Abstract
Introduction
Opioid-tolerant patients are more likely to deviate from recommended treatments and to experience inadequate analgesia than opioid-naive ones. The aim of this study was to examine whether pharmacist-led management could help improve treatment adherence and quality of life.
Methods
Eligible patients were randomized in a 1:1 ratio to control group and intervention group. The control group received routine education and support, while the intervention group received additional individualized pharmacist-led care. The primary endpoint was treatment adherence in the per-protocol analysis, as evaluated by blinded assessors. An interim analysis was planned when 30% patients completed the study. Alpha was divided into the interim analysis (0.015) and the final analysis (0.035).
Results
In the interim analysis (97 and 87 patients in the control and intervention groups, respectively), the primary endpoint was met. Pharmacist-led intervention significantly increased treatment adherence (93.3 vs. 79.8%; OR: 2.25; 95% CI 1.02, 4.94; P = 0.013), quality of life (0.81 ± 0.17 vs. 0.72 ± 0.25; P = 0.008), and reporting of adverse events (82.7 vs. 61.9%; OR: 1.88; 95% CI 1.16, 3.07; P = 0.004). The two groups did not differ in pain control rate (66.7 vs. 57.1%; OR: 1.25; 95% CI 0.87, 1.78; P = 0.218), breakthrough pain-free rate (66.7 vs. 61.9%; OR: 1.12; 95% CI 0.78, 1.59; P = 0.532) and pain score (1.97 ± 1.04 vs. 2.15 ± 1.24; P = 0.522).
Conclusions
Pharmacist-led management improved treatment adherence, quality of life, and the reporting of adverse events in opioid-tolerant patients with cancer pain.
Trial Registration
ClinicalTrials.gov, NCT03455023.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40122-021-00342-0.
Keywords: Pharmacist, Adherence, Opioid, Opioid tolerance, Cancer pain
Key Summary Points
| Poor treatment adherence is one of the main causes of insufficient analgesia in cancer pain patients. |
| The aim of this study was to examine whether pharmacist-led management could help improve treatment adherence and quality of life in opioid-tolerant patients with cancer pain. |
| This trial showed that pharmacist-led management could improve treatment adherence and quality of life vs. standard care. |
| Also, follow-up management by pharmacists improved the reporting of adverse opioid events. |
| These findings encourage proactive participation of pharmacists in managing cancer pain, both during and after hospitalization. |
Introduction
Pain is a common, distressing symptom among cancer patients. It occurs in 30% of all cancer patients at diagnosis, and its prevalence increases to as high as 90% when the disease reaches an advanced stage [1, 2]. Persistent cancer pain seriously affects patients' physical function, social activities, and quality of life [3, 4]. Despite the availability of various analgesics and carefully developed pain guidelines, cancer pain remains under-treated in 25–77% of patients [5, 6].
One of the main causes of insufficient analgesia is poor treatment adherence by patients [7–9], which may be due to a belief that opioid analgesics have numerous side effects and may lead to drug addiction and tolerance, or a belief that tumor-related pain is inevitable and must be tolerated as much as possible [8, 10]. Participation of pharmacists in the multidisciplinary management team of cancer pain could decrease non-adherence and improve pain control [11].
Pain control and treatment adherence in opioid-tolerant patients (defined as those who have received at least 60 morphine milligram equivalents (MME) for at least 1 week) [12], are particularly challenging [13, 14]. Whether pharmacist-led management can increase treatment adherence in opioid-tolerant patients with cancer pain is unclear.
In this multicenter, randomized controlled study, we evaluated the effect of pharmacist-led management on treatment adherence, treatment efficacy, adverse events, and quality of life in opioid-tolerant patients with cancer pain.
Methods
Patients
Opioid-tolerant patients with cancer pain at least 18 years-old and with a Karnofsky Performance Status score above 50 were recruited at six cancer hospitals in China from June 2018 to October 2019. Patients were excluded if they reported concurrent non-cancer pain such as toothache; if they were using an analgesic pump; if they had severe renal or hepatic insufficiency, defined as creatinine clearance less than 15 ml/min and levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) more than 10 times the upper normal limit; if they suffered health conditions that might compromise assessments of treatment efficacy or adverse reactions, such as pathological fractures, digestive tract obstruction, or non-opioid-related constipation; or if they were expected to survive fewer than 3 months from the start of the study. Patients provided written informed consent before enrollment in the study.
Study Design
The parallel, randomized trial was conducted at six tertiary cancer hospitals in China. The study protocol was approved by the Ethics Committees at all six participating sites (Table S1), and it has been registered on Clinicaltrials.gov (NCT03455023). The study was performed in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and with the principles of the Declaration of Helsinki.
Patients were randomly assigned to a control or intervention group in a 1:1 ratio by an independent statistician using a random number generator on a computer with simple randomization. Physicians responsible for enrolling patients and evaluating endpoints were blinded to group allocation. A pharmacist was responsible for the interventions.
The control group received only routine education and support, including instruction about why and how to take opioids, common adverse reactions and how to deal with them, what is breakthrough pain (BTP) and what should be done when it occurs, and treatments necessary to address BTP or adverse reactions. The intervention group received the same routine education and support as well as individualized pharmacist-led care, including one systematic intervention during hospitalization (evaluation and advice on adjustment of analgesics, education about pain and medications, education about treatment adherence), one intervention on the day of discharge (education on rational use of opioids, management of BTP, monitoring and treatment of adverse reactions, and situations requiring immediate medical attention) and four weekly follow-up support visits after discharge. During these support visits, which were conducted by telephone, pharmacists instructed the patients about what to do if treatment was ineffective, if they failed to adhere to the treatment regime, or if adverse reactions occurred. All participating pharmacists were first trained in the trial protocol and relevant areas of medical education.
Endpoints and Assessments
The primary endpoint was treatment adherence in the per-protocol (PP) population. Adherence was assessed using the self-report, four-item Medication Adherence Scale. It is a validated questionnaire with good internal consistency and retest reliability [15]. Scores range from 0 to 4, with higher scores representing better medication adherence. In this study, a score equal to 4 was judged as good adherence.
The secondary endpoints included pain control rate (PCR), defined as the percentage of patients who scored less than 3 on a numerical pain scale and who experienced BTP less than 3 times on the day of follow-up; BTP-free rate, defined as the percentage of patients without BTP on the day of follow-up; pain score, evaluated using a numeric rating scale (NRS); quality of life, evaluated using the European five-dimensional health scale (EQ-5D); and adverse events, which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0). All endpoints were evaluated at baseline (before any support or intervention) and at 30 days after discharge.
Statistical Analysis
Sample size requirement was calculated based on the following assumptions: (1) adherence rate of 60% in the control group and 75% in the intervention group, according to our preliminary study (data not shown); (2) power of 90%; (3) two-sided alpha level of 0.05. Anticipating a 20% dropout rate, 500 patients were required. An interim analysis was planned when 30% patients completed the study. Alpha was divided into the interim analysis (0.015) and the final analysis (0.035).
Categorical variables were reported as frequencies and proportions, and differences were assessed for significance using the Chi-square test if all expected frequencies ≥ 5 and total sample size ≥ 40; otherwise, differences were assessed using Fisher’s exact test. Continuous variables were reported as mean and standard deviation and analyzed using Student’s t test or Mann–Whitney U test, as appropriate. Odds ratios were calculated by Chi-square test.
All differences were assessed for significance using two-sided tests, at an α level of 0.05. Both intention-to-treat (ITT) and PP analyses were conducted. All analyses were performed using SPSS 22.0 (IBM, Chicago, IL, USA).
Results
Baseline Characteristics
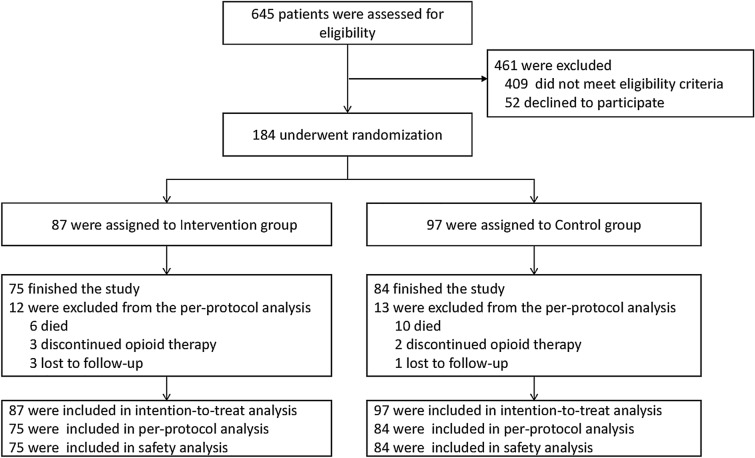
In the interim analysis (97 and 87 patients in the control and intervention groups, respectively), the primary endpoint was met, so the study was terminated by the Data Monitoring Committee. Patient flow through the trial is shown in Fig. 1. The baseline characteristics of the patients are listed in Table 1. Among 184 patients who were randomized, 111 (60.3%) were male, 165 (79.5%) were stage IV cancer, 69 (38.2%) had mixed pain, 97 (42.3%) had bone metastasis and 159 (86.4%) completed the trial.
Fig. 1.
Eligibility, randomization, and analysis. Patients were randomly assigned to control group and intervention group; the control group received routine education and support, while the intervention group received additional individualized pharmacist-led care (one systematic evaluation and intervention during the period of hospitalization, one education on discharge day, and four telephone follow-up intervention after discharge). This figure shows the efficacy and safety populations as of the data cut-off date
Table 1.
Baseline characteristics of patients randomized in the study
| Characteristic | Control group (n = 97) |
Intervention group (n = 87) |
P |
|---|---|---|---|
| Age, years | 57.9 ± 9.8 | 54.8 ± 9.5 | 0.009 |
| Sex | 0.176 | ||
| Male | 63 (64.9) | 48 (55.2) | |
| Female | 34 (35.1) | 39 (44.8) | |
| Smoking | 0.246 | ||
| Current or former smoker | 45 (46.4) | 33 (37.9) | |
| Never smoked | 52 (53.6) | 54 (62.1) | |
| Drinking | 0.293 | ||
| Current or former drinking | 35 (36.1) | 38 (43.7) | |
| Never drinking | 62 (63.9) | 49 (56.3) | |
| Marital status | 0.924 | ||
| Married | 96 (99.0) | 85 (97.9) | |
| Single | 1 (1.0) | 2 (2.3) | |
| Years of education | 0.363 | ||
| ≤ 6 | 44 (45.4) | 32 (36.8) | |
| 9–12 | 48 (49.5) | 52 (59.8) | |
| > 13 | 5 (5.2) | 3 (3.4) | |
| Monthly household income, USD | 0.186 | ||
| < 765 | 42 (43.3) | 43 (49.4) | |
| 765–1530 | 30 (30.9) | 32 (36.8) | |
| 1530–3060 | 15 (15.5) | 9 (10.3) | |
| > 3060 | 10 (10.3) | 3 (3.4) | |
| Cancer type | 0.427 | ||
| Lung | 31 (31.6) | 29 (34.1) | |
| Colorectal | 18 (18.4) | 12 (14.1) | |
| Breast | 8 (8.2) | 10 (11.8) | |
| Esophagus | 6 (6.1) | 3 (3.5) | |
| Pancreas | 11 (11.2) | 4 (4.7) | |
| Gastric | 7 (7.1) | 5 (5.9) | |
| Other | 17 (13) | 22 (25.9) | |
| Tumor stage | 0.867 | ||
| II | 1 (1.0) | 1 (1.1) | |
| III | 10 (1.3) | 7 (8.0) | |
| IV | 86 (88.7) | 79 (90.8) | |
| Karnofsky score | 0.391 | ||
| 60 | 15 (15.5) | 8 (9.2) | |
| 70 | 22 (22.7) | 21 (24.1) | |
| 80 | 42 (43.3) | 46 (52.9) | |
| 90 | 18 (18.6) | 12 (13.8) | |
| Pain type | 0.429 | ||
| Somatic | 34 (35.1) | 24 (27.6) | |
| Visceral | 24 (24.7) | 19 (21.8) | |
| Neuropathic | 8 (8.2) | 6 (6.9) | |
| Mixed | 31 (32.0) | 38 (43.7) | |
| Bone metastasis | 0.253 | ||
| Yes | 55 (56.7) | 42 (48.3) | |
| No | 42 (43.3) | 45 (51.7) | |
| Opioid dose: morphine milligram equivalents | 196.6 ± 33.7 | 205.1 ± 29.2 | 0.991 |
Values are n (%) or mean ± standard deviation
Primary Endpoints
Adherence
Pharmacist-led intervention significantly improved adherence in opioid-tolerant patients based on PP analysis (93.3 vs. 79.8%; OR: 2.25; 95% CI 1.02, 4.94; P = 0.013. Table 2 and Fig. 2). In ITT analysis, all patients who did not complete the study were counted as poor adherence. In this case, the intervention and control groups did not differ significantly in adherence (80.5 vs. 69.1%; OR: 1.41; 95% CI 0.93, 2.14; P = 0.077. Table 2).
Table 2.
Analysis of primary and secondary outcomes on a per-protocol (PP) or intention-to-treat (ITT) basis
| Outcome* | At baseline | OR (95% CI) | P value | 30 days after discharge (PP) | Odds ratio (95% CI) | P value | 30 days after discharge (ITT) | OR (95% CI) | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 97) |
Intervention (n = 87) | Control (n = 84) |
Intervention (n = 75) |
Control (n = 97) |
Intervention (n = 87) |
|||||||
| Adherence rate** | 63.9 (62) | 63.2 (55) | 0.98 (0.72–1.35) | 0.922 | 79.8 (67) | 93.3 (70) | 2.25 (1.02–4.94) | 0.013 | 69.1 (67) | 80.5 (70) | 1.41 (0.93–2.14) | 0.077 |
| Pain control rate** | 46.4 (45) | 39.1 (34) | 0.85 (0.62–1.17) | 0.317 | 57.1 (48) | 66.7 (50) | 1.25 (0.87–1.78) | 0.218 | 49.5 (48) | 57.5 (50) | 1.19 (0.87–1.62) | 0.278 |
| BTP-free rate** | 44.3 (43) | 42.5 (37) | 0.96 (0.71–1.31) | 0.806 | 61.9 (52) | 66.7 (50) | 1.12 (0.78–1.59) | 0.532 | 53.6 (52) | 57.5 (50) | 1.10 (0.80–1.48) | 0.599 |
| Pain score (NRS) | 2.38 ± 1.26 | 2.43 ± 1.15 | – | 0.623 | 2.15 ± 1.24 | 1.97 ± 1.04 | – | 0.522 | – | – | – | – |
| Qol (EQ-5D) | 0.73 ± 0.16 | 0.76 ± 0.17 | – | 0.129 | 0.72 ± 0.25 | 0.81 ± 0.17 | – | 0.008 | – | – | – | – |
Values are % (n) or mean ± standard deviation
BTP breakthrough pain, Qol quality of life, EQ-5D European five-dimensional health scale, OR odds ratio
*Patients who did not complete the study were counted as showing poor adherence or poor pain control
**Definitions: adherence rate was defined as the percentage of patients with a Medication Adherence Scale score equal to 4; Pain control rate was defined as the percentage of patients who scored less than 3 on a numerical pain scale and who experienced BTP less than 3 times on the day of follow-up; BTP-free rate was defined as the percentage of patients without BTP on the day of follow-up
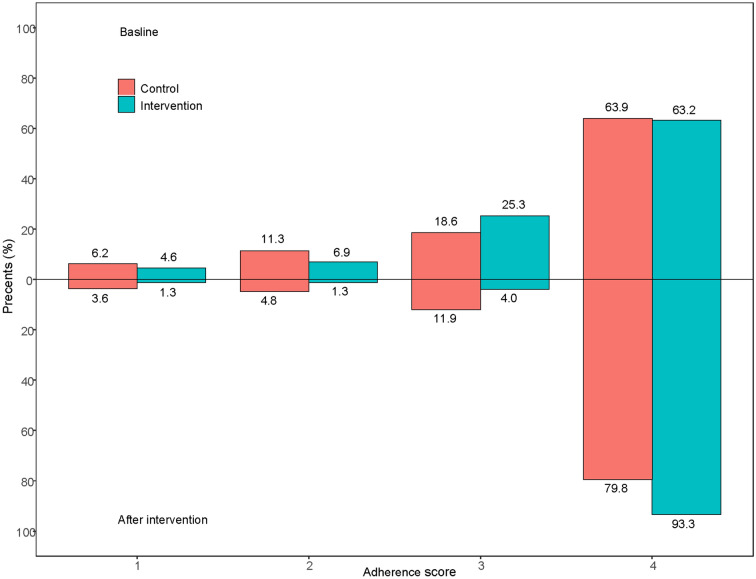
Fig. 2.
Adherence score distribution of control group and intervention group (per-protocol analysis). The baseline data was shown above the X-axis, and the 30 days after discharge data was displayed under the X-axis; The green column indicates the proportion of patients in the intervention group and red column indicates control group. In the baseline, 63.9% of patients scored 4, 18.6% scored 3, 11.3% scored 2, 6.2% scored 1 in control group and 63.2% of patients scored 4, 25.3% scored 3, 6.9% scored 2, 4.6% scored 1 in intervention group; 30 days after discharge, 79.8% of patients scored 4, 11.9% scored 3, 4.8% scored 2, 3.6% scored 1 in control group and 93.3% of patients scored 4, 4.0% scored 3, 1.3% scored 2, 1.3% scored 1 in intervention group. Scored 4 indicated the good adherence, and the lower score indicated the worse adherence
Treatment Efficacy
PCR tended to be higher in the intervention group in PP analysis (66.7 vs. 57.1%; OR: 1.25; 95% CI 0.87, 1.78; P = 0.218) and ITT analysis (57.5 vs. 49.5%; OR: 1.19; 95% CI 0.87, 1.62; P = 0.278). In the ITT analysis, all patients who did not complete the study were counted as showing poor pain control (Table 2).
At 30 days after discharge, the intervention and control groups showed similar BTP-free rates in PP analysis (66.7 vs. 61.9%; OR: 1.12; 95% CI 0.78, 1.59; P = 0.532) and ITT analysis (57.5 vs. 53.6%; OR: 1.10; 95% CI 0.80, 1.48; P = 0.599).
Pain scores did not differ between the two groups in PP analysis (1.97 ± 1.04 vs. 2.15 ± 1.24; P = 0.522). However, it decreased significantly 30 days after discharge compared to baseline in the intervention group (1.97 ± 1.04 vs. 2.43 ± 1.15; P = 0.013) while there was no significant change in the control group (2.15 ± 1.24 vs. 2.38 ± 1.26; P = 0.256).
Quality of Life
Based on PP analysis, quality of life at baseline was similar between the intervention and control groups (0.76 ± 0.17 vs. 0.73 ± 0.16; P = 0.129). However, at 30 days after discharge, quality of life was significantly better in the intervention group (0.81 ± 0.17 vs. 0.72 ± 0.25; P = 0.008).
Safety
All patients who finished the study were included in the safety analysis. The five most frequent opioid-related adverse events, regardless of grade, were constipation (40.3%), somnolence (8.8%), urinary retention (6.3%), nausea (5.7%), and vomiting (4.4%). Grade 3 opioid-related adverse events were seen in only one patient (constipation), and no grade 4 or 5 opioid-related adverse events were observed. The intervention group showed a significantly higher frequency of any adverse events of any grade (82.7 vs. 61.9%; OR: 1.88; 95% CI 1.16, 3.07; P = 0.004, Table 3).
Table 3.
Comparison of adverse events between the intervention and control groups
| Event | Control (n = 84) |
Intervention (n = 75) |
All patients | P value |
|---|---|---|---|---|
| Constipation | 34 (40.5) | 34 (45.3) | 64 (40.3) | 0.537 |
| Somnolence | 6 (7.1) | 8 (10.7) | 14 (8.8) | 0.434 |
| Nausea | 4 (4.8) | 5 (6.7) | 9 (5.7) | 0.861 |
| Vomiting | 4 (4.8) | 3 (4.0) | 7 (4.4) | 1.000 |
| Urinary retention | 3 (3.6) | 7 (9.3) | 10 (6.3) | 0.243 |
| Dry mouth | 1 (1.2) | 3 (4.0) | 4 (2.5) | 0.534 |
| Delirium | 1 (1.2) | 1 (1.3) | 2 (1.3) | 1.000 |
| Pruritus | 1 (1.2) | 2 (2.7) | 3 (1.9) | 0.912 |
| Dizziness | 2 (2.4) | 0 | 2 (1.3) | 0.533 |
| Abdominal distension | 0 | 1 (1.3) | 1 (0.6) | 0.949 |
| Sleep-talking | 0 | 1 (1.3) | 1 (0.6) | 0.949 |
| Any event of any grade | 52 (61.9) | 62 (82.7) | 114 (71.7) | 0.004 |
| Any event of grade 3–5 | 0 | 1 (1.3) | 1 (1.3) | 0.472 |
Values are n (%)
Discussion
The adherence rate among patients with cancer pain varies widely from 41 to 90.8% [11, 16–19]. This wide variation may reflect differences in pain management practices, tools for assessing adherence, as well as pain characteristics: one study reported adherence rates of 85–91% to around-the-clock opioids but rates of 22–27% to as-needed analgesics [18]. In our study, around 63% of patients in the control and intervention groups adhered to treatment at baseline, and pharmacist-led management significantly increase the treatment adherence of opioid-tolerant patients with cancer pain than routine support (93.3 vs. 79.8%; OR: 2.25; 95% CI 1.02, 4.94; P = 0.013). Similarly, a retrospective study in China involving 195 cancer pain patients found that adherence was 72.3% before pharmacist intervention and 91.8% afterward [12]. Our study substantially extends the literature because most previous prospective studies of pharmacist-led interventions have examined pain scores and quality of life, but not adherence [11].
However, pharmacist-led management improved adherence but did not improve the PCR in the current study. This contrasts with evidence linking better adherence with better pain management [10, 14]. This negative finding is most likely the result of early trial termination, which in turn was based on interim analysis of the primary outcome (treatment adherence). The early termination is based on pre-planned criteria, and thus justified, but could lead to insufficient sample size for PCR. Considering the existing literature on the association between poor adherence with low pain control rate, we decided to terminate the trial and started to implement pharmacist-led management in routine practice. Another possible confounding is the “spillover effect”, when patients in the control and intervention groups happened to be share the same ward, could also be contributed to the similar PCR. Nevertheless, consistent with previous studies [19, 20], pharmacist-led management resulted in higher quality of life (0.81 ± 0.17 vs. 0.72 ± 0.25; P = 0.008).
In our cohort, incidence of total adverse events was significantly higher in the intervention group, whereas previous studies have reported similar or lower incidence than in the control group [12, 21]. One explanation for this is that the intervention group was followed up once a week after discharge, which may have promoted the reporting of adverse events.
Our results should be interpreted with caution in light of several limitations. First, simple randomization was adopted instead of block randomization, which created risk of the above-mentioned “spillover effect”, potentially leading to underestimation of the effects of pharmacist-led intervention. Second, the four-item Medication Adherence Scale may overestimate adherence because it does not assess adherence to medications for which the patient adjusts the dose him- or herself. Third, the study included some patients with terminal cancer who could not take care of themselves, and 16 patients (8.74%) died before the end of the study, which not only reduced our statistical power but may also have compromised the generalizability of our results.
Conclusions
In summary, pharmacist-led management can significantly improve treatment adherence, quality of life and reporting of opioid adverse events in opioid-tolerant patients with cancer pain. Proactive participation of pharmacists should be encouraged both during and after hospitalization.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82003189), and Natural Science Foundation of Zhejiang Province (No. LQ17H310002). The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Study design, Ping Huang, Xiaowei Zheng, Haiying Ding and Luo Fang; Project coordination and supervision, Ping Huang, Yancai Sun, Qing Wei, Ruixiang Xie, Yuguo Liu, and Qing Zhai; Patient recruitment, Liyan Gong, Yiping Zhang, Zhengbo Song, Yang Yang, Shoubing Zhou, Chengsuo Huang, Jinyuan Lin and Chenchen Wang; Project execution and patient management, Silu Xu, Yinghui Tong, Jiao Sun, Wenxiu Xin, Nan Wu, Juan Chen, Wenna Shi, Ling Yang, Hui Li, Jingjing Shao, Yangkui Wang, Hui Yu, Bo Zhang, Qiong Du, Yezi Yang, Xiaodan Zhang, Cunxian Duan, Qiulin Zhao, Jing Shi, Jing Huang, Qing Fan, Huawei Cheng, Lingya Chen, Sisi Kong, Hui Zhang; Randomization and data statistics, Xianhong Huang. Data curation, Xiaowei Zheng, Haiying Din; Writing—original draft preparation, Xiaowei Zheng; Writing—review and editing, Ping Huang. All authors have read and agreed to the published version of the manuscript.
Disclosures
Xiaowei Zheng, Haiying Ding, Silu Xu, Ruixiang Xie, Yuguo Liu, Qing Zhai, Luo Fang, Yinghui Tong, Jiao Sun, Wenxiu Xin, Nan Wu, Juan Chen, Wenna Shi, Ling Yang, Hui Li, Jingjing Shao, Yangkui Wang, Hui Yu, Bo Zhang, Qiong Du, Yezi Yang, Xiaodan Zhang, Cunxian Duan, Qiulin Zhao, Jing Shi, Jing Huang, Qing Fan, Huawei Cheng, Lingya Chen, Sisi Kong, Hui Zhang, Liyan Gong, Yiping Zhang, Zhengbo Song, Yang Yang, Shoubing Zhou, Chengsuo Huang, Jinyuan Lin, Chenchen Wang, Xianhong Huang, Qing Wei, Yancai Sun, Ping Huang declare no conflicts of interest.
Compliance with Ethics Guidelines
The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments, and all research centers have received ethical approval (Table S1). All participants have signed informed consent forms.
Data Availability
The data are not publicly available due to ethical concerns. Requests for supplementary data and additional materials should be submitted directed to the corresponding author. Participant data will be provided after approval from the corresponding author and the Ethical Committee, only scientifically and methodologically ones will be considered.
Footnotes
Xiaowei Zheng and Haiying Ding contributed equally to this work.
Contributor Information
Qing Wei, Email: jsschwq@sina.com.
Yancai Sun, Email: 13349293359@163.com.
Ping Huang, Email: huangping1841@zjcc.org.cn.
References
- 1.Portenoy RK, Miransky J, Thaler HT, Hornung J, Bianchi C, Cibas-Kong I, Feldhamer E, Lewis F, Matamoros I, Sugar MZ, et al. Pain in ambulatory patients with lung or colon cancer. Prevalence, characteristics, and effect. Cancer. 1992;70:1616–1624. doi: 10.1002/1097-0142(19920915)70:6<1616::AID-CNCR2820700630>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Garcia de Paredes ML, Del Moral GF, Martinez Del Prado P, Marti Ciriquian JL, Enrech Frances S, Cobo Dols M, Esteban Gonzalez E, Ortega Granados AL, Majem Tarruella M, Cumplido Buron JD, Gasco Hernandez A, Lopez Miranda E, et al. First evidence of oncologic neuropathic pain prevalence after screening 8615 cancer patients. Results of the On study. Ann Oncol. 2011;22:924–930. doi: 10.1093/annonc/mdq449. [DOI] [PubMed] [Google Scholar]
- 3.Te Boveldt N, Vernooij-Dassen M, Burger N, Ijsseldijk M, Vissers K, Engels Y. Pain and its interference with daily activities in medical oncology outpatients. Pain Physician. 2013;16:379–389. doi: 10.36076/ppj.2013/16/379. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez C, Ji M, Wang HL, Padhya T, McMillan SC. Cancer pain and quality of life. J Hosp Palliat Nurs. 2019;21:116–123. doi: 10.1097/NJH.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 5.Reis-Pina P, Lawlor PG, Barbosa A. Adequacy of cancer-related pain management and predictors of undertreatment at referral to a pain clinic. J Pain Res. 2017;10:2097–2107. doi: 10.2147/JPR.S139715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh H, Banipal RPS, Singh B. Assessment of adequacy of pain management and analgesic use in patients with advanced cancer using the brief pain inventory and pain management index calculation. J Glob Oncol. 2017;3:235–241. doi: 10.1200/JGO.2016.004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon JH. Overcoming barriers in cancer pain management. J Clin Oncol. 2014;32:1727–1733. doi: 10.1200/JCO.2013.52.4827. [DOI] [PubMed] [Google Scholar]
- 8.Timmerman L, Stronks DL, Huygen FJ. The relation between patients' beliefs about pain medication, medication adherence, and treatment outcome in chronic pain patients: a prospective study. Clin J Pain. 2019;35:941–947. doi: 10.1097/AJP.0000000000000760. [DOI] [PubMed] [Google Scholar]
- 9.Ma X, Lu Y, Yang H, Yu W, Hou X, Guo R, Wang Y, Zhang Y. Relationships between patient-related attitudinal barriers, analgesic adherence and pain relief in Chinese cancer inpatients. Support Care Cancer. 2020;28:3145–3151. doi: 10.1007/s00520-019-05082-8. [DOI] [PubMed] [Google Scholar]
- 10.Kan E, Mustafa S, Chong WW, Premakumar CM, Mohamed SN. Relationship between adherence to opioid analgesics and pain beliefs among patients with cancer pain at tertiary care hospitals in Malaysia. Patient Prefer Adherence. 2020;14:1411–1419. doi: 10.2147/PPA.S255289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Wang C, Chen X, Luo J, Xie J, Li S, Hu J, Shi C. Evaluation of pharmacist interventions as part of a multidisciplinary cancer pain management team in a Chinese academic medical center. J Am Pharm Assoc. 2003;2020(60):76–80. doi: 10.1016/j.japh.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Mearis M, Shega JW, Knoebel RW. Does adherence to National Comprehensive Cancer Network guidelines improve pain-related outcomes? An evaluation of inpatient cancer pain management at an academic medical center. J Pain Symptom Manage. 2014;48:451–458. doi: 10.1016/j.jpainsymman.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Macintyre PE, Roberts LJ, Huxtable CA. Management of opioid-tolerant patients with acute pain: approaching the challenges. Drugs. 2020;80:9–21. doi: 10.1007/s40265-019-01236-4. [DOI] [PubMed] [Google Scholar]
- 14.Owodunni OP, Zaman MH, Ighani M, Grant MC, Bettick D, Sateri S, Magnuson T, Gearhart S. Opioid tolerance impacts compliance with enhanced recovery pathway after major abdominal surgery. Surgery. 2019;166:1055–1060. doi: 10.1016/j.surg.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence and long-term predictive validity of blood pressure control. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Valeberg BT, Miaskowski C, Hanestad BR, Bjordal K, Moum T, Rustoen T. Prevalence rates for and predictors of self-reported adherence of oncology outpatients with analgesic medications. Clin J Pain. 2008;24:627–636. doi: 10.1097/AJP.0b013e31816fe020. [DOI] [PubMed] [Google Scholar]
- 17.Tzeng JI, Chang CC, Chang HJ, Lin CC. Assessing analgesic regimen adherence with the Morisky medication adherence measure for Taiwanese patients with cancer pain. J Pain Symptom Manage. 2008;36:157–166. doi: 10.1016/j.jpainsymman.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Rhee YO, Kim E, Kim B. Assessment of pain and analgesic use in African American cancer patients: factors related to adherence to analgesics. J Immigr Minor Health. 2012;14:1045–1051. doi: 10.1007/s10903-012-9582-x. [DOI] [PubMed] [Google Scholar]
- 19.Miaskowski C, Dodd MJ, West C, Paul SM, Tripathy D, Koo P, Schumacher K. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J Clin Oncol. 2001;19:4275–4279. doi: 10.1200/JCO.2001.19.23.4275. [DOI] [PubMed] [Google Scholar]
- 20.Liang SY, Chen KP, Tsay SL, Wu SF, Chuang YH, Wang TJ, Tung HH, Cheng SF. Relationship between belief about analgesics, analgesic adherence and pain experience in Taiwanese cancer outpatients. Asian Pac J Cancer Prev. 2013;14:713–716. doi: 10.7314/APJCP.2013.14.2.713. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Lu XY, Wang WJ, Shen B, Ye Y, Jiang H, Wang QS, Cheng B. Impact of a clinical pharmacist-led guidance team on cancer pain therapy in China: a prospective multicenter cohort study. J Pain Symptom Manage. 2014;48:500–509. doi: 10.1016/j.jpainsymman.2013.10.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are not publicly available due to ethical concerns. Requests for supplementary data and additional materials should be submitted directed to the corresponding author. Participant data will be provided after approval from the corresponding author and the Ethical Committee, only scientifically and methodologically ones will be considered.