Abstract
Juvenile myoclonic epilepsy (JME) is a common idiopathic generalised epilepsy with variable seizure prognosis and sex differences in disease presentation. Here, we investigate the combined epidemiology of sex, seizure types and precipitants, and their influence on prognosis in JME, through cross-sectional data collected by The Biology of Juvenile Myoclonic Epilepsy (BIOJUME) consortium. 765 individuals met strict inclusion criteria for JME (female:male, 1.8:1). 59% of females and 50% of males reported triggered seizures, and in females only, this was associated with experiencing absence seizures (OR = 2.0, p < 0.001). Absence seizures significantly predicted drug resistance in both males (OR = 3.0, p = 0.001) and females (OR = 3.0, p < 0.001) in univariate analysis. In multivariable analysis in females, catamenial seizures (OR = 14.7, p = 0.001), absence seizures (OR = 6.0, p < 0.001) and stress-precipitated seizures (OR = 5.3, p = 0.02) were associated with drug resistance, while a photoparoxysmal response predicted seizure freedom (OR = 0.47, p = 0.03). Females with both absence seizures and stress-related precipitants constitute the prognostic subgroup in JME with the highest prevalence of drug resistance (49%) compared to females with neither (15%) and males (29%), highlighting the unmet need for effective, targeted interventions for this subgroup. We propose a new prognostic stratification for JME and suggest a role for circuit-based risk of seizure control as an avenue for further investigation.
Subject terms: Epilepsy, Prognosis
Introduction
Juvenile myoclonic epilepsy (JME) is the most common idiopathic generalised epilepsy (IGE) syndrome1 with estimated prevalence of 5% to 10% of all epilepsies and 18% of IGEs2. It is a complex genetic disorder with likely polygenic inheritance but its genetic architecture, and environmental components of its aetiology, are currently unknown. Seizure prognosis varies, with 20–40% of patients never achieving seizure remission3,4, and has remained so for decades. We currently have insufficient evidence to predict which patients are at differential risk for poorer outcomes, or who may benefit from alternative treatment approaches. Such stratification is urgently necessary to improve patient outcomes.
Some prognostic factors are well-known in JME, others are less well-validated. A recent meta-analysis confirms that experiencing absence seizures is the strongest negative prognostic factor for seizure remission4. Seizure precipitants are frequently self-reported by patients with JME and other epilepsies5–8, with stress and sleep deprivation being the most commonly reported precipitants in JME5,8. Small case series suggest that certain, less common precipitants are associated with anti-seizure medication (ASM) resistance: for example, eye closure sensitivity9 and praxis-induced seizures4,10 in mixed-sex cohorts, and catamenial seizures in female cohorts11. These studies are insufficiently replicated and not adjusted for other potential confounders and, because of the high prevalence of self-reported precipitants, require additional investigation. Further, little is known about the influence of frequently reported stress-related precipitants on seizure control. Photosensitivity, defined here as either seizures triggered by light/visual stimuli and/or a photoparoxysmal response (PPR) evoked during an EEG, is also commonly reported but there are conflicting data about its prognostic significance6,12,13. Overall, the relationship between precipitants and seizure control is unclear and may also be confounded by sex differences.
Sex differences in JME presentation are pervasive. In addition to the overall female preponderance in JME14,15, females have a greater frequency of absence seizures16, triggered seizures, and photosensitivity8,12,15,17, suggesting important sex differences in both seizure susceptibility and cortical excitability18–20. Yet evidence for sex-specific prognosis in JME is conflicting21. Moreover, regulations governing valproate prescription in females of childbearing age22 result in systematic differences in ASM exposure between the sexes. These considerations motivate careful and detailed investigation of sex-stratified prognosis, which may give insights into sex-specific effects and the complex aetiology of JME.
Here, we introduce the Biology of Juvenile Myoclonic Epilepsy (BIOJUME) Consortium, an international study spanning 72 sites from 12 countries focused on young people and adults with JME. This is the world’s largest JME dataset and is uniquely rich in phenotypic depth and breadth, including demographic, clinical, behavioural, treatment and EEG data, thereby allowing in-depth analysis of prognostic factors through multivariable analysis. In this study, we aimed to investigate (i) the epidemiology of seizure precipitants in JME; (ii) the relationship between patient-reported precipitants and the objective EEG measured PPR; (iii) lifestyle interventions and their relationship with reported precipitants; and (iv) factors influencing seizure control, including sex, seizure types and precipitants. We hypothesize that modifiers of seizure control, such as absence seizures and seizure precipitants, will have sex-specific effects.
Results
General demographics and clinical features
864 individuals were reviewed by the phenotyping panel, and of these, 80 did not meet strict inclusion criteria for JME, leaving 784 eligible individuals. Reasons for ineligibility were an epilepsy syndrome other than JME (n = 52), ineligible age (n = 8), not enough clinical data to determine phenotype (n = 8), no EEG information available (n = 3), no evidence of interictal generalised spikes/polyspike and waves on EEG (n = 2), abnormal background EEG (n = 2), ineligible age of seizure onset (n = 2), dysmorphic features (n = 2) and learning disability (n = 1). Self-reported ancestry was 87% European, 9% Asian, 3% mixed ethnicity and the remaining 1% either African, Middle Eastern or Indigenous American. Demographic and clinical information for participants are displayed in Table 1.
Table 1.
Demographics and clinical characteristics of the JME cohort.
| Male | Female | Total cohort | |
|---|---|---|---|
| N | 278 (36%) | 487 (64%) | 765 (100%) |
| Age (median, range) years | 22 (9–53) | 23 (6–53) | 23 (6–53) |
| Age at myoclonic seizure onset (mean ± SD) years | 14.7 ± 3.4 | 14.4 ± 3.2 | 14.5 ± 3.2 |
| Absence seizures | 98 (38%) | 213 (45%) | 311 (42%) |
| GTCS | 241 (90%) | 415 (88%) | 656 (89%) |
| Self-reported triggered seizures | 119 (50%)* | 240 (59%)* | 359 (56%) |
| Seizures triggered by stress-related precipitants | 82 (34%) | 168 (41%) | 250 (39%) |
| Percentage of seizures triggered (median) | 70% | 63% | 70% |
| Photosensitivity | 78 (37%)** | 206 (55%)** | 284 (49%) |
| (Any) Response to photic stimulation during EEG | 69 (29%)** | 186 (44%)** | 255 (38%) |
| Photoparoxysmal response | 58 (28%)** | 157 (42%)** | 215 (37%) |
| Seizures triggered by photic stimulation | 28 (12%)* | 82 (20%)* | 110 (17%) |
| Exclusively myoclonic seizures | 9 (32%) | 35 (43%) | 44 (40%) |
| Myoclonic and other seizure types | 6 (21%) | 6 (7%) | 12 (11%) |
| Other seizure types | 3 (11%) | 9 (11%) | 12 (11%) |
| Unknown seizure type | 10 (36%) | 32 (39%) | 42 (38%) |
| Seizure-free | 127 (71%) | 215 (66%) | 342 (68%) |
| Drug-resistant | 52 (29%) | 113 (35%) | 165 (33%) |
| Unknown/missing seizure control | 99 | 159 | 258 |
| History of valproate use | 183 (66%)** | 222 (46%)** | 405 (53%) |
For percentages, denominators are adjusted for missing data.
*Sex difference, p < 0.05.
**Sex difference, p < 0.001.
Epidemiology of triggered seizures and photoparoxysmal response
We investigated the concordance of triggered seizures in genetically-related individuals with JME. There were 36 genetically-related individuals in the cohort, of whom 11 sibling-pairs (n = 22) and two sibling-trios (n = 6) were concordant for not experiencing triggered seizures; one sibling-pair and one parent–child pair were concordant for experiencing triggered seizures; one sibling-pair was discordant in their response; and one sibling-pair unknown. For all further analyses we included only one individual per family (included n = 17, removed n = 19) leaving 765 in the final cohort (Table 1).
In our final cohort, 56% of individuals reported having triggered seizures, with a significant female excess (OR = 1.4 (95% CI = 1.1–2.0), p = 0.02) (Table 1). Individuals with triggered seizures were older at the time of recruitment than those without (median 25 vs 21 years, U = 38,368, p < 0.001).
Next, we investigated whether specific seizure types are associated with experiencing triggered seizures. Absence seizures were experienced by 42% of participants. Having triggered seizures was significantly associated with experiencing absence seizures in females (OR = 2.0 (95% CI = 1.4–3.1), p < 0.001, N = 404), but this was not statistically significant in males (OR = 1.4 (95% CI = 0.84–2.5), p = 0.19, N = 233). Experiencing generalised tonic–clonic seizures (GTCS) was associated with experiencing triggered seizures in males (OR = 2.7 (95% CI = 1.1–6.8), p = 0.03, N = 237) but not statistically significant in females (OR = 1.5 (95% CI = 0.9–2.7), p = 0.16, N = 402). There was no association between age of myoclonic (U = 42,196, p = 0.98), absence (U = 4411, p = 0.57), nor GTC (U = 32,279, p = 0.76) seizure onset and experiencing triggered seizures.
PPR was documented in 37% of the cohort and was more common in females (OR = 1.9 (95% CI = 2.3–2.8), p < 0.001, Table 1). 17% of individuals reported a history of seizures triggered by intermittent photic stimulation (IPS), mostly myoclonic seizures (see Table 1). Other seizure types reportedly triggered by IPS included absences, GTCS or eyelid flickering/myoclonia.
Epidemiology of seizure precipitants
The frequencies of reported seizure precipitants are shown in Table 2, with the most common being sleep deprivation. Females were more likely to report stress (OR = 1.6 (95% CI = 1.1–2.3), p = 0.01) and light/visual stimuli (OR = 1.7 (95% CI = 1.0–2.9), p = 0.04) as seizure precipitants than males, while more males reported playing games as a seizure precipitant (OR = 0.19 (95% CI = 0.1–0.7), p = 0.01). The majority of those reporting triggered seizures reported more than one precipitant, with 62% of precipitant-sensitive males (73/118) and females (147/239) reporting ≥ 2 precipitants.
Table 2.
Frequency of reported seizure precipitants by sex and the most common importance ranking.
| Trigger item | Frequencies | |||||
|---|---|---|---|---|---|---|
| Males | Females | Total | ||||
| N (%) | Rank | N (%) | Rank | N (%) | Rank | |
| 1. Sleep deprivation | 75 (31) | 1 | 147 (36) | 1 | 222 (34) | 1 |
| 2. Stress | 52 (22)* | 2 | 125 (31)* | 1 | 177 (27) | 1 |
| 3. Alcohol consumption | 31 (13) | 2 | 60 (15) | 2 | 91 (14) | 2 |
| 4. Light/visual patterns | 22 (9)* | 1 | 60 (15)* | 1 | 82 (13) | 1 |
| 5. Menstrual cycle | – | – | 50 (12) | 1 | 50 (8) | 1 |
| 6. Concentration | 14 (6) | 3 | 22 (5) | 3 | 36 (6) | 3 |
| 7. Hunger/thirst | 7 (3) | 3 | 9 (2) | 3 | 16 (3) | 3 |
| 8. Speaking in public | 3 (1) | 4 | 10 (3) | 3 | 13 (2) | 3 |
| 9. Manipulation (praxis) | 6 (3) | 2 | 7 (2) | 3 | 13 (2) | 4 |
| 10. Playing gamesa | 9 (4)* | 4 | 3 (1)* | 5 | 12 (2) | 4 |
| 11. Calculation | 3 (1) | 3 | 3 (1) | 2 | 6 (1) | 3 |
| 12. Writing | 1 (0) | 4 | 4 (1) | 2 | 5 (1) | 2 |
| 13. Listening to music | 2 (1) | 3 | 1 (0) | 3 | 3 (1) | 3 |
| 14. Reading | 1 (0) | 6 | 2 (1) | 6 | 3 (0.5) | 6 |
aNote that this may have been interpreted as either playing physical sports or playing video games.
*Sex difference, p < 0.05.
We next explored whether absence seizures are associated with a sensitivity to specific precipitants in females (there was no seizure/precipitant association in males), and found associations with having seizures provoked by stress (OR = 2.2 (95% CI = 1.4–3.4), p < 0.001), menstrual cycle (OR = 2.5 (95% CI = 1.3–4.6), p = 0.004), sleep deprivation (OR = 1.7 (95% CI = 1.1–2.5), p = 0.01), alcohol (OR = 1.8 (95% CI = 1.0–3.1), p = 0.04) or speaking in public (OR = 11 (95% CI = 1.4–87.9), p = 0.007) (N = 404 for all analyses).
A subset of male and female participants (n = 142) estimated the percentage of their total seizures they felt were precipitated. The median percentage reported was 70%, with 18% (25/142) of individuals reporting that 100% of their seizures had been triggered. In this small subgroup, the most frequent precipitants reported were similar to the overall precipitant ranking (Table 2): sleep deprivation (84%), stress (56%), alcohol (44%), menstrual cycle (20%), light/visual patterns (20%), concentration (12%), playing games (12%), praxis (8%) and speaking in public (4%).
Laboratory provocation and precipitants
We tested the association between self-reported triggered seizures and PPR, and observed a strong relationship between the two (OR = 2.8 (95% CI = 1.9–4.0), p < 0.001), with 71% (136/192) of those with PPR reporting triggered seizures. More specifically, 63% (46/73) of those who report light/visual patterns as a trigger also have PPR and 24% (46/192) of those with PPR report light/visual patterns as a trigger (OR = 3.9 (95% CI = 2.3–6.5), p < 0.001). The presence of PPR was associated to a lesser degree with other precipitants: stress (OR = 2.1 (95% CI = 1.2–3.3), p = 0.002), sleep deprivation (OR = 1.8 (95% CI = 1.1–2.8), p = 0.01), praxis (OR = 9.2 (95% CI = 1.1–77.3), p = 0.01) and concentration (OR = 3.1 (95% CI = 1.1–8.6), p = 0.02) in females (N = 349), and with alcohol (OR = 3.6 (95% CI = 1.5–8.6), p = 0.003, N = 202) in males.
Lifestyle modifications and precipitants
In the full cohort, 496 (78%) participants reported lifestyle modifications had been advised or applied to either reduce risk from seizures or mitigate the triggering of seizures, and this proportion did not differ by sex (χ2(1) = 1.4, p = 0.24). The frequencies of different lifestyle modifications are presented in Table 3. The most frequent lifestyle modifications parallel the most frequent seizure precipitants. However, the frequencies of those reporting lifestyle modifications that address the specific precipitant they experience are somewhat low (Table 3), ranging from 65% (individuals reporting sleep deprivation as a precipitant being recommended/undertaking sleep hygiene) to 2% (individuals reporting catamenial seizures being recommended/undertaking menstrual management). There was no difference in the percentage of individuals being advised/applying lifestyle modifications in those with triggered seizures (273/345, 79%) compared to those without (207/266, 78%) (χ2(1) = 0.15, p = 0.70).
Table 3.
Frequency of reported lifestyle interventions tabulated with reported seizure precipitants.
| Lifestyle interventions | Total | Reported seizure precipitants | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep deprivation | Stress | Alcohol | Light/visual patterns | Menstrual cycle | Playing gamesa | |||||||||
| N | Column % | N | Column % | N | Column % | N | Column % | N | Column % | N | Column % | N | Column % | |
| Sleep hygiene | 368 | 48 | 143 | 64 | 97 | 55 | 55 | 60 | 40 | 49 | 33 | 66 | 8 | 67 |
| Stress reduction | 221 | 29 | 74 | 33 | 69 | 39 | 21 | 23 | 27 | 33 | 26 | 52 | 8 | 67 |
| Alcohol consumption advice | 228 | 30 | 68 | 31 | 51 | 29 | 47 | 52 | 22 | 27 | 22 | 44 | 6 | 50 |
| Avoiding certain light conditions | 19 | 3 | 5 | 2 | 5 | 3 | 2 | 2 | 6 | 7 | 3 | 6 | 2 | 18 |
| Adjusting diet | 16 | 2 | 7 | 3 | 5 | 3 | 4 | 4 | 2 | 2 | 2 | 4 | 0 | 0 |
| Modified screen exposure | 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 17 |
| Exercise adjustment | 10 | 1 | 5 | 2 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 8 |
| Water/swimming precautions | 9 | 1 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Driving regulations | 8 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Avoiding illicit drugs | 5 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Smoking cessation | 4 | 1 | 4 | 2 | 3 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 8 |
| Menstrual management | 2 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 0 |
Frequency percentages given are the percent of individuals reporting that specific precipitant who are advised of the lifestyle intervention. Frequencies of lifestyle modifications relating to specific triggers are in bold.
aNote that this may have been interpreted as either playing physical sports or playing video games.
Predictors of seizure control
We tested the association of seizure precipitants and other clinical variables with seizure control, using a dichotomous variable of drug resistance/seizure freedom in males and females (Fig. 1a, b, seizure triggers with low frequencies were not considered). There was no difference in overall prevalence of drug resistance between males and females (Table 1, χ2(1) = 1.5, p = 0.22). Drug-resistant individuals were significantly older at the time of recruitment than seizure-free individuals (median age seizure-free 22 years vs drug-resistant 26 years, U = 19,626, p < 0.001).
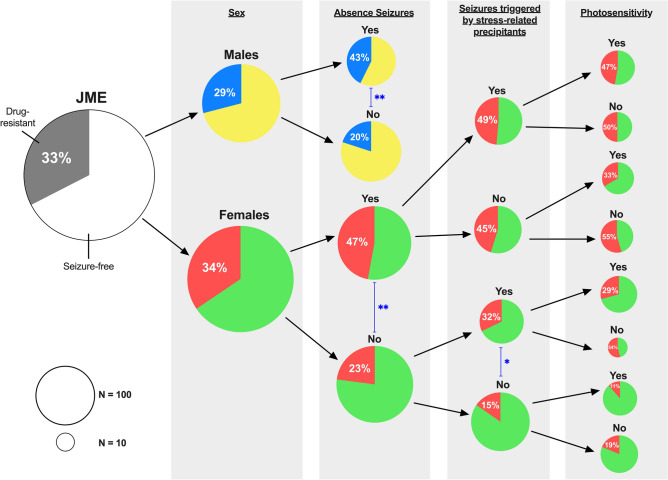
Figure 1.
Predictors of drug resistance in subgroups of JME. Odds of being drug-resistant in males (a) and females (b) with seizure precipitant predictors (* = p < 0.05 in univariate analysis). Odds of drug resistance in females was further stratified by a history of absence seizures due to its association with triggered seizures in females (c, d). Note the two scales of x-axis (top and bottom) in (d).
Seizure precipitants
In females, drug resistance was associated with experiencing seizures precipitated by menstrual cycle (OR = 4.9 (95% CI = 2.5–9.7), p < 0.001), stress (OR = 2.4 (95% CI = 1.4–3.9), p < 0.001) and concentration (OR = 3.3 (95% CI = 1.3–8.9), p = 0.012) in univariate analysis (N = 302). Experiencing PPR showed some indication of having a protective effect (better seizure control) in females (OR = 0.64 (95% CI = 0.4–1.1), p = 0.08, N = 269) (Fig. 1b). There was no association of any precipitant with seizure control in males (Fig. 1a). An increased number of seizure precipitants reported was associated with drug resistance in females (U = 8357, p = 0.02) but not males (U = 2661, p = 0.80). Reporting lifestyle modifications had no association with seizure outcome in males (OR = 0.8 (95% CI = 0.4–1.6), p = 0.49, N = 166) or females (OR = 1.1 (95% CI = 0.6–2.0), p = 0.73, N = 292).
General seizure characteristics
Drug resistance was highly associated with having absence seizures in both males (OR = 3.0 (95% CI = 1.5–5.9), p = 0.001, N = 173) and females (OR = 3.0 (95% CI = 1.9–4.9), p = < 0.001, N = 325) (Fig. 1a,b). Drug resistance was also associated with having GTCS (OR = 2.9 (95% CI = 1.2–7.3), p = 0.016, N = 324) (Fig. 1b) and earlier myoclonus onset age (p < 0.001) in females (age of myoclonus onset < 12 years increases risk of drug resistance (OR = 2.0 (95% CI = 1.1–3.6), p = 0.031, N = 297)).
Do absence seizures modify the influence of seizure precipitants on seizure control?
Above, we saw that triggered seizures are associated with absence seizures in females, and absence seizures are associated with drug resistance, therefore we further investigated the association of precipitants on drug resistance, stratified by absence seizures (Fig. 1c,d). Consequently, we found a marked difference in precipitant/seizure control associations, depending on whether females experience absence seizures. In females without absence seizures (N = 157), drug resistance was associated with experiencing seizures precipitated by concentration (OR = 31.6 (95% CI = 3.7–267.9), p = < 0.001), menstrual cycle (OR = 14.2 (95% CI = 4.2–48.6), p < 0.001), stress (OR = 3.4 (95% CI = 1.0–11.8), p = 0.002) and sleep deprivation (OR = 2.3 (95% CI = 1.1–5.1), p = 0.03) (Fig. 1d). In females with absence seizures (N = 143) no seizure precipitants were associated with drug resistance (Fig. 1c). Stratifying females based on absence seizures and susceptibility to stress-related precipitants (concentration, stress, sleep deprivation or menstrual cycle), we see that only 15% (15/98) of those without absence seizures or susceptibility to stress-related precipitants are drug-resistant compared to 49% (34/70) of those with both absence seizures and stress-related precipitants. A summary prognostic stratification based on these results is illustrated in Fig. 2.
Figure 2.
Differential rates of drug resistance in subgroups of JME. The right (white/green/yellow) segment of pie charts represent those seizure-free for over one year, and the left (grey/red/blue) segment represent those who are drug-resistant. Absence seizures moderate the effect of seizures triggered by stress precipitants in females, whereas photosensitivity (here defined as any response to photic stimulation during an EEG and/or reporting light/visual patterns as a trigger) is a protective factor for seizure control in all female strata. The area of the circle is proportional to the size of the subgroup in our cohort. **p < 0.01 and *p < 0.05 in chi-squared tests.
Multivariable analysis of seizure characteristics and precipitants on seizure control
Since experiencing absence seizures was the only variable associated with drug resistance in males, no multivariable analysis was carried out in males. We performed a logistic regression of drug resistance/seizure freedom including univariately associated precipitants in females (excluding concentration due to low frequency), as well as adjusting for other variables associated with seizure control (absence seizures, myoclonus onset age, GTCS and current age) (Table 4). In this model, absence seizures most significantly predicted poorer seizure control (OR = 6.0, p < 0.001), followed by having catamenial seizures (OR = 14.7, p = 0.001) and seizures precipitated by stress (OR = 5.3, p = 0.02). Effect modification of menstrual cycle precipitant on seizure control by absence seizures (p = 0.02) supported that catamenial seizures are only a predictor of poor seizure control in females without absence seizures. In contrast, PPR was associated with better seizure control (p = 0.03). A younger myoclonus onset age was also associated with drug resistance (p = 0.04).
Table 4.
Multivariable logistic regression of drug resistance in females (N = 226).
| Seizure-free/drug-resistant | OR (95%CI) | Coeff | Std. Err | Z | p value |
|---|---|---|---|---|---|
| (Intercept) | – | − 1.68 | 1.01 | − 1.66 | 0.10 |
| Absence sz | 5.97 (2.55–13.95) | 1.79 | 0.43 | 4.13 | 0.000037 |
| Menstrual cycle precipitant | 14.71 (2.82–76.70) | 2.69 | 0.84 | 3.19 | 0.0014 |
| Menstrual cycle precipitant*absence sz | 0.07 (0.01–0.61) | − 2.64 | 1.09 | − 2.41 | 0.016 |
| Stress precipitant | 5.31 (1.32–21.39) | 1.67 | 0.71 | 2.35 | 0.019 |
| PPR | 0.47 (0.24–0.94) | − 0.75 | 0.35 | − 2.15 | 0.031 |
| Myoclonus onset age | 0.89 (0.80–0.99) | − 0.12 | 0.06 | − 2.06 | 0.039 |
| GTCS | 2.91 (0.94–9.03) | 1.07 | 0.58 | 1.85 | 0.06 |
| Current age | 1.03 (0.99–1.07) | 0.03 | 0.02 | 1.63 | 0.10 |
| Sleep deprivation precipitant | 0.41 (0.09–1.82) | − 0.88 | 0.75 | − 1.17 | 0.24 |
| Sleep deprivation precipitant*absence sz | 0.49 (0.06–3.81) | − 0.72 | 1.05 | − 0.68 | 0.49 |
| Stress precipitant*absence sz | 0.86 (0.12–6.23) | − 0.16 | 1.01 | − 0.15 | 0.88 |
Seizure-free coded 0, drug resistant coded 1. OR = Odds ratio; sz = seizure; coeff = coefficient.
Bold p values are < 0.05.
*Indicates interaction terms.
The influence of valproate exposure on sex differences in seizure precipitants and seizure control
To investigate whether valproate efficacy is modulated by the presence of PPR, we stratified males based on the presence of PPR and history of valproate use. There was no difference in the frequency of drug-resistant individuals in these subgroups (χ2(3) = 1.5, p = 0.7, N = 136). Further, there was no association of any seizure precipitants on seizure control in a stratified group of males without a history of valproate use (N = 33, p > 0.3 for all precipitants) indicating that decreased valproate use in females compared to males (OR = 0.44 (95% CI = 0.32–0.59), p < 0.001, Table 1) is not responsible for sex differences we observe relating to seizure precipitants and seizure control.
Discussion
BIOJUME constitutes the largest, most comprehensively phenotyped JME-specific cohort to our knowledge, allowing us to carefully address questions about prognosis not possible with smaller or less well-characterized datasets. Our results demonstrate evidence for sex-stratified seizure prognosis in JME. The sex difference in prognosis mediated by precipitants suggests a novel concept in IGE of disease modification by stress-related mechanisms in females. We further propose a new evidence-based stratification scheme for clinicians to focus management according to prognosis and risk factors. Using this scheme, we observe a three-fold increase in ASM resistance in females with both absence seizures and seizures triggered by stress-related precipitants (49%) compared to females with neither (15%). Finally, we show that photosensitivity has a positive effect on seizure control not explained by valproate therapy, suggesting a subgroup whose aetiology is addressed by current treatment.
Seizure precipitants are not unique to epilepsy but common in other episodic or cyclical neurological disorders like migraine, in which there is also a female bias in disease prevalence, severity, the frequency of precipitants and transformation to chronic disease23. In epilepsy, precipitants are reported more commonly in JME than other IGE syndromes or focal epilepsies12,13. In this study, around half of all individuals with JME report triggered seizures, with a significant female excess (59% vs 50%). Moreover, this subgroup reports a large proportion (median 70%) of their seizures are triggered, with 1 in 5 estimating that all their seizures are precipitated. This has immediate clinical relevance since recommended lifestyle modifications do not align with individual seizure precipitants. PPR and photosensitivity are also more common in females, consistent with previous studies showing a female excess of 1.5–28,17,24. The female excess in PPR, triggered seizures and IGEs in general, implicates the influence of sex and steroid hormones, such as progestogens, androgens and oestrogens, on seizure susceptibility. A transcranial magnetic stimulation study in patients with JME showed differing patterns of fluctuating cortical excitability in females compared to males20, with studies in healthy controls indicating that the menstrual cycle contributes to these fluctuations25,26. The concordance for seizure precipitants among related individuals observed in this study suggests a genetic contribution to precipitant sensitivity.
Despite our exhaustive list, just five precipitants accounted for > 80% of the total: sleep deprivation, stress, alcohol, visual/lights and menstrual cycle, which are well-known in JME5,8 and other epilepsies6,7,27. Precipitants group according to two main neural circuits. First, stress-related precipitants: physiological states which influence neurobiological stress circuits, including stress itself, sleep deprivation, menstrual cycle, concentration and alcohol. Second, visual precipitants affecting circuits involved in visual/photosensitivity28–30, including both self-report and IPS; these were strongly associated, suggesting self-report represents a proxy measure of visual/photosensitivity.
Identifying risk factors for ASM resistance may inform alternate therapeutic approaches. The value of stratification is clear in this study where the overall sex difference in ASM resistance is non-significant, which likely contributes to inconsistent evidence for differential prognosis in females in previous studies21. In our study, we confirm that absence seizures are a strong independent risk factor for ASM resistance in both males (the only factor) and females. However, only in females do precipitants modify the odds of ASM resistance, depending on the presence of absence seizures. These differential odds are illustrated in Fig. 2. Briefly, stress-related precipitants negatively modify the odds of ASM resistance only in females without absence seizures; whilst visual/photosensitivity positively modifies the odds of ASM resistance in females regardless of absence seizures. While these seizure precipitants have been previously reported in females11, neither their sex-specific distribution nor their association with seizure control had been appreciated. Therefore, this is the first suggestion of environmental disease modification in IGE. A prospective longitudinal study would confirm the direction of the association between seizure precipitants and drug resistance, although there is no evidence to suggest that patients develop sensitivity to stress precipitants after failing to achieve seizure control31. Therefore, we hypothesise a differing genetic predisposition in females who are sensitive to stress-related precipitants, and that current ASMs do not address this susceptibility.
Individuals perceive and respond to stressors differently and can experience persistent sequelae depending on their level of stress resilience/vulnerability32. Brain anatomical and functional connectivity is an important determinant of individual stress resilience/vulnerability and is influenced by neurochemical and anatomical circuits including the neuroendocrine system (hypothalamic–pituitary–adrenal (HPA) axis), hippocampal pathways, the dopaminergic and serotonergic systems and medial prefrontal cortex (mPFC)32,33. Sexual dimorphism in stress susceptibility is linked to differential HPA axis activity via multiple neuroendocrine pathways32. Stress can alter structure and function in the prefrontal cortex34 while the mPFC also exerts strong negative control over stress pathways and mPFC lesions augment HPA axis response to emotional stress35. Previous studies indicate that individuals with stress-sensitive seizures exhibit blunted cortisol responses to acute stressors36 and have differential interactions of cortisol with brain functional connectivity37.
This novel understanding of prognostic classification (Fig. 2) focuses clinical and research implications onto: (i) females with stress-related precipitants, and (ii) more effective treatment of absence seizures. Almost half of females (49%) with both stress precipitants and absence seizures are ASM resistant, compared to 15% with neither. The overall figure of 33% ASM resistance conceals the large subgroup of females who do not respond to current ASMs. Future clinical trials should therefore consider stratifying participants with absence seizures and seizure precipitants. Also, stress-related precipitants should be routinely elicited in females. Our data show that an exhaustive list is superfluous—menstrual cycle, stress, sleep deprivation, concentration and visual sensitivity are sufficient to capture potential disease-modifying factors.
With the suggestion that sensitivity to stress-related precipitants may have a disease-modifying effect, we turn to data about lifestyle advice and interventions. We find a mismatch between the lifestyle advice given to patients and their reported precipitants, with an exceedingly small proportion of patients being recommended modifications to help with the seizure precipitants relevant to them (Table 3). This is especially true for catamenial seizures, which are the strongest independent risk factor for ASM resistance. We also found that lifestyle advice was not associated with seizure outcome, suggesting that current approaches are not effective in modifying disease course in females with precipitant-sensitive seizures. Overall, this indicates large gaps in applying targeted, evidence-based interventions for stress-related factors. Pharmacological and non-pharmacological interventions such as cognitive behavioural therapy, relaxation training, biofeedback and exercise should be evaluated38.
We also need to understand how visual pathway hypersensitivity acts as a protective factor in favour of seizure freedom, preferentially in females. Our finding suggests that failure to conduct a sex-stratified analysis explains the lack of overall association in a recent meta-analysis that found a protective effect of PPR on seizure freedom in four out of five studies4. One possibility is that the component of seizure susceptibility mediated via visual pathway hypersensitivity28–30 is effectively treated by current ASMs, a hypothesis we are unable to test in this study but merits further investigation.
The strong effect of absence seizures on ASM resistance, replicated multiple times4, and its strong association with trait impulsivity in JME39, mandates new thinking. Absence seizures are initiated by a distinct cortical network (involving occipital, prefrontal, precuneus, and medial parietal cortices40) from those involved in myoclonic seizures (prefrontal and motor cortices41,42 and cortico-striatal networks42,43), visual hypersensitivity28–30 and stress resilience32,37; however, propagation of activity across networks may explain seizure precipitation. Myoclonic and absence seizure types also have distinct genetic influences44,45. If absence seizures, visual sensitivity and stress-related precipitants in JME are not just clinical features but the instantiation of separate seizure susceptibility networks with their own distinct effect on seizure and behavioural outcomes46, then logically we should consider circuit-specific therapy47.
A cross-sectional study design with data collection from specialist clinics inevitably introduces ascertainment bias, as evidenced by a higher median age of drug-resistant individuals compared to seizure-free individuals, likely due to longer follow-up in clinics for those without seizure remission. A similar inflation in individuals reporting precipitants likely reflects their increased risk for drug resistance. However, we attempted to attenuate this ascertainment bias by including age in multivariable analyses. Self-reporting of seizures and precipitants is a common limitation in epilepsy studies. The utility of self-reporting seizure precipitants has previously been debated due to poor seizure-awareness and factors influencing self-perception of seizures, such as psychological state27. However, this study supports the credibility of self-perception of seizure precipitants due to their correlation with PPR, an objective EEG phenotype. There is also the possibility of attribution bias in recalling precipitants, which would falsely increase an association. Further, there may be subjectivity with participants/clinicians reporting seizure freedom, as we observed some individuals classified in this manner still reported infrequent myoclonic or absence seizures, as well as issues of medication non-compliance, which was not assessed here, contributing to a lack of seizure remission. Our assessment of lifestyle modifications was limited, and certain precipitants were only superficially characterised e.g., acute vs chronic stress, or the exact phase of menstrual cycle, were not distinguished. We did not assess comorbid psychiatric disorders and therefore could not include them in a multivariable model.
To conclude, there is a wide-ranging variability in prognosis between males and females with or without absence seizures in JME. Stress-related precipitants negatively modify the risk of ASM resistance in females, while visual/photosensitivity favours seizure freedom. Stratification reveals a lack of efficacy of current therapeutic approaches for large subgroups of females. Differential and complex neural stress responses may underlie this variability, which merit further research.
Methods
Participants and data collection
We collected cross-sectional data through the ongoing BIOJUME consortium study. Data for this study came from 864 individuals recruited retrospectively or prospectively from 58 sites across nine countries (Supplementary material). Inclusion criteria for this study are based on Avignon Class II consensus criteria for the diagnosis of JME48: (i) age of myoclonus onset 6–25 years; (ii) seizures comprising predominant or exclusive early morning myoclonus of upper extremities; (iii) EEG interictal generalised spikes/polyspike and waves with normal background. Participants aged between 6–55 years old were included. Exclusion criteria were: (i) myoclonus only associated with carbamazepine or lamotrigine therapy; (ii) EEG showing predominant focal interictal epileptiform discharges or abnormal background; (iii) any evidence of progressive or symptomatic myoclonus epilepsy or focal seizures; (iv) global learning disability; (v) dysmorphic features; (vi) unable to provide informed consent. We collected clinical data face-to-face through a structured questionnaire, augmented by clinical records and EEG reports. The dataset included general demographics and health information, epilepsy history, including seizure types, seizure frequency and drug/lifestyle interventions (Table 1).
Clinical data assurance
Sites uploaded clinical data onto a secure central REDCap (Research Electronic Data Capture) database49 and study coordinators ensured the most complete and accurate data possible for each participant through iterative feedback. A phenotyping panel, comprising seven epilepsy experts (CPB, KH, DKP, MR, GR, MS, RT), evaluated the diagnosis of JME according to inclusion criteria, through consensus where necessary. Missing data exist for some retrospective cases where certain clinical details were unknown.
Seizure precipitants
We compiled a list of seizure precipitants from the epilepsy literature5–7 and asked participants to answer in a binary response whether they identified any items from the list as precipitants of (myoclonic, absence or GTC) seizures. We then asked participants to rank the checked items in order of importance, with “1” being the most significant precipitant for that individual. We added a free field, “other”, to allow participants to include other precipitants not covered in the list. After data cleaning, we created a final list of 14 precipitants excluding null fields and including frequently mentioned items in the “other” field. For further analyses, we reduced the list to the most commonly reported items (frequency ≥ 2%) and grouped stress, menstrual cycle, concentration and sleep deprivation precipitants as stress-related precipitants. The occurrence of seizures or PPR provoked by intermittent photic stimulation (IPS) during an EEG recording was also reported, augmented from participant EEG reports. PPR was defined according to criteria in Kasteleijn-Nost Trenite et al.50. We operationally defined photosensitivity as anyone self-reporting seizures precipitated by light/visual patterns and/or PPR or seizures provoked by IPS.
Lifestyle modifications
Participants and researchers reported whether lifestyle modifications had been advised or applied to either reduce risk from seizures, or mitigate the triggering of seizures, as well as a free field to report specific interventions. We categorized reported interventions into (i) sleep hygiene, (ii) alcohol consumption advice, (iii) stress reduction, (iv) avoiding certain light conditions, (v) adjusting diet, (vi) modified screen exposure, (vii) exercise modification (including both exercise promotion and exercise precautions), (viii) driving regulations, (ix) water/swimming precautions, (x) avoiding psychoactive drugs, (xi) smoking cessation, and (xii) menstrual management.
Seizure control
To test associations of seizure precipitants with seizure control, we categorized participants based on their answers to two questions: (i) whether they had been free from seizures over the past year and (ii) current ASM therapy, categorized as either no drug therapy, monotherapy (not necessarily the first appropriate ASM), dual therapy, or drug-resistant (two or more ASM failures). Based on answers to these two questions, participants were categorized as either:
-
(i)
Seizure-free, defined as those who have not had a seizure of any type in over a year (whether on no drug therapy, monotherapy or dual therapy) (N = 351, 45%) or,
-
(ii)
Drug-resistant (either as reported or those who are not seizure-free on ≥ 2 ASMs) (N = 166, 21%).
Using this binary classification, 268 individuals were unable to be categorized, due to not fitting into either category (N = 129, 16%) or missing data (N = 139, 18%).
Analysis procedure and statistical methods
We carried out statistical analysis in SPSS51 and STATA52 software and produced graphics on GraphPad Prism53. Prior to statistical testing, we checked for violation of test assumptions and chose statistical tests accordingly. p < 0.05 was used to determine significance. Categorical variables were compared using a Chi-squared test, or a Fisher’s exact test if expected frequencies were less than five. Missing data were excluded from each analysis in a pairwise manner.
To investigate predictors of seizure control, we first performed univariate analysis stratified by sex, followed by post-hoc stratification by absence seizures. Odds ratios (OR) and 95% confidence intervals (CIs) of the association of seizure precipitants and other clinical variables with drug resistance were calculated. Continuous and ordinal variables were tested for associations with seizure outcome using Mann–Whitney tests. Based on results from univariate analysis, logistic regression analysis was carried out for females, with significantly associated variables (seizure precipitants: menstrual cycle, stress and sleep; PPR; age of myoclonus onset; current age; generalised tonic–clonic seizures (GTCS); absence seizures) tested against the seizure freedom/drug resistance outcome.
To ensure results were not confounded by sex differences in valproate prescribing, we stratified males into four categories based on the presence of PPR and valproate use (since it is more frequently used in males and is effective in controlling seizures in photosensitive individuals24) and tested for any association with drug resistance. Further, we tested for associations of any precipitant variable with drug resistance in the sub-group of males without a history of valproate use.
Study approval
BIOJUME is funded by the Canadian Institutes of Health Research (MOP-142405, FRN-167282) and received ethical approval from the National Health Service (NHS) Health Research Authority (South Central-Oxford C Research Ethics Committee, reference 16/SC/0266) and the Research Ethics Board of the Hospital for Sick Children, Toronto (REB#1000033784). Local ethical approvals were also held for all international sites. All procedures complied with appropriate regulatory requirements and ethical principles in line with the Declaration of Helsinki. Informed consent was obtained and documented for all participants. Assent was obtained from minors (under 16), and informed consent was obtained on their behalf by a parent or legally appropriate guardian. All clinical data from participants were de-identified before entry into the central database.
Supplementary Information
Acknowledgements
The authors thank all members of the BIOJUME consortium (Supplementary material) for their involvement in the study.
Author contributions
A.S., N.P., C.P.B., K.H., J.K., G.R., K.K.S., P.S., M.S., R.T., M.P.R., L.J.S. and D.K.P. contributed to conception and study design. A.S., N.P., A.C., H.C., A.H., L.J.S. and D.K.P. contributed to BIOJUME data management and project administration. D.M.A., C.P.B., C.Y.F., E.G., J.G., D.A.G., K.H., J.K., K.S.L., R.S.M., C.C.N., A.O., M.I.R., G.R., K.K.S., P.S., M.S., R.T., J.Z., M.P.R. and D.K.P. contributed to acquisition of study data. A.S., L.J.S. and D.K.P. contributed to analysis and interpretation of data and drafting of the manuscript. All authors reviewed the manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research: Biology of Juvenile Myoclonic Epilepsy 201503MOP‐342469 (DKP, LJS) and 201809FDN-407295 (LJS); UK Medical Research Council, Centre for Neurodevelopmental Disorders MR/N026063/1 (DKP, MPR); UK Medical Research Council, Programme Grant MR/K013998/1, (MPR); PhD stipend from UK Medical Research Council and the Sackler Institute for Translational Neurodevelopment (AS); NIHR Specialist Biomedical Research Centre for Mental Health of South London and Maudsley National Health Service Foundation Trust (DKP, MPR); UK Engineering and Physical Sciences Research Council, Centre for Predictive Modelling in Healthcare (EP/N014391/1 (MPR)); DINOGMI Department of Excellence of MIUR 2018–2022 (legge 232 del 2016 (PS)); Wales BRAIN Unit and Research Delivery Staff funded by Welsh Government through Health and Care Research Wales (KH); Biomarin srl, ENECTA srl, GW Pharmaceuticals, Kolfarma srl. and Eisai (PS); South-Eastern Regional Health Authority, Norway (Project Number 2016129) (JK); The Research Council of Norway (Project Number 299266 (MS)); Epilepsy Research UK (RT, MR); Health & Care Research Wales (MR), Wales Gene Park (MR), Abertawe Bro Morgannwg University NHS R&D (MR); UCB (GR); Nationwide Children’s Hospital (DAG); Odense University Hospital (JG); University of Southern Denmark (17/18517 (CPB)).
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
AS, NP, AC, HC, AH, CPB, CYF, EG, JG, DAG, KH, JK, KSL, RSM, CCN, AO, MIR, GR, JZ, MPR, LJS and DKP report no conflicts of interest. DA reports a grant and honoraria from Biocodex, honoraria from Eisai, royalties from UpToDate and grants from Ontario Brain Institute, McLaughlin Foundation and Dravet Syndrome Foundation, outside the present work. KKS reports a travel grant from UCB Nordic outside the present work. PS reports grants from Proveca and personal fees from Kolfarma, outside the present work. MS reports honoraria from Eisai. RHT reports honoraria from Sanofi, Eisai, GW Pharma, UCB Pharma, Zogenix, Arvelle and Bial and meeting support from LivaNova and Novartis outside the present work.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lisa J. Strug, Email: Lisa.Strug@utoronto.ca
Deb K. Pal, Email: deb.pal@kcl.ac.uk
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-06324-2.
References
- 1.Asadi-Pooya AA, Emami M, Sperling MR. A clinical study of syndromes of idiopathic (genetic) generalized epilepsy. J. Neurol. Sci. 2013;324:113–117. doi: 10.1016/j.jns.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Camfield CS, Striano P, Camfield PR. Epidemiology of juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S15–17. doi: 10.1016/j.yebeh.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 3.Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: A population-based study. Neurology. 2009;73:1041–1045. doi: 10.1212/WNL.0b013e3181b9c86f. [DOI] [PubMed] [Google Scholar]
- 4.Stevelink R, Koeleman BPC, Sander JW, Jansen FE, Braun KPJ. Refractory juvenile myoclonic epilepsy: A meta-analysis of prevalence and risk factors. Eur. J. Neurol. 2019;26:856–864. doi: 10.1111/ene.13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Silva Sousa P, Lin K, Garzon E, Sakamoto AC, Yacubian EM. Self-perception of factors that precipitate or inhibit seizures in juvenile myoclonic epilepsy. Seizure. 2005;14:340–346. doi: 10.1016/j.seizure.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Nakken KO, et al. Which seizure-precipitating factors do patients with epilepsy most frequently report? Epilepsy Behav. 2005;6:85–89. doi: 10.1016/j.yebeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Ferlisi M, Shorvon S. Seizure precipitants (triggering factors) in patients with epilepsy. Epilepsy Behav. 2014;33:101–105. doi: 10.1016/j.yebeh.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Kasteleijn-Nolst Trenite DG, de Weerd A, Beniczky S. Chronodependency and provocative factors in juvenile myoclonic epilepsy. Epilepsy Behav. 2013;28(Suppl 1):S25–29. doi: 10.1016/j.yebeh.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 9.Uchida CGP, et al. Prognosis of Juvenile myoclonic epilepsy with eye-closure sensitivity. Seizure. 2018;62:17–25. doi: 10.1016/j.seizure.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Uchida CGP, et al. Phenotyping juvenile myoclonic epilepsy. Praxis induction as a biomarker of unfavorable prognosis. Seizure. 2015;32:62–68. doi: 10.1016/j.seizure.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 11.Choi H, et al. Development and validation of a predictive model of drug-resistant genetic generalized epilepsy. Neurology. 2020 doi: 10.1212/WNL.0000000000010597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolf P, Goosses R. Relation of photosensitivity to epileptic syndromes. J. Neurol. Neurosurg. Psychiatry. 1986;49:1386–1391. doi: 10.1136/jnnp.49.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiraishi H, Fujiwara T, Inoue Y, Yagi K. Photosensitivity in relation to epileptic syndromes: A survey from an epilepsy center in Japan. Epilepsia. 2001;42:393–397. doi: 10.1046/j.1528-1157.2001.05300.x. [DOI] [PubMed] [Google Scholar]
- 14.Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46:956–960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- 15.Kishk N, et al. Sex differences among epileptic patients: A comparison of epilepsy and its impacts on demographic features, clinical characteristics, and management patterns in a tertiary care hospital in Egypt. Egypt. J. Neurol. Psychiatry Neurosurg. 2019;55:39. doi: 10.1186/s41983-019-0078-7. [DOI] [Google Scholar]
- 16.Martinez-Juarez IE, et al. Juvenile myoclonic epilepsy subsyndromes: Family studies and long-term follow-up. Brain. 2006;129:1269–1280. doi: 10.1093/brain/awl048. [DOI] [PubMed] [Google Scholar]
- 17.Poleon S, Szaflarski JP. Photosensitivity in generalized epilepsies. Epilepsy Behav. 2017;68:225–233. doi: 10.1016/j.yebeh.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 18.Galimberti CA, et al. Seizure frequency and cortisol and dehydroepiandrosterone sulfate (DHEAS) levels in women with epilepsy receiving antiepileptic drug treatment. Epilepsia. 2005;46:517–523. doi: 10.1111/j.0013-9580.2005.59704.x. [DOI] [PubMed] [Google Scholar]
- 19.van Campen JS, et al. Cortisol fluctuations relate to interictal epileptiform discharges in stress sensitive epilepsy. Brain. 2016;139:1673–1679. doi: 10.1093/brain/aww071. [DOI] [PubMed] [Google Scholar]
- 20.Puri V, Sajan PM, Chowdhury V, Chaudhry N. Cortical excitability in drug naive juvenile myoclonic epilepsy. Seizure. 2013;22:662–669. doi: 10.1016/j.seizure.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Giuliano L, et al. Long-term prognosis of juvenile myoclonic epilepsy: A systematic review searching for sex differences. Seizure. 2021;86:41–48. doi: 10.1016/j.seizure.2021.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Tomson T, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia. 2015;56:1006–1019. doi: 10.1111/epi.13021. [DOI] [PubMed] [Google Scholar]
- 23.Vetvik KG, Macgregor EA. Sex differences in the epidemiology, clinical features, and pathophysiology of migraine. Lancet Neurol. 2017;16(1):76–87. doi: 10.1016/S1474-4422(16)30293-9. [DOI] [PubMed] [Google Scholar]
- 24.Jain S, Tripathi M, Srivastava AK, Narula A. Phenotypic analysis of juvenile myoclonic epilepsy in Indian families. Acta Neurol. Scand. 2003;107:356–362. doi: 10.1034/j.1600-0404.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 25.Inghilleri M, et al. Ovarian hormones and cortical excitability. An rTMS study in humans. Clin. Neurophysiol. 2004;115:1063–1068. doi: 10.1016/j.clinph.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Ann. Neurol. 2002;51:599–603. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- 27.Sperling MR, Schilling CA, Glosser D, Tracy JI, Asadi-Pooya AA. Self-perception of seizure precipitants and their relation to anxiety level, depression, and health locus of control in epilepsy. Seizure. 2008;17:302–307. doi: 10.1016/j.seizure.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Bartolini E, et al. Abnormal response to photic stimulation in Juvenile Myoclonic Epilepsy: An EEG-fMRI study. Epilepsia. 2014;55:1038–1047. doi: 10.1111/epi.12634. [DOI] [PubMed] [Google Scholar]
- 29.Moeller F, et al. Representation and propagation of epileptic activity in absences and generalized photoparoxysmal responses. Hum. Brain Mapp. 2013;34:1896–1909. doi: 10.1002/hbm.22026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vollmar C, et al. Altered microstructural connectivity in juvenile myoclonic epilepsy: The missing link. Neurology. 2012;78:1555–1559. doi: 10.1212/WNL.0b013e3182563b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Campen JS, Jansen FE, de Graan PN, Braun KP, Joels M. Early life stress in epilepsy: A seizure precipitant and risk factor for epileptogenesis. Epilepsy Behav. 2014;38:160–171. doi: 10.1016/j.yebeh.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron. 2012;75:747–761. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Wu G, et al. Understanding resilience. Front. Behav. Neurosci. 2013;7:10. doi: 10.3389/fnbeh.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Campen JS, et al. Relation between stress-precipitated seizures and the stress response in childhood epilepsy. Brain. 2015;138:2234–2248. doi: 10.1093/brain/awv157. [DOI] [PubMed] [Google Scholar]
- 37.den Heijer JM, et al. The relation between cortisol and functional connectivity in people with and without stress-sensitive epilepsy. Epilepsia. 2018;59:179–189. doi: 10.1111/epi.13947. [DOI] [PubMed] [Google Scholar]
- 38.Leeman-Markowski BA, Schachter SC. Cognitive and behavioral interventions in epilepsy. Curr. Neurol. Neurosci. Rep. 2017;17:42. doi: 10.1007/s11910-017-0752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shakeshaft A, et al. Trait impulsivity in Juvenile Myoclonic Epilepsy. Ann. Clin. Transl. Neurol. 2021;8:138–152. doi: 10.1002/acn3.51255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tangwiriyasakul C, et al. Dynamic brain network states in human generalized spike-wave discharges. Brain. 2018;141:2981–2994. doi: 10.1093/brain/awy223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vollmar C, et al. Motor system hyperconnectivity in juvenile myoclonic epilepsy: A cognitive functional magnetic resonance imaging study. Brain. 2011;134:1710–1719. doi: 10.1093/brain/awr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caeyenberghs K, et al. Hyperconnectivity in juvenile myoclonic epilepsy: A network analysis. Neuroimage Clin. 2015;7:98–104. doi: 10.1016/j.nicl.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Ramos C, et al. Progressive dissociation of cortical and subcortical network development in children with new-onset juvenile myoclonic epilepsy. Epilepsia. 2018;59:2086–2095. doi: 10.1111/epi.14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pal DK, et al. Complex inheritance and parent-of-origin effect in juvenile myoclonic epilepsy. Brain Dev. 2006;28:92–98. doi: 10.1016/j.braindev.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durner M, et al. Genome scan of idiopathic generalised epilepsy: Evidence for major susceptibility gene and modifying genes influencing the seizure type. Ann. Neurol. 2001;49:328–335. doi: 10.1002/ana.69. [DOI] [PubMed] [Google Scholar]
- 46.Crunelli V, et al. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain. 2020;143:2341–2368. doi: 10.1093/brain/awaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Chen Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol. Ther. 2019;201:77–93. doi: 10.1016/j.pharmthera.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Kasteleijn-Nolst Trenité DGA, et al. Consensus on diagnosis and management of JME: From founder’s observations to current trends. Epilepsy Behav. 2013;28:S87–S90. doi: 10.1016/j.yebeh.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 49.Harris PA, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasteleijn-Nolst Trenite DG, et al. Methodology of photic stimulation revisited: Updated European algorithm for visual stimulation in the EEG laboratory. Epilepsia. 2012;53:16–24. doi: 10.1111/j.1528-1167.2011.03319.x. [DOI] [PubMed] [Google Scholar]
- 51.IBM Corp. IBM SPSS Statistics for Macintosh, Version 25.0. (IBM Corp, 2017).
- 52.StataCorp. Stata Statistical Software: Release 16. (StataCorp LLC, 2019).
- 53.GraphPad Prism Version 9.3.0 for Mac. GraphPad, San Diego, California USA,. www.graphpad.com. (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.