Abstract
Introduction
The necessity for an equal distribution of the COVID-19 vaccination is critical. Lower-middle and lower income countries may not be able to manufacture their vaccines, nor may they be able to afford to buy them for every inhabitant. Furthermore, the vaccination's potency may wane over time. A booster dosage is recommended. Despite this, certain areas or groups of people are still waiting for their first vaccine dosage.
Objectives
The purposes of this study were to assess the safety and tolerability of patients who received a fractionated intradermal administration (ID) of PFE-BNT as a booster dose in a group of people who had previously finished full doses of Verocell and to determine the antibody response after the injection.
Methods
An open-label experiment was carried out. Participants were at least 18 years old. Participants received 6 ug of PFE-BNT vaccination through intradermal injection. The safety and adverse reactions were monitored at immediate after injection, 30 min later, day 1, day 7, and day 30. Venous blood tests for specific IgG concentration against SARS-CoV-2 spike S1 were received prior to injection and day 30.
Results
42 participants completed the study. The mean age was 48 (the range; 23–62). The average duration after completing the 2nd dose of Verocell was 78.3 days (95% CI; 73.9–82.8). There was no serious adverse event. Almost 50% of participants reported minor adverse reactions on day 1 and roughly 30% still reporting on day 7. Systemic reactions were found less than 5%. The antibody level at day 30 was 16669.8 (95% CI; 3692.6–51238.9), which was 40 times higher.
Conclusion
PFE-BNT at a dose of 6 ug (1/5 of the typical dose) was shown to be safe and well tolerated when given intradermally. The antibody reaction was very strong. The ID administration could potentially save vaccine doses.
Keywords: Antibody response after intradermal vaccination, Completed two-doses of Verocell (inactivated vaccine), Safety, Tolerability
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also known as coronavirus disease 2019 (COVID-19), originated in December 2019 in Wuhan, China. It has ever since rapidly spread worldwide, causing morbidity and mortality in its way. In March 2020, the World Health Organization (WHO) designated the COVID-19 outbreak as a pandemic [1].
To end the COVID-19 pandemic, equitable access to effective vaccine is imperative. Currently, 7.38 billion doses of vaccines have been administered across 184 countries. It will take another six months to fully vaccine 75% of the world's population and for the herd immunity to kick in. Population in countries and regions with the highest incomes are getting injected 20 times more than those in the lowest incomes areas [2]. This disparity in vaccinations is giving rise to emergence of new mutant strains which might make the vaccinations ineffective [1].
Previous research has found that the immune response waned over time, particularly in inactivated vaccines [3]. According to a study's predicted model, the immune response of an inactivated vaccine with a 50% efficacy at first will decrease to half efficacy after 130 days and less than 20% efficacy at 240 days [3]. A joint study of 500 people from Thammasat University's faculty of medicine, Thailand and the National Centre for Genetic Engineering and Biotechnology (Biotec) reported that the level of antibodies of who received two doses of Sinovac has dropped by 50% every 40 days [4]. People who received a second dosage after more than 60 days had lower mean antibody levels than those who received it after less than 60 days. Furthermore, the vaccine's effectiveness against the original strain was reduced from 60% to 70% in the first 60 days to roughly 50% after that [4]. The study with the real subjects showed an accelerated rate of decline in vaccine efficacy than a predictive model in the previous study.
WHO with support of the Strategic Advisory Group of Experts (SAGE) on Immunization and its COVID-19 Vaccines Working Group suggested that (1) waning of the immunity overtime, (2) reduction of the efficacy in protection against severe disease and variant(s) of concern, and (3) insufficiency of the immunity in protection in some risk groups are reasons to support the booster dose for covid-19 vaccine. The management for vaccine booster doses may differ by vaccine product, epidemiological setting, risk group, and vaccine coverage rates [5].
According to an interim study from a randomized, double-blind, placebo-controlled phase 2 clinical research involving 540 adults, a third dose of Sinovac given 6 months or longer after the second dose could raise neutralizing antibodies by around threefold [6]. Another randomized, double-blind, placebo-controlled phase 1/2 clinical research including 303 healthy people aged 60 and up found that giving the third dosage of Sinovac 8 months after the second dose increased the geometric mean titer (GMT) of serum neutralizing antibodies by 7 times [7]. An observation study (unpublished data) from Chulalongkorn University's Center of Excellence in Clinical Virology found that people who completed two doses of Sinovac and received AstraZeneca as a third dose in a Sinovac and Astra Zeneca mix-and-match regimen increased their level of immunity by 131.5 times after two weeks [8]. The increase in antibody levels after a booster dosage has shown that those who have completed the basic Covid-19 vaccine's recommended dose should receive a booster dose as time passes.
Despite the WHO's recommendation for a booster dosage, vaccine shortage, distribution, and equity remain key issues in low- and middle-income countries. The intradermal (ID) delivery of Covid-19 vaccination was recommended as a solution to these problems. [9]. The papillary dermis, which is the ID route's target layer, is thought to be densely packed with antigen-presenting cells (APC). Due to the prevalence of APCs in the dermis, the dose of ID administration could be reduced to 1/10 or 1/5 of conventional IM or SC doses [10], [11].
According to MedRxiV, a research in the Netherlands indicated that ID delivery of 10 ug and 20 ug of mRNA-1273 vaccination, which are 1/10 and 1/5 dosages of IM route, was well tolerated and safe, and resulted in a significant antibody response in 38 healthy volunteers (median age is 25) [12]. Another unpublished study from Thailand found that the antibody levels of a booster dose of Astrazenaca IM route and Astrazenaca ID, which is a 1/5 dose of IM route, were similar in participants aged 18–59 who had already received two doses of Sinovac vaccines. The mean antibody levels of IM Vs.ID were 17,214.1 AU/ml and 17,662.3 AU/ml, respectively [13].
Given a very few studies have been done to prove the tolerability, safety, and immunogenicity of the ID route and no study has been done with the Pfizer-BioNTech (PFE-BNT) booster dose, we investigated the safety and tolerability and immunogenicity of the PFE-BNT vaccine delivered intradermally as a booster dose.
2. Methods
2.1. Study design and participants
This is an open-label research study. Eligible participants were adults 18 years and older who completed 2 doses of a three-week schedule of inactivated Verocell's Covid-19 vaccine(Sinovac®), had finished 2 doses of Verocell for more than 8 weeks, and have not had SARS-CoV-2 infection. Participants were excluded from the study if they had a history of severe adverse or allergic reactions to previous vaccinations, were pregnant or planning to become pregnant within 4 weeks of vaccination, used systemic or topical corticosteroids, had bleeding diathesis, received another Covid-19 vaccine, or were infected with SARS-CoV-2 after enrollment.
The study protocol was approved by Lampang hospital institutional review board (No.100/64). A written consent was provided by all participants. The vaccine manufacturer was not involved in this study. The trail was registered at Thai Clinical Trials Registry at TCTR20211023002.
Antibody titers were compared between pre ID vaccination (the day that subjects received PFE-BNT immunization) and post ID vaccination on day 30.
2.2. Sample size
The sample size was calculated using data from a previous study, which found that participants who completed two doses of BNT162b2 had an antibody level of 1108 u/ml, or 8027.5 Au/ml, and participants who previously had Covid-19 infection and completed two doses of BNT162b2 after the infection had an antibody level of 8174 u/ml, or 59218.3 Au/ml [14], [24]. The antibody titer of subjects who had previously been infected with Covid-19 was 7.3 times higher than that of their counterparts. We used the ID delivery route, which used only 1/5 dose of IM vaccination and only one booster shot, therefore we predicted the participants' antibody titers to be roughly 5 times higher. We employed a single sample group, with a statistical power of 80% and a type I error of 5%. The estimated required sample size was 32. We used 42 participants in our study.
2.3. Procedures
PFE-BNT vaccine lot 30125BA was utilized in the study. The vaccine was procured and distributed from ministry of public health, Thailand. Each vaccine was prepared according to Thailand's Department of Disease Control's PFE-BNT vaccine preparation recommendations, which is the same recommendation aligns with the vaccine manufacturer [15]. The PFE-BNT vaccine vial is diluted with 1.8 ml of sterile 0.9% NaCl solution. To prepare ID administration dosage, 0.06 ml (6ug/dose) was withdrawn from the vial using the low dead-space (LDS) technique with a syringe insulin ultrafine II. The 6 ug/dose is 1/5 of the 30 ug/dose of IM PFE-BNT. A skilled pharmacist prepared each dose of vaccination and ensured that it met quality standards. The vaccine was kept refrigerated at 3–8 degrees Celsius and used within 3–4 h after preparation. The intradermal administration approach, using syringe insulin ultrafine II, was performed during injection. The injection site was at the upper arm.
2.4. Monitoring of safety and tolerability
Adverse events were observed immediately after injection, 30 min later, and on day 1, 7 and 30. Participants were monitored from the moment of injection to 30 min after the injection at the research location. On day 1, 7, and 30 after ID injection, participants answered an online side effect monitoring survey that included local and systemic side effects. The official account chat box was provided during the trial for participants to use direct message to contact PI with any further adverse effects or concerns.
2.5. Antibody level after ID injection
Each individual had 4 ml of whole blood taken from them. The whole blood was collected using gel and clot activator tubes. After centrifuging the whole blood in the tube at 2000 rounds per minute for 10 min, sera were isolated from the whole blood. 25 ul of serum were used for each test [16]. Sera were obtained from participants before ID injection and on day 30 after ID injection. Sera were tested by using an automated AdviseDx SARS-CoV-2 IgG II assay, Architect. This machine detects immunoglobulin class G (IgG) antibodies to the receptor binding domain (RBD) of the S1 component of the spike protein of SARS-CoV-2 using the chemiluminescent microparticle immunoassay (CMIA) approach. The AdviseDx SARS-CoV-2 IgG II assay's result unit is AU/ml (Arbitary unit), and the cutoff is 50 Au/ml. The AU/ml can be converted to WHO unit (Binding antibody unit --BAU/ml) by multiplying the result with 0.142. The AdviseDx assay sensitivity is 92.7% (95% CI 90.2–94.8) and specificity is 99.9% (99.4–100%) [22], [23].
2.6. Statistical analysis
Descriptive statistic and independent t-test were used for data analysis.
2.7. Duration
The study was conducted during August–September 2021.
3. Results
3.1. Participants’ characteristics
42 participants completed the study. The age range of participants was 23 to 62 years old. Their characteristics were shown in Table 1.
Table 1.
Characteristics of participants.
| Characteristics | Mean (95% CI)/N (%) | |
|---|---|---|
| Age | 48 (45.4–51.4) | |
| Sex | M | 12 (28.3) |
| F | 30 (71.4) | |
| Days after completing full dose of Verocell | 78.3 (73.9–82.8) | |
3.2. Safety & tolerability
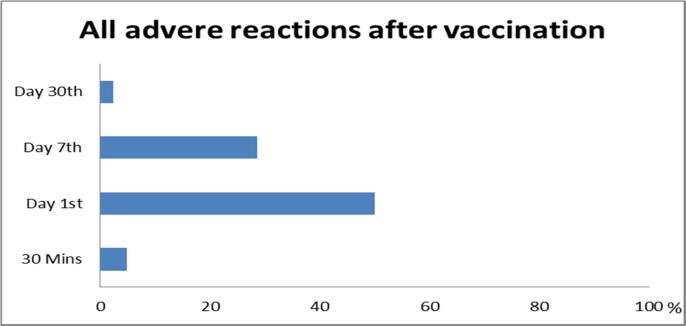
There was no serious adverse event. An overall of vaccine-related adverse reactions is shown in Fig. 1. There was no acute reaction reported at immediately after injection. There were some minor reactions discovered at 30 min, day 1, day 7, and day 30.
Fig. 1.
Total adverse reaction.
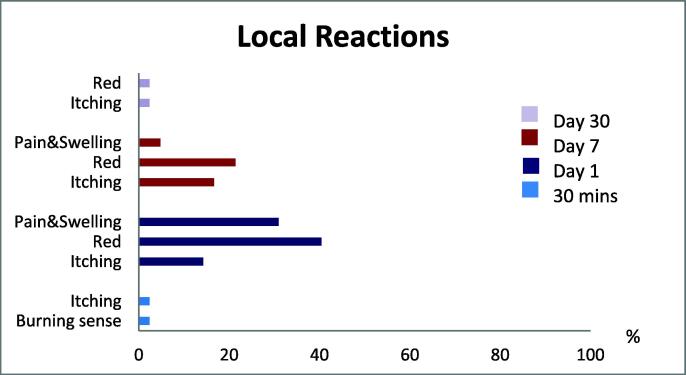
Within 30 min of injection, local adverse effects began to appear, and some participants still had some of the local reactions on day 30. On day 1, the majority of local reactions were red (40.5%), pain and swelling (30.9%), and itching (14.2%); on day 7, the majority of local reactions were red (21.4%), itching (16.7%), and pain and swelling (4.7%). Majority of local reactions might occur as early as the 1st day after injection and can last up to 7 days. On the day 30, just one participant out of 42 (2.4%) felt itching and redness at the injection site (Fig. 2).
Fig. 2.
Local reactions.
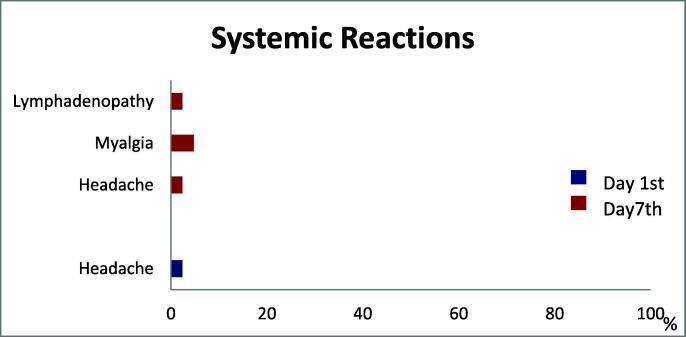
Systemic reactions were found on the report on day 1 and day 7. All of them were minor systemic reactions. 2.4% of participants had headache on the day 1. 4.8% of participants had myalgia, 2.4% had headache, and 2.4% had lymphadenopathy at day 7. There were no subjects who reported a fever (Fig. 3).
Fig. 3.
Systemic reactions.
3.3. Antibody response after ID injection
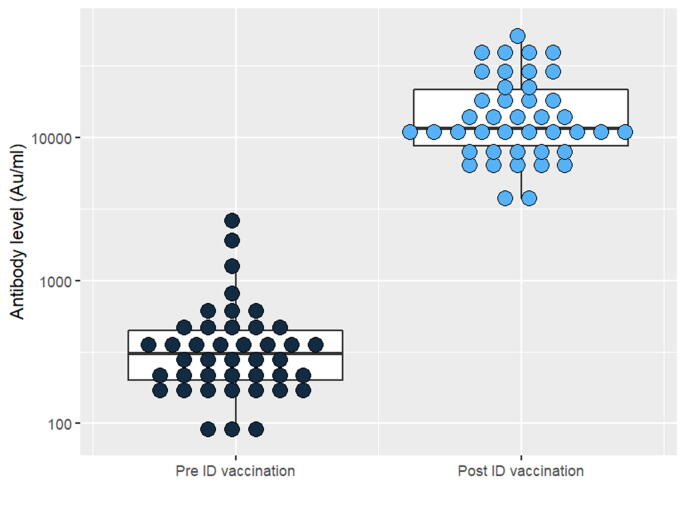
The mean of the antibody level before and after ID injection was shown on Table 2. After ID injection, antibody response was about 40 times higher on average. Each participant's antibody response following ID injection ranged from 17.4 to 90.2 times greater. The antibody level of each participant pre/post ID injection was shown in Fig. 4.
Table 2.
The means of antibody level of Pre-ID vaccination and Post-ID vaccination.
| Mean of Antibody level (Au/ml) | 95% CI (Au/ml) | Range (Au/ml) | |
|---|---|---|---|
| Pre-ID vaccination | 432.1 | 284.2–580 | 85.1–2627.1 |
| Post-ID vaccination | 16669.8 | 13020.9–20318.7 | 3692.6–51238.9 |
Fig. 4.
Antibody response of each participant pre Vs. post ID vaccination.
4. Discussion
The fractional dose of ID administration, which is 6 μg/dose (1/5 of the IM dose), was found to be safe, well tolerated, and immunogenic as a booster dose in adults who had already received two doses of inactivated vaccine. This finding was backed up by prior research on ID administration on Astrazeneca in Thailand [13] and a study on mRNA-1273 in the Netherlands [12], which found that ID administration on Covid-19 vaccination is safe and can boost immune response in adults.
In our study, the mean age of participants was 48, which was significantly greater than the previous study, which involved participants aged 18–30. As a result, fractional ID injection as a booster dose could be utilized safely in adults over 30 years old.
There were no major negative effects in patients who got an ID booster dose of PFE-BNT immunization. The majority of ID injectable side effects were minor, such as burning, itching, redness, pain, and swelling at the injection site. Some participants' local effects could linger until the 7th day. The local reactions described in this investigation were similar to those described in an mRNA-1273 analysis [12]. Local reactions in the mRNA-1273 ID administration research, on the other hand, appeared within 1–2 days and lasted no more than 7 days, with the exception of hyperpigmentation at the injection site, which remained until day 44 [12]. The local reactions to PFE-BNT vaccine ID injection were likewise similar to numerous fractional ID delivery vaccines that had previously been employed, such as rabies and influenza [17]. In our investigation, the systemic effects were mild. During the 30-day follow-up period, no participants had a fever or experienced cardiac adverse effects. Only 1 out of 42 participants (2.4%) had a headache, and 1 to 2 persons out of 42 (2.3–4.8%) had myalgia, headache, and lymphadenopathy. When compared to a study that evaluated the risks and safety of BNT162b2 mRNA vaccine at 42 days on participants who received IM administration, the systemic reactions were minor. BNT162b2 mRNA IM approach was most strongly associated with an elevated risk of myocarditis (risk ratio, 3.24), 2.7 events per 100,000)) persons; lymphadenopathy ((risk ratio, 2.43), 78.4 events per 100,000 persons); appendicitis (risk ratio, 1.40) 5.0 events per 100,000)), and herpes zoster infection ((risk ratio, 1.43), 15.8 events per 100,000 persons) [18].
The antibody levels of subjects who received a booster dose of PFE-BNT by ID injection increased between 17.4 and 90.2 times from baseline on day 30 after vaccination, with an average antibody response level of 16669.8 Au/ml (95% CI; 13020.9–20318.7). In a previous investigation [19], antibody levels in patients infected with SAR-CoV-2 who had received two doses of PFE-BNT rose from 334 (IQR; 103–1070 Au/ml) to 18,047 Au/ml (95% CI; 10884–22413 AU/ml) on day 42 after vaccination. The antibody levels increased approximately 54 times.
Anti-spike antibody titers that we used to measure antibody level in our study are associated with neutralizing activity. A previous study showed that neutralization titers significantly correlated with spike-binding titers (Spearman ρ = 0.87, P < 0.0001) [20]. Also, the anti-spike antibody titers has positive percent agreement with PRNT (plaque reduction neutralization test) at 100% (95% CI 95.2–100) [16], [23]. Despite the importance and high level of neutralization antibodies after immunization [19], [21] or in convalescent persons [21], the effectiveness of neutralizing antibody or anti-spike antibody titer in protecting and preventing COVID-19 infection, hospitalization, death, or transmission remains unknown. As a result, future research or follow-up studies in people who have already been vaccinated via any route will be needed to see if antibody levels are linked to protection or may be used as a marker.
The limitations of our study included; the method that we used is not a gold standard method, which is the neutralizing activity method. However, the anti-spike antibody assay method, which we employed in this investigation to quantify pre and post-vaccination, is commercially accessible, has a wide acceptability, and is well calibrated. It also has a perfect agreement (100%) with the gold standard measure [16], [23]. Another drawback is that we did not compare antibody levels after ID delivery to those who received a booster dose of conventional injection. As a result, there may be some uncertainty about the antibody levels after IM and ID routes. More research is needed to compare both IM and ID administration. Next, in our trial, we exclusively employed the PFE-BNT vaccine, despite the fact that different vaccines might be administered via the ID method. Finally, we did not investigate the adverse reactions and antibody level's longevity after day 30.
In conclusion, the administration of 6 ug of PFE-BNT ID as a booster in people over the age of 18 who have received two doses of inactivated vaccine is safe and well tolerated, and could result in a more robust immune response. Local adverse effects were modest, while systemic side effects were insignificant. In nations or regions where vaccination distribution is insufficient, ID administration could be safely employed and suggested as a dose-sparing method.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The author would like to acknowledge support from the director and the administering committees of Lampang Hospital.
Funding source
This work was supported by Lampang medical educational center. [grant number 1/64]
References
- 1.Koritala T., Hussain A., Pleshkova Y., Dondapati L., Tirupathi R., Rabaan A.A., et al. A narrative review of emergency use authorization versus full FDA approval and its effect on COVID-19 vaccination hesitancy. Infez Med. 2021;29(3):339–344. doi: 10.53854/liim-2903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bloomberg.com. More than 7.38 billion shots given: Covid-19 tracker [Internet]. [cited 2021 Nov 12]; Available from: https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/.
- 3.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 4.Thammasart today. Faculty of Medicine, Thammasart University and BIOTEC. The study of SV. [online]. 2021; [Retrived 18 Aug 2021]; [1 page].Available from: URL: https://www.facebook.com/thammasattoday/photos/371218451004514?_rdc=1&_rdr.
- 5.World Health Organization: WHO. Interim statement on COVID-19 vaccine booster doses [Internet]. [update 2021 Aug 10; cited 2021 Oct 21]. Available from: https://www.who.int/news/item/10-08-2021-interim-statement-on-covid-19-vaccine-booster-doses.
- 6.Pan H, Wu Q, Zeng G, Yang J, Jiang D, Deng X, et al. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18-59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. [cited 2021 Oct 21]; Available from: https://www.medrxiv.org/content/10.1101/2021.07.23.21261026v1.
- 7.Shumei L. Sinovac booster shot gives big increase in neutralizing antibodies: study [Internet]. [update 2021 Aug 9; cited 2021 Oct 21]. Available from: https://www.globaltimes.cn/page/202108/1231014.shtml.
- 8.Thaipbs “The antibody response after Sinovac- Astrazenaca” [online]. 2021; [Retrived 9 Aug 2021]; [3 pages]. Available at: URL: https://news.thaipbs.or.th/content/304162.
- 9.Migliore A., Gigliucci G., Di Marzo R., Russo D., Mammucari M. Intradermal Vaccination: A Potential Tool in the Battle Against the COVID-19 Pandemic? RMHP. 2021;14:2079–2087. doi: 10.2147/RMHP.S309707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaumburg F., De Pijper C.A., Grobusch M.P. Intradermal travel vaccinations when less means more. Trav Med Infect Dis. 2019;28:3–5. doi: 10.1016/j.tmaid.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization and Programme for Appropriate Technology in Health (Path). Intradermal Delivery of Vaccines: A Review of the Literature and the Potential for Development for Use in Low-And Middle-Income Countries. Geneva, Switzerland: World Health Organization; 2009. [Internet]. [cited 2021 Oct 21]; Available from: https://www.path.org/resources/intradermal-delivery-of-vaccines-a-review-of-the-literature-and-the-potential-for-development-for-use-in-low-and-middle-income-countries/.
- 12.Roozen GVT, Prins MLM, Binnendijk R van, Hartog G den, Kuiper VP, Prins C, et al. Tolerability, safety and immunogenicity of intradermal delivery of a fractional dose mRNA-1273 SARS-CoV-2 vaccine in healthy adults as a dose sparing strategy [Internet]. [cited 2021 Oct 21]. Available from: https://www.medrxiv.org/content/10.1101/2021.07.27.21261116v1.
- 13.Workpoint today. Antibody response after Intradermal vaccination as a booster dose [internet]. [cited 2021 Oct 21]; Available from: https://workpointtoday.com/covid19-127/.
- 14.Steensels D., Pierlet N., Penders J., Mesotten D., Heylen L. Comparison of SARS-CoV-2 antibody response following vaccination with BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533–1535. doi: 10.1001/jama.2021.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trinity-health.org. Pfizer-vaccine-storage-handling-administration [internet]. [cited 5 February 2022]. Available from: https://www.trinity-health.org/covid-19-resources/_assets/documents/clinical-guidance/vaccine/guidebook/pfizer-vaccine-storage-handling-administration.pdf.
- 16.Abbot. AdviseDx SARS-CoV-2 IgG II for use with architect [Internet].[update 2021 Feb; cited 2021 Oct 21]. Available from: https://www.fda.gov/media/146371/download.
- 17.Side effects of Fluzone Intradermal Quadrivalent (Influenza Vaccine), warnings, uses [Internet]. [update 2016 Oct 19; cited 2021 Oct 21]. Available from: https://www.rxlist.com/fluzone-intradermal-quadrivalent-2016-2017-formula-side-effects-drug-center.htm.
- 18.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eyre D.W., Lumley S.F., Wei J., Cox S., James T., Justice A., et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin Microbiol Infect. 2021;27(10):1516.e7–1516.e14. doi: 10.1016/j.cmi.2021.05.041. Epub 2021 Jun 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson N.A.C., Kester K.E., Casimiro D., Gurunathan S., DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. npj Vaccines. 2020;5(1):11. doi: 10.1038/s41541-020-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National SARS-CoV-2 Serology Assay Evaluation Group Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed]
- 23.Bradley B.T., Bryan A., Fink S.L., Goecker E.A., Roychoudhury P., Huang M.L., Zhu H., Chaudhary A., Madarampalli B., Lu J.Y.C., Strand K., Whimbey E., Bryson-Cahn C., Schippers A., Mani N.S., Pepper G., Jerome K.R., Morishima C., Coombs R.W., Wener M., Cohen S., Greninger A.L. Anti-SARS-CoV-2 Antibody Levels Measured by the AdviseDx SARS-CoV-2 Assay Are Concordant with Previously Available Serologic Assays but Are Not Fully Predictive of Sterilizing Immunity. J Clin Microbiol. 2021;59(9):8921. doi: 10.1128/JCM.00989-21. Epub 2021 Aug 18. PMID: 34165323; PMCID: PMC8373027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukaszuk K, Kiewisz J, Rozanska K, Podolak A, Jakiel G, Woclawek-Potocka I, et al. Is WHO International Standard for Anti-SARS-CoV-2 Immunoglobulin Clinically Useful? [Internet]. Infectious Diseases (except HIV/AIDS); 2021 May [cited 2022 Feb 6]. Available from: http://medrxiv.org/lookup/doi/10.1101/2021.04.29.21256246.