Abstract
Recognition of Hepatozoon canis and Hepatozoon americanum as distinct species was supported by the results of Western immunoblotting of canine anti-H. canis and anti-H. americanum sera against H. canis gamonts. Sequence analysis of 368 bases near the 3′ end of the 18S rRNA gene from each species revealed a pairwise difference of 13.59%.
Canine hepatozoonosis is an emerging protozoal tick-borne infection of dogs that was first reported in the United States from Texas (8) and Louisiana (10) and according to recent reports has spread to Alabama, Georgia (14), and Oklahoma (17). The causative agent of canine hepatozoonosis in North America is Hepatozoon americanum, an apicomplexan parasite of leukocytes and muscular tissues that induces severe myositis and gait abnormalities (20). Before 1997, H. americanum was considered a strain of Hepatozoon canis, a protozoan with morphologically similar gamonts in leukocytes. H. canis, first reported in India in 1905 (12), is the cause of Old World canine hepatozoonosis, which usually causes a milder disease that affects the spleen, lymph nodes, and bone marrow, resulting in anemia and lethargy (1). It has been reported from southern Europe, the Middle East, Africa, and the Far East. The main vector for the disease is the tick Rhipicephalus sanguineus (5). In the United States, H. americanum-like oocysts were found in hemocoel smears made from Amblyomma maculatum ticks found on dogs with hepatozoonosis (20). This tick has recently been shown to be capable of transmitting H. americanum, whereas R. sanguineus failed to transmit this parasite (15).
The classification of H. americanum as a new species was based mainly on the clinical signs, tissue tropism, and pathological and morphological findings from dogs with hepatozoonosis in North America, which were different from those reported for H. canis infections from other parts of the world (20). The aims of this study were to provide further evidence at the molecular and antigenic levels for the recent species differentiation between H. americanum and H. canis.
Blood was drawn in an EDTA tube from a 2-year-old Yorkshire Terrier dog infected with H. americanum admitted to the College of Veterinary Medicine at Auburn University in Alabama. In Israel, blood was sampled similarly from a 10-year-old Yorkshire Terrier infected with H. canis at the Hebrew University School of Veterinary Medicine. Gamonts of Hepatozoon were observed in neutrophils from both dogs by light microscopy of Giemsa-stained blood smears prior to DNA extraction. Genomic DNA from gamont-infected neutrophils was extracted and purified using the IsoQuick Kit (Orca Research Incorporated, Bothell, Wash.). A portion of the 3′ end of the small-subunit (SSU) rRNA gene was amplified by PCR using internal primer 5′-CCAGGTCCAGACATGG-3′ (designated Cocci A) and P3 of Clark and Diamond (6). PCR mixtures consisted of 10 ng of template DNA, 5 mM KCl, 1 mM Tris-HCl, 0.1% Triton X-100, 1.5 mM MgCl2 (the last four reagents from Promega, Madison, Wis.), 200 μM (each) deoxynucleoside triphosphate (Pharmacia Biotech, Piscataway, N.J.), 1 μM (each) primer, and 2.5 U of Taq DNA polymerase (Gibco BRL, Life Technologies Inc., Gaithersburg, Md.) in 100-μl reaction volumes. PCR was performed by using the following parameters and a thermal cycler (Perkin Elmer Cetus Co., Wellesley, Mass.): 94°C for 30 s (melting), 56°C for 1 min (annealing), and 72°C for 2 min (extension). The resulting PCR products were electrophoresed on a 1% agarose gel and stained with ethidium bromide. Product bands were excised from the gel, and DNA was recovered from gel slices using the GeneClean II Kit (Bio 101, Vista, Calif.). The PCR products were cloned using the Zero Background/Kan Cloning Kit (Invitrogen Corporation, Carlsbad, Calif.) and sequenced in both directions with M13 forward and reverse primers using the ABI 377 Prism automated sequencer.
Heparinized peripheral blood (82 ml) was obtained by venipuncture from a dog naturally infected with H. canis. The infected blood was layered onto a test tube with a sintered filter (Uni-Sep10+; Novamed, Jerusalem, Israel) that prevents mixing of the blood and the Ficoll-Hypaque density-gradient medium. After centrifugation at 800 × g for 20 min at room temperature, a fraction containing leukocytes was collected, suspended in 30 ml of phosphate-buffered saline (PBS) (pH 7.2), and washed three times with PBS by centrifugation at 800 × g for 20 min. The leukocytes were then resuspended in 30 ml of PBS, equilibrated in a nitrogen cavitation chamber at 500 lbs/in2 for 10 min, and disrupted by releasing the pressure (11). The material containing cell-free gamonts and debris was collected in a centrifuge tube and centrifuged for 10 min at 800 × g. The pellet containing purified parasites was resuspended in PBS and washed three times with PBS by centrifugation at 800 × g for 20 min at 4°C. The final pellet containing released gamonts was resuspended in 1 ml of PBS, and the number of purified parasites was determined in a Neubauer hemocytometer with 0.5% trypan blue. The purified and counted gamonts were frozen at −70°C, and at each stage of purification, the material was examined by Nomarski phase microscopy.
Positive anti-H. canis serum samples were obtained from a naturally infected dog and an experimentally infected dog with H. canis parasitemia from Israel. The serum from the experimentally infected dog was collected 63 days postinfection. Sera from three dogs naturally infected with H. americanum from Alabama diagnosed by muscle biopsy (9) were used to test reactivity with H. canis gamont antigen by Western blotting and indirect fluorescent-antibody testing (IFAT). Negative-control sera were obtained from a tick-free laboratory-raised dog prior to infection with H. canis and from a blood donor dog from the College of Veterinary Medicine at Auburn, Alabama. The experimentally infected dog was inoculated with H. canis as previously described (2). Briefly, a 3-month-old laboratory-raised dog was inoculated with 30 adult R. sanguineus ticks that were repleted as nymphs on a naturally infected dog. The dog developed hepatozoonosis with parasites which were identified in blood smears and bone marrow aspirates by light microscopy.
The frozen suspension of purified H. canis gamonts was thawed at room temperature, and after centrifugation at 800 × g for 5 min, the protein concentration of the supernatant was determined by the Bradford method (4). The material was further solubilized in sample buffer (0.025 M Tris-glycine [pH 6.8], 2% [wt/vol] sodium dodecyl sulfate [SDS], 15% [wt/vol] glycerol and bromphenol blue) at 100°C for 3 min. The gamont antigen at 10 μg of protein/lane was subjected to SDS-polyacrylamide gel electrophoresis (7.5 to 17.5% gradient gel) under nonreducing conditions (13). The resolved polypeptides were transferred overnight onto nitrocellulose membranes in Tris-glycine buffer containing 20% methanol at constant 35 mA and 4°C (19). The membranes were blocked with PBS containing 3% casein for 2 h at room temperature, followed by five successive washings with PBS containing 0.5% Tween 20 (PT). Dog sera were diluted in PT, applied to individual strips, and incubated overnight at 4°C. The nitrocellulose strips were washed seven times as described above, and the bound antibody was detected with rabbit anti-dog immunoglobulin G (IgG) conjugated to horseradish peroxidase at 1:3,000 dilution in PBS containing 0.2% bovine serum albumin (BSA). After incubation for 1 h at 37°C, the nitrocellulose strips were washed eight times with PT and developed with 0.005% 3,3′-diaminobenzidine (DAB) (Sigma, St. Louis, Mo.) supplemented with 0.03% cobalt chloride and 0.3% H2O2.
Serum samples were analyzed by IFAT for IgG antibodies reactive with H. canis antigen (18). Antigen slides were prepared with blood from a naturally infected dog with high parasitemia. The buffy coat was washed with PBS by centrifugation three times, and the final pellet was resuspended in a mixture of equal volumes of PBS and 3% BSA. Thin smears of buffy coat were made on glass slides and dried at room temperature. The slides were immersed for 10 min in acetone and then stored at −80°C. Before use, antigen slides, stored at −80°C, were warmed and dried at 37°C for 30 min. A series of successive twofold dilutions of serum in PBS, from 1:16 to 1:4,096, were applied to the smears and incubated for 30 min at 37°C in a humid chamber. The slides were washed three times in PBS and blotted dry. Fluorescein-conjugated rabbit anti-dog IgG was applied to the wells at 1:70 dilution. The slides were then incubated at 37°C for 30 min, washed as described above, and dried. The smears were mounted under coverslips in PBS-buffered glycerol (pH 8.5) and examined under a fluorescence microscope.
A partial sequence for the 18S SSU rRNA was obtained from H. americanum and H. canis using cycle sequencing of PCR fragments generated using a combination of a coccidium-specific primer (Cocci A) and a universal primer (6, 16). The sequences have been submitted to GenBank under accession numbers AF206669 (H. canis) and AF206668 (H. americanum). A region of 368 bases from the two Hepatozoon species infecting dogs near the 3′ end of their 18S rRNA genes was used for further analysis. This region spans three hypervariable regions of the 18S rRNA gene. These partial sequences from the 18S rRNA gene from H. americanum and H. canis were aligned by eye against the same region from two other Hepatozoon species, H. catesbianae (GenBank accession no. AF206670) and H. sipedon (GenBank accession no. AF206671), gamonts of which occur in the blood of frogs and snakes, respectively. The alignment (Fig. 1) demonstrated relatively few differences between the two canine parasites and the Hepatozoon species infecting frog and snake hosts. Pairwise differences among taxa in this region of the 18S rRNA gene range from 7.64 to 14.47% (Table 1). The pairwise difference between H. canis and H. americanum was 50 of 368 bases (13.59%).
FIG. 1.
Alignment of a portion of the 18S rRNA gene from H. americanum (United States) and H. canis (Israel) and two Hepatozoon species, H. sipedon from a snake and H. catesbianae from a frog. Only the nucleotides that are different from those of H. sipedon are shown.
TABLE 1.
Pairwise differences between pairs of Hepatozoon species for a 368-base aligned section flanking the 3′ end of the 18S rRNA gene
| Species | Pairwise differencea (% different) between:
|
||
|---|---|---|---|
| H. canis | H. americanum | H. sipedon | |
| H. americanum | 13.59 | ||
| H. sipedon | 7.64 | 12.85 | |
| H. catesbianae | 10.10 | 14.47 | 9.86 |
These pairwise differences have been adjusted for the presence of gaps in the alignment.
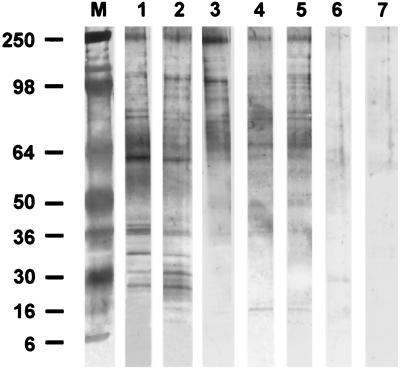
Western blot analysis of anti-H. canis and anti-H. americanum sera reacted with H. canis gamont antigens revealed that multiple bands were recognized by sera from dogs infected with either Hepatozoon species (Fig. 2). A similarity in the pattern of reactivity of sera from dogs with H. canis or H. americanum infections was observed for protein bands ranging from 250 to 36 kDa with distinct bands of relative molecular mass (rMM) of 250, 107, 88, 63, and 37 kDa. Reactivity with the 63- and 37-kDa bands was weaker with anti-H. americanum sera than with anti-H. canis sera. A triplet of antigenic bands with lower rMM of 32, 30, and 28 that were reactive with anti-H. canis were not reactive with anti-H. americanum sera. Minimal weak nonspecific reactivity was observed with the control and preinfection sera.
FIG. 2.
Western blot analysis of H. canis gamont antigens recognized by sera from dogs infected with H. canis and H. americanum. Reactions of sera from different dogs are shown as follows: lane 1, serum from a dog experimentally infected with H. canis; lane 2, serum from a dog with a naturally occurring H. canis infection; lanes 3 to 5, sera from three dogs that were naturally infected with H. americanum; lanes 6 and 7, control serum from a blood donor dog at Auburn University and from preinfection serum from the experimentally infected dog, respectively. Lane M contains molecular mass markers. Note the triplet of bands with relative rMM of 32 to 28 kDa that are reactive only with anti-H. canis sera.
By IFAT, serum samples from the two dogs infected with H. canis and the three dogs infected with H. americanum were all reactive with H. canis gamont antigen. Both dogs infected with H. canis demonstrated high titers of 1:1,024 for the experimentally infected dog and 1:4,096 for the naturally infected dog, while those infected with H. americanum had low titers ranging between 1:32 and 1:64. The two control sera showed no reactivity with H. canis antigen by IFAT.
The findings presented in this study demonstrate that the geographically distinct H. canis and H. americanum, previously thought to be strains of the same species (7, 8) and recently classified separately mainly on the basis of different pathological, clinical, and morphological findings (20), also differ at the genetic and antigenic levels.
The genetic distances among the four Hepatozoon species in a portion of the coding region of the 18S rRNA gene were consistent with their recognition as distinct species (20). The pairwise difference between the two parasites of dogs, H. canis and H. americanum, was 13.59% in the short region of the 18S rRNA gene sampled. This interspecific variation was comparable to the differences between the two Hepatozoon species infecting frogs and snakes (e.g., 9.86% between H. catesbianae and H. sipedon). The differences in this region were comparable to the interspecific variation observed among various Eimeria species infecting poultry (3). Since the portion of the coding region sequenced from these four Hepatozoon species contains at least three hypervariable regions, the percentage differences between the various Hepatozoon species for the entire gene are likely to be considerably lower.
The Western blotting patterns observed in this study for reactivity of anti-H. canis and anti-H. americanum sera with H. canis antigen show that although some similarities in reactivity were seen for higher-molecular-mass proteins, three molecular mass bands in the 32- to 28-kDa region detected by anti-H. canis sera were not recognized by anti-H. americanum sera. In addition, the anti-H. canis serum reacted strongly with the 63- and 37-kDa bands of H. canis gamont antigen, while sera from H. americanum-infected dogs recognized these antigens weakly. These findings support the hypothesis that H. canis and H. americanum are antigenically related and show a degree of cross-reactivity by Western blotting, but H. canis is substantially different from H. americanum, as detected by the Western blotting reactivity to certain protein bands only by anti-H. canis serum and the weaker antibody response to other protein bands by anti-H. americanum serum. Similarly, high antibody titers (up to 1:4,096) were obtained in homologous IFAT reactions with the sera used for Western blotting and significantly lower titers (up to 1:64) in heterologous reactions. The low cross-reactive IFAT antibody titers to H. canis antigen demonstrated in the sera of H. americanum-infected dogs are in agreement with the findings of Vincent-Johnson et al. (20), who reported a low to negative reactivity of H. americanum-infected canine sera with H. canis antigen from Israel by IFAT. The low IFAT titers of anti-H. americanum sera may provide another indication that H. americanum shares some antigenic epitopes with H. canis, as seen by Western blotting, but anti-H. americanum serum is distinct and elicits an antibody response that detects only some H. canis antigens.
In conclusion, the genetic and antigenic analyses described in this study provide additional support for the differentiation of H. canis and H. americanum as different species of Hepatozoon that are the causative agents of unique and distinct clinical diseases.
REFERENCES
- 1.Baneth G, Weigler B. Retrospective case-control study of hepatozoonosis in dogs in Israel. J Vet Intern Med. 1997;11:365–370. doi: 10.1111/j.1939-1676.1997.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 2.Baneth G, Shkap V, Samish M, Pipano E, Savitsky I. Antibody response to Hepatozoon canis in experimentally infected dogs. Vet Parasitol. 1998;74:299–305. doi: 10.1016/s0304-4017(97)00160-x. [DOI] [PubMed] [Google Scholar]
- 3.Barta J R, Martin D S, Liberator P A, Dashkevicz M, Anderson J W, Feighner S D, Elbrecht A, Perkins-Barrow A, Jenkins M C, Danforth H D, Ruff M D, Profous-Juchelka H. Phylogenetic relationships among eight Eimeria species infecting the domestic fowl inferred using complete small subunit ribosomal DNA sequences. J Parasitol. 1997;83:262–271. [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 5.Christophers S R. The sexual life cycle of Leucocytozoon canis in the tick. Sci Mem Off Med Sanit Dep Gov India. 1907;28:1–11. [Google Scholar]
- 6.Clark C G, Diamond L S. The Laredo strain and other ‘Entamoeba histolytica-like’ amoebae are Entamoeba moshkovskii. Mol Biochem Parasitol. 1991;46:11–18. doi: 10.1016/0166-6851(91)90194-b. [DOI] [PubMed] [Google Scholar]
- 7.Craig T M. Hepatozoonosis. In: Greene C E, editor. Clinical microbiology and infectious diseases of the dog and cat. W. B. Philadelphia, Pa: Saunders; 1990. pp. 778–785. [Google Scholar]
- 8.Craig T M, Smallwood J E, Knauer K W, McGrath J P. Hepatozoon canis infection in dogs: clinical, radiographic, and hematological findings. J Am Vet Med Assoc. 1978;173:967–972. [PubMed] [Google Scholar]
- 9.Craig T M, Jones L P, Nordgren R M. Diagnosis of Hepatozoon canis by muscle biopsy. J Am Anim Hosp Assoc. 1984;20:301–303. [Google Scholar]
- 10.Gosset K A, Gaunt S D, Aja D S. Hepatozoonosis and ehrlichiosis in a dog. J Am Anim Hosp Assoc. 1985;21:265–267. [Google Scholar]
- 11.Hunter M J, Commerford S L. Pressure homogenization of mammalian tissues. Biochim Biophys Acta. 1961;47:580–586. doi: 10.1016/0006-3002(61)90553-4. [DOI] [PubMed] [Google Scholar]
- 12.James S P. On a parasite found in the white corpuscles of the blood of dogs. Sci Mem Off Med Sanit Dep Gov India. 1905;14:1–12. [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Macintire D K, Vincent-Johnson N, Dillon A R, Blagburn B L, Lindsay D S, Whitley E M, Banfield C. Hepatozoonosis in dogs: 22 cases (1989–1994) J Am Vet Med Assoc. 1997;210:916–922. [PubMed] [Google Scholar]
- 15.Mathew J S, Ewing S A, Panciera R J, Woods P J. Experimental transmission of Hepatozoon americanum Vincent-Johnson et al., 1997 to dogs by the Gulf Coast tick, Amblyomma maculatum Koch. Vet Parasitol. 1998;80:1–14. doi: 10.1016/s0304-4017(98)00189-7. [DOI] [PubMed] [Google Scholar]
- 16.Medlin L, Elwood H J, Stickel S, Sogin M L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 17.Panciera R J, Gatto N T, Crystal M A, Helman R G, Ely R W. Canine hepatozoonosis in Oklahoma. J Am Anim Hosp Assoc. 1997;33:221–225. doi: 10.5326/15473317-33-3-221. [DOI] [PubMed] [Google Scholar]
- 18.Shkap V, Baneth G, Pipano E. Circulating antibodies to Hepatozoon canis demonstrated by immunofluorescence. J Vet Diagn Investig. 1994;6:121–123. doi: 10.1177/104063879400600127. [DOI] [PubMed] [Google Scholar]
- 19.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent-Johnson N A, Macintire D K, Lindsay D S, Lenz S D, Baneth G, Shkap V, Blagburn B L. A new Hepatozoon species from dogs: description of the causative agent of canine hepatozoonosis in North America. J Parasitol. 1997;83:1165–1172. [PubMed] [Google Scholar]