Abstract
Objective
Despite their possible importance in the design of novel neuromodulatory approaches and in understanding status epilepticus, the dynamics and mechanisms of seizure termination are not well studied. We examined intracranial recordings from patients with epilepsy to differentiate seizure termination patterns and investigated whether these patterns are indicative of different underlying mechanisms.
Methods
Seizures were classified into one of two termination patterns: (a) those that end simultaneously across the brain (synchronous), and (b) those whose termination is piecemeal across the cortex (asynchronous). Both types ended with either a burst suppression pattern, or continuous seizure activity. These patterns were quantified and compared using burst suppression ratio, absolute energy, and network connectivity.
Results
Seizures with electrographic generalization showed burst suppression patterns in 90% of cases, compared with only 60% of seizures which remained focal. Interestingly, we found similar absolute energy and burst suppression ratios in seizures with synchronous and asynchronous termination, while seizures with continuous seizure activity were found to be different from seizures with burst suppression, showing lower energy during seizure and lower burst suppression ratio at the start and end of seizure. Finally, network density was observed to increase with seizure progression, with significantly lower densities in seizures with continuous seizure activity compared to seizures with burst suppression.
Significance
Based on this spatiotemporal classification scheme, we suggest that there are a limited number of seizure termination patterns and dynamics. If this bears out, it would imply that the number of mechanisms underlying seizure termination is also constrained. Seizures with different termination patterns exhibit different dynamics even before their start. This may provide useful clues about how seizures may be managed, which in turn may lead to more targeted modes of therapy for seizure control.
Keywords: seizure termination, burst suppression, synchronous ending, asynchronous ending
1. Introduction
Seizures arise from a multitude of etiologies and manifest a wide variety of electrographic dynamics1–3. Most studies of these dynamics focus on seizure initiation; seizure termination has been correspondingly neglected. Yet, understanding seizure cessation may provide valuable information about physiological mechanisms of seizures and open up entirely new approaches in designing treatments for seizures and status epilepticus4,5.
The literature on seizure termination is sparse, and despite a few studies in the field6–8, no study has considered seizure termination in the context of different seizure types and patterns of spread. At first glance, we might assume that all seizures terminate identically, especially compared with seizure initiation where the transition from normal to ictal activity can be so varied. However, this assumption is an oversimplification and is ultimately inaccurate. For example, while some seizures do terminate simultaneously across the brain (synchronous termination), others do not8–10. Seizures can also be differentiated by their bursting patterns. Some seizures exhibit a termination pattern that resembles those found in recordings of neural dynamics in anesthetized patients or those with impaired consciousness11,12. This pattern, sometimes referred to as “interclonic intervals” or “clonic bursts”12, is characterized by high amplitude, high frequency bursts of activity interrupted by a period of suppressed activity with low amplitude13. That said, it is unclear how many termination patterns can be identified and how these patterns may differ from one another.
The primary goal of this study, therefore, was to quantify the relationship between patterns at seizure termination and seizure focality, with an eye towards establishing a baseline for mechanistic studies. More specifically, we sought to evaluate the incidence of different burst patterns at seizure termination in four different seizure categories, distinguished by their termination timing (synchronous versus asynchronous) and spread of neuronal activity (focal versus electrographic generalization). We hypothesize that certain bursting patterns (burst suppression versus continuous seizure activity) manifesting at the termination of both focal seizures and seizures with electrographic generalization not only reflect the involvement of different neural circuitry, but also can be indicative of the spread of network recruitment that eventually leads to seizure termination.
2. Materials and methods
2.1. Data Acquisition and seizure classification
We analyzed seizures from patients with medication-refractory epilepsy who underwent clinical monitoring to locate their seizure onset zone at Massachusetts General Hospital from 2006 to 2019 and at Brigham and Women’s Hospital from 2009 to 2018. Patients were implanted with depth electrodes and/or grids and strips as determined by clinicians independent of this study. All data acquisition and analyses in this study were approved by the Institutional Review Board (IRB) covering the two hospitals (Mass General Brigham and Partners Human Research Committee).
Data were recorded using XLTEK/Natus clinical EEG equipment (Natus Medical Inc., Oakville, Canada), or a Blackrock Cerebus system (Blackrock Microsystems). Data were sampled at a range of frequencies from 250 Hz to 2 kHz and were subsequently downsampled to 200 Hz for further analysis. Epileptologists, blind to this study, identified the seizure onset regions. Seizures were reviewed (bipolar montage) in MATLAB (R2020a; MathWorks) using the FieldTrip browser14. We excluded from analysis all seizures from a patient if either: a) the patient’s seizure onset area could not be identified; b) more than three seizure onset areas were identified; or c) the patients’ seizures were extremely short (<30 s). We visually classified 710 seizures from 104 patients. Each seizure was categorized based on focality and termination pattern. Seizures were labeled as focal if the ictal activity could only be seen in one recorded area and did not propagate to other brain regions; otherwise, the seizure was classified as having electrographic generalization, meaning that the seizure started in one or multiple area(s) and later propagated to other areas (e.g., starting in temporal regions and propagating to frontal regions; Fig. 1–2). Next, we labeled seizures based on their termination patterns: synchronous termination (ST), where seizure activity ended in all channels within one second, or asynchronous termination (AT), where seizure activity terminated on some channels while continuing on others. To account for seizure count variability across patients, we randomly selected one representative seizure for each unique (focality, termination pattern, onset zone) label combination for each patient, after excluding those deemed to have excessive noise or persistent, high-amplitude artifacts. For example, if one patient had three focal seizures initiating from the hippocampus and all classified as ST type, only one seizure out of these three was selected for the analysis. More than one seizure was selected for a given patient only when they differed in their focality/spread, termination type, or onset. Seizures that developed into status epilepticus (N=7) were also excluded. Seizure end-time and patterns were identified by two reviewers and discussed until agreement was reached. Where agreement could not be reached, the seizure was marked as unclassifiable (Supp. Fig. 1; N=14) and excluded. This resulted in a total of 207 seizures across all patients. Finally, seizures received an additional label based on their burst pattern at termination: a) seizures with synchronous (phase-locked) burst suppression activity across more than 75% of channels at seizure termination (sBS), b) seizures that show burst suppression activity before seizure termination in most channels, but the burst suppression activity was synchronous in less than 75% of the channels (aBS), c) seizures with continuous seizure activity patterns at termination in all channels with ictal activity (CSA). We note that this burst suppression channel threshold (75%) is arbitrary and selected solely for practical purposes. For most seizures with burst suppression activity, virtually all channels showed bursting activity (either synchronous or asynchronous), but for a select group of seizures (reported in section 3.2), the mesial temporal channels exhibited bursting activity while other channels were suppressed. We determined that the proportion of these channels did not exceed 25%.
Figure 1. Seizures exhibit different patterns at termination.
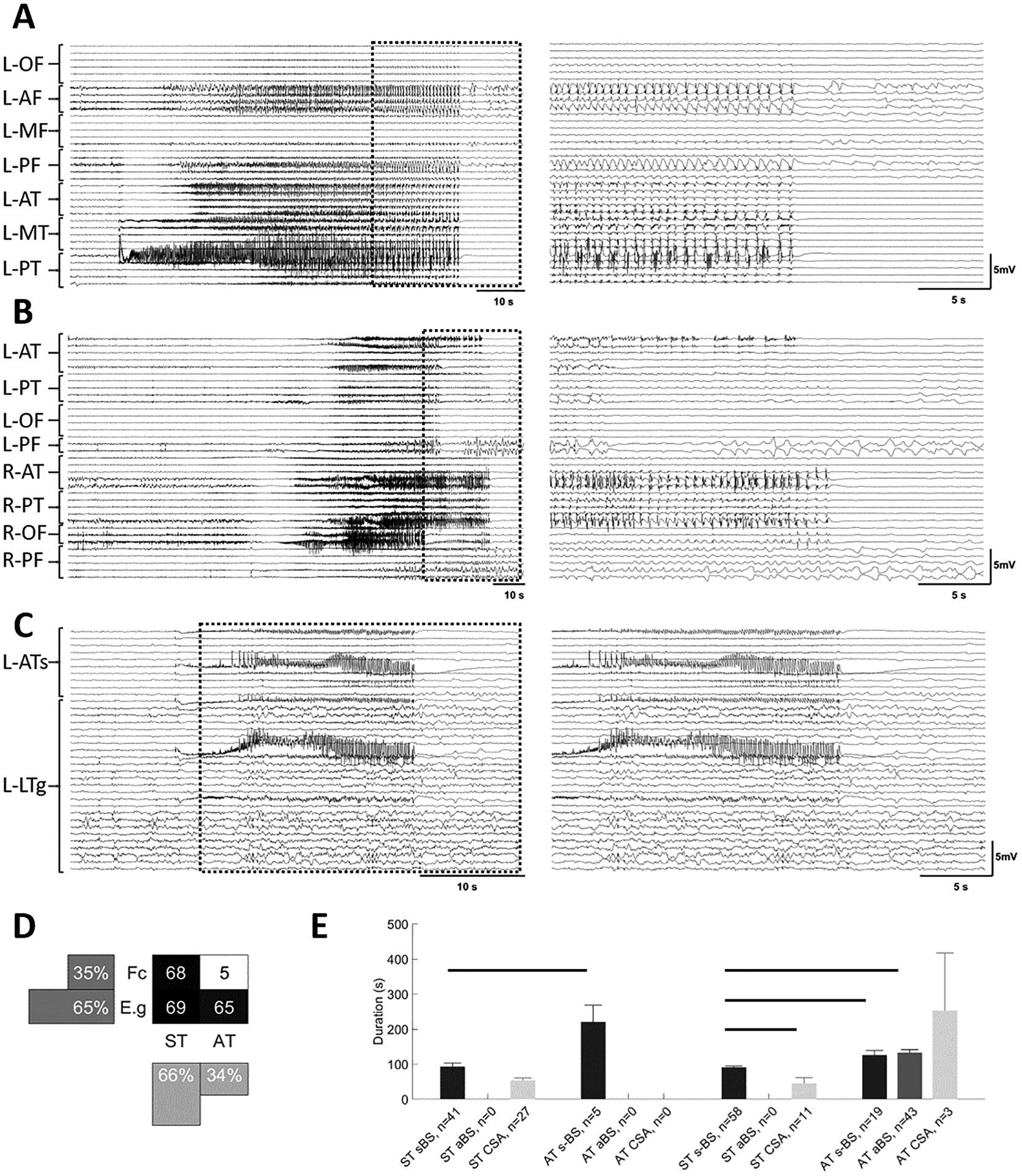
Examples of seizures with different focality and termination patterns. Seizure traces from 10 s before the onset until 10 s after their termination (A-C, left panels). An expanded view of these traces (A-C, right panels) shows the last 20 s of each seizure and the 10 s post termination period. This 30 s period is identified on the left with dashed boxes. A) Seizure with onset in left posterior temporal area with synchronous termination (ST). In the right panel, note the burst suppression activity before the termination. B) Seizure with onset in right anterior temporal area that propagates to the rest of the brain, terminating in some channels while continuing in others (AT). C) Seizure that starts focally in left anterior temporal area and remains focal for the duration of the seizure with synchronous termination (ST) with bursting activity at termination. Seizures in each group were further classified into three subgroups: seizures with synchronous burst and suppression activity across almost all channels at the end of the seizure (sBS, A), and seizures that show burst and suppression activity before seizure termination in most channels, but with burst suppression activity not synchronized across all channels (aBS, B), seizures with continuous seizure activity patterns (CSA, C). D) A distribution of seizures based on focality and the termination pattern (ST and AT). E) The duration of seizures in different groups. In both focal and electrographic generalization groups, CSA seizure had significantly shorter duration compared to sBS seizures. Horizontal bars indicate significance between the pairs (p<0.05). L: left; R: right; OF: orbitofrontal; AF: anterior frontal; MF: middle frontal; PF: posterior frontal; AT: anterior temporal; MT: middle temporal; PT: posterior temporal; ATs: anterior temporal strip; LTg: lateral temporal grid; Fc: focal; E.g: electrographic generalization.
Figure 2. Ictal activity in asynchronous termination, unlike seizure initiation and spread, appears to end abruptly within different regions (A-B).
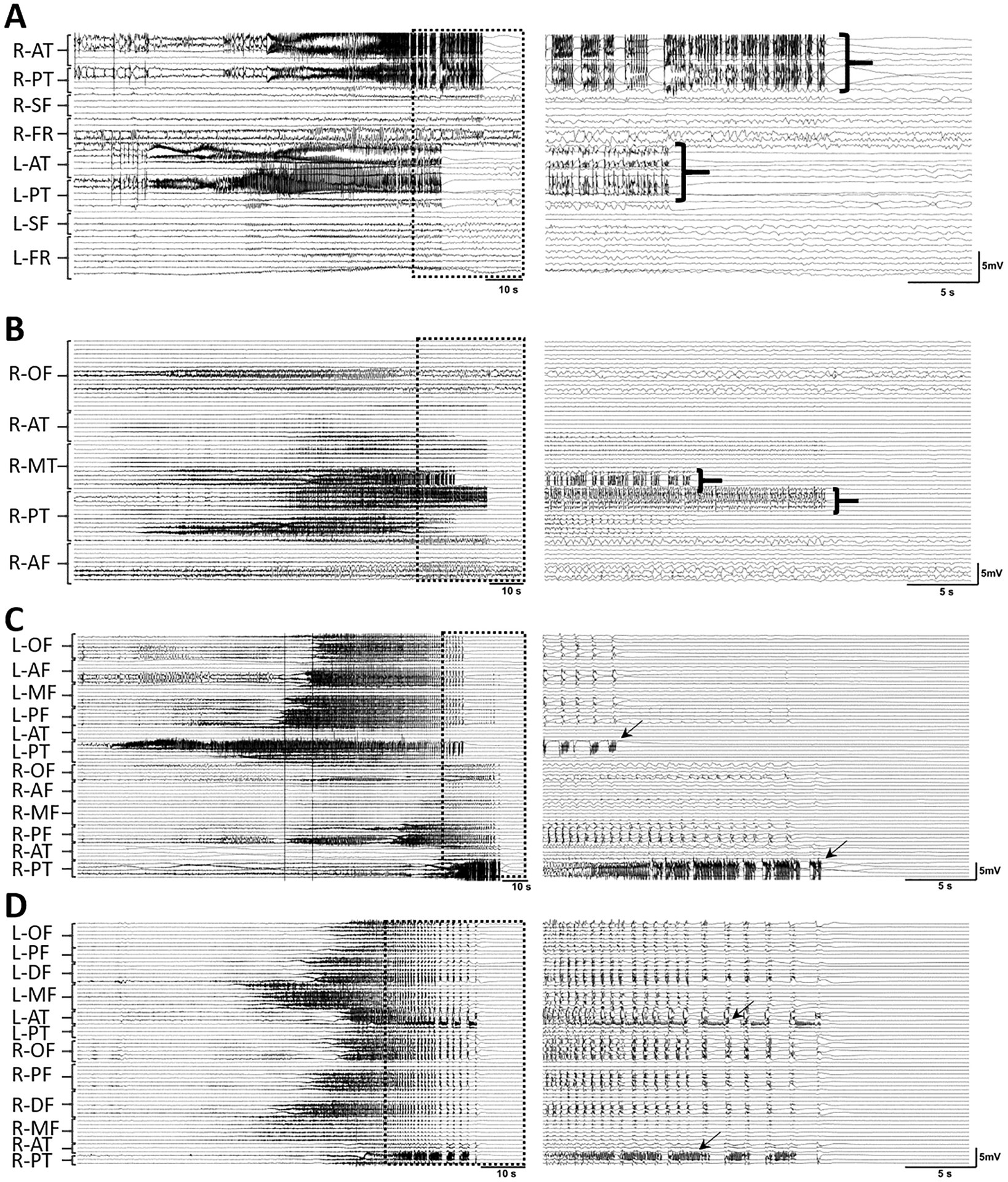
Two seizures with asynchronous termination and asynchronous bursting patterns (aBS) from two different patients with onset in left posterior temporal (A), and right lateral temporal (B) regions. Seizures are presented as per Fig. 1. Note that ictal events from different regions end in groups. In some seizures with burst suppression activity at their termination, the mesial temporal area exhibits bursting activity while other regions are suppressed (C-D). Two seizures with burst suppression activity from two different patients. C) Seizure with an onset in left posterior temporal and asynchronous termination. The arrows are indicating the bursting activities in the left and right hippocampus while the rest of the regions are suppressed. D) Seizure with an onset in left middle frontal that propagates to the rest of the brain. The arrows are showing the bursting activity in left and right hippocampus. The arrows showing the bursting activity in the right hippocampal regions. L: left; R: right; AT: anterior temporal; MT: middle temporal; PT: posterior temporal; SF: sub-frontal; FR: frontal; OF: orbitofrontal; AF: anterior frontal; MF: middle frontal; PF: posterior frontal; DF: dorsal frontal.
The seizures that had spiking patterns did not always have spike wave activity in all channels. These were classified in the “continuous seizure activity” group when there was no suppressed period observed between the spikes, otherwise they were classified in the “burst suppression” group (Supp. Fig. 2). The burst suppression pattern has been referred to as “interclonic interval” or “clonic bursts” in other studies12, and has recently been used to describe the analogous pattern seen at termination15. Here, correspond with the terminology used in encephalography research and to acknowledge the similarity between this pattern (albeit without knowing if the mechanisms are identical) and what is seen in anesthesia, we use the term “burst suppression”.
2.2. Average of absolute energy and burst suppression detection
Seizures were visually analyzed to identify the channels involved in their propagation. The channels that exhibited ictal activities were selected to identify their changes in the average absolute energy (AAE) and burst suppression ratio (BSR; 47.6±3.24 channels per seizure; min=2; max=201). All seizures were analyzed from 10 s before seizure-onset until 10 s after termination.
To calculate the AAE, each bipolar channel involved in the seizure was high-pass filtered above 2 Hz to remove the effect of DC changes; the absolute value of the amplitude was measured and averaged across the channels as a representation of the energy of each seizure (Fig. 3A).
Figure 3. Evaluating AAE and BSR in different seizure types.
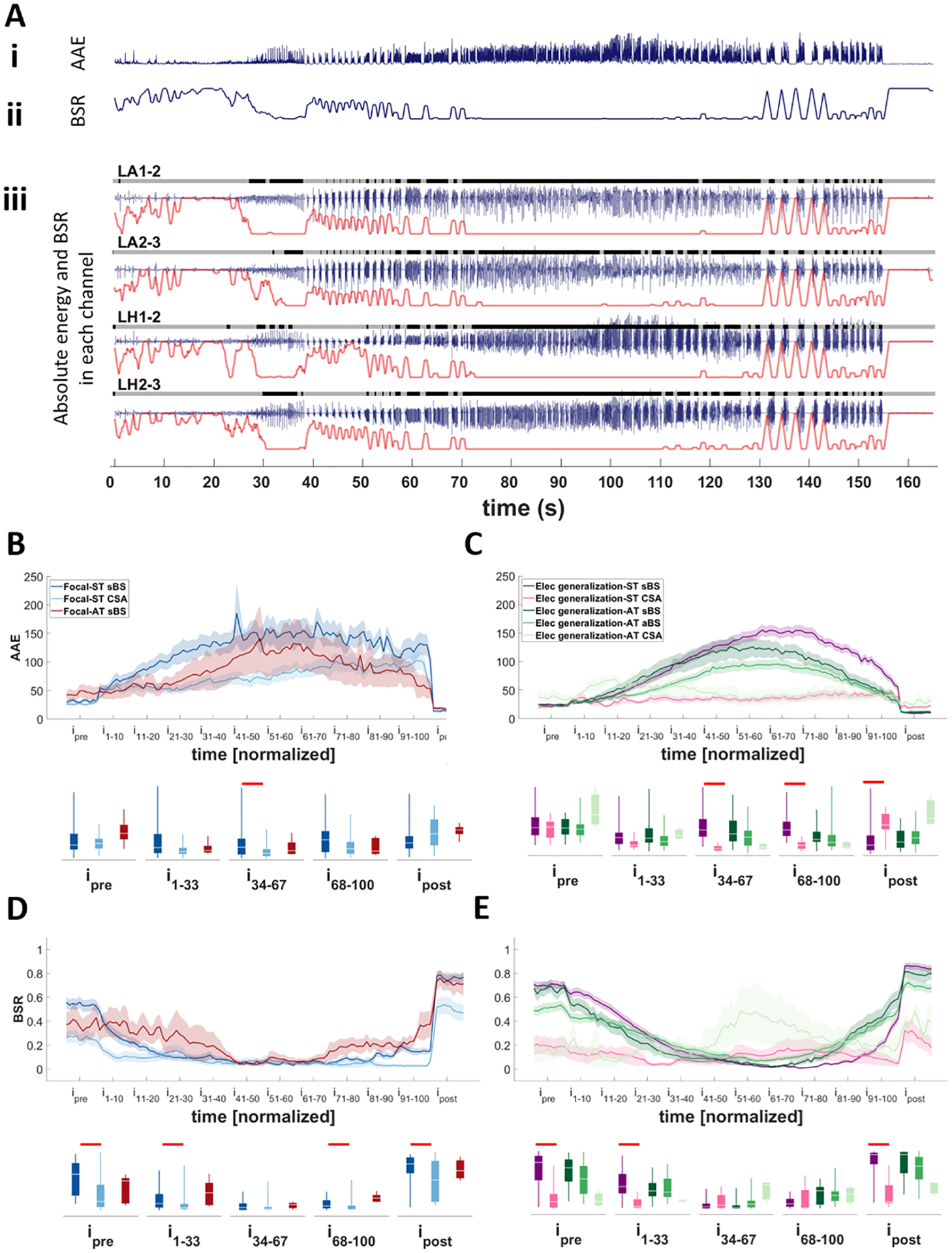
A) An example of a focal seizure that starts in mesial temporal areas and remains focal throughout the seizure duration. Ai) The average absolute energy (AAE) of seizure calculated by averaging the absolute amplitude of channels with seizure spread (four depicted channels for this seizure). Aii) The average value of burst suppression ratio (BSR) of all channels with seizure spread. Aiii) For each channel burst (black) and suppression (gray) intervals were identified over time. Note the gray and black bars on top of the signal trace (blue trace) of each channel. The value of the BSR was then calculated for each channel over time (red trace underneath each signal of each channel). These values were then averaged for all channels with seizure activity (here only four channels exhibited seizure activity) B and C) Changes in the AAE over time in focal seizures (B) and seizures with electrographic generalization (C) seizures with synchronous and asynchronous termination. Note that in both groups the seizures with CSA activity at the termination maintain lower energy throughout the seizure compared to the seizures with BS activity. D and E) Changes in the BSR in focal seizures (D) and seizures with electrographic generalization (E) seizures with synchronous and asynchronous termination. The box-and-whisker plots underneath the traces indicate summarized measures (AAE or BSR) within different time periods. Traces in B-D indicate averages with a spread of plus/minus one standard error (shaded area). Horizontal bars indicate significance between the pairs (p<0.05). LA: left amygdala; LH: left hippocampus; ST: synchronous termination; AT: asynchronous termination; sBS: synchronous burst suppression; aBS: asynchronous burst suppression; CSA: continuous seizure activity; i: interval.
BSR was computed using an automated method adapted from Westover et al.16 Band-pass filtered signals were normalized by subtracting the signal mean and dividing by its standard deviation to account for amplitude differences. The method then labels each sample as either burst or suppression using previous data in each signal and applies the following equations:
Here, xt is the value of the normalized signal of one channel at time t, μt and σt2 are current values of the recursively estimated local mean and variance, respectively. Finally, zt is an indicator function that labels each data point as either a burst (zero) or suppression (one). The value of β determines the balance between the effect of recent and past data; this was set to the value obtained from previously trained data (β = 0.9534)16. The classification threshold θ (points above this threshold are classified as burst) chosen by Westover et al.16 was evaluated using data recorded from patients in the Intensive Care Unit (ICU). We determined through a preliminary analysis that this value of θ is not sufficient to classify the data recorded from epileptic data. We therefore evaluated our dataset visually with values of θ = 0.1, 0.5, and 1.75. The choice of theta was made by two experts who reviewed selected intervals to identify burst and suppression using each possible theta value. The value of θ = 0.1 was selected to reliably identify ictal burst and suppression.
The BSR for each channel was evaluated as the proportion of suppression-labeled samples in a moving window (1 s duration, no overlap). The BSR for each seizure was evaluated as the average over all channels that exhibited the ictal activity (Fig. 3A).
2.3. Localizing the region for each contact with seizure propagation
We identified the anatomical location of all bipolar contacts (see: Salami et al.3 for more detail) that exhibited ictal activity and were determined to be involved in seizure propagation. Channels were classified into five distinct groups based on proximity and structural similarity: Region 1) hippocampus; 2) mesial temporal structures; 3) lateral temporal area; 4) frontoparietal including pre-central, post-central and inferior parietal; 5) all other areas.
2.4. Functional connectivity and network dynamics
Functional connectivity was measured between all intracranial recording channels that contained no artifacts for each seizure (98.8±3.57 channels per seizure; min=26; max=205) and was not limited to the channels of seizure spread, utilizing linear cross-correlation strength between channels. Connectivity was evaluated on low-pass filtered data (200 Hz cutoff) using a moving window (1 s duration, 0.5 s overlap). Each bipolar channel was considered as a node and connectivity was measured between each pair. We used a bootstrap method to assess the statistical significance of each connectivity measurement17, such that edge assignment was constrained by statistical significance irrespective of direction. This resulted in the creation of a connectivity network for each moving window, which was analyzed using network density (Fig. 6B); density=NE/NPE, the number of edges (NE) as a fraction of the total number of possible edges, NPE = n(n-1)/2, where n is the number of nodes, or channels.
2.5. Statistical analysis
We used the Kruskal-Wallis test to identify significant differences between the duration of seizures of different groups. This was followed by the Tukey-Kramer method for multiple comparisons with the level of significance set at p=0.05. To compare measures of AAE, BSR and network density between seizures, the differences in seizure duration need to be considered. The duration of each seizure was divided into 100 subintervals of equal duration to normalize the time during the seizure. This normalization over time allowed us to directly compare different seizures which naturally have different durations. The value representing each interval was the average of the measures of all samples within that interval. All seizure measures were then averaged and plotted in this normalized time scale. To test differences between groups over time, seizures were compared in five time periods: pre-seizure, beginning of the seizure (subintervals 1–33), middle of the seizure (subintervals 34–66), end of the seizure (subintervals 67–100), and post-seizure periods. For each time period, the Kruskal-Wallis test was used to identify significant differences in AAE and BSR between seizures with similar focality but different termination patterns. This was followed by the Tukey-Kramer method for multiple comparisons with the level of significance set at p=0.05. To compare the differences between network density of seizures with different bursting patterns, we used Wilcoxon rank-sum test with the level of significance set at p= 0.05. Results in the text and bar graphs are reported as mean ± standard error.
2.6. Data availability
The data of this study are available upon request from the corresponding author. The data are not publicly available as they contain information that could compromise the privacy of the participants.
3. Results
3.1. Individual patients can have seizures with different termination types
We analyzed a total of 710 seizures recorded from 104 patients. The majority of seizures ended synchronously (82%) with the remainder showing asynchronous termination (AT, 18%). Among the 104 patients, six had status epilepticus only or seizures whose termination was unclassifiable. Fifty-nine patients had only one type of termination (81% ST-only and 19% AT-only), five of which had at least one extra unclassifiable seizure or seizures that lead to status epilepticus. Thirty-nine patients had seizures of both ST and AT types, and four had at least one unclassifiable seizure or a seizure that led to status epilepticus. Among the 44 patients that exhibited AT seizures, eight patients had only AT seizures. Of the remaining patients that exhibited both ST and AT types, 39% of their seizures were AT type (min=7%; max=88%).
Out of the initial 710 seizures, seven seizures lasted longer than five minutes and were classified as status epilepticus. The majority (6/7) of these seizures had electrographic generalization: one with synchronous termination and asynchronous burst suppression pattern; two with synchronous termination and synchronous burst suppression; three had asynchronous termination with two of them having asynchronous burst suppression pattern and one with synchronous burst suppression pattern. The remaining seizure was focal, with seizure activity continuing in some channels longer than others (asynchronous termination with CSA). After selecting representative seizures, we had a total of 207 seizures for analysis across all patients (2±0.1 seizures per patient; min=1; max=7). Most (65%) of these seizures had electrographic generalization. Of the 35% of seizures that were focal, most had ST (93%); 51% with electrographic generalization had ST, with the rest having AT (Fig. 1D).
Even in seizures with AT, the ictal activity typically appeared to end abruptly within different regions. In contrast with the gradual recruitment of regions at seizure initiation, during termination, the seizure either ends across all channels at the same time (ST) or the termination happens in groups of channels belonging to the same region (AT; Fig. 2A–B).
3.2. Seizures manifest different bursting patterns at termination
In addition to focality of termination patterns, seizures were also distinguished by their bursting patterns. We identified three subgroups: synchronous burst suppression (sBS; Fig. 1A), asynchronous burst suppression (aBS; Fig. 1B), and seizures with continuous seizure activity (CSA; Fig. 1C). Focal seizures with ST (N=68) either had CSA (40%) or sBS (60%) patterns. This was also true for ST-type seizures with electrographic generalization (N=69; 16% CSA, 84% sBS). Only five seizures manifested as focal with AT, with all of these having the sBS pattern. Only seizures with electrographic generalization that were AT-type manifested with all three burst patterns (66% aBS, 30% sBS, 4% CSA). Focal seizures with ST and sBS patterns had significantly shorter durations than those of AT. Seizures with electrographic generalization with sBS patterns were significantly longer than seizures with CSA patterns in the ST group, and were significantly shorter compared with AT seizures with either sBS or aBS patterns (Fig. 1E; p<0.05).
Interestingly, in some seizures with aBS and sBS patterns, the mesial temporal area occasionally exhibited bursting activity while other regions were suppressed. This type of activity was seen mostly in the hippocampus, but other times appeared in amygdala and adjacent regions (Fig. 2C–D).
3.3. Seizures with burst suppression patterns at termination show higher amplitudes
Signal amplitude may serve as a measure of synchrony in the network18. We therefore calculated changes in average absolute energy (AAE) during the seizure. For each seizure, we computed the absolute energy over time per channel and then averaged over all channels that showed ictal activity (Fig. 3A, top trace). We then summarized each energy average as a 100-element sequence in which the first and last values represent the AAE at the beginning and end of the seizure, respectively.
There were no differences in AAE between seizures with synchronous and asynchronous endings. However, seizures with sBS reached significantly higher AAE during the seizure compared to seizures with CSA in both focal (Fig. 4B; p<0.02) seizures and those with electrographic generalization (Fig. 4C; p<0.0005). In contrast, the AAE of the post-ictal period of seizures with electrographic generalization and sBS endings was significantly lower than that of seizures with CSA termination (Fig. 4C; p<0.001).
Figure 4. Changes in the average absolute energy (AAE) of different regions during different seizure types.
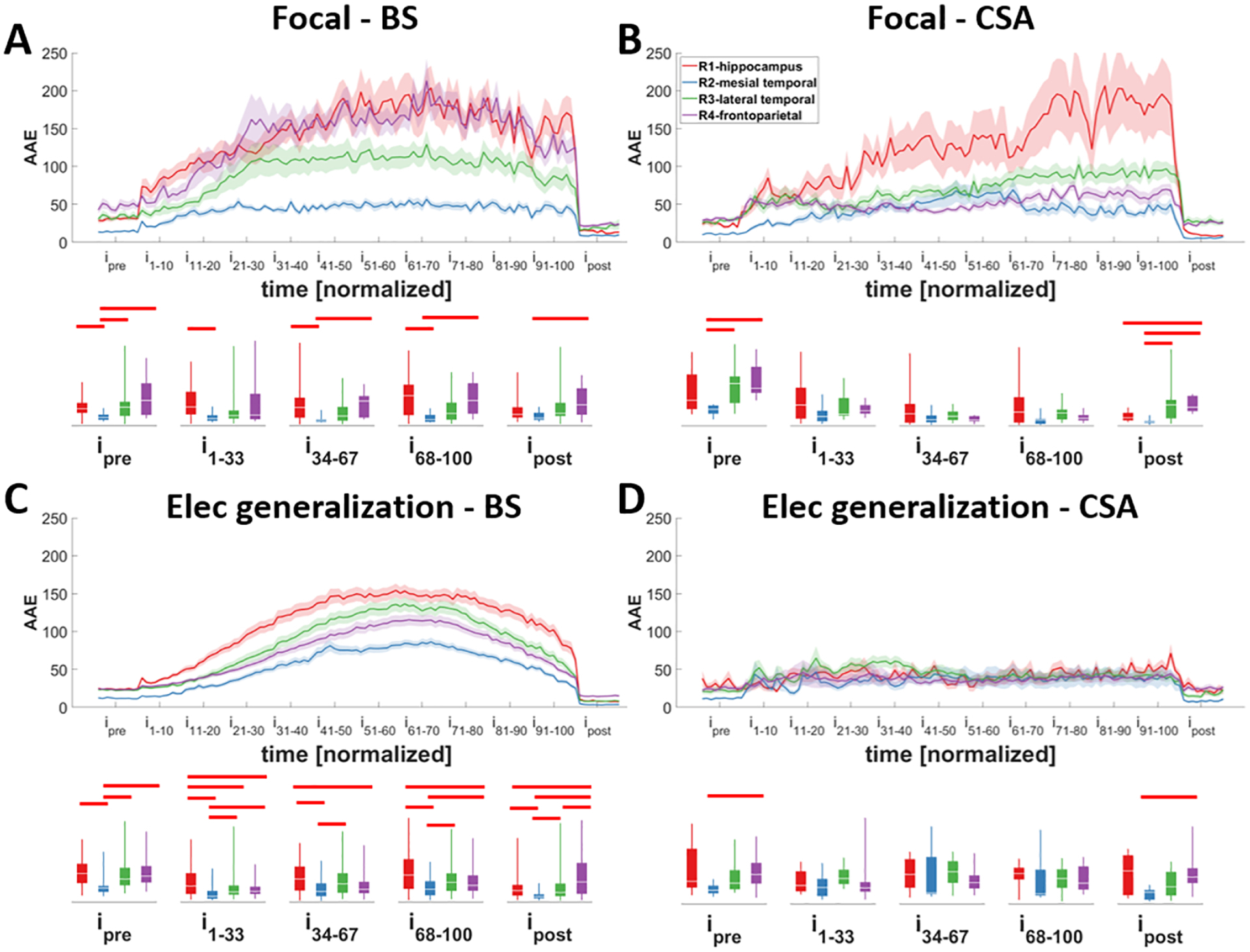
Seizures were classified into different groups based on their focality: A and B) focal; C and D) with electrographic generalization, and the bursting pattern at their termination: A and C) asynchronous burst suppression (aBS) and synchronous burst suppression (sBS); B and D) continuous seizure activity (CSA). Hippocampus tends to have higher AAE in seizures with burst suppression activity at their termination (A and C). This difference has reached significance in multiple intervals in seizures with electrographic generalization and with burst suppression (aBS and sBS). Traces indicate averages with a spread of plus/minus one standard error (shaded area). The box-and-whisker plots underneath the traces indicate summarized measure (AAE) within different time periods. Horizontal bars indicate significance between the pairs (p<0.05; i: interval).
We have also compared the measures recorded from depth versus grid/strip electrodes in patients that received both electrode types. This comparison was performed based on the recording region. In general, we observed the same dynamic changes in AAE and BSR measures in signals recorded via grids/strips or depth electrodes (Supp. Fig. 3).
3.4. Seizures which terminate with burst suppression exhibit more suppression during pre- and post-ictal periods
The burst suppression ratio (BSR) indicates the proportion of time in which a signal is suppressed and is equal to zero (one) if the entire period consists of bursting (suppression). The BSR for each seizure was computed in a similar fashion to AAE (Fig. 3A). We did not identify any significant differences in BSR dynamics between seizures with synchronous and asynchronous termination (p>0.05). In all groups the pre-seizure period featured bursting and suppressed activity, with bursting activity increasing (and BSR falling) on seizure onset. Towards the end of the seizure, the bursting activity became interrupted by periods of suppression and the BSR value started to increase until termination. In seizures with CSA patterns, the BSR remained relatively stable from pre-seizure to post-seizure, and it tended to be significantly lower than that of sBS seizures in the pre- and post-seizure periods in both focal (Fig. 3D; p<0.02) seizures and those with electrographic generalization (Fig. 3E; p<0.00001). It is important to note in the group of seizures with electrographic generalization and AT, there were only three seizures exhibiting the CSA pattern in our dataset. Therefore, even though the BSRs of these seizures were different from those with the BS pattern, this difference did not reach significance (Fig. 3E).
3.5. Hippocampus activity is of high amplitude in seizures with burst suppression pattern
In each seizure, the channels that exhibited seizure spread were located in different regions, which were classified into five distinct groups based on proximity and structural similarity. For each seizure type, we compared the AAE and BSR in different regions to determine whether all regions behave in similar ways. To perform this comparison, channels situated in the same region were grouped and averaged together for each seizure. We compared only the activity in the four major regions where seizures occurred most frequently. Thus, all areas that were classified into the ‘leftover’ Region 5 group were excluded from this analysis due to low case count. In both focal seizures and the ones with electrographic generalization that had BS pattern, hippocampal regions in general demonstrated higher AAE. Frontoparietal areas also showed higher AAE compared to other regions except the hippocampus (Fig. 4; p<0.05).
When comparing BSR over time in seizures with different focality and termination patterns, we found that most regions behave similarly except for frontoparietal regions. Before the seizure starts, all regions had a baseline consisting of both burst and suppression activities; however, frontoparietal regions exhibited more bursting activity (and less suppression), especially compared to hippocampus and mesial temporal areas. This behavior persisted after the seizure ended where all regions entered a suppressed period, except frontoparietal regions which exhibited more bursting (Fig. 5B–D; p<0.05).
Figure 5. Changes in the burst suppression ratio (BSR) of different regions during different seizure types.
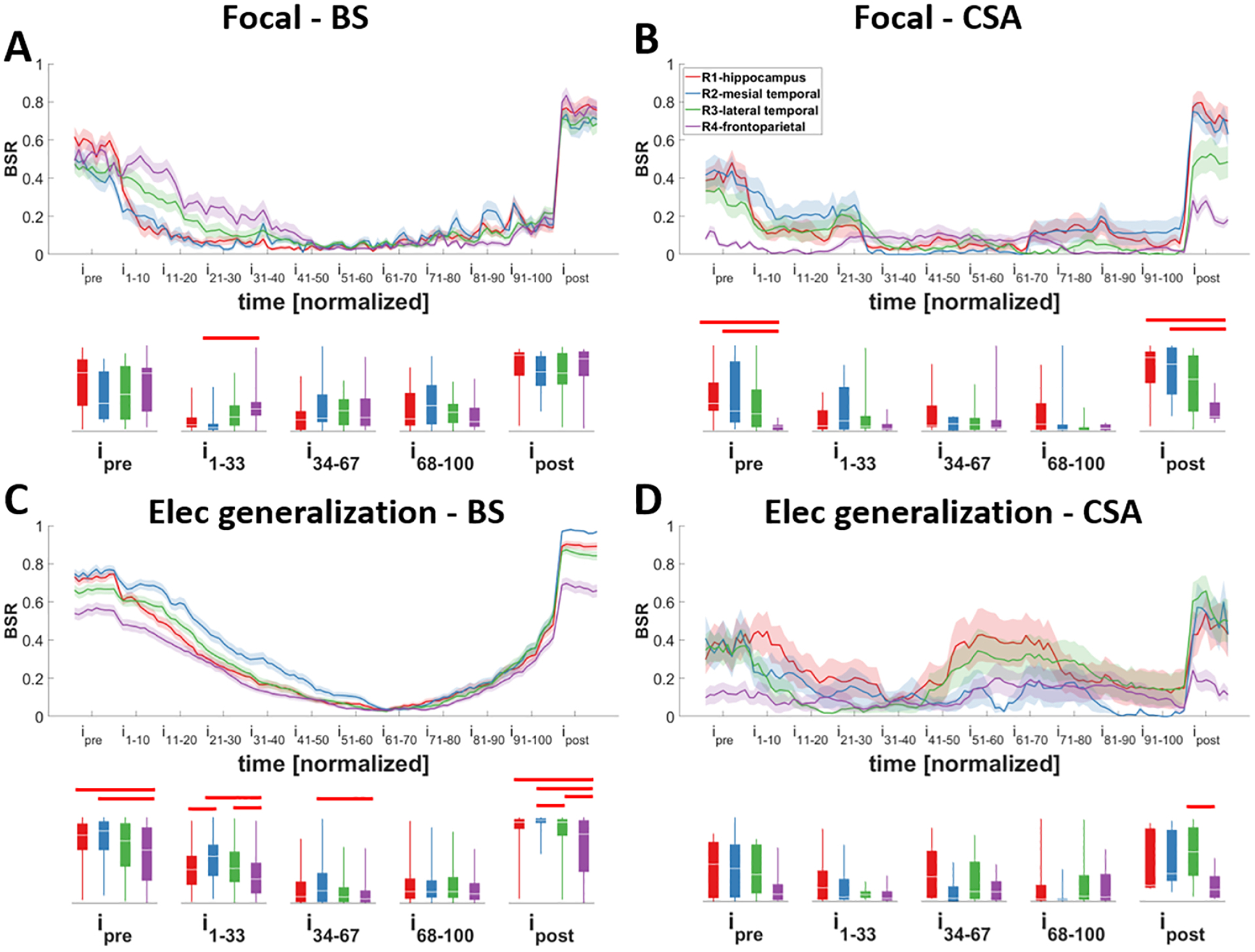
Seizures were classified into different groups based on their focality: A and B) focal; C and D) with electrographic generalization, and the bursting pattern at their termination: A and C) asynchronous burst suppression (aBS) and synchronous burst suppression (sBS); B and D) continuous seizure activity (CSA). Note that most regions behave similarly except for frontoparietal regions. Except for focal-BS seizures, before the seizure starts and after the seizure ends, frontoparietal regions show more bursting activity (and less suppression) especially compared to hippocampus (B, C). Traces indicate averages with a spread of plus/minus one standard error (shaded area). The box-and-whisker plots underneath the traces indicate summarized measure (BSR) within different time periods. Horizontal bars indicate significance between the pairs (p<0.05; i: interval).
To confirm that the differences in AAE between the seizures with burst suppression pattern and seizures with continuous seizure activity is not influenced by the proportion of seizures with mesial temporal onset in the group with the burst suppression pattern, we excluded all the seizures with mesial temporal onset and redid the analysis. We observed the same pattern in the dynamics of energy changes over time in seizures that did not have a mesial temporal onset (Supp. Fig. 4).
3.6. Network density at the termination of seizures with burst suppression activity is higher than the seizures with continuous seizure activity
In both CSA seizures and BS seizures, we did not identify any significant differences in AAE or BSR between the ST and AT seizures; however, CSA seizures tend to have lower AAE and a significantly lower BSR over the entire course of the seizure compared to BS seizures (Fig. 3C–D). To better understand these differences, we evaluated the changes in the network connectivity of CSA and BS seizures. For seizures with focal onsets, no differences between the functional connectivity network density of the two types were identified (Fig. 6C). This result was somewhat predictable; the density of functional connectivity in focal seizures tends to be low since the areas that are involved in seizure propagation are confined to a small region of the brain. However, in seizures with electrographic generalization, those with BS pattern exhibited an increase in network density with progression of the seizure (Fig. 6D; p=0.002). The peak network density of BS seizures (occurring in the last interval of the seizure) was significantly higher than that of CSA seizures showing that there is more synchronization in seizures with BS patterns compared to seizures with CSA patterns.
Figure 6. Changes in network density in different seizure types.
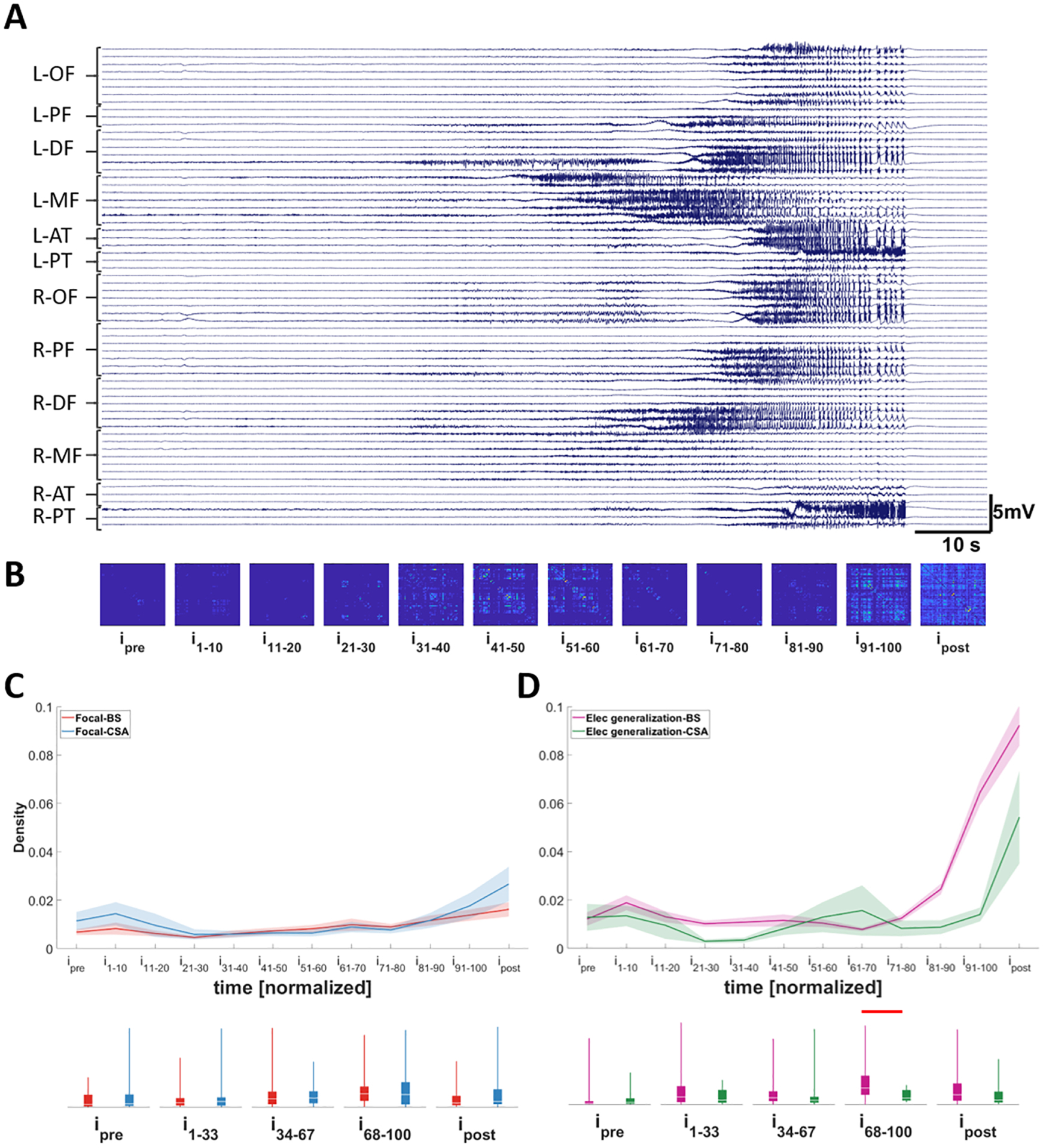
A) Seizure with onset in left posterior temporal area; synchronous termination (ST). B) Average connectivity matrix over time for the seizure shown in A. C and D) Differences in average density between seizures with different bursting activity at their termination in focal seizures (C) and seizures with electrographic generalization (D). Note that there is a transient increase in the density over time in all types of seizures. The density increases significantly in seizures with electrographic generalization and with burst suppression activity at their termination indicating an increase in the synchrony in these seizures. Traces in C-D indicate averages with a spread of plus/minus one standard error (shaded area). The box-and-whisker plots underneath the traces indicate summarized measure (density) within different time periods. Horizontal bars indicate significance between the pairs (p<0.05). L: left; R: right; OF: orbitofrontal; PF: posterior frontal; DF: dorsal frontal; MF: middle frontal; AT: anterior temporal; PT: posterior temporal; BS: burst suppression; CSA: continuous seizure activity; i: interval.
4. Discussion
Seizure termination occurs through complex mechanisms that remain unknown. To address this knowledge gap, the main goal in this study was to explore the different ways in which seizures can terminate. We hypothesized that the dynamics at termination fall into a limited number of categories and that different types of onset and propagation dynamics may still end the same way. Consistent with that hypothesis, we found that different seizure termination patterns can manifest within a single patient, even in seizures with similar onsets, using a classification scheme based on spatiotemporal features. Within this classification approach, we found that most seizures terminate with patterns of burst suppression (BS), regardless of their generalization; seizures with electrographic generalization show BS patterns in 90% of cases and most seizures terminate synchronously and all seizures (ST and AT) show block-like termination patterns. Whether the seizure terminates synchrnously or not, the AAE and BSR is similar. In contrast, seizures with a continuous activity pattern (CSA) have lower AAE during the seizure compared to seizures with bursting patterns. AAE in seizures with electrographic generalization and burst suppression recorded from hippocampus tends to be higher compared to those in other regions; and finally, network density increases with seizure progression (reaching a maximum at termination), with significantly lower densities in CSA seizures compared to seizures with BS activity.
To our knowledge this is the first study that compares the interactions between different channels (brain regions) to establish a scheme for seizure termination classification. Other studies have proposed differentiating seizures by their temporal dynamics6. Unlike that approach, which focuses on changes in frequency or amplitude in one channel before seizure termination, we propose in our study a different classification method that considers the interaction between different channels. While there are advantages in considering seizure termination through either method, we believe that our classification can help understand how interregional seizure spread can influence the specific pathways (mechanisms) by which they terminate. We anticipate that our classification will provide a framework for future studies investigating the different mechanisms that might underlie seizure termination.
4.1. Termination classification and mechanistic implications
Theories about seizure termination can be grouped into three basic frameworks. The first of these focuses on how metabolic processes change gradually during a seizure. A second theory posits that increases in inhibition coming from some structure(s) work as a compensatory break on the hyperactivity of the seizures19. Alternatively, a third theory suggests that termination relates to a transient increase in network synchrony13,20–24 and that termination is an emergent property of that network level change. Which of the proposed mechanisms could explain these different results and dynamics?
During a seizure with sustained paroxysmal discharges, metabolic mechanisms must be changing25. Changes in pH caused by sustained synaptic transmission26,27, adenosine28, and extracellular volume along with neurotransmitter depletion29 as well as changes in ionic concentration30,31 have been supported by findings from animal studies. Studies in both animals and humans have shown that there are changes in oxygen and glucose as well – though these appear to be brief and not necessarily at the end of the seizure, arguing against their role in termination32. Most relevant for the work here, it is not clear how metabolic changes could explain the ubiquitous feature of abrupt termination. It would seem more plausible that gradual metabolic changes would lead to gradual cessation. In most seizures, at the beginning of the seizure a high-frequency, low-amplitude pattern can be identified whose amplitude is relatively small at initiation3. From this point, the amplitude gradually increases as the seizure progresses and more networks of neurons are recruited. We observed that CSA seizures have significantly lower AAE compared to sBS seizures. At the LFP level, the amplitude indicates the level of synchrony between focal groups of neurons18. If more neurons activate simultaneously, the recorded signal will register with a higher amplitude. Since the AAE in CSA seizures remains low, we hypothesize that their recruited networks are relatively small and do not change over the course of a seizure. In addition, the BSR of CSA seizures was significantly lower than that of BS seizures even during the pre-seizure period. Additionally, the high degree of synchrony observed toward the end of seizures, especially those with burst suppression, would also be hard to derive from purely metabolic factors that are going to be patchy and dependent of activity in a given location. That being said, it does seem reasonable that metabolic factors might work to either speed up or slow down discharges especially in CSA seizures thus affecting dynamics of the seizure but not directly responsible for termination itself.
In contrast, subcortical structures including the thalamus, basal ganglia, and brain stem nuclei may play important roles in synchronizing brain activity between different regions, which may facilitate seizure propagation33. Two brainstem nuclei – the dorsal raphe nuclei and locus coeruleus – are known to produce global neuromodulatory effects through the release of their respective neurotransmitters, serotonin and norepinephrine. Serotonin, in particular, is known to have an effect on seizure occurrence, with increases in extracellular serotonin inhibiting both focal and generalized seizures34. In addition, previous studies have demonstrated that the locus coeruleus projects bilaterally35 and its stimulation can attenuate seizures36–39. Further, lesions of the locus coeruleus reduce the possible benefits of vagal nerve stimulation and may even facilitate the pathway to status epilepticus40,41.
The synchronous activity exhibited in seizures13,22,23, especially with BS at the end, could be mediated by subcortical regions including (but not limited to) thalamus, which would induce inhibitory effects throughout the brain19. The BS pattern can be compared to the synchronized pattern seen in seizures of idiopathic generalized epilepsy, where all regions exhibit synchronized voltage fluctuations and suggests that thalamocortical loops play an important role during seizure termination42,43.
Additionally, burst suppression activity can be recorded during anesthesia or impaired consciousness11. In the decades since its description44, researchers have investigated the neurological correlates of this activity. It seems reasonable to assume that cortical networks enter a brief ‘silent mode’ between bursts, however studies45,46 in cats showed that this was not the case for subcortical regions including thalamus and hippocampus. Steriade et al.45 showed that at least 30% of thalamic neurons are active during suppression. The thalamus plays an important role during synchronization, as evidenced by various animal studies and human imaging studies33,47. This notion is further supported by the finding that thalamic stimulation can be used to control seizures48. Thus, thalamic activity may be perfectly poised to mediate the largely synchronous and abrupt terminations that are seen especially in generalized seizures with BS patterns.
Thalamus alone is not the only subcortical structure that could play such a role. Kroeger et al.46 demonstrated that hippocampal activity increases with higher levels of network synchrony, a finding that we observed in a subset of our seizures with BS at their termination (Fig. 2C–D). Further, we showed that hippocampus seizures tend to have higher AAE compared to other regions in seizures with BS at their termination. Thus, it is possible that the hippocampus may also be able to mediate the synchrony of seizure termination.
Ultimately, the increase in the synchronization and in the network density may suggest that the evolution in network synchrony can lead to seizure termiantion. As previously suggested22, as the seizure approaches termination, the underlying neuronal activity becomes more chaotic, which may lead to more synchronized bursting dynamics. This, in turn, results in more network edges that serve to further amplify synchronization until the edges between recruited subnetworks merge and the bursting dynamics cease.
4.2. Seizure termination is characterized by a discontinuous process
While seizure initiation and spread tend to progress gradually, seizure termination is usually abrupt, even in seizures with asynchronous endings (AT; Fig. 1B and Fig. 2A–B). While this feature might be obvious (particularly to encephalographers), its importance has rarely been commented on. Even in the case of AT, localized groups of channels are seen to terminate together, demonstrating that ictal activity, either within a single region or shared across functionally-connected regions, ends all at once.
Additionally, roughly a fifth of seizures in both focal and electrographic generalization types exhibit no suppressed activity throughout the seizure (CSA seizures). Although we identified a small number (10%) of seizures with electrographic generalization of this nature, the majority of such seizures were focal. Furthermore, where these CSA seizures spread to other areas, they propagated to relatively fewer regions. But, even in these seizures where the ictal pattern is continuous (not showing burst suppression) termination is characterized by abrupt suppression or reversion to the baseline. Crucially, seizure termination, is therefore, not the reverse of initiation regardless of overall dynamics of the seizure.
Even though our findings do not directly specify which mechanisms underlie seizure termination, we hypothesize that there are a limited number of mechanisms through which a seizure might end. Even in the case of asynchronous termination we have observed that the termination is localized to a group of channels. This might indicate the involvement of multiple drivers for the termination of these seizures. For instance, assuming thalamic engagement in termination of some seizures, we need to consider that it consists of many subnuclei, with the role of each in epilepsy remaining unclear. For example, anterior nucleus of the thalamus (ANT) is part of the limbic circuit which connects to the hippocampus and it is believed to be involved in emotional processing and seizure propagation49. Therefore, ANT has commonly been targeted for the management of temporal lobe epilepsy by therapeutic stimulation. However, the efficacy of ANT stimulation is lower in cases of non-temporal epilepsy48. Another studied nucleus, the centromedian nucleus of the thalamus projects to cortical regions such as frontal and insula. We therefore hypothesize that subcortical regions and their various projections may influence the variability in termination. To reiterate, this hypothesis is solely based on our comparison with a limited number of measures, and further studies are needed to identify the precise mechanisms underlying seizure termination. The role of different cortical and subcortical regions should be assessed to understand whether the observed synchronization at seizure end is necessary and how it can be achieved.
In summary, our qualitative and quantitative analysis of seizure terminations leads to a simple classification scheme which characterizes the vast majority of seizures and therefore suggests that there is a limited number of dynamical patterns at seizure termination. We believe that, ultimately, this framework can be useful in understanding how the propagation of seizures may influence their termiantion and lead to new avenues of therapy.
Supplementary Material
Most seizures end synchronously, and show block-like termination patterns.
Most seizures terminate with burst suppression, regardless of their generalization.
Network density is lower in seizures with continuous seizure activity.
Limited patterns of seizure termination suggest a limit to the number of termination mechanisms.
Acknowledgements
The authors wish to thank Alex Hadjinicolaou, Angelique Paulk, Dan Soper, and Rina Zelmann for their invaluable insight on drafts of this manuscript. We are also grateful to the clinical team, technicians and our participants who selflessly help us further our knowledge of the brain.
Funding
MAK was supported by NIH NINDS R01-NS110669. MBW was supported by the Glenn Foundation for Medical Research and American Federation for Aging Research (Breakthroughs in Gerontology Grant); American Academy of Sleep Medicine (AASM Foundation Strategic Research Award); Football Players Health Study (FPHS) at Harvard University; Department of Defense through a subcontract from Moberg ICU Solutions, Inc; and NIH R01-NS102190, R01-NS102574, R01-NS107291, RF1-AG064312. SSC was supported by K24-NS088568, R01-2NS062092.
Abbreviations
- IRB
Institutional Review Board
- ST
synchronous termination
- AT
asynchronous termination
- sBS
synchronous burst suppression
- aBS
asynchronous burst suppression
- BS
burst suppression
- CSA
continuous seizure activity
- AAE
average of absolute energy
- BSR
burst suppression ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
MBW and SSC are co-founders of Beacon Biosignals. Other authors report no competing interests.
Supplementary Material
A word document containing four figures is attached.
References
- 1.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x [DOI] [PubMed] [Google Scholar]
- 2.Perucca P, Dubeau F, Gotman J. Intracranial electroencephalographic seizure-onset patterns: effect of underlying pathology. Brain J Neurol. 2014;137(Pt 1):183–196. doi: 10.1093/brain/awt299 [DOI] [PubMed] [Google Scholar]
- 3.Salami P, Peled N, Nadalin JK, et al. Seizure onset location shapes dynamics of initiation. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2020;131(8):1782–1797. doi: 10.1016/j.clinph.2020.04.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lado FA, Moshé SL. How do seizures stop? Epilepsia. 2008;49(10):1651–1664. doi: 10.1111/j.1528-1167.2008.01669.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubler F, Steimer A, Gast H, Schindler KA. Chapter Eight - Seizure Termination. In: Jiruska P, de Curtis M, Jefferys JGR, eds. International Review of Neurobiology. Vol 114. Modern Concepts of Focal Epileptic Networks. Academic Press; 2014:187–207. doi: 10.1016/B978-0-12-418693-4.00008-X [DOI] [PubMed] [Google Scholar]
- 6.Saggio ML, Crisp D, Scott JM, et al. A taxonomy of seizure dynamotypes. eLife. 9. doi: 10.7554/eLife.55632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boido D, Gnatkovsky V, Uva L, Francione S, de Curtis M. Simultaneous enhancement of excitation and postburst inhibition at the end of focal seizures. Ann Neurol. 2014;76(6):826–836. doi: 10.1002/ana.24193 [DOI] [PubMed] [Google Scholar]
- 8.Afra P, Jouny CC, Bergey GK. Termination Patterns of Complex Partial Seizures: An Intracranial EEG Study. Seizure. 2015;32:9–15. doi: 10.1016/j.seizure.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trinka E, Walser G, Unterberger I, et al. Asymmetric termination of secondarily generalized tonic-clonic seizures in temporal lobe epilepsy. Neurology. 2002;59(8):1254–1256. doi: 10.1212/01.WNL.0000032105.00984.77 [DOI] [PubMed] [Google Scholar]
- 10.Proix T, Jirsa VK, Bartolomei F, Guye M, Truccolo W. Predicting the spatiotemporal diversity of seizure propagation and termination in human focal epilepsy. Nat Commun. 2018;9. doi: 10.1038/s41467-018-02973-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amzica F What does burst suppression really mean? Epilepsy Behav EB. 2015;49:234–237. doi: 10.1016/j.yebeh.2015.06.012 [DOI] [PubMed] [Google Scholar]
- 12.Bauer PR, Thijs RD, Lamberts RJ, et al. Dynamics of convulsive seizure termination and postictal generalized EEG suppression. Brain. 2017;140(3):655–668. doi: 10.1093/brain/aww322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evangelista E, Bénar C, Bonini F, et al. Does the Thalamo-Cortical Synchrony Play a Role in Seizure Termination? Front Neurol. 2015;6. doi: 10.3389/fneur.2015.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Computational Intelligence and Neuroscience. doi: 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shokooh LA, Toffa DH, Pouliot P, Lesage F, Nguyen DK. Intracranial EEG seizure onset and termination patterns and their association. Epilepsy Res. 2021;176:106739. doi: 10.1016/j.eplepsyres.2021.106739 [DOI] [PubMed] [Google Scholar]
- 16.Brandon Westover M, Shafi MM, Ching S, et al. Real-time segmentation of burst suppression patterns in critical care EEG monitoring. J Neurosci Methods. 2013;219(1):131–141. doi: 10.1016/j.jneumeth.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer MA, Eden UT, Cash SS, Kolaczyk ED. Network inference with confidence from multivariate time series. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79(6 Pt 1):061916. doi: 10.1103/PhysRevE.79.061916 [DOI] [PubMed] [Google Scholar]
- 18.Warren CP, Hu S, Stead M, Brinkmann BH, Bower MR, Worrell GA. Synchrony in Normal and Focal Epileptic Brain: The Seizure Onset Zone is Functionally Disconnected. J Neurophysiol. 2010;104(6):3530–3539. doi: 10.1152/jn.00368.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutton JT, Frost JD, Foster J. The Influence of the Cerebellum in Cat Penicillin Epilepsy. Epilepsia. 1972;13(3):401–408. doi: 10.1111/j.1528-1157.1972.tb04580.x [DOI] [PubMed] [Google Scholar]
- 20.Topolnik L, Steriade M, Timofeev I. Partial cortical deafferentation promotes development of paroxysmal activity. Cereb Cortex N Y N 1991. 2003;13(8):883–893. doi: 10.1093/cercor/13.8.883 [DOI] [PubMed] [Google Scholar]
- 21.Guye M, Régis J, Tamura M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain J Neurol. 2006;129(Pt 7):1917–1928. doi: 10.1093/brain/awl151 [DOI] [PubMed] [Google Scholar]
- 22.Kramer MA, Eden UT, Kolaczyk ED, Zepeda R, Eskandar EN, Cash SS. Coalescence and Fragmentation of Cortical Networks during Focal Seizures. J Neurosci. 2010;30(30):10076–10085. doi: 10.1523/JNEUROSCI.6309-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truccolo W, Donoghue JA, Hochberg LR, et al. Single-neuron dynamics in human focal epilepsy. Nat Neurosci. 2011;14(5):635–641. doi: 10.1038/nn.2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer MA, Cash SS. Epilepsy as a Disorder of Cortical Network Organization. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry. 2012;18(4):360–372. doi: 10.1177/1073858411422754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2001;21(10):1133–1145. doi: 10.1097/00004647-200110000-00001 [DOI] [PubMed] [Google Scholar]
- 26.Ziemann AE, Schnizler MK, Albert GW, et al. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11(7):816–822. doi: 10.1038/nn.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinning A, Hübner CA. Minireview: pH and synaptic transmission. FEBS Lett. 2013;587(13):1923–1928. doi: 10.1016/j.febslet.2013.04.045 [DOI] [PubMed] [Google Scholar]
- 28.Lovatt D, Xu Q, Liu W, et al. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A. 2012;109(16):6265–6270. doi: 10.1073/pnas.1120997109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kullmann DM, Semyanov A. Glutamatergic modulation of GABAergic signaling among hippocampal interneurons: novel mechanisms regulating hippocampal excitability. Epilepsia. 2002;43 Suppl 5:174–178. doi: 10.1046/j.1528-1157.43.s.5.12.x [DOI] [PubMed] [Google Scholar]
- 30.Fröhlich F, Bazhenov M, Timofeev I, Sejnowski TJ. Maintenance and termination of neocortical oscillations by dynamic modulation of intrinsic and synaptic excitability. Thalamus Relat Syst. 2005;3(2):147–156. doi: 10.1017/S1472928807000155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnan GP, Bazhenov M. Ionic Dynamics Mediate Spontaneous Termination of Seizures and Postictal Depression State. J Neurosci. 2011;31(24):8870–8882. doi: 10.1523/JNEUROSCI.6200-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman AG, Meldrum BS, Siesiö BK. Cerebral Metabolic Changes During Prolonged Epileptic Seizures in Rats. J Neurochem. 1977;28(5):1025–1035. doi: 10.1111/j.1471-4159.1977.tb10665.x [DOI] [PubMed] [Google Scholar]
- 33.Norden AD, Blumenfeld H. The role of subcortical structures in human epilepsy. Epilepsy Behav. 2002;3(3):219–231. doi: 10.1016/S1525-5050(02)00029-X [DOI] [PubMed] [Google Scholar]
- 34.Bagdy G, Kecskemeti V, Riba P, Jakus R. Serotonin and epilepsy. J Neurochem. 2007;100(4):857–873. doi: 10.1111/j.1471-4159.2006.04277.x [DOI] [PubMed] [Google Scholar]
- 35.Nagai T, Satoh K, Imamoto K, Maeda T. Divergent projections of catecholamine neurons of the locus coeruleus as revealed by fluorescent retrograde double labeling technique. Neurosci Lett. 1981;23(2):117–123. doi: 10.1016/0304-3940(81)90027-6 [DOI] [PubMed] [Google Scholar]
- 36.Libet B, Gleason CA, Wright EW, Feinstein B. Suppression of an eplieptiform type of electrocortical activity in the rat by stimulation in the vicinity of locus coeruleus. Epilepsia. 1977;18(4):451–462. doi: 10.1111/j.1528-1157.1977.tb04991.x [DOI] [PubMed] [Google Scholar]
- 37.Faber J, Vladyka V. Antiepileptic effect of electric stimulation of the locus coeruleus in man. Act Nerv Super (Praha). 1983;25(4):304–308. [PubMed] [Google Scholar]
- 38.Neuman RS. Suppression of penicillin-induced focal epileptiform activity by locus ceruleus stimulation: mediation by an alpha 1-adrenoceptor. Epilepsia. 1986;27(4):359–366. doi: 10.1111/j.1528-1157.1986.tb03554.x [DOI] [PubMed] [Google Scholar]
- 39.Jimenez-Rivera C, Voltura A, Weiss GK. Effect of locus ceruleus stimulation on the development of kindled seizures. Exp Neurol. 1987;95(1):13–20. doi: 10.1016/0014-4886(87)90002-1 [DOI] [PubMed] [Google Scholar]
- 40.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39(7):709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x [DOI] [PubMed] [Google Scholar]
- 41.Giorgi FS, Ferrucci M, Lazzeri G, et al. A damage to locus coeruleus neurons converts sporadic seizures into self-sustaining limbic status epilepticus. Eur J Neurosci. 2003;17(12):2593–2601. doi: 10.1046/j.1460-9568.2003.02692.x [DOI] [PubMed] [Google Scholar]
- 42.Bernasconi A, Bernasconi N, Natsume J, Antel SB, Andermann F, Arnold DL. Magnetic resonance spectroscopy and imaging of the thalamus in idiopathic generalized epilepsy. Brain J Neurol. 2003;126(Pt 11):2447–2454. doi: 10.1093/brain/awg249 [DOI] [PubMed] [Google Scholar]
- 43.Kim JB, Suh SI, Seo WK, Oh K, Koh SB, Kim JH. Altered thalamocortical functional connectivity in idiopathic generalized epilepsy. Epilepsia. 2014;55(4):592–600. doi: 10.1111/epi.12580 [DOI] [PubMed] [Google Scholar]
- 44.Derbyshire AJ, Rempel B, Forbes A, Lambert EF. The effects of anesthetics on action potentials in the cerebral cortex of the cat. Am J Physiol-Leg Content. 1936;116(3):577–596. doi: 10.1152/ajplegacy.1936.116.3.577 [DOI] [Google Scholar]
- 45.Steriade M, Amzica F, Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol. 1994;90(1):1–16. doi: 10.1016/0013-4694(94)90108-2 [DOI] [PubMed] [Google Scholar]
- 46.Kroeger D, Florea B, Amzica F. Human Brain Activity Patterns beyond the Isoelectric Line of Extreme Deep Coma. PLOS ONE. 2013;8(9):e75257. doi: 10.1371/journal.pone.0075257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aracri P, de Curtis M, Forcaia G, Uva L. Enhanced thalamo-hippocampal synchronization during focal limbic seizures. Epilepsia. 2018;59(9):1774–1784. doi: 10.1111/epi.14521 [DOI] [PubMed] [Google Scholar]
- 48.Li MCH, Cook MJ. Deep brain stimulation for drug-resistant epilepsy. Epilepsia. 2018;59(2):273–290. doi: 10.1111/epi.13964 [DOI] [PubMed] [Google Scholar]
- 49.Child ND, Benarroch EE. Anterior nucleus of the thalamus: Functional organization and clinical implications. Neurology. 2013;81(21):1869–1876. doi: 10.1212/01.wnl.0000436078.95856.56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data of this study are available upon request from the corresponding author. The data are not publicly available as they contain information that could compromise the privacy of the participants.


