Abstract
Purpose
In this article, we aimed to investigate the influences of luteolin on inflammatory injury to cardiomyocytes induced by lipopolysaccharide (LPS).
Materials and Methods
H9c2 cells were pretreated with different concentrations of luteolin (10, 20, and 50 µM) for 12 h and then stimulated with 10 µg/mL LPS or no LPS for 6 h. Cell viability was detected by CCK-8 assay. Cell apoptosis was determined by flow cytometry. QRT-PCR and Western blotting were utilized to examine mRNA and protein levels. ELISA was used to determine the levels of monocyte chemoattractant protein-1, tumor necrosis factor-alpha, interleukin (IL)-6, IL-1β, and IL-18 in cell supernatants among different groups of H9c2 cells. Immunofluorescence was applied to evaluate reactive oxygen species formation in H9c2 cells. M-mode images of echocardiography, the ejection fraction test, fractional shortening test, end-systolic volume test, and end-diastolic volume test of mouse heart function were obtained by ultrasonic electrocardiogram.
Results
Luteolin could alleviate inflammatory damage and inflammatory factor expression among LPS-induced H9c2 cells. Additionally, we found that luteolin decreased LPS-stimulated inflammatory damage in H9c2 cells by down-regulating NOD-like receptor family pyrin domain containing 3 (Nlrp3). Luteolin also improved myocardial function in mice treated with LPS and reduced myocardial relaxation. Luteolin reversed myocardial histological abnormalities in mice and reduced inflammation and cardiomyocyte apoptosis. Additionally, luteolin inhibited oxidative stress-mediated myocardial and systemic tissue damage in mice. Finally, luteolin reduced LPS-induced inflammatory damage in mouse cardiomyocytes by down-regulating Nlrp3.
Conclusion
We found that luteolin could reduce inflammatory damage to cardiomyocytes induced by LPS by down-regulating Nlrp3.
Keywords: Luteolin, lipopolysaccharide, H9c2 cells, Nlrp3
INTRODUCTION
Myocarditis refers to focal or diffuse inflammatory lesions of the myocardium caused by multiple risk factors, such as viral infections, bacterial infections, toxins, autoimmune diseases, and chemical factors.1 It is characterized by dyspnea, chest pain, decreased exercise capacity, and arrhythmia. The most common cause of sudden cardiac death is viral myocarditis infection or immune-mediated response after virus. The symptoms and signs of myocarditis are diverse. While there is no obvious clinical manifestation in early stages, severe arrhythmia, cardiac insufficiency, and sudden death may occur in later stages.2 Cardiac magnetic resonance imaging has been used to diagnose myocarditis, albeit biopsy is the gold standard for diagnosing myocarditis. Recently, immunosuppression and immunomodulation have proven to be useful in the treatment of myocarditis.2 However, great efforts are still needed to develop new and effective myocarditis treatment strategies, as currently, the incidence of myocarditis is growing every year because of a lack of effective treatment.
Luteolin is a natural flavonoid compound widely present in various plants, including mint, rosemary, thyme, pine, and fern.3 Flavonoids are some of the most abundant secondary metabolites in plants and have a variety of pharmacological activities. As a falconoid medicinal plant, luteolin displays antimicrobial, immunomodulatory, anticancer, and anti-lipase activity.4,5 Luteolin also exhibits beneficial pharmacological effects in allergy,6 osteoporosis,7 diabetes,8 and liver toxicity,9 and studies have shown that luteolin has cardiac effects in ischemia/reperfusion injury.10,11 Additionally, luteolin has been found to fortify Bcl-2 expression and to reduce the Bax and cleaved caspase-3 expression following injury.12 Another article revealed that luteolin can be applied as a chemotherapy sensitizer to elevate the therapeutic impact of tamoxifen in drug-resistant breast cancer cells by inhibiting the expression of cyclin E2.13
Previous studies have shown that inflammasomes play an important role in clearing infections and tailoring host immune responses.14,15 NOD-like receptor family pyrin domain containing 3 (Nlrp3) is one of the most well-characterized inflammasomes, along with apoptosis-associated speck-like protein (ASC) and caspase-1. Accumulating evidence indicates that Nlrp3 expression is increased in coxsackievirus B3 (CVB3)-infected myocardiocytes.16,17 Tong, et al.18 indicated that miR-15 inhibition alleviates myocardial inflammation and cell injury, which might be involved in Nlrp3 inflammasome inactivation. Moreover, Zhang, et al.19 found that luteolin exerts protective effects on myocardial ischemia reperfusion injury, which might be related to the downregulation of the toll-like receptor-4 (TLR4)-meidated NF-kB/Nlrp3 inflammasome. However, whether luteolin regulates Nlrp3 inflammasome activation requires further investigation in myocarditis.
In this study, we aimed to examine the influences of luteolin on inflammatory injury to cardiomyocytes induced by lipopolysaccharide (LPS) and potential mechanisms thereof. Our results indicated that luteolin may be a potential option for treating myocarditis.
MATERIALS AND METHODS
Cell cultivation
H9c2 cardiomyocytes were obtained from the American Type Culture Collection. H9c2 cells were cultured in DMEM supplied with 100 µg/mL of streptomycin, 100 U/mL of penicillin, and 10% FBS. H9c2 cells were treated with different concentrations of luteolin (10, 20, and 50 µM) for 12 h and then excited with 10 µg/mL of LPS or no LPS for 6 h. The cells were split into five groups, including control, LPS, LPS+luteolin 10 µM, LPS+luteolin 20 µM, and LPS+luteolin 50 µM.
Small-interfering RNA transfection
Nlrp3 small-interfering RNA (siRNA) and negative control were designed and synthesized by GenePharma (Shanghai, China). H9c2 cells were cultured in six-well plates and then transfected with si-Nlrp3 or si-NC using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Cell viability
Cell viability was determined by CCK-8 assay (Sigma, St. Louis, MO, USA). Briefly, cells (1× 103 cells/well) were placed in 96-well plates. After indicated treatment for 24 h, cells in each well were treated with 10 µL of CCK-8 reagent. Afterwards, cells were incubated for 1.5 h in 37℃ incubator. Finally, OD450 absorbance was tested using a microplate reader.
Analysis of apoptotic cells
The H9c2 cells were gathered and washed twice with 1× phosphate buffered saline (PBS). The cells were then dyed with Annexin V fluorescein isothiocyanate and propidium iodide. Lastly, apoptosis rate was detected using FlowJo software (Becton, Dickinson, Franklin Lake, NJ, USA).
qRT-PCR
TRIzol reagent (Invitrogen) was employed to separate total RNA from the cells and tissues, and cDNA was synthesized by a SuperScript III reverse transcriptase (TransGen Biotech, Beijing, China). qPCR was conducted using an ABI Prism 7500 with SYBR Green qPCR Master Mix (Toyobo, Osaka, Japan), and GAPDH was included as an internal control. The relative expression levels of Nlrp3 were detected according to the formula of 2−ΔΔct. Primers for qPCR are presented in Table 1.
Table 1. Primers Utilized in qRT-PCR.
| Name | Sequences |
|---|---|
| Nlrp3 forward | 5'-ATTACCCGCCCGAGAAAGG-3' |
| Nlrp3 reverse | 5'-TCGCAGCAAAGATCCACACAG-3' |
| GAPDH forward | 5'-TGTGTCCGTCGTGGATCTGA-3' |
| GAPDH reverse | 5'-CCTGCTTCACCACCTTCTTGA-3' |
Western blotting
Extracted protein was isolated using RIPA lysate (with protease suppressor). The concentration was detected by BCA reagent and heated at 95℃ for 5 min. Protein samples were seperated by SDS-PAGE and transferred onto a PVDF membrane. Then, the membrane was put into 5% skim milk for 1 h and incubated with primary antibody for all night at 4℃. Then the membrane was incubated with second antibody at room temperature for 1 h. The membrane was cleaned and subjected to enhanced chemiluminescence for development. QUANTITY ONE software was used to evaluate gray value.
Reactive oxygen species measurement
According to the oxidation of 2′7′-dichlorodihydrofluorescein diacetate (DCFH-DA), intracellular reactive oxygen species (ROS) was tested. After oxidation, ROS could pass into cells and be converted into the fluorescent product 2′7′-dichlorofluorescein. After pretreatment with luteolin (0, 10, 20, and 50 µM) for 12 h, LPS (10 µg/mL) was added, and the H9c2 cells were incubated for another 6 h. Next, DMEM was replaced with DCFH-DA solution, which was attenuated with DMEM (10 µmol/L, without FBS). After cultivation for another 20 min at normal conditions, the extracellular DCFH-DA was removed and cleaned with DMEM (serum-free). Then, under a microscope, the intracellular fluorescence intensity of ROS was detected using Image J Analysis Software V.1.31 (National Institutes of Health, Bethesda, MD, USA).
Enzyme linked immune sorbent assay
Enzyme linked immune sorbent assay (ELISA) was performed to confirm the levels of monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-18, and IL-6. H9c2 cells were cultivated on 24-well plates. After indicated treatment, the cells were dissolved by RIPA lysis buffer and separated by centrifugation at 14000×g for 5 min. Then, the supernatant was gathered for ELISA. The levels of MCP-1, TNF-α, IL-1β, IL-18, and IL-6 were measured by a commercially available assay kit following the manufacturer’s protocols.
Animal models
Male C57BL/6 mice (aged 8 weeks, weighing 20–23 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All mice were maintained in a specific pathogen-free room (24±1℃, 55±10% humidity) and fed a standard laboratory diet and had free access to water. After acclimation for l week, the mice were divided into control (without treatment, n=6), LPS (n=6), LPS+luteolin 10 mg/kg (n=6), LPS+luteolin 20 mg/kg (n=6), and LPS+luteolin 50 mg/kg (n=6) groups. Mice in the LPS group received intraperitoneal injections with 10 mg/kg of LPS. The mice in the LPS+luteolin groups were administered intraperitoneal injections with different does of luteolin (10, 20, and 50 mg/kg), and after 30 minutes, they received intraperitoneal injections with 10 mg/kg of LPS. After injection with LPS for 18 h, the mice were anesthetized using 50 mg/kg of pentobarbital sodium to evaluate cardiac function. Subsequently, heart and blood samples were collected for subsequent experiments. Animal experiments were approved by the Ethics Committee of Jinan Central Hospital.
Echocardiographic evaluation
Echocardiographic evaluation was conducted to assess the influences of luteolin on myocarditis in mice. In brief, the mice were put into the left lateral decubitus position, and an M-mode ultrasonic cardiogram system with a supersonic probe was applied to acquire ultrasonic cardiogram images. At the level of the mitral valve, the ultrasonic probe was placed in the parasternal view for long-axis image formation of the heart. Afterwards, the M-mode images were obtained to estimate the ejection fraction (EF) of the left ventricle (LV), fractional shortening (FS), end-diastolic volume (EDV), end-systolic volume (ESV), LV internal diameter at diastole (LVIDd), and LV internal diameter at systole (LVIDs). The computational formula for EF was (EDV-ESV)/EDV, and that for FS was (LVIDd-LVIDs)/LVIDd.
Immunohistochemical analysis
The mice heart tissues were fixed with 4% paraformaldehyde, dehydrated, and embedded in paraffin. Next, the paraffin-embedded tissue samples were deparaffinized in xylene and then rehydrated using graded alcohol. The sections were immersed in 3% H2O2 to block endogenous peroxidase activity and then heated at 100℃ for antigen retrieval with 0.1 M sodium citrate. After washing with PBS, the sections were incubated with anti-8-hydroxy-2′-deoxyguanosine (8-OHdG) antibodies overnight at 4℃ and then incubated with secondary antibody for 1 h. Finally, the sections were stained with 3, 3-diaminobenzidine, and the 8-OHdG positive cells were detected under a light microscope.
Malondialdehyde and superoxide dismutase activity analysis
Malondialdehyde (MDA) and superoxide dismutase (SOD) are deemed as markers of systemic oxidative stress. To probe the effects of luteolin on the myocardial tissue mediated by oxidative stress in mice, plasma concentrations of MDA and SOD in mice were detected. On the basis of MDA and thiobarbituric acid yielding a red product with diverse absorption, MDA detection was performed on a spectrophotometer. A standard curve, which was constructed on the basis of reference standards, was used to quantify MDA levels. In plasma, the catalytic disproportionation reactions of SOD were tested using the WST-8 method. The procedures were carried out in accordance with the manufactures’ instructions for each assay kit.
Statistical analysis
GraphPad V.8.0 (GraphPad Software Inc., San Diego, CA, USA) was utilized to conduct the statistical analyses. One-way analysis of variance followed by Dunnett’s post hoc test was applied to compare multiple groups. p<0.01 was regarded as a significant difference. All data are displayed as the mean± SD from three experiments.
RESULTS
Luteolin elevates cardiac function
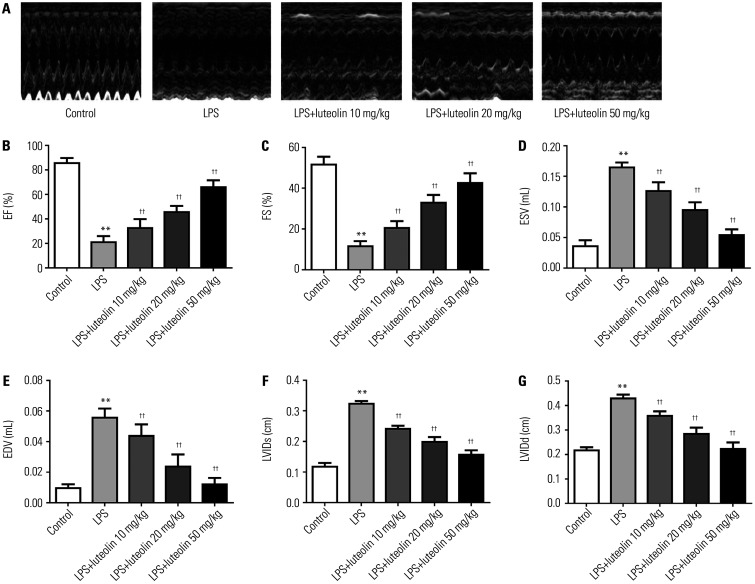
To explore the impact of luteolin on heart function, many cardiac parameters, including EF, FS, ESV, EDV, LVIDs, and LVIDd, were evaluated through ultrasonic cardiogram in vivo (Fig. 1A). As illustrated in Fig. 1B and C, EF and FS decreased in the LPS group relative to those in control group. Both low (10 mg/kg) and high (20, 50 mg/kg) doses of luteolin increased EF and FS, compared to that in the LPS group. Also, ESV and EDV were increased in the LPS group relative to those in control group, and luteolin treatment decreased these values (Fig. 1D and E). Similarly, LVIDs and LVIDd were higher in myocarditic mice, and luteolin effectively reduced these values (Fig. 1F and G). Together, these results indicated that luteolin improves cardiac function and relieves cardiac dilation in LPS-stimulated myocarditis.
Fig. 1. Luteolin promotes cardiac function and eases cardiac dilation in mice with LPS-damaged myocarditis. (A) Ultrasonic cardiogram images in each group were analyzed. Quantitative test of the cardiac function and indices, including (B) EF, (C) FS, (D) ESV, (E) EDV, (F) LVIDs, and (G) LVIDd. **p<0.01 vs. control group, ††p<0.01 vs. LPS group. LPS, lipopolysaccharide; EF, ejection fraction; FS, fractional shortening; ESV, end-systolic volume; EDV, end-diastolic volume, LVIDs, LV internal diameter at systole; LVIDd, LV internal diameter at diastole.
Luteolin mitigates oxidative stress responses in vivo
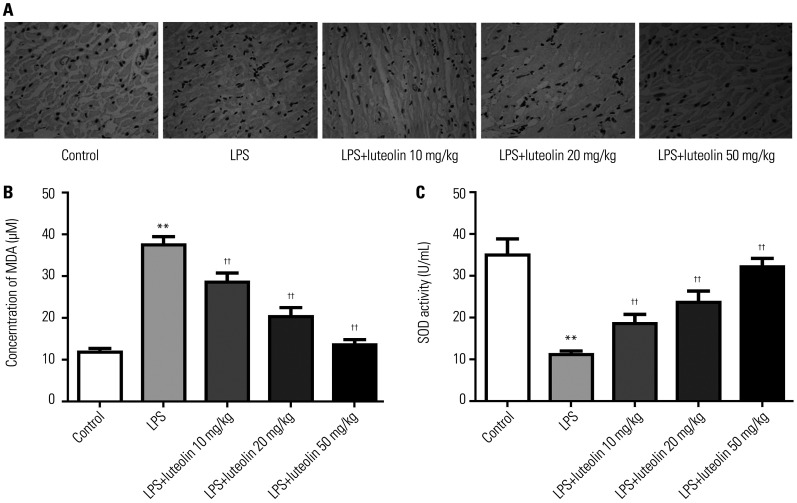
To investigate the impact of luteolin on oxidative stress, immunohistochemistry assay was used to detect 8-OHdG-labeled positive cells. As presented in Fig. 2A, the results indicated that LPS induced oxidative stress-mediated damage in the myocardium. Treatment with luteolin significantly improved oxidative stress-mediated damage. Plasma concentrations of MDA were reduced and SOD changes were higher in the luteolin group relative to those in LPS group (Fig. 2B and C).
Fig. 2. Luteolin represses oxidative stress in vivo. (A) Immunohistochemical assessment of 8-OHdG-positive cells in myocardial tissues (magnification×400). The concentration of MDA (B) and the activity of SOD (C) in mice plasma. **p<0.01 vs. control group, ††p<0.01 vs. LPS group. LPS, lipopolysaccharide; MDA, malondialdehyde; SOD, superoxide dismutase.
Luteolin reduces inflammatory factors in myocardial tissue
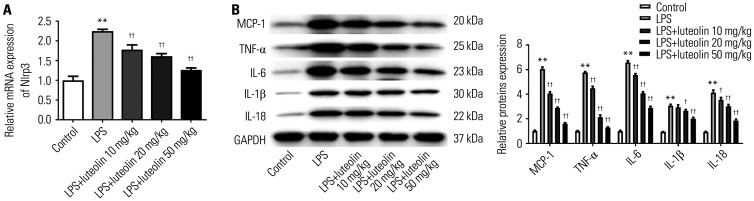
The mRNA expression of Nlrp3 in myocardial tissues was detected using qRT-PCR analysis. The results showed that LPS up-regulated Nlrp3 expression, compared with the control group, while luteolin reduced Nlrp3 expression (Fig. 3A). To detect alterations in inflammatory factors, the protein levels of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 in myocardial tissues were examined by Western blotting. As displayed in Fig. 3B, the expression of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 in myocardial tissues was altered in the LPS group, compared with that in control group. After luteolin treatment, the expression of the above mentioned cytokines was reduced relative to those in the LPS group. These results indicated that luteolin could down-regulate the expression of inflammatory factors in LPS-damaged myocarditis.
Fig. 3. Luteolin reduces inflammatory factors in the myocardial tissues of mice. (A) Nlrp3 mRNA in myocardial tissues of mice was detected using qRT-PCR assay. (B) The protein levels of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 were detected using Western blotting. **p<0.01 vs. control group, ††p<0.01 or †p<0.05 vs. LPS group. LPS, lipopolysaccharide; MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor; IL, interleukin.
Luteolin accelerates H9c2 cell viability and represses cell apoptosis
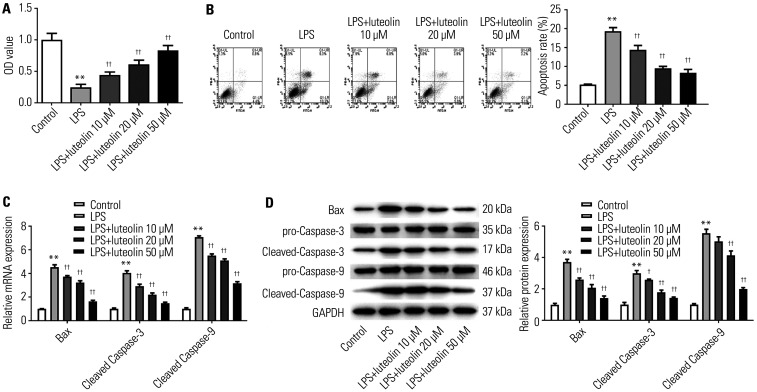
To examine the properties of luteolin on myocardium cells, CCK-8 and flow cytometry assays were employed. First, we demonstrated that the viability of H9c2 cells was inhibited by LPS relative to control; however, pretreatment with luteolin (10, 20, 50 µM) fortified cell viability in a dose-dependent manner (Fig. 4A). Meanwhile, H9c2 cell apoptosis was boosted by LPS, which was gradually reduced as the concentration of luteolin increased (Fig. 4B). As presented in Fig. 4C and D, the mRNA and protein levels of Bax and cleaved caspase-3 and -9 in H9c2 cells were fortified by LPS, while their levels were reduced with increasing concentrations of luteolin. These findings indicated that luteolin could promote H9c2 cell viability and inhibit cell apoptosis.
Fig. 4. Luteolin weakens LPS-induced injury in H9c2 cells. (A) Cell viability was detected by CCK-8 assay in H9c2 cells. (B) Cell apoptosis was analyzed by flow cytometry in H9c2 cells. The mRNA (C) and protein (D) levels of Bax and cleaved-caspase-3 and -9 were tested by qRT-PCR and Western blotting. **p<0.01 vs. control group, ††p<0.01 or †p<0.05 vs. LPS group. LPS, lipopolysaccharide.
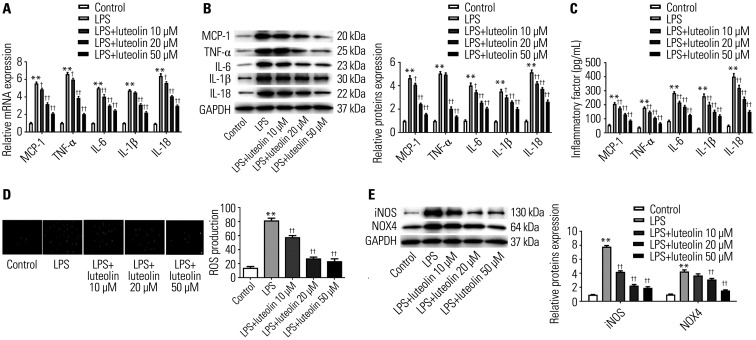
Luteolin affects inflammatory factors and oxidative stress responses in H9c2 cells
To assess changes in inflammatory factors, the mRNA and protein expression of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 was examined in H9c2 cells. As illustrated in Fig. 5A and B, mRNA and protein expression of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 was stimulated by LPS, while their levels were reduced as the concentration of luteolin increased in H9c2 cells. Next, we used the ELISA method to further test the levels of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 in the cell supernatants of H9c2 cells. As shown in Fig. 5C, the levels of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 were fortified by LPS, while their levels were decreased as the concentration of luteolin increased in H9c2 cells. These results demonstrated that luteolin could reduce the expression of inflammatory factors in LPS-elicited myocarditis.
Fig. 5. Luteolin alleviates inflammatory responses in H9c2 cells. The mRNA (A) and protein (B) levels of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 were detected by qRT-PCR and Western blotting. (C) The ELISA method was utilized to assess the levels of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 in H9c2 cells. (D) Immunofluorescence method was utilized to detect the formation of ROS in H9c2 cells. (E) Western blotting was utilized to analyze the protein levels of iNOS and NOX4. **p<0.01 vs. control group, ††p<0.01 or †p<0.05 vs. LPS group. LPS, lipopolysaccharide; MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor; IL, interleukin.
To examine the antioxidant properties of luteolin, DCFH-DA was utilized to label intracellular ROS in H9c2 cells. As displayed in Fig. 5D, fluorescence of ROS was visible after LPS stimulation, hinting that ROS was induced in H9c2 cells in the LPS group. This was repressed by luteolin treatment. Then, we analyzed protein expression of iNOS and NOX4 by Western blotting. Fig. 5E shows that the protein levels of iNOS and NOX4 were suppressed by luteolin. These findings demonstrated that luteolin inhibits oxidative stress responses in H9c2 cells in vitro.
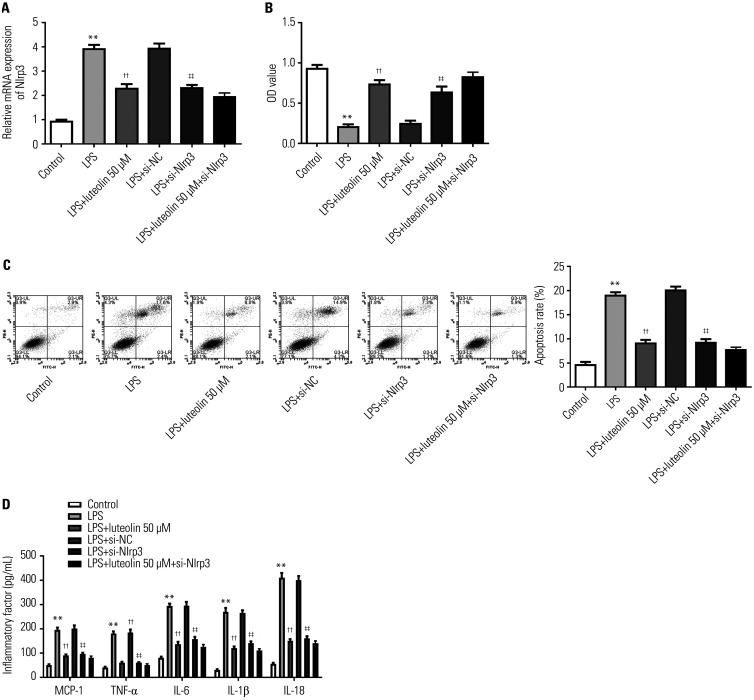
Luteolin alleviates LPS-induced injury in H9c2 cells by inhibiting Nlrp3
To determine whether Nlrp3 is involved in the protection of luteolin against oxidative stress in H9c2 cells, Nlrp3 siRNA was used to inhibit Nlrp3 expression. As shown in Fig. 6A, Nlrp3 mRNA expression was reduced after transfection of Nlrp3 siRNA into H9c2 cells. Knockdown of Nlrp3 enhanced cell viability in H9c2 cells treated with LPS and decreased LPS-induced cell apoptosis and inflammatory factors (Fig. 6B-D). Further treatment with 50 µM luteolin, which showed the strongest protective effect, elicited almost not change in cell viability, apoptosis, or inflammatory factors in Nlrp3 siRNA-transfected cells, compared with LPS+si-Nlrp3 cells (Fig. 6B-D). These findings demonstrated that luteolin alleviates LPS-induced injury in H9c2 cells by inhibiting Nlrp3.
Fig. 6. Luteolin alleviates LPS-induced injury in H9c2 cells by inhibiting Nlrp3. (A) Nlrp3 mRNA in H9c2 cells was detected using qRT-PCR assay. (B) Cell viability was detected using CCK-8 assay in H9c2 cells. (C) Cell apoptosis was analyzed by flow cytometry in H9c2 cells. (D) ELISA assay was used to assess the levels of MCP-1, TNF-α, IL-6, IL-1β, and IL-18 in H9c2 cells. **p<0.01 vs. control group, ††p<0.01 vs. LPS group, ‡‡p<0.01 vs. LPS+si-NC group. LPS, lipopolysaccharide; MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor; IL, interleukin.
DISCUSSION
Lipopolysaccharide, also known as endotoxin, is the chief constituent of the cell walls of gram-negative bacteria. LPS promotes the secretion of proinflammatory cytokines, nitric oxide, and eicosanoids, and it is regarded as an important inducer of multiorgan failure in septic shock.20,21 Previous studies have reported that TNF-α, IL-6, and elevated TLR4 mediate LPS-induced myocardial dysfunction and have a close relationship with LPS-triggered various organ failure.22,23 Therefore, LPS is widely used to reproduce inflammatory diseases, including myocarditis, in the laboratory. Herein, both in vitro and in vivo experiments were conducted to explore the effects of luteolin on LPS-induced injury in mice.
As one member of the NLR family, Nlrp3 can be enabled by pathogen-concerned molecular patterns and damage-associated molecular patterns. As a momentous inflammatory marker, Nlrp3 inflammasome has garnered vast attention in inflammatory diseases. Nlrp3 inflammasome plays a vital role in the innate immune system. Recently, research has indicated that the Nlrp3 inflammasome is related to several immune diseases, including myocarditis.24 Additionally, studies have shown that Nlrp3 oligomerization and recruitment of ASC and procaspase-1 trigger autoactivation of caspase-1, leading to the secretion of pro-inflammatory cytokines, such as IL-1β and IL-18.25 By inhibiting the Nlrp3 inflammasome pathway, luteolin has been shown to weaken high glucose-stimulated podocyte injury.26 Moreover, via a TLR4/NF-kB/Nlrp3 inflammasome pathway, luteolin has been found to exert a protective influence on myocardial I/R injury.19 Consistent with the above reports, we discovered that luteolin plays a protective effect on myocarditis injury by repressing Nlrp3 expression.
MCP-1, TNF-α, and IL-6 are thought to be key cytokines involved in triggering inflammation. As one member of C–C class of the β chemokine supergene family, MCP-1 is associated with inflammatory properties and with myocarditis and I/R injury in the heart.27 Reported, luteolin effectively inhibits the expression of MCP-1.28 Meanwhile, as a cytokine, TNF-α is central to the inflammatory cascade that regulates the immune response, both humoral and cellular immunity. Interestingly, targeting TNF-α has bolstered myocarditis therapy.29 In human vascular cells and mice, a combination of luteolin and curcumin suppressed TNF-α-stimulated vascular inflammation.30 Interestingly, in rheumatoid arthritis, TNF-α has been shown to induce the activation of Nlrp3 inflammasome.31 IL-6 is a pleiotropic inflammatory cytokine that plays crucial roles in vascular disease, the neuroendocrine system, and communication between the central nervous and immune system. In one study by Poffenberger and Horwitz,32 IL-6 was found to have a vital impact on myocarditis. In human iPSC-derived neural aggregates, luteolin receded IL-6-medicted astrogliosis.33 In the current study, we found a markedly repressive effect of luteolin on Nlrp3, MCP-1, TNF-α, IL-1β, IL-18, and IL-6 in LPS-treated H9c2 cells.
There are some limitations in our study. First, we did not detect the plasma levels of luteolin in animal experimental groups. In addition, matrix metalloproteinases (MMPs) and cathepsins (CatS/CatK) families have been found to play a significant role in cardiovascular cell apoptosis, matrix metabolism, and vascular remodeling.34,35,36 Therefore, further studies are required to investigate the effects of luteolin on MMPs and CatS/CatK in LPS-treated H9c2 cells and mice.
In this study, luteolin was found to protect cardiomyocytes cells against LPS-induced apoptosis and inflammatory damage. While the expression of Nlrp3 was increased by LPS, it was reversed to a low level after pre-treating cells with luteolin in LPS-treated H9c2 cells. Moreover, LPS-induced inflammatory damage was relieved by the depletion of Nlrp3. These findings support the application of luteolin in myocarditis therapy and Nlrp3 as potential therapeutic target in myocarditis.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Zhongfen Liu.
- Data curation: Zhongfen Liu and Shaohua Gao.
- Formal analysis: Zhongfen Liu and Shaohua Gao.
- Funding acquisition: Ying Bu.
- Investigation: Ying Bu.
- Methodology: Zhongfen Liu.
- Project administration: Zhongfen Liu and Xiaoyan Zheng.
- Resources: Zhongfen Liu and Shaohua Gao.
- Software: Zhongfen Liu and Ying Bu.
- Supervision: Xiaoyan Zheng.
- Validation: Ying Bu.
- Visualization: Zhongfen Liu, Shaohua Gao, and Xiaoyan Zheng.
- Writing—original draft: Zhongfen Liu.
- Writing—review & editing: Zhongfen Liu, Shaohua Gao, and Xiaoyan Zheng.
- Approval of final manuscript: all authors.
AVAILABILITY OF DATA AND MATERIAL
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Huber SA. Viral myocarditis and dilated cardiomyopathy: etiology and pathogenesis. Curr Pharm Des. 2016;22:408–426. doi: 10.2174/1381612822666151222160500. [DOI] [PubMed] [Google Scholar]
- 2.Ammirati E, Veronese G, Cipriani M, Moroni F, Garascia A, Brambatti M, et al. Acute and fulminant myocarditis: a pragmatic clinical approach to diagnosis and treatment. Curr Cardiol Rep. 2018;20:114. doi: 10.1007/s11886-018-1054-z. [DOI] [PubMed] [Google Scholar]
- 3.Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019;112:108612. doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- 4.Zheng CD, Duan YQ, Gao JM, Ruan ZG. Screening for anti-lipase properties of 37 traditional Chinese medicinal herbs. J Chin Med Assoc. 2010;73:319–324. doi: 10.1016/S1726-4901(10)70068-X. [DOI] [PubMed] [Google Scholar]
- 5.Ramirez G, Zamilpa A, Zavala M, Perez J, Morales D, Tortoriello J. Chrysoeriol and other polyphenols from Tecoma stans with lipase inhibitory activity. J Ethnopharmacol. 2016;185:1–8. doi: 10.1016/j.jep.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Kritas SK, Saggini A, Varvara G, Murmura G, Caraffa A, Antinolfi P, et al. Luteolin inhibits mast cell-mediated allergic inflammation. J Biol Regul Homeost Agents. 2013;27:955–959. [PubMed] [Google Scholar]
- 7.Jing Z, Wang C, Yang Q, Wei X, Jin Y, Meng Q, et al. Luteolin attenuates glucocorticoid-induced osteoporosis by regulating ERK/Lrp-5/GSK-3β signaling pathway in vivo and in vitro. J Cell Physiol. 2019;234:4472–4490. doi: 10.1002/jcp.27252. [DOI] [PubMed] [Google Scholar]
- 8.Li L, Luo W, Qian Y, Zhu W, Qian J, Li J, et al. Luteolin protects against diabetic cardiomyopathy by inhibiting NF-κB-mediated inflammation and activating the Nrf2-mediated antioxidant responses. Phytomedicine. 2019;59:152774. doi: 10.1016/j.phymed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Tan X, Yang D, Lu J, Liu B, Baiyun R, et al. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-κB/P53 signaling pathway in rats. Oncotarget. 2017;8:40982–40993. doi: 10.18632/oncotarget.17334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao C, Xia ML, Wang J, Zhou XR, Lou YY, Tang LH, et al. Luteolin attenuates cardiac ischemia/reperfusion injury in diabetic rats by modulating Nrf2 antioxidative function. Oxid Med Cell Longev. 2019;2019:2719252. doi: 10.1155/2019/2719252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JT, Wang J, Zhou XR, Xiao C, Lou YY, Tang LH, et al. Luteolin alleviates cardiac ischemia/reperfusion injury in the hypercholesterolemic rat via activating Akt/Nrf2 signaling. Naunyn Schmiedebergs Arch Pharmacol. 2018;391:719–728. doi: 10.1007/s00210-018-1496-2. [DOI] [PubMed] [Google Scholar]
- 12.Qi L, Pan H, Li D, Fang F, Chen D, Sun H. Luteolin improves contractile function and attenuates apoptosis following ischemia-reperfusion in adult rat cardiomyocytes. Eur J Pharmacol. 2011;668:201–207. doi: 10.1016/j.ejphar.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Tu SH, Ho CT, Liu MF, Huang CS, Chang HW, Chang CH, et al. Luteolin sensitises drug-resistant human breast cancer cells to tamoxifen via the inhibition of cyclin E2 expression. Food Chem. 2013;141:1553–1561. doi: 10.1016/j.foodchem.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 14.Hayward JA, Mathur A, Ngo C, Man SM. Cytosolic recognition of microbes and pathogens: inflammasomes in action. Microbiol Mol Biol Rev. 2018;82:e00015–e00018. doi: 10.1128/MMBR.00015-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi H, Morley SC. Cells under stress: the mechanical environment shapes inflammasome responses to danger signals. J Leukoc Biol. 2019;106:119–125. doi: 10.1002/JLB.3MIR1118-417R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miteva K, Pappritz K, Sosnowski M, El-Shafeey M, Müller I, Dong F, et al. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of Coxsackievirus B3-induced inflammatory cardiomyopathy. Sci Rep. 2018;8:2820. doi: 10.1038/s41598-018-20686-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Gao B, Xiong S. Involvement of NLRP3 inflammasome in CVB3-induced viral myocarditis. Am J Physiol Heart Circ Physiol. 2014;307:H1438–H1447. doi: 10.1152/ajpheart.00441.2014. [DOI] [PubMed] [Google Scholar]
- 18.Tong R, Jia T, Shi R, Yan F. Inhibition of microRNA-15 protects H9c2 cells against CVB3-induced myocardial injury by targeting NLRX1 to regulate the NLRP3 inflammasome. Cell Mol Biol Lett. 2020;25:6. doi: 10.1186/s11658-020-00203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, et al. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed Pharmacother. 2017;91:1042–1052. doi: 10.1016/j.biopha.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Balija TM, Lowry SF. Lipopolysaccharide and sepsis-associated myocardial dysfunction. Curr Opin Infect Dis. 2011;24:248–253. doi: 10.1097/QCO.0b013e32834536ce. [DOI] [PubMed] [Google Scholar]
- 21.McDonald TE, Grinman MN, Carthy CM, Walley KR. Endotoxin infusion in rats induces apoptotic and survival pathways in hearts. Am J Physiol Heart Circ Physiol. 2000;279:H2053–H2061. doi: 10.1152/ajpheart.2000.279.5.H2053. [DOI] [PubMed] [Google Scholar]
- 22.Liaunardy-Jopeace A, Gay NJ. Molecular and cellular regulation of toll-like receptor-4 activity induced by lipopolysaccharide ligands. Front Immunol. 2014;5:473. doi: 10.3389/fimmu.2014.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Xu X, Ceylan-Isik AF, Dong M, Pei Z, Li Y, et al. Ablation of Akt2 protects against lipopolysaccharide-induced cardiac dysfunction: role of Akt ubiquitination E3 ligase TRAF6. J Mol Cell Cardiol. 2014;74:76–87. doi: 10.1016/j.yjmcc.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao J, Sun T, Yue Y, Xiong S. Macrophage NLRP3 inflammasome activated by CVB3 capsid proteins contributes to the development of viral myocarditis. Mol Immunol. 2019;114:41–48. doi: 10.1016/j.molimm.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Nunes T, de Souza HS. Inflammasome in intestinal inflammation and cancer. Mediators Inflamm. 2013;2013:654963. doi: 10.1155/2013/654963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Q, Zhang M, Qian L, Wen D, Wu G. Luteolin attenuates high glucose-induced podocyte injury via suppressing NLRP3 inflammasome pathway. Life Sci. 2019;225:1–7. doi: 10.1016/j.lfs.2019.03.073. [DOI] [PubMed] [Google Scholar]
- 27.Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 2009;117:95–109. doi: 10.1042/CS20080581. [DOI] [PubMed] [Google Scholar]
- 28.Jia Z, Nallasamy P, Liu D, Shah H, Li JZ, Chitrakar R, et al. Luteolin protects against vascular inflammation in mice and TNF-alpha-induced monocyte adhesion to endothelial cells via suppressing IKBα/NF-κB signaling pathway. J Nutr Biochem. 2015;26:293–302. doi: 10.1016/j.jnutbio.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Yu J, Sun H, Zhao Y, Wang J, Zhang J, et al. Effects of ubiquitin-proteasome inhibitor on the expression levels of TNF-α and TGF-β1 in mice with viral myocarditis. Exp Ther Med. 2019;18:2799–2804. doi: 10.3892/etm.2019.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Wang X, Zhang L, Virgous C, Si H. Combination of curcumin and luteolin synergistically inhibits TNF-α-induced vascular inflammation in human vascular cells and mice. J Nutr Biochem. 2019;73:108222. doi: 10.1016/j.jnutbio.2019.108222. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Wei W, Wang Y, Wan C, Bai Y, Sun X, et al. TNF-α/calreticulin dual signaling induced NLRP3 inflammasome activation associated with HuR nucleocytoplasmic shuttling in rheumatoid arthritis. Inflamm Res. 2019;68:597–611. doi: 10.1007/s00011-019-01244-w. [DOI] [PubMed] [Google Scholar]
- 32.Poffenberger MC, Horwitz MS. IL-6 during viral-induced chronic autoimmune myocarditis. Ann N Y Acad Sci. 2009;1173:318–325. doi: 10.1111/j.1749-6632.2009.04850.x. [DOI] [PubMed] [Google Scholar]
- 33.Zuiki M, Chiyonobu T, Yoshida M, Maeda H, Yamashita S, Kidowaki S, et al. Luteolin attenuates interleukin-6-mediated astrogliosis in human iPSC-derived neural aggregates: a candidate preventive substance for maternal immune activation-induced abnormalities. Neurosci Lett. 2017;653:296–301. doi: 10.1016/j.neulet.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Ogasawara S, Cheng XW, Inoue A, Hu L, Piao L, Yu C, et al. Cathepsin K activity controls cardiotoxin-induced skeletal muscle repair in mice. J Cachexia Sarcopenia Muscle. 2018;9:160–175. doi: 10.1002/jcsm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu L, Huang Z, Ishii H, Wu H, Suzuki S, Inoue A, et al. PLF-1 (proliferin-1) modulates smooth muscle cell proliferation and development of experimental intimal hyperplasia. J Am Heart Assoc. 2019;8:e005886. doi: 10.1161/JAHA.117.005886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pauschinger M, Chandrasekharan K, Schultheiss HP. Myocardial remodeling in viral heart disease: possible interactions between inflammatory mediators and MMP-TIMP system. Heart Fail Rev. 2004;9:21–31. doi: 10.1023/B:HREV.0000011391.81676.3c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.