A current challenge in the fight against the SARS-CoV-2 virus is the assessment of the long-term effectiveness of vaccination, in particular against the variants of concern and in vulnerable populations. One of the key questions is whether biomarkers of humoral and cellular immunities are reliable surrogates for the clinical effectiveness of vaccination, that is, protection against SARS-CoV-2 infection, COVID-19, and mortality. In the general population, a significant waning of antibody levels within 6 months of vaccination was observed.1 Protection against SARS-CoV-2 infection decreased rapidly within 6 months of vaccination, but protection against hospitalization and death persisted at robust levels.2 The latter data were generated in Qatar from January 2021 to September 2021 when the B.1.351 (beta) and B.1.617.2 (delta) variants were dominant. A study in health care workers revealed lower peak and peri-infection neutralizing antibody titers in individuals with breakthrough infections, 85% of which were caused by the B.1.1.7 (alpha) variant, as compared with noninfected matched controls.3 In the dialysis population, data are currently limited. A gradual waning of antibody levels was observed 2 to 3 months4 and 6 months5 after vaccination, but direct comparisons with the decay trajectory in healthy volunteers have not been made. In addition, immunologic characteristics that may predict breakthrough infections in dialysis patients have not been identified.
In this prospective multicenter study, we assessed the longevity of the humoral and cellular immune responses to BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) vaccination in a large cohort of hemodialysis patients and in COVID-19–naive healthy volunteers and analyzed predictors of breakthrough infections. The short methods are described in the Supplementary Methods.6,S1–S3
Results
A total of 569 hemodialysis patients and 76 healthy volunteers were included (Supplementary Figure S1). After exclusion of dropouts, missing samples, and breakthrough infections, the final analysis of the humoral and cellular immune responses at 24 weeks was conducted in 492 hemodialysis patients, of whom 436 were COVID-19 naive, and in 75 COVID-19–naive healthy volunteers (Supplementary Table S1).
At 24 weeks after vaccination, antibody geometric mean titer and QuantiFERON geometric mean concentration were substantially lower in COVID-19–naive hemodialysis patients than in COVID-19–naive healthy volunteers. The geometric mean titer and geometric mean concentration were markedly higher in COVID-19–experienced than in COVID-19–naive hemodialysis patients at all time points (Table 1, Supplementary Table S2, and Supplementary Figure S2).
Table 1.
Humoral and cellular immune responses at 24 weeks
| Hemodialysis n = 492) |
Healthy volunteers (n = 75) |
|||||
|---|---|---|---|---|---|---|
| BNT162b2 recipients (n = 289) | mRNA-1273 recipients (n = 203) | P | BNT162b2 recipients (n = 37) | mRNA-1273 recipients (n = 38) | P | |
| Humoral response | ||||||
| GMT (95% CI), AU/ml | ||||||
| Overall | 323 (262–399) | 906 (719–1141) | <0.001 | 1521 (1241–1863) | 4046 (3167–5168) | <0.001 |
| COVID-19 naive | 226 (190–268) | 702 (565–871) | <0.001 | 1521 (1241–1863) | 4046 (3167–5168) | <0.001 |
| COVID-19 experienced | 5220 (2339–11,649) | 6671 (3183–13,982) | 0.676 | — | — | — |
| %>3560 AU | ||||||
| Overall, % | 9.0 | 20.2 | 0.0005 | 10.8 | 60.5 | <0.001 |
| COVID-19 naïve, % | 2.0 | 13.3 | <0.001 | 10.8 | 60.5 | <0.001 |
| COVID-19 experienced, % | 63.6 | 73.9 | 0.563 | — | — | — |
| Cellular response (Ag2a) | ||||||
| GMC (95% CI), IU/mla | ||||||
| Overall | 0.126 (0.101–0.156) | 0.157 (0.124–0.198) | 0.181 | 0.134 (0.097–0.185) | 0.301 (0.197–0.459) | 0.0041 |
| COVID-19 naive | 0.106 (0.085–0.133) | 0.136 (0.107–0.172) | 0.147 | 0.134 (0.097–0.185) | 0.301 (0.197–0.459) | 0.0041 |
| COVID-19 experienced | 0.447 (0.224–0.893) | 0.464 (0.207–1.038) | 0.947 | — | — | — |
| % ≥0.15 IU/ml | ||||||
| Overall, % | 47.2 | 49.8 | 0.643 | 43.2 | 71.1 | 0.020 |
| COVID-19 naive, % | 43.5 | 47.7 | 0.429 | 43.2 | 71.1 | 0.020 |
| COVID-19 experienced, % | 75.8 | 65.2 | 0.549 | — | — | — |
AU, Abbott unit; GMC, geometric mean concentration; GMT, geometric mean titer.
Results for Ag1 are similar.
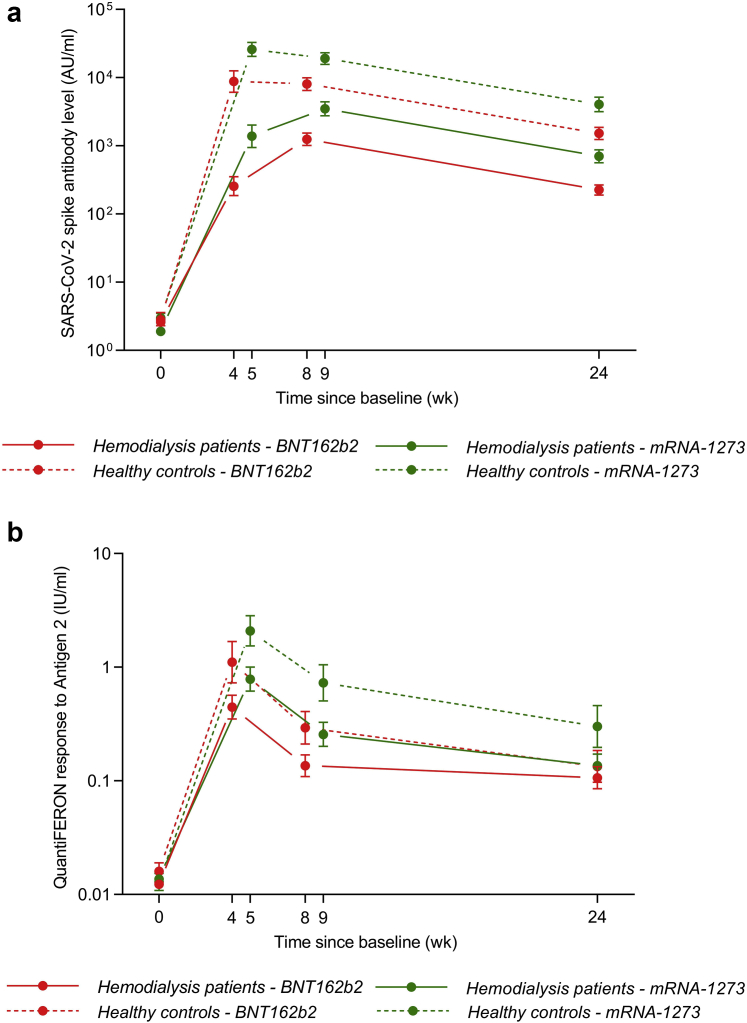
The serologic responses elicited by the mRNA-1273 vaccine were significantly greater than those induced by the BNT162b2 vaccine in hemodialysis patients and healthy volunteers at all time points (Figure 1a and Table 1). Similarly, the cellular response was greater in mRNA-1273 recipients than in BNT162b2 recipients from the control group at all time points. However, the superior effect of the mRNA-1273 vaccine on the cellular response was lost at 24 weeks in hemodialysis patients (Figure 1b and Table 1). In a separate analysis of the COVID-19–experienced hemodialysis patients, the immunogenicity of the BNT162b2 and mRNA-1273 vaccines was not significantly different (Table 1 and Supplementary Figure S2).
Figure 1.
Humoral and cellular immune responses. (a) Geometric mean titers (95% CIs) of the SARS-CoV-2 spike antibody after vaccination in COVID-19–naive patients: hemodialysis BNT162b2 recipients (solid red line), healthy BNT162b2 recipients (dashed red line), hemodialysis mRNA-1273 recipients (solid green line), and healthy mRNA-1273 recipients (dashed green line). (b) Geometric mean concentrations (95% CIs) of the QuantiFERON response to antigen 2 after vaccination in COVID-19–naive patients: hemodialysis BNT162b2 recipients (solid red line), healthy BNT162b2 recipients (dashed red line), hemodialysis mRNA-1273 recipients (solid green line), and healthy mRNA-1273 recipients (dashed green line). The response to antigen 1 follows a similar course (data not found).
A multivariate analysis revealed COVID-19 experience, use of immunosuppressive drugs, serum albumin level, hepatitis B nonresponder status, and lymphocyte count as independent predictors of humoral and cellular responses at 24 weeks, while vaccine type was an independent predictor of the humoral response only (Supplementary Table S3).
The decay trajectory of the humoral response was similar in all groups, with a decline in antibody levels of around 80% between 8/9 weeks and 24 weeks. In contrast, the decline of the QuantiFERON levels was around 20% in BNT162b2 recipients on hemodialysis versus approximately 50% in the other groups (Supplementary Table S2).
In hemodialysis patients, 7 breakthrough infections were identified, 5 of which were documented by polymerase chain reaction (80% alpha, 20% delta) and 2 by appearance of anti-N antibodies at 24 weeks. All breakthrough infections occurred in COVID-19–naive BNT162b2 recipients. Furthermore, 3 patients had severe disease requiring hospitalization and 2 patients died. As compared with the matched COVID-19–naive hemodialysis patients without breakthrough infection, peak humoral response was numerically but not significantly lower in breakthrough cases (Table 2). However, cellular responses at 8/9 weeks were significantly lower in the patients with breakthrough infections than in matched noninfected patients (Table 2).
Table 2.
Immunologic responses in breakthrough infections and matched controls
| Breakthrough cases n = 7 | Matched controls n = 21 | Significancea | |
|---|---|---|---|
| Age, yr | 76.4 (49.1–79.2)a | 76.4 (51.8–79.1)a | P = 0.937 |
| Male, % | 71.4% (5) | 71.4% (15) | P = 0.999 |
| mRNA-1273 recipient, % | 0.0% (0) | 33.3% (7) | P = 0.141 |
| Dialysis vintage, yr | 2.62 (0.87–2.88)b | 3.54 (1.59–5.91)b | P = 0.160 |
| Immunosuppressive drugs, % | 0.0% (0) | 19.1% (4) | P = 0.545 |
| Hepatitis B vaccine nonresponder, % | 14.3% (1) | 10.0% (2) | P = 0.999 |
| Serum albumin, g/l | 40.2 (39.6–41.4)b | 39.8 (38.4–41.0)b | P = 0.448 |
| Ln (lymphocyte count), n/μl | 1000 (900–1300)b | 1200 (1000–1650)b | P = 0.577 |
| Humoral response at 8/9 wk | |||
| GMT (95% CI), AU/ml | 1097 (309–3892) | 1888 (756–4715) | P = 0.564 |
| % >3560 AU/ml | 28.6% (2) | 33.3% (7) | P = 0.816 |
| Cellular response (Ag2) at 8/9 wkc | |||
| GMC (95% CI), IU/ml | 0.052 (0.034–0.081) | 0.261 (0.124–0.552) | P = 0.014 |
| % ≥0.15 IU/ml | 0.0% (0) | 57.1% (12) | P = 0.013 |
AU, Abbott unit; GMC, geometric mean concentration; GMT, geometric mean titer.
According to Fisher exact test, Mann-Whitney U test, or exact conditional logistic regression analysis (mid-P value).
Median (interquartile range).
Results for Ag1 are similar.
Discussion
A large body of evidence has documented impaired short-term immune responses to SARS-CoV-2 vaccination in hemodialysis patients.7 Emerging data reveal waning antibody levels in hemodialysis patients,4,5 but comparisons with healthy volunteers were not made. In a large cohort of hemodialysis patients, we demonstrate that hemodialysis patients maintain suboptimal humoral and cellular immunities 24 weeks after vaccination as compared with healthy volunteers. The superior immunogenicity of the mRNA-1273 vaccine versus the BNT162b2 vaccine is well preserved in healthy volunteers, but gradually disappears in hemodialysis patients, such that there is no longer a difference in cellular response between mRNA-1273 and BNT162b2 recipients at 24 weeks. COVID-19 experience results in strikingly better vaccine-induced immune responses, overruling the effect of vaccine type. Other independent predictors of the immune responses were similar as those reported at 8/9 weeks6: use of immunosuppressive drugs, serum albumin level, lymphocyte count, and hepatitis B nonresponder status.
Despite these impaired immune responses, only few breakthrough infections (0.01%, 7/569) were recorded in our hemodialysis population. However, these favorable results need to be interpreted against the background of extremely high vaccination coverage among hemodialysis patients (98%) and health care workers (nearing 100%) in the participating dialysis centers, including in the general population in Flanders (80% of the entire population), resulting in low circulation of the virus among close patient contacts. In addition, data were collected between April 2021 and October, 2021, when the epidemic was slowing down, and the delta variant only became the dominant strain in early July. The risk of breakthrough infections in the dialysis population may increase substantially with greater circulation of the virus resulting from general waning of immunity, dominance of the highly contagious delta and omicron variants, and indoor activities during the winter months. The identification of immunologic predictors of breakthrough infections is therefore critical to optimize the vaccination strategy. The cellular immune responses at week 8/9 were remarkably different between breakthrough cases and matched controls. In contrast, peak antibody levels were numerically but not significantly different, most likely because of low patient numbers.
Taken together, humoral and cellular immunities to SARS-CoV-2 vaccination remain significantly lower in hemodialysis patients than in healthy volunteers 6 months after vaccination, although the overall pattern of decay is similar. Robust cellular immunity appears paramount to prevent breakthrough infections.
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors are indebted to Tessa Acke, Manuela Caster, Evelyne Deglorie, Mirjam Demesmaecker, Suzanne Driessens, Inne Hoebrekx, Annelien Leunen, Carine Lowis, Isabel Moyaert, Danny Pauwels, Joris Penders, Melissa Renders, Carmen Reynders, Sofie Tombeur, Katrien Uyttersprot, Femke Van Den Berg, Kristel Van Varenbergh, Tine Verheyen, Manon Verhulst, and Sophie Vleeschouwers for their invaluable help in the collection of the patient data and analysis of the samples. This research was supported by a grant of Amgen (DONATION-331036). The funding source had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
Footnotes
Supplementary Methods.
Supplementary References.
Table S1. Demographic and clinical characteristics at baseline.
Table S2. (A) Changes in humoral response between 8/9 and 24 weeks. (B) Changes in cellular response (Ag 2∗∗) between 8/9 and 24 weeks.
Table S3. (A) Multivariate analysis of factors associated with humoral immune response at 24 weeks. (B) Multivariate analysis of factors associated with cellular immune response at 24 weeks.
Figure S1. Study flowchart.
Figure S2. Humoral and cellular responses in hemodialysis patients.
Supplementary Material
Table S1. Demographic and clinical characteristics at baseline.
Table S2. (A) Changes in humoral response between 8/9 and 24 weeks. (B) Changes in cellular response (Ag 2∗∗) between 8/9 and 24 weeks.
Table S3. (A) Multivariate analysis of factors associated with humoral immune response at 24 weeks. (B) Multivariate analysis of factors associated with cellular immune response at 24 weeks.
Figure S1. Study flowchart.
Figure S2. Humoral and cellular responses in hemodialysis patients.
Supplementary Reference.
References
- 1.Levin E.G., Lustig Y., Cohen C., et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chemaitelly H., Tang P., Hasan M.R., et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergwerk M., Gonen T., Lustig Y., et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385:1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angel-Korman A., Peres E., Bryk G., et al. Diminished and waning immunity to COVID-19 vaccination among hemodialysis patients in Israel: the case for a third vaccine dose. Clin Kidney J. 2021;15:226–234. doi: 10.1093/ckj/sfab206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidovic T., Schimpf J., Abbassi-Nik A., et al. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: time for a boost. Kidney Int. 2021;100:1334–1335. doi: 10.1016/j.kint.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Praet J., Reynders M., De Bacquer D., et al. Predictors and dynamics of the humoral and cellular immune response to SARS-CoV-2 mRNA vaccines in hemodialysis patients: a multicenter observational study. J Am Soc Nephrol. 2021;32:3208–3220. doi: 10.1681/ASN.2021070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Karoui K., De Vriese A.S. COVID-19 in dialysis: clinical impact, immune response, prevention and treatment. Kidney Int. 2022 doi: 10.1016/j.kint.2022.01.022. , pii: S0085-2538(22)00099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.