We had previously reported on the immunogenicity of the BNT162b2 messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccine (tozinameran) in a large cohort of patients with cancer after the first and second doses1 and we subsequently showed the rapid decline of humoral response over time until 6 months of follow-up.2 , 3 Herein, we describe the serological response to the third dose (also called booster or additional dose) of the vaccine in this frail population.
Study design and methods were reported in Supplementary materials, available at https://doi.org/10.1016/j.annonc.2022.02.006.1
From September to November 2021, 407 patients received the additional third dose at 4-6 months after the completion of their primary vaccine series. The median age was 67 (range 24-89) years. Breast cancer (28.5%) and lung cancer (19.9%) were the most common tumor subtypes. Most patients (366/407, 89.9%) were on active anticancer treatment during the 28 days before the administration. Chemotherapy alone or in combination with monoclonal antibodies (anti-HER2 and antiangiogenic drugs, immune checkpoint inhibitors) was the most used treatment (32.2%), followed by targeted therapy (22.8%) and anti-cytotoxic T-lymphocyte–associated antigen-4 and anti-programmed death-ligand 1 checkpoint inhibitors (15.7%). Chronic steroid use (daily assumption started at least 30 days before the vaccination) was reported in 48 (11.8%) patients.
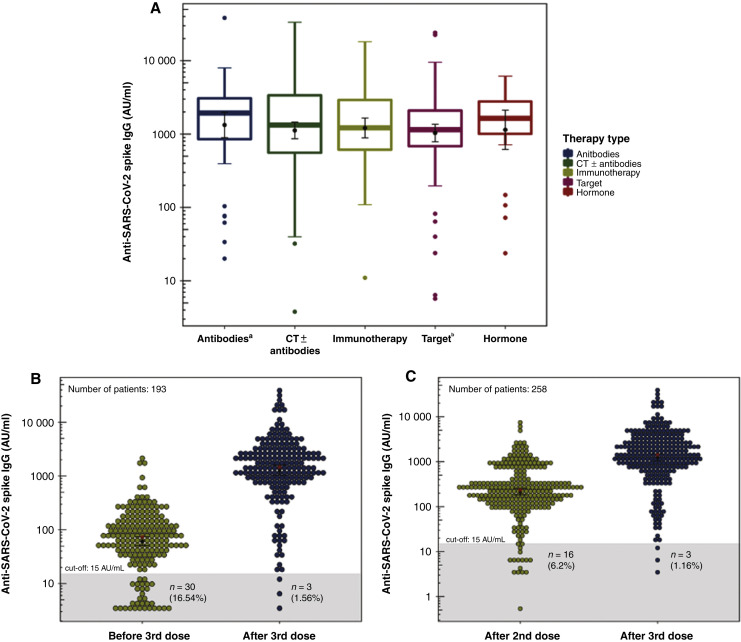
Patients with a positive serological status after the third dose were 402/407 (98.8%). All the five nonresponder patients had a negative serological status also at predose assessment, although one patient was seropositive after the second dose. Of the five patients remaining seronegative after the additional dose, four (80%) were on active treatment (one with chemotherapy, two with targeted therapy, and one with immunotherapy). The geometric mean concentration (GMC) of anti-S immunoglobulin G (IgG) reached after the booster was 1054.5 AU/ml (95% confidence interval 909.4-1238.2 AU/ml). Analysis of postdose IgG titer according to clinical characteristics showed that only chronic use of steroids was significantly associated with lower antibodies level (P = 0.035). Nevertheless, the type of anticancer treatment did not significantly affect the IgG titer (P > 0.05; Figure 1 A).
Figure 1.
(A) Box plots of anti-S IgG titer after the third dose according to the active anticancer treatment. A logarithm scale was used for IgG titer. Inside each box plot, the geometric mean concentrations ± standard errors were represented by a black point with error bars; the median is depicted as a thick horizontal line. No statistically significant differences were found according to the type of treatment using analysis of variance (P = 0.065). aTherapy with monoclonal antibodies including anti-angiogenics and anti-HER-2. bTarget therapy is referred to the use of tyrosine kinases inhibitors. Dot plots of anti-S IgG titer assessed before and after the additional dose (B) and after the second and third doses (C). A logarithm scale was used for IgG titer. The gray area is the area below the prefixed cut-off of positivity. Inside each dot plot, the geometric mean concentrations ± standard errors were represented by a black point with error bars; the median is depicted as a red asterisk. All comparison were significant at P < 0.001. IgG, immunoglobulin G; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
A total of 193 patients were evaluated for IgG titer just before and at 4 weeks after the third dose. The GMC increased from 61.3 to 1280.4 AU/ml with a statistically significant increase of 20.9-fold (P < 0.001; Figure 1B). Of the 30 seronegative patients before the additional dose, 27/30 (90%) acquired a positive serological status after the booster and only 3/30 (10%) patients remained seronegative. Considering the 258 patients who were evaluated for IgG titer at 4 weeks after the second and third doses, the GMC significantly increased 5.77-fold, from 215.2 AU/ml (95% confidence interval 181.7-255 AU/ml) after the second dose to 1240.6 AU/ml (95% 1229.5-1471.2 AU/ml) after the booster (P < 0.001; Figure 1C).
No severe adverse events after the third dose were observed.
The present study is properly powered to evaluate humoral response to the third dose of the vaccine in patients with cancer. Previous studies were strongly limited by small sample sizes.4, 5, 6 Our results showed a globally positive response to the additional dose of BNT162b2 mRNA COVID-19 vaccine in patients affected by solid cancer with a relevant potentiation of the humoral response initially acquired after the two-dose cycle. Although a very high seroconversion rate followed the additional dose, there was a very small subgroup of patients with no detectable humoral response even after the third dose. For these patients, further studies are warranted to assess the existence of other types of immunity (i.e. T-cell immunity) and to explore its correlation with protection from infection.
Nevertheless, the active anticancer treatment did not affect the serological immune response to the third dose and patients receiving chemotherapy seems not have weaker humoral response compared with patients undergoing other types of treatment, in contrast with our previous observations regarding the two doses of the vaccine.1, 2, 3 The chronic use of steroids was associated with a weaker humoral response also for the third dose.
The present study is still ongoing to evaluate the durability of immune response to the additional dose in a long-term period.
Acknowledgements
We thank medical oncologists (Paolo Carlini, Gianluigi Ferretti, Maria Bassanelli Fabiana Letizia Cecere, Elvira Colella, Consuelo D’Ambrosio, Emanuela Dell’Aquila, Loretta D’Onofrio, Virginia Ferraresi, Alberto Fulvi, Simona Gasparro, Paola Malaguti, Elisa Onesti, Domenicangela Pellegrini, Michelangelo Russillo, Antonella Savarese, Sabrina Vari, Massimo Zeuli), residents (Antonella Cosimati, Vittoria Barberi, Mattia Di Civita, Federica Riva, Maria Teresa Maccallini, Gariazzo Ludovica), nurses of Medical Oncology 1 Unit, hospital pharmacists, medical direction members, and data managers (Elisabetta Bozzoli, Alessandra Zambardi, Viviana Cangiano, Barbara Conforti) of IRCCS Regina Elena National Cancer Institute in Rome, Italy, for their commitment to the COVID-19 vaccination campaign for patients with cancer. We thank the patients referred to our Unit and their families.
Funding
None declared.
Disclosure
VDN received speakers’ fee from AstraZeneca, MSD, BMS, Istituto Gentili, and Boehringer Ingelheim; grant consultancies from AstraZeneca, MSD, BMS, and Boehringer Ingelheim; travels’ fee from MSD and Boehringer Ingelheim; and also received institutional research grants from Roche. FC is a member of the advisory board of GSK, Roche, AstraZeneca, and Eli-Lilly; received speakers’ fee from GSK, Roche, AstraZeneca, Eli-Lilly, Novartis, Amgen, Pfizer, MSD, BMS, Astellas, and Eli-Lilly. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Di Noia V., Pimpinelli F., Renna D., et al. Immunogenicity and safety of COVID-19 vaccine BNT162b2 for patients with solid cancer: a large cohort prospective study from a single institution. Clin Cancer Res. 2021;27:6815–6823. doi: 10.1158/1078-0432.CCR-21-2439. [DOI] [PubMed] [Google Scholar]
- 2.Di Noia V., Pimpinelli F., Renna D., et al. Clinical characteristics limiting the durability of humoral response to BNT162b2 in patients with solid cancer. Ann Oncol. 2022;33:350–352. doi: 10.1016/j.annonc.2021.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Di Noia V., Pimpinelli F., Renna D., et al. Rapid decline of humoral response to two doses of BNT162b2 vaccine in patients with solid cancer after 6-months: the urgent need of the additional dose! EJC. 2022;165:169–173. doi: 10.1016/j.ejca.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Rottenberg Y., Grinshpun A., Ben-Dov I.Z., Oiknine Djian E., Wolf D.G., Kadouri L. Assessment of response to a third dose of the SARS-CoV-2 BNT162b2 mRNA vaccine in patients with solid tumors undergoing active treatment. JAMA Oncol. 2022;8:300–301. doi: 10.1001/jamaoncol.2021.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro L.C., Thakkar A., Campbell S.T., et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2022;40:3–5. doi: 10.1016/j.ccell.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenioux C., Teixeira L., Fourati S., et al. SARS-CoV-2 Antibody response to 2 or 3 doses of the BNT162b2 vaccine. JAMA Oncol. 2022 doi: 10.1001/jamaoncol.2021.7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.