Abstract
A wound-inducible promoter facilitates the regulated gene expression at the targeted site during the time of mechanical stress or infestation by the pathogen. The present work has aimed to identify a wound-inducible promoter that expresses at early time points preceding wound-stress treatment in Arabidopsis thaliana. The computational analysis of microarray data (GSE5627) resulted in the identification of five early inducible genes, viz., AT1G17380, AT1G80440, AT2G43530, AT3G48360, and AT5G13220. The RT-PCR analysis showed AT5G13220 (JASMONATE-ASSOCIATED 1) gene induced at a significantly higher level post 30 min of wounding. Thus, the promoter of the highly induced and early expressed wound-inducible gene, AT5G13220 (named PW220), was characterized by fusing with β-glucuronidase (gusA) reporter or Cry1EC genes. The fluorometric analysis and histochemical staining of the gusA gene and quantitative estimation of Cry1EC protein in Nicotiana tabacum transgenic lines confirmed wound-induced expression characteristic of the selected promoter. Insect bioassay suggested that wound-inducible and constitutive expression of Cry1EC protein in transgenic lines showed a similar level of protection against different instar Spodoptera litura larvae. Furthermore, we identified that abscisic acid influenced the wound-specific expression of the selected PW220 promoter in the transgenic lines, which correlates with the presence of conserved cis-regulatory elements associated with dehydration and abscisic acid responses. Altogether, our results suggested that the wound-inducible promoter PW220 provides an excellent alternative for developing insect-tolerant transgenic crops in the future.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-022-03143-0.
Keywords: Wounding, Chewing pest, Gene regulation, Inducible promoter, Insecticidal protein
Introduction
Plants are sessile organisms and are bound to live under unavoidable biotic and abiotic stress conditions. Among the biotic stresses, herbivorous insects damage plants by feeding on them are one of the major causes for the loss in crop yield and quality. More than a million phytophagous insect species consume plants as their food source. These insects take diet either by chewing aerial or underground parts of the plants or by sucking sap from aerial parts. Both insects and plants evolved a variety of interactions with each other during the co-evolution of ~ 350 million years (Mithofer and Boland 2008). The interactions are either beneficial (insect mediated pollination and seed dispersion) or harmful (insect feeding on plant part) to plants. During long co-existence in close interaction with the insects, the plants have evolved various mechanisms to combat insect infestation (Kempema et al. 2007; Wei et al. 2007). The mechanisms involve either inherent constitutive defense (formation of physical barriers like cell wall, cuticle, etc.) or induced defense (Kempema et al. 2007). Physical wounding, other than insects or pathogen attack, may also potentially induce systemic acquired resistance (SAR) response as a consequence of the accumulation of salicylic acid, jasmonic acid, and ethylene (Kato et al. 2000; Kessler and Baldwin 2002; Savatin et al. 2014; Zhu et al. 2014). The physical damage caused by wounding is a primary mode for any pathogen to invade the plant cell and alone can induce SAR. Furthermore, the insect saliva at the site of wounding provides the base for the infection by other pathogens, thus inducing distinct transcriptional programming (Savatin et al. 2014).
The promoter is an important molecular tool to express transgenes in plants, which consists of several cis-regulatory elements and control its expression in response to several molecular and physiological stimuli (Chaturvedi et al. 2007; Kiran et al. 2006; Lodhi et al. 2008; Pandey et al. 2019; Srivastava et al. 2014a, b, 2018). Most commonly, constitutive promoters are deployed to achieve higher expression of insecticidal toxin proteins in almost all the tissues and all the time to develop insect-resistant plants. However, constitutive expression of insecticidal proteins is neither always required nor desired as they may not get properly metabolized in plants and also diverts significant cellular resources in expression that may result in a yield penalty (Chakrabarti et al. 2006). Additionally, the emergence of insect resistance against insecticidal protein due to constitutive expression has also been reported (Ferré et al. 1991; Tabashnik et al. 2011). Thus, considering the disadvantages related to the use of constitutive promoters, there is an unmet need to identify promoters that express the insecticidal protein at the site of insect bite and at the early time point of insect infestation to ensure effective protection against insects. Thus, wound-inducible protein expression would be a better and safer strategy to manage insect pests in developing transgenic insect-resistant plants.
In the present study, we analyzed microarray data for identifying wound-induced genes and their promoter. We characterized one such wound-inducible promoter by fusing it with reporter gene gusA and insecticidal gene Cry1EC for developing transgenic tobacco lines expressing these constructs. Our study thus validates and provides potential use of a wound-inducible promoter for developing insect-resistant transgenic plants.
Materials and methods
Plant material and growth conditions
Nicotiana tabacum cv. Petit Havana (NTPH) and Arabidopsis thaliana (Col-0) plants were grown and maintained in the glass/culture house. NTPH was used to generate the transgenic plants, which had been grown at 24 ± 2 °C for 16-h light/8-h dark conditions. Agrobacterium tumefaciens strain LBA 4404 containing helper plasmid PAL 4404 (laboratory stock) was used for the tobacco transformation experiments. For Arabidopsis growth, Col-0 seeds were sown in soil and kept for 2–3 days at 4 °C in dark. After 2–3 days, the sown seeds were moved to a culture house maintained at 16-h light/8-h dark conditions (100–120 μmol m–2 s–1) at temperature 20 ± 2 °C. Three-to-four-week-old Arabidopsis plants were chosen for further experiments.
Microarray experiment data analysis for the selection of the wound-inducible genes
The microarray DataSet GSE5627 available from NCBI Gene Expression Omnibus (GEO) was selected for identifying significantly induced genes after mechanical wound treatment (Supplementary Table 1). The microarray experiment was performed at different time points (15 min, 30 min, and 1 h) post-wound treatment in A. thaliana as per the information provided in GEO data set GSE69995 (BioProject PRJNA96933) (Kilian et al. 2007). We used time point 0 h as a control in our analysis. Student’s t test was applied to determine statistically significant differentially expressed genes using Array Assist Software 5.2.2 (Stratagene, La Jolla, California, USA). The significantly induced genes were further filtered based on the conditions of fold change (≥ 2.0) with a p-value (≤ 0.01).
Quantitative RT-PCR (qRT-PCR)
The real-time quantitation was performed using the SYBR Green PCR Master Mix on Applied Biosystems ABI-7500 Real-Time PCR. For wound treatment, A. thaliana was pricked repeatedly several times with fine forceps, and thereafter, leaves were harvested after 15 min, 30 min, 1 h, and 3 h for extracting total RNA. The qRT-PCR was performed with gene-specific primers using cDNA prepared from RNA isolated at a different time point after wound treatment and leaves without wounding used as control. Primer sequences used in qRT-PCR are listed in Supplementary Table 2. The relative fold of the gene expression was calculated by the 2−ΔΔCT process. The actin gene (Locus ID AT3G18780) was used as an internal control for the data normalization.
Development of expression constructs and transgenic plants
A. thaliana genomic DNA was used as a template for amplification of promoter using AccuPrime™ Pfx DNA Polymerase (Invitrogen). Primers for the PCR amplification of promoters used in this study are given in Supplementary Table 2. Amplified promoters comprising SalI and BamHI sites were first cloned into pBluescriptSK+ and sequenced, and thereafter sub-cloned into a pBI101 vector with a GUS-A (β-glucuronidase) gene and in vector pPK203 with Cry1EC gene. Tobacco transformation was performed according to an early published protocol (Horsch et al. 1985). Transgenic (T1) tobacco seeds were harvested and grown for identification and selection of positive plants using kanamycin antibiotic (300 mg/l).
Fluorimetric estimation of β-glucuronidase (GUS)
The leaf (100 mg) samples were taken from mechanically wounded and control (non-wounded) plants, which were grounded in liquid nitrogen into a fine powder and mixed with 0.5 ml of GUS extraction buffer (GEB) for proper homogenization (Jefferson 1987). Cellular debris from the samples was settled down by centrifuging at 13,000 rpm, for 20 min at 4 °C, and the supernatant was transferred into a fresh tube. Ninety microliters of the supernatant were mixed with 10 µl of 10X GUS assay buffer (10 mM MUG in GEB buffer) in fresh tubes. To reduce the GUS-like background fluorescence existing in non-transformed and transformed samples, methanol (20%) was mixed in the solution comprising the extract and MUG. The tubes were incubated at 55 °C for 5 min in dark conditions, followed by chilling on ice and the addition of 20% methanol with mixing. After mixing, the tubes were kept at 37 °C for 45 min in dark conditions, and the reaction was stopped by mixing 900 µl of stop buffer (0.2 M Na2CO3). Relative fluorescence of 4-methyl umbelliferone (MU) was measured using TECAN 200 infinite spectro-fluorimeter with at 365 nm excitation and 455 nm emission wavelength. Bradford Dye-binding assay was used to quantify the protein concentration in the 5 µl sample from the same extract used in GUS determination. The specific activity of GUS was calculated as nM 4-MU/µg protein/min.
Histochemical GUS staining analysis of transgenic plants
Wounded and control (non-wounded) leaf samples were placed in X-Gluc (5-Bromo-4-chloro-3-indolylglucuronide) solution for 16 h at 37 °C in buffer containing 50 mM sodium phosphate buffer pH 7.0, 3 mM potassium ferrocyanide, 3 mM potassium ferricyanide, 0.2% Triton X-100, and 20% methanol (Singh et al. 2010). After the development of color, the chlorophyll of the leaf samples was removed by repeated incubation with absolute ethanol in 4–5 consecutive days.
Protein expression analysis by DAC-ELISA
T1 transgenic plants of PW220:Cry1EC were analyzed for the detection of the expression of the Cry1EC in terms of specific δ-endotoxin activity by ELISA. The 200 mg leaf from control and wound-treated T1 transgenic plants (60 days old) were crushed in 1 ml bicarbonate buffer pH 9.6 containing insoluble PVP (1%, w/v), Triton X-100 (0.1%, v/v), Tween-20 (0.05%, v/v), sodium azide (0.02% w/v), and 100 mM PMSF. The mixture was settled down by spin at 10,000 rpm at 4 °C for 10 min. A total soluble protein in the supernatant was measured by the Bio-Rad dye-binding assay. The protein extract (100 µl) from control or wound-treated transgenic plant lines had been taken into the 96-well microtitre plate. Two additional controls were considered in the test; one was an extraction buffer treated directly on wells (negative buffer control) and the second were dilutions of purified Cry1EC protein expressed in E. coli and dissolved in extraction buffer used as a standard positive control. The plates were kept in the dark for 2 h at 37 °C. The 0.5% BSA was used to block free binding sites. The plates were washed thrice with buffer PBS-T. After blocking and washing, 10,000-fold diluted antiserum (primary antibody) in PBS-T was added to all sample wells. The plates were incubated at 37 °C for 2 h. The primary antibody was prepared in rabbits by injecting purified δ-endotoxin Cry1EC made from E. coli. The goat anti-rabbit IgG (alkaline phosphatase labeled) was diluted to 30,000-fold in buffer PBS-T-BSA and was added as a secondary antibody. After 2 h of incubation at 37ºC, 200 µl pNPP substrate prepared freshly in an alkaline 0.1 M di-ethanolamine (pH 9.8) was added to each well. Subsequently, the reaction was stopped by mixing 50 µl of 3 M NaOH and incubating for 35 min at 37 °C. The 96-well microtitre plate was measured at wavelength 405 nm and the Cry1EC protein level was calculated with purified Cry1EC protein on a linear standard curve plotted.
Insect bioassay
Spodoptera litura larvae were maintained on semi-synthetic food in laboratory conditions. Leaves from the second to the fourth node of 8 week transgenic plants or non-transformed (control) tobacco plants were used for insect bioassay using the first instar (3 days old) and third instar (6 days old) of S. litura larvae. Ten first instar and five third instar larvae were placed on each leaf. Each insect bioassay experiment was repeated twice with three biological leaf samples. Mortality of larvae was documented at five different time points (6, 12, 18, 24, and 48 h) for first instar larvae and three different time points (24, 48, and 72 h) for third instar larvae.
Cis-regulatory element analysis
The cis-regulatory element-binding site analysis of the promoter PW220 sequence was performed using the PLACE database (http://www.dna.affrc.go.jp/PLACE/) (Higo et al. 1998).
Phyto-hormone treatment
Hoagland media containing 1 mM SA, 100 µM meJA, and 100 µM ABA (Zhang et al. 2008) and controlled media without hormone were sprayed on transgenic tobacco plants. Leaf samples were collected after 30 min of treatment for fluorometric estimation of GUS. All the experiments were performed twice with three biological replicates.
Results
Identification and validation of the early wound-inducible genes
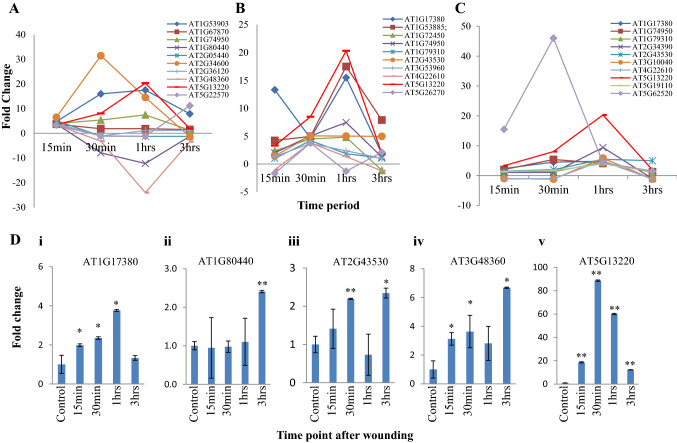
For the identification of the early wound-inducible promoter, expression profile GSE5627 from NCBI GEO-DataSets was analyzed at the different time points from 15 min to 24 h in Arabidopsis (Kilian et al. 2007) (Supplementary Fig. 1A). The differentially expressed genes (fold change ≥ 2.0 and p ≤ 0.05) were selected after 15 min, 30 min, and 1 h early time point of wound induction (Fig. 1A–C, Supplementary Fig. 1A, and Supplementary Table 1). The RNA was prepared from wound-treated Arabidopsis leaf according to as suggested on GSE5627 and used for qRT-PCR validation of selected genes (Fig. 1D and Supplementary Fig. 1B). Out of the five genes randomly selected, only AT5G13220 was found significantly induced by wound treatment early point (15 min, 30 min, and 1 h) (Fig. 1D). The qRT-PCR results showed that AT5G13220 was 20- and 88-fold up-regulated after 15 min and 30 min of wound induction as compared to control treatment, respectively. However, the other four genes were induced at later time points (1 or 3 h) of wound induction or were not highly induced like AT5G13220 (Fig. 1D). Based on these results obtained from the microarray profile and qRT-PCR, the wound-inducible gene AT5G13220 was eventually selected for promoter cloning and its characterization in transgenic tobacco plants.
Fig. 1.
Differential expression profiles were established by Microarray and qRT-PCR for the selection of genes at different time points of wounding. A Top 10 up-regulated genes after 15 min of wounding. B Top 10 up-regulated genes after 30 min of wounding. C Top 10 up-regulated genes after 1 h of wounding. D Validation of wound-inducible nature of selected genes in A. thaliana with qRT-PCR at four different early time points after wound treatment. The values above the bars indicate the SE from the means of three independent experiments
Molecular evaluation wound-inducible pattern of AT5G13220 promoter in the transgenic tobacco plants
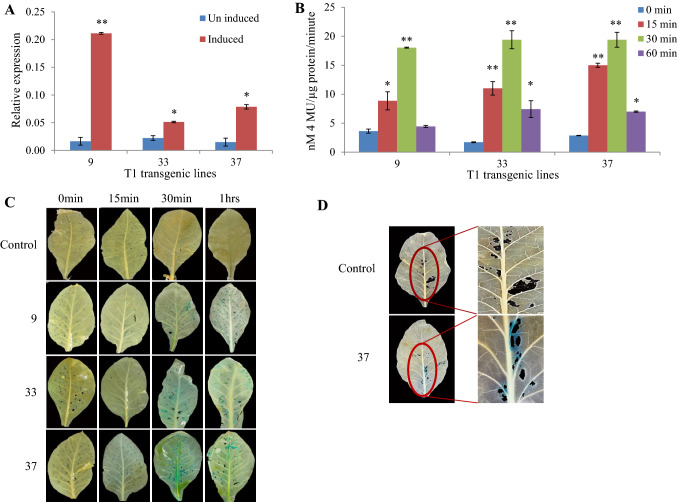
To investigate the effect of the wound-inducible promoter and its expression pattern, 1 kb upstream promoter sequence from transcription start site along with the 113 bp of 5′UTR of AT5G13220 gene (named hereafter, Promoter Wound AT5G13220, PW220) was cloned upstream to gusA gene in the pBI101 binary expression vector (Supplementary Fig. 2A). The expression construct was transformed into N. tabacum using Agrobacterium-mediated transformation to obtain several independent transgenic lines. Three T1 transgenic lines were selected randomly to determine the GUS expression (Fig. 2A). All selected transgenic plants expressing the gusA gene driven by PW220 showed an increase in GUS expression after 30 min of in-planta wounding as compared to the control wild-type plant. The GUS expression in T1 transgenic line no. 9 was highly induced (12.8-fold) with the lowest level of the basal level of the expression (Fig. 2A). Thus, qRT-PCR analysis of the reporter gusA gene suggests that promoter PW220 regulates the wound-inducible GUS expression among the transgenic lines.
Fig. 2.
Evaluation of PW220 wound-inducible promoter in the transgenic plants. A The qRT-PCR estimation of GUS transcript in PW220:gusA T1 transgenic lines after 30 min of wound treatment. Graphical presentation of induced versus un-induced expression of GUS among the lines under evaluation. The values above the bars indicate the SE from the means of three independent experiments. The mark symbols denote **p ≤ 0.001; *p ≤ 0.05. B Fluorimetric quantitation of GUS protein in three independent transgenic lines at four different time points. A one-tailed t test was applied to determine the significant differences in the expression among different time points of evaluation. The values above the bars indicate the SD from the means of three independent experiments. The mark symbols denote **p ≤ 0.001; *p ≤ 0.05. C The GUS histochemical staining of three independent T1 transgenic lines at four different time points after wound treatment. D The GUS staining of a (PW220:gusA) T1 transgenic line after 12 h of S. litura first instar larvae release
To examine how PW220 expresses during insect infestation, we performed the quantitative (fluorimetric) and qualitative (histochemical) expression of the GUS protein in the transgenic lines. For GUS quantification, three T1 transgenic lines of PW220:gusA (same as used for GUS transcript estimation) were selected for wound-inducible GUS activity measured at four different time points (0, 15, 30, and 60 min) (Fig. 2B). The samples harvested at 0 min of the treatment were used as a control for the estimation of fold changes in later time points. All three transgenic lines of PW220:gusA were significantly induced by wound treatment at 15 min and reached the highest level of GUS activity approximately from 5- to 12-fold at 30 min as compared to the starting point (0 min) (Fig. 2B). The GUS activity level of all selected transgenic lines was declined at 1 h of the wound treatment (Fig. 2B). These three PW220:gusA T1 transgenic lines were further selected for histochemical staining of GUS enzyme at different early time points (0, 15, 30, and 60 min) after mechanical wounding. Histochemical GUS staining revealed maximum and specifically wound localized expression of GUS after 30 min, whereas GUS activity was not observed in non-transformed control plants (Fig. 2C). T1 transgenic line no. 9 showed strong wound-inducible GUS expression with no background among all the lines evaluated. However, T1 transgenic line no. 33 showed strong expression of reporter gene at wounded places with some non-specific stains (in the vasculature), which could be possibly the accumulation of excess precipitates (Fig. 2C). Also, histochemical GUS staining in all three selected T1 transgenic lines was evaluated under stereo-microscope at two different time points (15 and 30 min). A relatively stronger and specific GUS activity was observed after 30 min of wound treatment in comparison to 15 min in all the selected lines (Supplementary Fig. 3). Furthermore, GUS histochemical staining after first instar larval feeding of S. litura was also performed in both transgenic and non-transgenic fully expanded leaves. The blue color of GUS activity was detected at the site of chewing of S. litura first larval stage in T1 transgenic plant, suggesting that chewing of insects also induced the PW220 promoter activity (Fig. 2D). Thus, these results validate that promoter PW220 has wound-inducible activity during the early time point of wounding or chewing effect of insect attack.
PW220 promoter is comparable to the constitutive CaMV 35S promoter for insecticidal protein activity
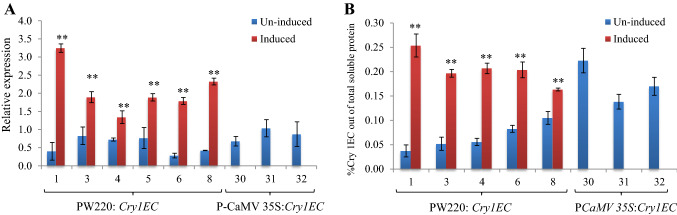
To evaluate the expression of insecticidal protein driven by PW220 promoter and CaMV 35S promoter, we cloned the insecticidal Cry1EC gene with PW220 and CaMV 35S promoters in pBI101 and developed several independent tobacco transgenic lines (Supplementary Fig. 2B and 2C). Primarily, the Cry1EC gene expression was estimated in PW220:Cry1EC and PCaMV 35S:Cry1EC tobacco transgenic plants. All T1 transgenic plants PW220:Cry1EC examined showed significant induction in Cry1EC gene after 30 min wound leaf as compared to un-wounded plant samples (Fig. 3A). The fold induction among transgenic lines PW220:Cry1EC ranges from 1.8- to 8.1-fold as compared to the un-induced plant samples. However, analysis of Cry1EC expression in PCaMV 35S:Cry1EC transgenic lines showed a little higher level than an un-induced condition of PW220:Cry1EC transgenic lines (Fig. 3A). The condition of wound induction was not applied in PCaMV 35S:Cry1EC transgenic plants as the promoter is known to express constitutively regardless of any induction, stage, or tissue (Kumar et al. 2009; Kay et al. 1987).
Fig. 3.
Comparative expression analysis of wound-inducible PW220 and constitutive PCaMV 35S promoters in the transgenic plants. A The qRT-PCR of Cry1EC transcripts in PW220:Cry1EC and PCaMV 35S:Cry1EC T1 transgenic plants after 30 min of the wound treatment. The values above the bars indicate the SE from the means of three independent experiments. The mark symbols denote **p ≤ 0.05. B The ELISA-based estimation of Cry1EC protein in T1 transgenic tobacco plants. A one-tailed t test was applied on the replicates of data to determine the statistically significant differences among un-induced versus induced expression of the protein in transgenic plants. Highly significant differences **p ≤ 0.005 in expression, among the lines evaluated were marked as a double star
Next, the DAC-ELISA-based estimation of Cry1EC protein was carried out in five PW220:Cry1EC and three PCaMV 35S:Cry1EC lines of T1 transgenic plants. The Cry1EC protein in plant samples was estimated in terms of percent Cry1EC out of total soluble protein by DAC-ELISA using purified Cry1EC protein dilutions as a standard (Fig. 3B). The wound-induced Cry1EC protein activity in PW220:Cry1EC after 30 min of wounding ranges from 0.16 to 0.25% of total soluble protein. The wound induction of Cry1EC protein in PW220-derived transgenic lines ranges from 1.6 to 8.3 folds as compared to the un-induced plant. While in the case of constitutive the Cry1EC transgenic lines, 0.13% to 0.22% of total soluble protein of Cry1EC was accumulated (Fig. 3B). These data revealed that the wound-induced Cry1EC protein activity was approximately equivalent to constitutively Cry1EC expressing lines. The Cry1EC protein activity in transgenic line 1 of PW220:Cry1EC plant was found highest among all the lines studied with a minimal level of un-induced expression. The results suggested that PW220 wound-induced expression of the Cry1EC protein was better as compared to the best constitutive expressing line.
Insect bioassay was further performed to examine the insecticidal activity by feeding first instar larvae on a fully expanded detached leaf for both wound-inducible and constitutively Cry1EC expressing lines. Leaf area damaged by larval feeding and % mortality of insect larvae was calculated at five different time points (6, 12, 18, 24, and 48 h) after insect’s release (Fig. 4A). Leaf samples of the transgenic plants with constitutive (PCaMV 35S:Cry1EC) or wound-inducible promoter (PW220:Cry1EC) were found protected as compared to the non-transformed plant. Complete (100%) first instar larval mortality was achieved within 48 h of insect release in all transgenic plants, while significant leaf area damage was found in non-transformed leaf samples (Fig. 4A). The first instar larval mortality dynamics were established at different time intervals on both types of plants and are shown in Fig. 4B. The mortality percentage of the first instar larvae was observed similarly in both PCaMV 35S:Cry1EC and PW220:Cry1EC transgenic plants during the insect bioassay (Fig. 4B).
Fig. 4.
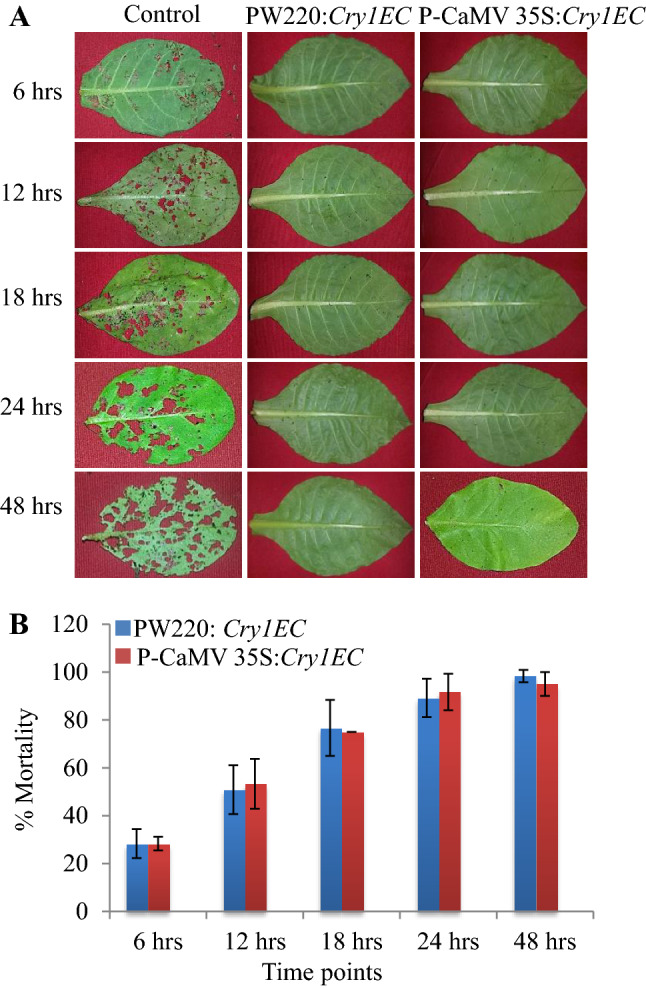
Comparative first instar insect mortality analysis of wound-inducible PW220 and constitutive PCaMV 35S promoters in the transgenic plants. A Insect bioassay with first instar larvae of the S. litura at 6, 12, 18, 24, and 48 h. B Mortality assay of the first instar larvae of the S. litura at 6, 12, 18, 24, and 48 h. The values above the bars indicate the SD from the means of three independent experiments
Detached leaf insect bioassay was also executed with third instar larvae of S. litura using T1 transgenic lines. The third instar larvae are the voracious eaters and chew a single fully expanded leaf of tobacco within 12 h of release. Detached leaf samples of both constitutive (PCaMV 35S:Cry1EC) or wound-inducible promoter (PW220:Cry1EC) lines were observed protected with minimal leaf area damage in comparison to non-transformed control (Fig. 5A). However, Complete (100%) mortality was achieved in both of the transgenic lines within 72 h of release, while during the assay period 3 to 4 control leaves were fully chewed by insects (Fig. 5A). The third instar larval mortality was recorded at three different time points of insect release are plotted as graphical presentation in Fig. 5B. The mortality dynamics of third instar larval insecticidal behavior in both types of transgenic plants was observed to be similar among evaluated lines (Fig. 5B).
Fig. 5.
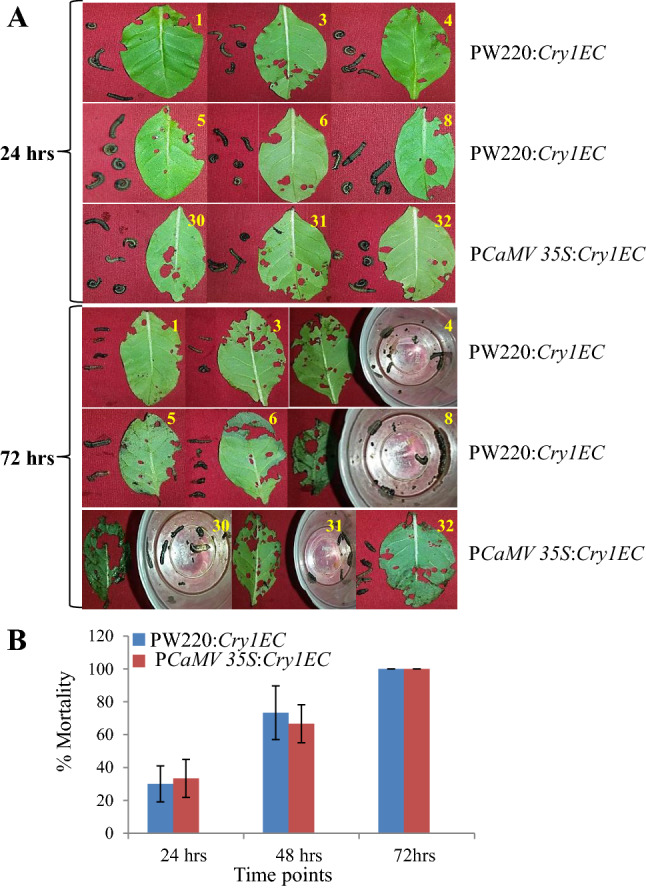
Comparative third instar insect mortality analysis of wound-inducible PW220 and constitutive PCaMV 35S promoters in the transgenic plants. A Insect bioassay with third instar larvae of the S. litura at 24 and 72 h. B Mortality assay of the third instar larvae of the S. litura at 24, 48, and 72 h. The values above the bars indicate the SD from the means of three independent experiments
Stress hormones influence the cis-regulatory motif of the wound-inducible PW220 promoter
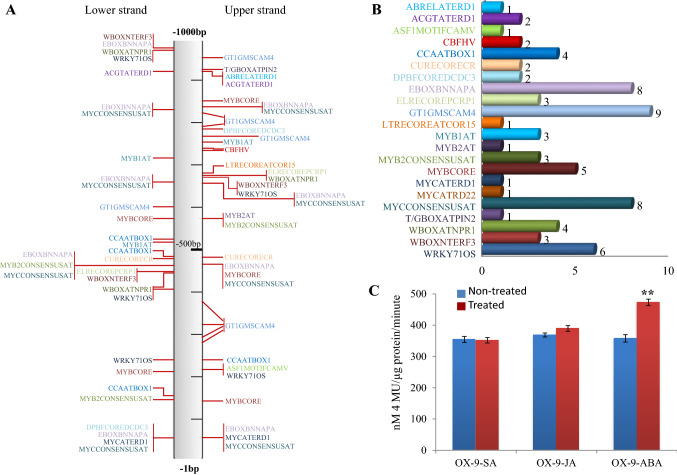
We were further interested in understanding the regulation of the PW220 promoter under influence of stress hormones. Therefore, the conserved cis-regulatory presence in the PW220 promoter was identified. In-silico identification of the cis-acting regulatory elements in the selected PW220 promoter has been carried out using the PLACE database (Higo et al. 1999). The 71 cis-acting regulatory DNA elements were identified as having a role in biotic and abiotic stress responses in the PW220 promoter sequence (Fig. 6A, Supplementary Table 3). Several different cis-acting dehydration and ABA-responsive elements, including DPBFCOREDCDC-3, MYB1AT, MYBCORE, MYCCONSENSUSAT, WBOXATNPR1, and WRKY710S, are found on 1000 bp promoter sequence in varying numbers. Around 69% of the total cis-acting elements are associated with dehydration and ABA-responsiveness (Fig. 6A, B) (Abe et al. 1997, 2003). To examine the effect of different stress hormone responses due to the presence of cis-regulatory elements in PW220 promoter, leaves were exogenously treated with jasmonic acid (JA, 100 µM), salicylic acid (SA, 1 mM), and abscisic acid (ABA, 100 µM). The GUS protein estimation was carried out after 30 min of JA, SA, and ABA hormonal treatments in the PW220:gusA T1 transgenic plants. ABA treatment significantly induced GUS protein expression in the PW220 transgenic plants after 30 min (Fig. 6C). However, no statistically significant GUS activity was observed in JA- and SA-treated transgenic plants (Fig. 6C). Moreover, this result complemented the findings of in-silico identification of cis-acting elements and revealed the association with ABA and dehydration responses.
Fig. 6.
Influence wound-induced gene expression by cis-acting elements present on the PW220. A The cis-acting elements site present in PW220 promoter identified from PLACE database. B The number of different cis-acting elements present in PW220 promoter. C Influence of different stress hormones on the expression of PW220:gusA in T1 transgenic tobacco plants. The values above the bars indicate the SD from the means of three independent experiments. The mark symbols denote **p ≤ 0.05
Discussion
In the present study, the early wound-inducible genes were identified by analyzing publically available dataset profile GSE5627. This dataset profile GSE5627 revealed that wound affects the global gene expression of several processes including plant growth and development, transcription, cell cycle, and other signaling and metabolic pathway (Kilian et al. 2007). We finally identified the early and highly wound- and insect-inducible promoter PW220, which regulates a protein JAS1 (AT5G13220), a member of the JAZ (JASMONATE ZIM-domain) proteins acting as a jasmonate (JA) signaling repressors (Chung and Howe 2009). The regulated and inducible transgene expression has significant advantages in the development of insect-resistant plants. Several transgenic plants with elevated resistance to pests and disease agents are under commercial agricultural practices. However, in most cases, constitutive promoters have been used successfully to express a variety of transgenes for developing transgenic plants, which are known to always be expressed at high levels even in the absence of induced signals. Moreover, the constant production and high accumulation of transgene products interfere with plants' metabolism, growth, and development and growth. In addition, protein accumulation is not compulsory for plant development irrelevantly turn away from the cellular resources of the plant, which can put a yield penalty to the plant to compromise its health (Chakrabarti et al. 2006), environmental magnification (Saxena et al. 2004), and undesirably targets beneficial insects. The unregulated or constitutive expression of insecticidal proteins has the risk of exposing non-target organisms (Hilbeck and Schmidt 2006; Prütz and Dettner 2004; Chen et al. 2008; Baur and Boethel 2003; Liu et al. 2005). Constitutive expression of insecticidal genes raises long-term safety concerns in the consumption of edible plant parts (Heinemann and Traavik 2004; Séralini et al. 2007; Kılıç and Akay 2008; Finamore et al. 2008). Also, the use of natural constitutive plant promoters may potentially cause transgene silencing. In contrast, the use of plant-derived inducible promoters has distinct advantages of being triggered by pathogens invasion. The early induction of protein after wound or insect bite makes sure availability of good enough toxin to kill the pest at the attacked site before pest consumes most of the photosynthetic area (defoliation). The most desirable and safe strategy is the expression of the transgene in genetically modified crops which must be limited to the tissues needing the programmed action. A different resistance/tolerance management exercise, therefore, needs be to use in temporal protections where pest tolerance/resistance genes could be expressed under the tight regulation of an inducible promoter that generates toxins only when they are required at a particular specific site (Gould 1998).
The subtractive hybridization, differential display technologies. Macro/microarrays, genome sequences, and most recently transcriptome sequences (differential gene expression profiles under various treatments) have opened new alleys toward a better understanding of plant–insect interactions at the transcriptomic level. Accumulating studies suggested that different plant and insect interactions have been studied using microarray technology, for example, Arabidopsis infested with aphid, whitefly, wound and chewing, cucumber (Cucumis sativus) with mites, and tomato (Lycopersicon esculentum) with mites (Kant et al. 2004; Kempema et al. 2007; Mercke et al. 2004; Moran et al. 2002; De Vos et al. 2005). In particular, the induced promoter PW220 will be a better alternative to the CaMV 35S promoter, because the wound-inducible gene expression is observed (in promoter-reporter fusion construct lines) around mechanical wounds only, with no expression in un-induced parts (Fig. 2). The wound-inducible transgenic lines, PW220 promoter-driven expression CryI EC was observed to be similar to constitutive promoter regulation in terms of mortality and percent expression of the insecticidal protein. Notably, it is practically hard to monitor the toxicity in the insects (in terms of mortality) after such a short time of induction like 15–30 min, because insects recover successfully with no significant mortality or weight loss on non-transformed plants after such a short feeding on transgenic plants. However, wound-inducible expression and protein activity at early time points were clearly observed in the case of PW220 promoter Cry1EC transgenic plants (Fig. 3).
The expression of insecticidal proteins in a tissue-specific and insect-inducible manner is supposed to be a safer and eco-friendly policy for transgenic development. Phloem-specific promoters like Commelina yellow mottle virus (Matsuda et al. 2002), Maize sucrose synthase-1 (Yang and Russell 1990), and Pumpkin (Cucurbita moschata) PP2 gene (Guo et al. 2004) are reported to express proteins in targeted tissues. Similarly, wound-inducible synthetic promoters developed by fusion of natural minimal promoter with multimers of defined regulatory elements are reported to govern pathogen inducible expression in Arabidopsis (Rushton et al. 2002) and wound/insect-inducible promoters of potato proteinase inhibitor II are also reported (Godard et al. 2007). Phloem-specific expression of insecticidal proteins under rolC and RSs1 promoters is also reported for the management of sap-sucking insects (Saha et al. 2007). Although these promoters are tissue-specific (exclusively expressed in a particular tissue), but leaky or background expression makes them constitutive in nature and not suitable for insecticidal protein expression.
The wound is a probable site for infection of the pathogens; thus, defense-related gene expression at the infestation site of the wound works as an obstacle against harmful microbes and pests. Plants respond to the wound by the induction of several defense/stress genes. The first report of plant wound-inducible defense-related genes was proteinase inhibitors I and II from tomato and potato (Graham et al. 1986; Ryan 1990). Regulated expression at the site and time of wound would be a safer strategy to minimize the risks related to constitutive expression of insecticidal proteins. Identification of novel wound promoters with the ability of expression after the early time point of wounding would enrich our toolbox for the expression of insecticidal proteins against pests with different feeding behavior dynamics. Physical wounding is frequently considered to imitate insect infestation as it is easy to regulate the treatment. The cis-regulatory elements investigation in the wound-inducible promoters reveals the identification of several motifs. Rushton et al. (2002) confirmed that these motifs considered a wound-induced expression by evaluating synthetic promoters comprising the defined regulatory elements in Arabidopsis (Rushton et al. 2002). They indicated that four copies of the S, DRE, GCC, JERE, W, and GST motifs fused with the minimal 35S promoter insured wound-induced GUS expression. Moreover, promoter of jasmonic acid (JA) inducible genes also contain motifs such as G-box, GCC-box, H-box, and TGA, which is commonly related to the wound (Rouster et al. 1997; Nishiuchi et al. 2004; Mason et al. 1993; Takeda et al. 1999). The regulatory element W-box has also been observed in many wound-inducible gene promoters (Hara et al. 2000).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Devesh Kumar Mishra obliged to the CSIR and Bhoopendra K. Pandey obligated for ICMR for their fellowship support. We thank Dr. P. K. Singh (CSIR-NBRI) for providing the construct of Cry1-EC.
Funding
This work was supported by grants from the Council of Scientific and Industrial Research (CSIR), Government of India, under the institutional OLP0079 project. Institute Manuscript Number is CSIR-NBRI_MS/2021/12/03.
Declarations
Conflict of interest
The authors declared no conflict of interest concerning the authorship and publication of this article.
References
- Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. Role of Arabidopsis MYC and MYB homologs in drought-and abscisic acid-regulated gene expression. Plant Cell. 1997;9(10):1859–1868. doi: 10.1105/tpc.9.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell. 2003;15(1):63–78. doi: 10.1105/tpc.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur ME, Boethel DJ. Effect of Bt-cotton expressing Cry1A(c) on the survival and fecundity of two hymenopteran parasitoids (Braconidae, Encyrtidae) in the laboratory. Biol Control. 2003;26(3):325–332. doi: 10.1016/S1049-9644(02)00160-3. [DOI] [Google Scholar]
- Chakrabarti SK, Lutz KA, Lertwiriyawong B, Svab Z, Maliga P. Expression of the cry9Aa2 Bt gene in tobacco chloroplasts confers resistance to potato tuber moth. Transgenic Res. 2006;15(4):481. doi: 10.1007/s11248-006-0018-z. [DOI] [PubMed] [Google Scholar]
- Chaturvedi CP, Lodhi N, Ansari SA, Tiwari S, Srivastava R, Sawant SV, Tuli R. Mutated TATA-box/TATA binding protein complementation system for regulated transgene expression in tobacco. Plant J. 2007;50(5):917–925. doi: 10.1111/j.1365-313X.2007.03089.x. [DOI] [PubMed] [Google Scholar]
- Chen M, Zhao J-Z, Collins HL, Earle ED, Cao J, Shelton AM. A critical assessment of the effects of Bt transgenic plants on parasitoids. PLoS ONE. 2008;3(5):e2284. doi: 10.1371/journal.pone.0002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Howe GA. A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell. 2009;21(1):131–145. doi: 10.1105/tpc.108.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Van Oosten VR, Van Poecke RMP, Van Pelt JA, Pozo MJ, Mueller MJ, Buchala AJ, Métraux J-P, Van Loon LC, Dicke M, Pieterse CMJ. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microbe Interact. 2005;18(9):923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- Ferré J, Real MD, Van Rie J, Jansens S, Peferoen M. Resistance to the Bacillus thuringiensis bioinsecticide in a field population of Plutella xylostella is due to a change in a midgut membrane receptor. Proc Natl Acad Sci USA. 1991;88(12):5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finamore A, Roselli M, Britti S, Monastra G, Ambra R, Turrini A, Mengheri E. Intestinal and peripheral immune response to MON810 maize ingestion in weaning and old mice. J Agric Food Chem. 2008;56(23):11533–11539. doi: 10.1021/jf802059w. [DOI] [PubMed] [Google Scholar]
- Godard K-A, Byun-McKay A, Levasseur C, Plant A, Séguin A, Bohlmann J. Testing of a heterologous, wound-and insect-inducible promoter for functional genomics studies in conifer defense. Plant Cell Rep. 2007;26(12):2083–2090. doi: 10.1007/s00299-007-0417-5. [DOI] [PubMed] [Google Scholar]
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol. 1998;43(1):701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- Graham JS, Hall G, Pearce G, Ryan CA. Regulation of synthesis of proteinase inhibitors I and II mRNAs in leaves of wounded tomato plants. Planta. 1986;169(3):399–405. doi: 10.1007/BF00392137. [DOI] [PubMed] [Google Scholar]
- Guo H, Chen X, Zhang H, Fang R, Yuan Z, Zhang Z, Tian Y. Characterization and activity enhancement of the phloem-specific pumpkin PP2 gene promoter. Transgenic Res. 2004;13(6):559–566. doi: 10.1007/s11248-004-2738-2. [DOI] [PubMed] [Google Scholar]
- Hara K, Yagi M, Kusano T, Sano H. Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol Gen Genet MGG. 2000;263(1):30–37. doi: 10.1007/PL00008673. [DOI] [PubMed] [Google Scholar]
- Heinemann JA, Traavik T. Problems in monitoring horizontal gene transfer in field trials of transgenic plants. Nat Biotechnol. 2004;22(9):1105–1109. doi: 10.1038/nbt1009. [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Higo H. PLACE: a database of plant cis-acting regulatory DNA elements. Nucleic Acids Res. 1998;26(1):358–359. doi: 10.1093/nar/26.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999;27(1):297–300. doi: 10.1093/nar/27.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbeck A, Schmidt JE. Another view on Bt proteins—how specific are they and what else might they do. Biopestic Int. 2006;2(1):1–50. [Google Scholar]
- Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5(4):387–405. doi: 10.1007/BF02667740. [DOI] [Google Scholar]
- Kant MR, Ament K, Sabelis MW, Haring MA, Schuurink RC. Differential timing of spider mite-induced direct and indirect defenses in tomato plants. Plant Physiol. 2004;135(1):483–495. doi: 10.1104/pp.103.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Hayakawa Y, Hyodo H, Ikoma Y, Yano M. Wound-induced ethylene synthesis and expression and formation of 1-aminocyclopropane-1-carboxylate (ACC) synthase, ACC oxidase, phenylalanine ammonia-lyase, and peroxidase in wounded mesocarp tissue of Cucurbita maxima. Plant Cell Physiol. 2000;41(4):440–447. doi: 10.1093/pcp/41.4.440. [DOI] [PubMed] [Google Scholar]
- Kay R, Chan A, Daly M, McPherson J. Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science. 1987;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- Kempema LA, Cui X, Holzer FM, Walling LL. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol. 2007;143(2):849–865. doi: 10.1104/pp.106.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT. Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol. 2002;53(1):299–328. doi: 10.1146/annurev.arplant.53.100301.135207. [DOI] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D'Angelo C, Bornberg-Bauer E, Kudla J, Harter K. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J. 2007;50(2):347–363. doi: 10.1111/j.1365-313X.2007.03052.x. [DOI] [PubMed] [Google Scholar]
- Kılıç A, Akay MT. A three generation study with genetically modified Bt corn in rats: biochemical and histopathological investigation. Food Chem Toxicol. 2008;46(3):1164–1170. doi: 10.1016/j.fct.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Kiran K, Ansari SA, Srivastava R, Lodhi N, Chaturvedi CP, Sawant SV, Tuli R. The TATA-box sequence in the basal promoter contributes to determining light-dependent gene expression in plants. Plant Physiol. 2006;142(1):364–376. doi: 10.1104/pp.106.084319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Shukla AK, Singh H, Tuli R. Development of insect resistant transgenic cotton lines expressing cry1EC gene from an insect bite and wound inducible promoter. J Biotechnol. 2009;140(3–4):143–148. doi: 10.1016/j.jbiotec.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang Q, Zhao J-Z, Cai Q, Xu H, Li J. Effects of the Cry1Ac toxin of Bacillus thuringiensis on Microplitis mediator, a parasitoid of the cotton bollworm, Helicoverpa armigera. Entomol Exp Appl. 2005;114(3):205–213. doi: 10.1111/j.1570-7458.2005.00248.x. [DOI] [Google Scholar]
- Lodhi N, Ranjan A, Singh M, Srivastava R, Singh SP, Chaturvedi CP, Ansari SA, Sawant SV, Tuli R. Interactions between upstream and core promoter sequences determine gene expression and nucleosome positioning in tobacco PR-1a promoter. Biochim Biophys Acta (BBA) Gene Regul Mechanisms. 2008;1779(10):634–644. doi: 10.1016/j.bbagrm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Mason HS, DeWald DB, Mullet JE. Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell. 1993;5(3):241–251. doi: 10.1105/tpc.5.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Liang G, Zhu Y, Ma F, Nelson RS, Ding B. The Commelina yellow mottle virus promoter drives companion-cell-specific gene expression in multiple organs of transgenic tobacco. Protoplasma. 2002;220(1):51–58. doi: 10.1007/s00709-002-0027-6. [DOI] [PubMed] [Google Scholar]
- Mercke P, Kappers IF, Verstappen FW, Vorst O, Dicke M, Bouwmeester HJ. Combined transcript and metabolite analysis reveals genes involved in spider mite induced volatile formation in cucumber plants. Plant Physiol. 2004;135(4):2012–2024. doi: 10.1104/pp.104.048116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithofer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008;146(3):825–831. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran PJ, Cheng Y, Cassell JL, Thompson GA. Gene expression profiling of Arabidopsis thaliana in compatible plant-aphid interactions. Arch Insect Biochem Physiol. 2002;51(4):182–203. doi: 10.1002/arch.10064. [DOI] [PubMed] [Google Scholar]
- Nishiuchi T, Shinshi H, Suzuki K. Rapid and transient activation of transcription of the ERF3 Gene by Wounding in tobacco leaves possible involvement OF NtWRKYs AND autorepression. J Biol Chem. 2004;279(53):55355–55361. doi: 10.1074/jbc.M409674200. [DOI] [PubMed] [Google Scholar]
- Pandey B, Prakash P, Chandra Verma P, Srivastava R. Current developments in biotechnology and bioengineering. Amsterdam: Elsevier; 2019. Regulated gene expression by synthetic modulation of the promoter architecture in plants; pp. 235–255. [Google Scholar]
- Prütz G, Dettner K. Effect of Bt corn leaf suspension on food consumption by Chilo partellus and life history parameters of its parasitoid Cotesia flavipes under laboratory conditions. Entomol Exp Appl. 2004;111(3):179–187. doi: 10.1111/j.0013-8703.2004.00166.x. [DOI] [Google Scholar]
- Rouster J, Leah R, Mundy J, Cameron-Mills V. Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain. Plant J. 1997;11(3):513–523. doi: 10.1046/j.1365-313X.1997.11030513.x. [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Reinstädler A, Lipka V, Lippok B, Somssich IE. Synthetic plant promoters containing defined regulatory elements provide novel insights into pathogen-and wound-induced signaling. Plant Cell. 2002;14(4):749–762. doi: 10.1105/tpc.010412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA. Protease inhibitors in plants: genes for improving defenses against insects and pathogens. Annu Rev Phytopathol. 1990;28(1):425–449. doi: 10.1146/annurev.py.28.090190.002233. [DOI] [Google Scholar]
- Saha P, Chakraborti D, Sarkar A, Dutta I, Basu D, Das S. Characterization of vascular-specific RSs1 and rolC promoters for their utilization in engineering plants to develop resistance against hemipteran insect pests. Planta. 2007;226(2):429–442. doi: 10.1007/s00425-007-0493-3. [DOI] [PubMed] [Google Scholar]
- Savatin DV, Gramegna G, Modesti V, Cervone F. Wounding in the plant tissue: the defense of a dangerous passage. Front Plant Sci. 2014;5:470. doi: 10.3389/fpls.2014.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena D, Stewart CN, Altosaar I, Shu Q, Stotzky G. Larvicidal Cry proteins from Bacillus thuringiensis are released in root exudates of transgenic B. thuringiensis corn, potato, and rice but not of B. thuringiensis canola, cotton, and tobacco. Plant Physiol Biochem. 2004;42(5):383–387. doi: 10.1016/j.plaphy.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Séralini G-E, Cellier D, de Vendomois JS. New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Arch Environ Contam Toxicol. 2007;52(4):596–602. doi: 10.1007/s00244-006-0149-5. [DOI] [PubMed] [Google Scholar]
- Singh SP, Pandey T, Srivastava R, Verma PC, Singh PK, Tuli R, Sawant SV. BECLIN1 from Arabidopsis thaliana under the generic control of regulated expression systems, a strategy for developing male sterile plants. Plant Biotechnol J. 2010;8(9):1005–1022. doi: 10.1111/j.1467-7652.2010.00527.x. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Rai KM, Srivastava M, Kumar V, Pandey B, Singh SP, Bag SK, Singh BD, Tuli R, Sawant SV. Distinct role of core promoter architecture in regulation of light-mediated responses in plant genes. Mol Plant. 2014;7(4):626–641. doi: 10.1093/mp/sst146. [DOI] [PubMed] [Google Scholar]
- Srivastava R, Srivastava R, Singh UM (2014b) Understanding the patterns of gene expression during climate change. In: Climate change effect on crop productivity. CRC Press, Taylor & Francis Group, Print ISBN: 978-1-4822-2920-2 eBook ISBN, pp 279–328
- Srivastava R, Rai KM, Srivastava R. Biosynthetic technology and environmental challenges. Singapore: Springer Singapore; 2018. Plant biosynthetic engineering through transcription regulation: an insight into molecular mechanisms during environmental stress; pp. 51–72. [Google Scholar]
- Tabashnik BE, Huang F, Ghimire MN, Leonard BR, Siegfried BD, Rangasamy M, Yang Y, Wu Y, Gahan LJ, Heckel DG, Bravo A, Soberón M. Efficacy of genetically modified Bt toxins against insects with different genetic mechanisms of resistance. Nat Biotechnol. 2011;29(12):1128–1131. doi: 10.1038/nbt.1988. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sugimoto K, Otsuki H, Hirochika H. A 13-bp cis-regulatory element in the LTR promoter of the tobacco retrotransposon Tto1 is involved in responsiveness to tissue culture, wounding, methyl jasmonate and fungal elicitors. Plant J. 1999;18(4):383–393. doi: 10.1046/j.1365-313X.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- Wei J, Wang L, Zhu J, Zhang S, Nandi OI, Kang L. Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE. 2007;2(9):e852. doi: 10.1371/journal.pone.0000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Russell D. Maize sucrose synthase-1 promoter directs phloem cell-specific expression of Gus gene in transgenic tobacco plants. Proc Natl Acad Sci. 1990;87(11):4144–4148. doi: 10.1073/pnas.87.11.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu W, Li Z, Deng XW, Wu W, Xue Y. F-box protein DOR functions as a novel inhibitory factor for abscisic acid-induced stomatal closure under drought stress in Arabidopsis. Plant Physiol. 2008;148(4):2121–2133. doi: 10.1104/pp.108.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Xi DH, Yuan S, Xu F, Zhang DW, Lin HH. Salicylic acid and jasmonic acid are essential for systemic resistance against tobacco mosaic virus in Nicotiana benthamiana. Mol Plant Microbe Interact. 2014;27(6):567–577. doi: 10.1094/MPMI-11-13-0349-R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.