4/80 either with M1 macrophage marker iNOS (inducible nitric oxide synthase), or with M2 macrophage marker MMR/CD206 (Bastian et al., 2018, Klar et al., 2018). Treatment with α-IL-10 significantly increased both M1 and M2 macrophages in diabetic wounds on days 1 and 3 (Figure 5f-i and Figure S8). However, M1 macrophages were significantly reduced in α-IL-10-treated day 10 diabetic wounds and the remaining macrophages were of the M2 type, as assessed by M1/M2 ratios (Figure 5i-j).
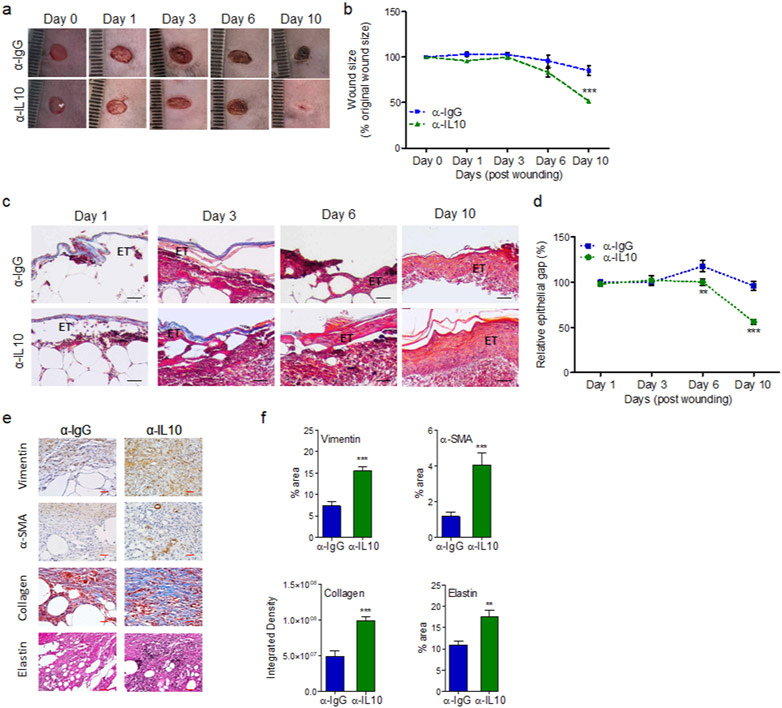
Importantly, treatment with α-IL-10 antibody significantly improved healing in diabetic wounds, as assessed by digital photography and histological analyses of re-epithelization and epidermal thickening assessment using H&E staining (Figure 6a-d and Figure S9a). Persistent inflammatory environment in diabetic chronic wounds adversely affects fibroblast and myofibroblasts functions resulting in reduced collagen and elastin extracellular matrix deposition in diabetic chronic wounds (Augustine et al., 2014, Diegelmann and Evans, 2004, Yue et al., 1986). α-IL-10-treated diabetic wounds showed significant increases in fibroblast, myofibroblast, elastin, and collagen healing markers in day 10 diabetic wounds (Figure 6e-f and Figure S9b), as assessed by IHC, using their respective markers Vimentin, α-SMA, Elastin, and Masson’s Trichrome staining (Goldufsky et al., 2015, Hinz, 2006, Wilgus et al., 2008).
Figure 6. Blocking IL-10 signaling stimulates wound healing in diabetic wound.
db/db wounds were treated with either α-IgG or α-IL10 (10μg/wound) and assessed for wound healing by digital photography (a-b); by the histochemical assessment of re-epithelization and epidermal thickening, using H&E staining (c & d); and by histochemical assessments of the Vimentin, α-SMA, Masson’s Trichrome staining, and Elastin healing markers (e-f). Representative images are shown in (a, c, & e) and the corresponding data are plotted as mean ± SEM in (b, d, & f). Black scale bars=100μm, red scale bars=50μm. (N ≥ 5 mice/group, ≥9 random fields/wound/mouse, *p<0.05, ** p<0.01, ***p<0.001. Statistical analyses between groups were performed by One-way ANOVA, and pair-wise comparisons within groups were performed or by unpaired Student’s t-test).
Corroborating these data, blocking IL10 signaling by treating diabetic wounds with anti-IL10 receptor antibody (α- IL10R) also yielded similar results, showing significant increases in representative TLR components (TLR4 and MyD88) in day 1 diabetic wound (Figure S10a-b), and higher levels of CCL2 in day 1 and day 3 wounds, but significantly lower levels of CCL2 in day 6 and day 10 old wounds (Figure S10c). Diabetic wounds treated with α-IL10R antibody also contained significantly more leukocytes, as assessed by H&E staining, and more macrophages as assessed by IHC using macrophage marker anti-CD68 antibody (Wood et al., 2014), on days 1 and 3 (Figure S10d-g) and healed significantly better than (α-IgG-treated diabetic wounds (Figure S11). Collectively, these data indicated that diabetic wounds will not develop persistent non-resolving inflammation and heal significantly better, if IL-10 signaling is disrupted in these wounds early after injury.
DISCUSSION
Here we report that acute exposure (1h) to HG causes a rapid transformation of human and mouse monocytes into an anti-inflammatory phenotype, manifested by increased expression and secretion of IL-10, which in an autocrine/paracrine fashion, causes reduction in TLR signaling and proinflammatory cytokines production in hPBMCs and mBMDMs. We posit that the rapid rise in the IL-10 expression and secretion in response to acute exposure to HG is a protective measure taken by monocytes to reduce glucose uptake (as our data show) in order to prevent cellular and organelle damage which has been shown to occur after prolonged exposure to HG (Allen et al., 2003, Kumar and Sitasawad, 2009, Vanhorebeek et al., 2009).
Our data indicate that inadequate inflammatory responses in diabetic wound during the acute phase of healing early after injury is a major contributing factor to impaired healing in diabetic wound. Consistent with this notion, prospective studies involving diabetic individuals with healing and non-healing foot ulcers have found elevated proinflammatory characteristics, (e.g., higher M1/M2 macrophage and enhanced NF-κB signaling network), at initial visit (early after injury) as prognostic markers for healing DFUs, whereas these markers were lower at the initial visit but continued to increase in non-healing DFUs (Nassiri et al., 2015, Theocharidis et al., 2020).
Our data further indicate that increased IL-10 expression in diabetic wound early after injury is a major factor, contributing to impaired healing in diabetic wound. IL-10 knockout mice exhibit accelerated healing, albeit healing is associated with increased extracellular deposition and scarring (Eming et al., 2007, Liechty et al., 2000), suggesting that IL-10 is a negative regulator of scarring during wound healing. Interestingly, diabetic chronic wounds exhibit inadequate extracellular matrix deposition and reduced scarring (Augustine et al., 2014, Diegelmann and Evans, 2004, Yue et al., 1986), suggesting that reduced scarring in DFUs may be due to increased IL-10.
There appears to be a disconnect between our data showing increased IL-10 expression and reduced proinflammatory cytokine production resulting from short-term exposure to HG and the clinical reports indicating decreased IL-10 levels and increased proinflammatory cytokines in the blood of diabetic individuals, and proinflammatory phenotype in monocytes/macrophages after prolonged exposure to HG (Hotamisligil et al., 1995, Keane et al., 2017, Yaghini et al., 2011). Circulating classical monocytes have 24h lifespan (Daley et al., 2010, Patel et al., 2017), thus they have prolonged exposure to HG in diabetic individuals. We also find that 24h exposure to HG transforms monocytes proinflammatory but interestingly, no IL-10 could be detected in these cells or in their culture supernatants. We propose that despite initial increases in IL-10 expression and secretion in monocytes exposed to HG, prolonged exposure to HG allows for gradual increases in cytosolic glucose levels, which in turn causes mitochondrial damage (Devi et al., 2013, Freemerman et al., 2014, Ip et al., 2017, Park and Park, 2013), which eventually shuts down IL-10 expression and drives monocytes/macrophages toward proinflammatory phenotype. More studies are needed to determine the underlying causes of monocyte’s biphasic and opposite responses to short-term versus long-term exposure to HG, and how these responses may lead to insufficient inflammatory responses in diabetic wounds early after injury, and persistent non-resolving inflammation as these become chronic.
Previous reports by Dasu et al., (Dasu and Jialal, 2013, Dasu et al., 2010b) have shown increased expression of TLR2, TLR4, TLR6 and proinflammatory cytokines in streptozotocin (STZ)-induced type 1diabetic mice at day 10 post-wounding. They also provided bar graphs to show accelerated healing in STZ-induced TLR2−/− or TLR4−/− diabetic mice. Disappointingly, in these reports there were no histological or photographical data to corroborate their accelerated healing data. Our data support their finding with respect to the heightened inflammatory environment in day 10 diabetic wounds. However, our data highlight the need for proinflammatory responses during the acute phase of healing for proper healing in diabetic wounds. Our findings are in line with the preponderance of evidences that point to a central role for inflammatory responses and TLR signaling in mediating effective wound healing (Chen and DiPietro, 2017, Goren et al., 2009, Leibovich and Ross, 1975, Lucas et al., 2010, Maruyama et al., 2007, Mirza et al., 2009, Portou et al., 2015).
Our data indicates that blocking IL-10 signaling resulted in significant increases in both M1 and M2 macrophages early after injury, as assessed by F4/80 staining in combination with iNOS or MMR/CD206 respectively. It remains unclear whether the monocytes trafficking into diabetic wounds after IL-10 signaling blockade originated from circulation (hematopoietic origin) or from skin resident monocytes (yolk-sac origin). Previously, circulating monocytes/macrophages from hematopoietic origin were shown to be F4/80lo whereas skin resident monocytes originating from yolk sac were found to be F4/80high (Schulz et al., 2012), thus suggesting that these macrophages may be originating from skin. However, more recent studies have shown that circulating monocytes from hematopoietic origin populate wound initially after injury and these monocytes/macrophages also express F4/80 (Castela et al., 2017, Crane et al., 2014, Hopkinson-Woolley et al., 1994, Li et al., 2008, Maruyama et al., 2007, Wood et al., 2014). Interestingly, Francke et al., reported that while bone marrow monocytes are initially F4/80lo, they become F4/80high after adhering to the surface in the presence of M-CSF (Francke et al., 2011), suggesting that bone marrow/hematopoietic monocytes have the capacity to become F4/80high after populating the wound. More detailed studies are needed to determine the source(s) of monocytes populating the diabetic wound after IL-10 signaling blockade.
Encouragingly, we found that one-time topical treatment with anti-IL-10 or anti- IL-10R antibodies significantly enhanced inflammatory responses during the acute phase of healing and substantially improved healing in diabetic wounds. Given that diabetic foot ulcers suffer from persistent inflammation, one might question the therapeutic value of anti-IL-10 treatments in diabetic foot ulcers. We propose that anti-IL-10 strategies may have therapeutic potential in diabetic wound care, (at least in a subset of obese type 2 diabetic individuals which our model represents), if applied topically after the surgical wound debridement, which is performed as a standard of care weekly or biweekly to reset a chronic non-healing ulcer into an acute fresh wound (Cardinal et al., 2009, Golinko et al., 2008, Lebrun et al., 2010), which we propose to be more similar to the day 1 wound environment in diabetic mice.
MATERIALS AND METHODS (Additional experimental details are provided in Supplementary Materials & Methods)
Animals:
We have an approval from the Rush University Medical Center Institutional Animal Care and Use Committee (IACUC No: 18-037), which allow us to conduct research as indicated. We obtained 8-10 weeks old C57BL/6 (normal) and their diabetic littermates, C57BLKS-m leprdb (db/db), mice from the Jackson Laboratories (Bar Harbor, ME). Wounding was carried out as we described previously (Goldufsky et al., 2015, Wood et al., 2014).
MONOCYTES ISOLATION FROM HUMAN AND MOUSE:
We have an Institutional Review Board (IRB)- approved protocol (ORA #: 16120704-IRB02) from Rush University Medical Center which allows us to collect blood samples from volunteers with their informed written consents for these studies. Human monocytes from healthy subjects (both male and female) were purified from peripheral blood using the EasySep™ Human Monocytes Enrichment Kit (STEMCELL Technologies), according to manufacturer’s protocol. Mouse monocytes were extracted from either bone marrow using the EasySep™ Mouse Monocytes Enrichment Kit (STEMCELL Technologies), as per manufacture’s protocol.
HISTOPATHOLOGICAL EVALUATION by H&E Staining:
Leukocytes in wounds were identified by their rounded or polymorphonuclear morphology as described previously (Goldufsky et al., 2015, Kroin et al., 2016). Macrophages were assessed by immunohistochemical (IHC) analysis using monocyte/macrophage markers F4/80 with iNOS (M1 macrophages) or F4/80 with MMR/CD206 (M2 macrophages), or macrophage marker CD68 as described (Lucas et al., 2010, Wood et al., 2014). Wound tissues’ contents of IL-1β, TNF-α and CCL2 were assessed by ELISA, as described (Gupta et al., 2017). Wound healing was assessed by digital photography, by re-epithelization assessment using H&E staining, and by histological assessments of elastin, vimentin, α-SMA, and collagen matrix deposition, as described (Almine et al., 2012, Goldufsky et al., 2015, Wilgus et al., 2008, Wood et al., 2014).
GLUCOSE UPTAKE:
Glucose uptake in monocytes was assessed by EZCell™ Glucose Uptake Assay Kit (Cat. No. K924 from BioVision) according to the manufacturer’s protocol.
WESTERN BLOTTING:
We performed Western immunoblotting on cell lysates or on tissue lysates, using the indicated antibodies, as we described previously (Kroin et al., 2016, Shafikhani and Engel, 2006).
GENE EXPRESSION ANALYSIS BY REAL-TIME POLYMERASE CHAIN REACTION (RT-PCR):
Gene expression at transcriptional level in wound tissues was assessed by mRNA analysis of indicated genes using RT-PCR, as described (Gupta et al., 2017, Wood et al., 2014).
STATISTICAL ANALYSIS
was performed as we described previously (Goldufsky et al., 2015, Gupta et al., 2017, Wood et al., 2014). Briefly, we applied One-way ANOVA with Bonferroni post hoc testing for statistical analyses involving multiple groups. For pair-wise comparisons between any two groups, we used unpaired Student’s t-test. Tabulated data were presented as the Mean ± SEM. P-values less than or equal to 0.05 were taken as significant.
Supplementary Material
RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| NF-κB p65 (D14E12) XP® Rabbit mAb | Cell Signaling Technology | Cat. No. 8242 |
| IL-1β (3A6) Mouse mAb | Cell Signaling Technology | Cat. No. 12242 |
| F4/80 (D2S9R) XP® Rabbit mAb | Cell Signaling Technology | Cat. No. 70076 |
| Mouse (G3A1) mAb IgG1 Isotype Control | Cell Signaling Technology | Cat. No. 5415 |
| GAPDH Antibody Rabbit Polyclonal | Proteintech | Cat. No. 1094-I-AP |
| MCP-1 Antibody (Mouse Specific) | Cell Signaling Technology | Cat. No. 2029 |
| TLR1 Rat anti-Mouse, Clone: 285923 | R &D systems | Cat. No. MAB1475 |
| TLR2 Goat anti-Mouse, Polyclonal | R &D systems | Cat. No. AF1530 |
| TLR4 Antibody (25) | Santa Cruz Biotechnology | Cat. No. 293072 |
| IL-10 Antibody (E-10) | Santa Cruz Biotechnology | Cat. No. 8438 |
| TLR2 Antibody (TL2.1) | Santa Cruz Biotechnology | Cat. No. 21759 |
| TNF alpha Antibody | Santa Cruz Biotechnology | Cat. No. 52746 |
| Anti-MyD88 antibody | Abcam | Cat. No. ab135693 |
| Anti-IL-10 antibody [JES5-2A5] | Abcam | Cat. No. ab33471 |
| InVivoMAb anti-mouse IL-10R (CD210), clone: 1B1.3A | InVivoMab Antibodies | Cat. No. BE0050 |
| Recombinant Anti-G-CSF antibody [EPR3203(N)(B)] | Abcam | Cat. No. ab181053 |
| TRAF6 Polyclonal Antibody | Invitrogen | Cat. No. 38-0900 |
| GM-CSF Monoclonal Antibody (22E9) | Invitrogen | Cat. No. MM500C |
| IL-10 Monoclonal Antibody (JES3-9D7) | Invitrogen | Cat. No. AHC0102 |
| Mouse (MOPC-21) mAb IgG1 Isotype Control | Cell Signaling Technology | Cat. No. 4097 |
| InVivoMAb anti-mouse IL-10, Clone: JES5-2A5 | InVivoMab Antibodies | Cat. No. BE0049 |
| anti-horseradish peroxidase, Clone: HRPN | InVivoMab Antibodies | Cat. No. BE0088 |
| F4/80 (D2S9R) XP® Rabbit mAb | Cell Signaling Technology | Cat. No. 70076 |
| MMR/CD206/Mannose Receptor Antibody | Novus Biologicals | Cat no. NBP1-90020 |
| iNOS Antibody | Novus Biologicals | Cat no. NBP2-22119 |
| F4/80 Antibody (C-7) | Santa Cruz Biotechnology | Cat no. 377009 |
| Goat anti-Rabbit IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Thermo Fisher | Cat no. A11-012 |
| Alexa Fluor® 488 AffiniPure Goat Anti-Mouse IgG (H+L) | Jackson Immuno Research | Cat no. 115-545-062 |
| Anti-α-SMA antibody | Abcam | Cat. No. ab5694 |
| Anti-vimentin antibody | Abcam | Cat. No. ab92547 |
| Reagents and kits | ||
| Hematoxylin | Thermo Fisher | Cat. No. 7111L |
| Eosin Y | Thermo Fisher | Cat. No. 7211L |
| Bluing Reagent | Thermo Fisher | Cat. No. 7301L |
| Masson’s Trichrome stain | Abcam | Cat. No. ab150686 |
| EasySep Human Monocytes Enrichment Kit | STEMCELL Technologies | Cat. No. 19359 |
| EasySep Mouse monocytes Enrichment Kit | STEMCELL Technologies | Cat. No. 19861 |
| EasySep Buffer | STEMCELL Technologies | Cat. No. 20144 |
| SYBR™ Green PCR Master Mix | Thermo Fisher | Cat. No. 4309155 |
| SuperScript™ III First-Strand Synthesis System | Thermo Fisher | Cat. No. 18080051 |
| Elastic connective tissue stain | Abcam | Cat. No. ab150667 |
| Critical Commercial Assays | ||
| CCL2 ELISA kit | Thermo Fisher | Cat. No. 88-7391-22 |
| IL-1β ELISA kit | Thermo Fisher | Cat. No. 88-7013-88 |
| TNF-α ELISA kit | Thermo Fisher | Cat. No. 88-7324-88 |
| EZCell™ Glucose Uptake Assay kit | BioVision | Cat. No. K924 |
| Experimental Models: Organisms | ||
| Mouse: C57BL/6J | Jackson laboratories | 000664 |
| Mouse: C57BLKS-m leprdb(db/db) | Jackson laboratories | 000642 |
| Oligonucleotides | ||
| Human: | ||
| Tlr2 Forward: GAAGAGTGAGTGGTGCAAGTAT, Reverse: AATGGGCTCCAGAAGAATGAG; | Integrated DNA Technologies | N/A |
| Tlr4 Forward: TTTCAGCTCTGCCTTCACTAC, Reverse: GACACCACAACAATCACCTTTC | Integrated DNA Technologies | N/A |
| MYD88 Forward: CTGTGTCTGGTCTATTGCTAGTG, Reverse: TTCCTTGCTCTGCAGGTAATC | Integrated DNA Technologies | N/A |
| IL1 Forward: CAAAGGCGGCCAGGATATAA, Reverse: CTAGGGATTGAGTCCACATTCAG | Integrated DNA Technologies | N/A |
| TNF Forward: GCAGGTCTACTTTGGGATCATT, Reverse: AGAAGAGGTTGAGGGTGTC | Integrated DNA Technologies | N/A |
| RelA Forward: CTGTCCTTTCTCATCCCATCTT, Reverse: TCCTCTTTCTGCACCTTGTC | Integrated DNA Technologies | N/A |
| RelB Forward: CTGCGGATTTGCCGAATTAAC, Reverse: ACACCACTGATATGTCCTCTTTC | Integrated DNA Technologies | N/A |
| Ccl2 Forward: TCATAGCAGCCACCTTCATTC, Reverse: CTCTGCACTGAGATCTTCCTATTG | Integrated DNA Technologies | N/A |
| Ccl3 Forward: GGCAGATTCCACAGAATTTCATAG, Reverse: TCGCTTGGTTAGGAAGATGAC | Integrated DNA Technologies | N/A |
| IL10 Forward: TCCTTGCTGGAGGACTTTAAGGGT, Reverse: TGTCTGGGTCTTGGTTCTCAGCTT | Integrated DNA Technologies | N/A |
| 18s Forward: CACGGACAGGATTGACAGATT, Reverse: GCCAGAGTCTCGTTCGTTATC | Integrated DNA Technologies | N/A |
| GAPDH Forward: GGTGTGAACCATGAGAAGTATGA, Reverse: GAGTCCTTCCACGATACCAAAG | Integrated DNA Technologies | N/A |
| Mice: | ||
| granulocyte (Csf3) Forward: TGTTCCCAAACTGGGTTCTT, Reverse: TGGCTGCCACTGTTTCTT | Integrated DNA Technologies | N/A |
| GM-CSF Forward: AGCTCTGAATCCAGCTTCTC, Reverse: CCACATCTCTTGGTCCCTTTA | Integrated DNA Technologies | N/A |
| Ccl2 Forward: CTCACCTGCTGCTACTCATTC, Reverse: ACTACAGCTTCTTTGGGACAC | Integrated DNA Technologies | N/A |
| Ccl3 Forward: TCACTGACCTGGAACTGAATG, Reverse: CAGCTTATAGGAGATGGAGCTATG | Integrated DNA Technologies | N/A |
| Rela Forward: CCGACTTGTTTGGGTGATCT, Reverse: TCCGTCTCCAGGAGGTTAAT | Integrated DNA Technologies | N/A |
| Relb Forward: TGCCGAATCAACAAGGAGAG, Reverse: TGCTGAACACCACGGATATG | Integrated DNA technologies | N/A |
| Tlr2 Forward: CACTATCCGGAGGTTGCATATC, Reverse: GGAAGACCTTGCTGTTCTCTAC | Integrated DNA Technologies | N/A |
| Tlr4 Forward: GAGCAAACAGCAGAGGAAGA, Reverse: CCAGGTGAGCTGTAGCATTTA | Integrated DNA Technologies | N/A |
| Myd88 Forward: AGCAACTAGGACTGCCTTTC, Reverse: GAACTCTTCCACTCAGCTATCC | Integrated DNA Technologies | N/A |
| IL-1 Forward: GCACTACAGGCTCCGAGATGAAC; Reverse: TTGTCGTTGCTTGGTTCTCCTTGT | Integrated DNA Technologies | N/A |
| Tnf Forward: TTGTCTACTCCCAGGTTCTCT, Reverse: GAGGTTGACTTTCTCCTGGTATG | Integrated DNA Technologies | N/A |
| GAPDH Forward: TTGGGTTGTACATCCAAGCA, Reverse: CAAGAAACAGGGGAGCTGAG | Integrated DNA Technologies | N/A |
| Cell lines | ||
| Mouse Primary Epidermal Keratinocytes | Cell Biologics | Cat No: C57-6066K |
| Mouse Primary Dermal Lymphatic Endothelial Cells | Cell Biologics | Cat No: C57-6064L |
| Software and Algorithms | ||
| GraphPad Prism | GraphPad | https://graphpad.com/scientific-software/prism/ |
ACKNOWLEDGMENTS:
We would like to thank Dr. Luisa DiPietro (University of Illinois Chicago) and Dr. Aristatis Veves (Beth Israel Deaconess Medical Center) for their feedbacks and insights into these projects. We would like to thank the rest of Shafikhani lab for their valued opinions on these studies. This work was supported by the National Institutes of Health (NIH) grants RO1DK107713 to (S.H.S.), F31DK118797 to (J.Z.), and the institutional NIH training grant R25109421.
Footnotes
CONFLICT OF INTEREST: Rush University Medical Center has filed a patent on these findings. Drs. Sasha Shafikhani and Ruchi Roy are the listed inventors on this application.
Dr. Reiser reports personal fees from Biomarin, grants from National Institute of Health, grants from Nephcure, other from TRISAQ, grants from Thermo BCT, personal fees from Astellas, personal fees from Massachusetts General Hospital, personal fees from Genentech, personal fees from Up to Date, personal fees from Merck, personal fees from Incepetionsci, personal fees from GLG, Personal fees from Visterra outside the submitted work; In addition, Dr. Reiser has a patent US20110212083- Role of soluble uPAR inthe Pathogenesis of Proteinuric Kidney Disease with royalties paid to TRISAQ, a patent US9867923-Reducing Solucble rokinase Receptor in the Circulation with royalties paid to Miltenyi, a patent JP2016530510-Non-Glycoslyated suPAR Biomarkers and Uses thereof with royalties paid to TRISAQ, a patent US20160296592-Methods/Compositions for the Treatment of Proteinuric Diseases with royalties paid to TRISAQ, a patent US9144594-Dynamin Mediated Diseases with royalties paid to TRISAQ, and a patent US8809386-Dynamin Ring Stabilizers with royalties paid to TRISAQ.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY STATEMENT:
No datasets were generated or analyzed during this study.
REFERENCES
- Abke S, Neumeier M, Weigert J, Wehrwein G, Eggenhofer E, Schäffler A, et al. Adiponectin-induced secretion of interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1, CCL2) and interleukin-8 (IL-8, CXCL8) is impaired in monocytes from patients with type I diabetes. Cardiovascular diabetology 2006;5(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter N, Hasan A, Shenouda S, Wilson A, Kochumon S, Ali S, et al. TLR4/MyD88-mediated CCL2 production by lipopolysaccharide (endotoxin): Implications for metabolic inflammation. Journal of Diabetes & Metabolic Disorders 2018;17(1):77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DA, Harwood SM, Varagunam M, Raftery MJ, Yaqoob MM. High glucose- induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. The FASEB journal 2003;17(8):1–21. [DOI] [PubMed] [Google Scholar]
- Almine JF, Wise SG, Weiss AS. Elastin signaling in wound repair. Birth Defects Research Part C: Embryo Today: Reviews 2012;96(3):248–57. [DOI] [PubMed] [Google Scholar]
- Augustine R, Kalarikkal N, Thomas S. Role of wound dressings in the management of chronic and acute diabetic wounds. Diabetes Mellit Hum Health Care Holist Approach Diagn Treat 2014:273–314. [Google Scholar]
- Bastian C, Zaleski J, Stahon K, Parr B, McCray A, Day J, et al. NOS3 inhibition confers post-ischemic protection to young and aging white matter integrity by conserving mitochondrial dynamics and miro-2 levels. Journal of Neuroscience 2018;38(28):6247–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnsholt T, Kirketerp-Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, et al. Why chronic wounds will not heal: a novel hypothesis. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 2008;16(1):2–10. [DOI] [PubMed] [Google Scholar]
- Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006;23(6):594–608. [DOI] [PubMed] [Google Scholar]
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. The Journal of clinical investigation 2007;117(5):1219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal M, Eisenbud DE, Armstrong DG, Zelen C, Driver V, Attinger C, et al. Serial surgical debridement: a retrospective study on clinical outcomes in chronic lower extremity wounds. Wound Repair and Regeneration 2009;17(3):306–11. [DOI] [PubMed] [Google Scholar]
- Castela M, Nassar D, Sbeih M, Jachiet M, Wang Z, Aractingi S. Ccl2/Ccr2 signalling recruits a distinct fetal microchimeric population that rescues delayed maternal wound healing. Nature communications 2017;8(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, DiPietro LA. Toll-Like Receptor Function in Acute Wounds. Adv Wound Care (New Rochelle) 2017;6(10):344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL Jr, Albina JE. The monocyte to macrophage transition in the murine sterile wound. PloS one 2014;9(1):e86660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtale G, Mirolo M, Renzi TA, Rossato M, Bazzoni F, Locati M. Negative regulation of Toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proceedings of the National Academy of Sciences of the United States of America 2013;110(28):11499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley JM, Brancato SK, Thomay AA, Reichner JS, Albina JE. The phenotype of murine wound macrophages. Journal of leukocyte biology 2010;87(1):59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care 2010a;33(4):861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Jialal I. Amelioration in wound healing in diabetic toll-like receptor-4 knockout mice. Journal of diabetes and its complications 2013;27(5):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Thangappan RK, Bourgette A, DiPietro LA, Isseroff R, Jialal I. TLR2 expression and signaling-dependent inflammation impair wound healing in diabetic mice. Lab Invest 2010b;90(11):1628–36. [DOI] [PubMed] [Google Scholar]
- Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabetic Medicine 1997;14(1):29–34. [DOI] [PubMed] [Google Scholar]
- Devi TS, Hosoya K-I, Terasaki T, Singh LP. Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: implications for diabetic retinopathy. Experimental cell research 2013;319(7):1001–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004;9:283–9. [DOI] [PubMed] [Google Scholar]
- Dipietro LA, Reintjes MG, Low QE, Levi B, Gamelli RL. Modulation of macrophage recruitment into wounds by monocyte chemoattractant protein-1. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 2001;9(1):28–33. [DOI] [PubMed] [Google Scholar]
- Eming SA, Werner S, Bugnon P, Wickenhauser C, Siewe L, Utermöhlen O, et al. Accelerated wound closure in mice deficient for interleukin-10. The American journal of pathology 2007;170(1):188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Advances in wound care 2012;1(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino DF, Bond MW, Mosmann T. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. The Journal of experimental medicine 1989;170(6):2081–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke A, Herold J, Weinert S, Strasser RH, Braun-Dullaeus RC. Generation of mature murine monocytes from heterogeneous bone marrow and description of their properties. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society 2011;59(9):813–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. The Journal of biological chemistry 2014;289(11):7884–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldufsky J, Wood SJ, Jayaraman V, Majdobeh O, Chen L, Qin S, et al. Pseudomonas aeruginosa uses T3SS to inhibit diabetic wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 2015;23(4):557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golinko MS, Joffe R, Maggi J, Cox D, Chandrasekaran EB, Tomic-Canic RM, et al. Operative debridement of diabetic foot ulcers. Journal of the American College of Surgeons 2008;207(6):e1–e6. [DOI] [PubMed] [Google Scholar]
- Goren I, Allmann N, Yogev N, Schurmann C, Linke A, Holdener M, et al. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. The American journal of pathology 2009;175(1):132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Hogg JC, Suwa T, Quinlan KB, van Eeden SF. A novel method to quantify the turnover and release of monocytes from the bone marrow using the thymidine analog 5′-bromo-2′-deoxyuridine. American Journal of Physiology-Cell Physiology 2003;285(2):C253–C9. [DOI] [PubMed] [Google Scholar]
- Gupta KH, Goldufsky JW, Wood SJ, Tardi NJ, Moorthy GS, Gilbert DZ, et al. Apoptosis and Compensatory Proliferation Signaling Are Coupled by CrkI-Containing Microvesicles. Dev Cell 2017;41(6):674–84 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H, Augustine N, Rallison M, Santos J. Defective monocyte chemotactic responses in diabetes mellitus. J Clin Immunol 1983;3(1):70–7. [DOI] [PubMed] [Google Scholar]
- Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. European journal of cell biology 2006;85(3-4):175–81. [DOI] [PubMed] [Google Scholar]
- Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. Journal of cell science 1994;107(5):1159–67. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. The Journal of clinical investigation 1995;95(5):2409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang Q, Liu J, Hao H, Jiang C, Han W. Granulocyte-Colony Stimulating Factor (G-CSF) Accelerates Wound Healing in Hemorrhagic Shock Rats by Enhancing Angiogenesis and Attenuating Apoptosis. Medical science monitor : international medical journal of experimental and clinical research 2017;23:2644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of proinflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine 1996;8(7):548–56. [DOI] [PubMed] [Google Scholar]
- Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017;356(6337):513–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y, Kuninaka Y, Nosaka M, Furuta M, Kimura A, Taruya A, et al. CCL2-Mediated Reversal of Impaired Skin Wound Healing in Diabetic Mice by Normalization of Neovascularization and Collagen Accumulation. Journal of Investigative Dermatology 2019;139(12):2517–27.e5. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Frontiers in immunology 2014;5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane KN, Calton EK, Carlessi R, Hart PH, Newsholme P. The bioenergetics of inflammation: insights into obesity and type 2 diabetes. European journal of clinical nutrition 2017;71(7):904–12. [DOI] [PubMed] [Google Scholar]
- Klar AS, Michalak-Mićka K, Biedermann T, Simmen-Meuli C, Reichmann E, Meuli M. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatric surgery international 2018;34(2):129–35. [DOI] [PubMed] [Google Scholar]
- Kluwe J, Mencin A, Schwabe RF. Toll-like receptors, wound healing, and carcinogenesis. J Mol Med (Berl) 2009;87(2):125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knödler A, Schmidt SM, Bringmann A, Weck MM, Brauer KM, Holderried TAW, et al. Post-transcriptional regulation of adapter molecules by IL-10 inhibits TLR-mediated activation of antigen-presenting cells. Leukemia 2009;23(3):535–44. [DOI] [PubMed] [Google Scholar]
- Kroin JS, Li J, Goldufsky JW, Gupta K, Moghtaderi M, Buvanendran A, et al. Perioperative high inspired oxygen fraction therapy reduces surgical site infection with Pseudomonas aeruginosa in rats. Journal of medical microbiology 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Frontiers in physiology 2018;9:419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sitasawad SL. N-acetylcysteine prevents glucose/glucose oxidase-induced oxidative stress, mitochondrial damage and apoptosis in H9c2 cells. Life sciences 2009;84(11-12):328–36. [DOI] [PubMed] [Google Scholar]
- Lebrun E, Tomic- Canic M, Kirsner RS. The role of surgical debridement in healing of diabetic foot ulcers. Wound repair and regeneration 2010;18(5):433–8. [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infection and immunity 1999;67(9):4435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Huang L, Sung S-SJ, Vergis AL, Rosin DL, Rose CE Jr, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia–reperfusion injury. Kidney international 2008;74(12):1526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10–deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg 2000;35(6):866–73. [DOI] [PubMed] [Google Scholar]
- Lin Q, Fang D, Fang J, Ren X, Yang X, Wen F, et al. Impaired wound healing with defective expression of chemokines and recruitment of myeloid cells in TLR3-deficient mice. The Journal of Immunology 2011;186(6):3710–7. [DOI] [PubMed] [Google Scholar]
- Loegl J, Hiden U, Nussbaumer E, Schliefsteiner C, Cvitic S, Lang I, et al. Hofbauer cells of M2a, M2b and M2c polarization may regulate feto-placental angiogenesis. Reproduction 2016;152(5):447–55. [DOI] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, et al. Differential roles of macrophages in diverse phases of skin repair. Journal of immunology 2010;184(7):3964–77. [DOI] [PubMed] [Google Scholar]
- Macedo L, Pinhal-Enfield G, Alshits V, Elson G, Cronstein BN, Leibovich SJ. Wound healing is impaired in MyD88-deficient mice: a role for MyD88 in the regulation of wound healing by adenosine A2A receptors. Am J Pathol 2007;171(6):1774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann A, Niekisch K, Schirmacher P, Blessing M. Granulocyte–macrophage colony-stimulating factor is essential for normal wound healing. Journal of Investigative Dermatology Symposium Proceedings: Elsevier; 2006. p. 87–92. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol 2007;170(4):1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. The American journal of pathology 2009;175(6):2454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology 2001;19(1):683–765. [DOI] [PubMed] [Google Scholar]
- Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proceedings of the National Academy of Sciences 2005;102(24):8686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassiri S, Zakeri I, Weingarten MS, Spiller KL. Relative expression of proinflammatory and antiinflammatory genes reveals differences between healing and nonhealing human chronic diabetic foot ulcers. The Journal of investigative dermatology 2015;135(6):1700. [DOI] [PubMed] [Google Scholar]
- Park E-Y, Park J-B. High glucose-induced oxidative stress promotes autophagy through mitochondrial damage in rat notochordal cells. International orthopaedics 2013;37(12):2507–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Barbul A. Understanding the role of immune regulation in wound healing. American journal of surgery 2004;187(5A):11S–6S. [DOI] [PubMed] [Google Scholar]
- Park S, Rich J, Hanses F, Lee JC. Defects in innate immunity predispose C57BL/6J-Leprdb/Leprdb mice to infection by Staphylococcus aureus. Infection and immunity 2009;77(3):1008–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. The Journal of experimental medicine 2017;214(7):1913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portou M, Baker D, Abraham D, Tsui J. The innate immune system, toll-like receptors and dermal wound healing: a review. Vascular pharmacology 2015;71:31–6. [DOI] [PubMed] [Google Scholar]
- Repine JE, Clawson CC, Goetz FC. Bactericidal function of neutrophils from patients with acute bacterial infections and from diabetics. The Journal of infectious diseases 1980;142(6):869–75. [DOI] [PubMed] [Google Scholar]
- Ridiandries A, Tan JTM, Bursill CA. The Role of Chemokines in Wound Healing. Int J Mol Sci 2018;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Yamamoto M, Shimosato T, Klinman DM. Accelerated wound healing mediated by activation of Toll- like receptor 9. Wound repair and regeneration 2010;18(6):586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Perdiguero EG, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 2012;336(6077):86–90. [DOI] [PubMed] [Google Scholar]
- Scully IL, McNeil LK, Pathirana S, Singer CL, Liu Y, Mullen S, et al. Neutrophil killing of Staphylococcus aureus in diabetes, obesity and metabolic syndrome: a prospective cellular surveillance study. Diabetology & metabolic syndrome 2017;9(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 2009;17(6):763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Shi C, Pamer EG. Monocyte-mediated immune defense against murine Listeria monocytogenes infection. Advances in immunology 2012;113:119–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafikhani SH, Engel J. Pseudomonas aeruginosa type III-secreted toxin ExoT inhibits host-cell division by targeting cytokinesis at multiple steps. Proceedings of the National Academy of Sciences of the United States of America 2006;103(42):15605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sima AA, O'Neill SJ, Naimark D, Yagihashi S, Klass D. Bacterial phagocytosis and intracellular killing by alveolar macrophages in BB rats. Diabetes 1988;37(5):544–9. [DOI] [PubMed] [Google Scholar]
- Snyder RJ, Lantis J, Kirsner RS, Shah V, Molyneaux M, Carter MJ. Macrophages: a review of their role in wound healing and their therapeutic use. Wound Repair and Regeneration 2016;24(4):613–29. [DOI] [PubMed] [Google Scholar]
- Suga H, Sugaya M, Fujita H, Asano Y, Tada Y, Kadono T, et al. TLR4, rather than TLR2, regulates wound healing through TGF-β and CCL5 expression. Journal of dermatological science 2014;73(2):117–24. [DOI] [PubMed] [Google Scholar]
- Theocharidis G, Baltzis D, Roustit M, Tellechea A, Dangwal S, Khetani RS, et al. Integrated Skin Transcriptomics and Serum Multiplex Assays Reveal Novel Mechanisms of Wound Healing in Diabetic Foot Ulcers. Diabetes 2020;69(10):2157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhorebeek I, Ellger B, De Vos R, Boussemaere M, Debaveye Y, Vander Perre S, et al. Tissue-specific glucose toxicity induces mitochondrial damage in a bum injury model of critical illness. Critical care medicine 2009;37(4):1355–64. [DOI] [PubMed] [Google Scholar]
- Wang P, Wu P, Siegel M, Egan R, Billah M. Interleukin (IL)-10 inhibits nuclear factor B activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem 1995;270:9558–63. [DOI] [PubMed] [Google Scholar]
- Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, DiPietro LA. Regulation of scar formation by vascular endothelial growth factor. Laboratory investigation 2008;88(6):579–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S, Jayaraman V, Huelsmann EJ, Bonish B, Burgad D, Sivaramakrishnan G, et al. Proinflammatory chemokine CCL2 (MCP-1) promotes healing in diabetic wounds by restoring the macrophage response. PloS one 2014;9(3):e91574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaghini N, Mahmoodi M, Asadikaram GR, Hassanshahi GH, Khoramdelazad H, Kazemi Arababadi M. Serum levels of interleukin 10 (IL-10) in patients with type 2 diabetes. Iranian Red Crescent medical journal 2011;13(10):752. [PMC free article] [PubMed] [Google Scholar]
- Yue D, Swanson B, McLennan S, Marsh M, Spaliviero J, Delbridge L, et al. Abnormalities of granulation tissue and collagen formation in experimental diabetes, uraemia and malnutrition. Diabetic medicine 1986;3(3):221–5. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zou W, Du J, Zhao Y. The origins and homeostasis of monocytes and tissue- resident macrophages in physiological situation. Journal of cellular physiology 2018;233(10):6425–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analyzed during this study.