Over the past 40 years, the most successful pharmacologic approach to cardiovascular disease prevention has been aggressive lowering of LDL cholesterol. Yet, cardiologists and vascular biologists now have hard evidence that inhibition of the NLRP3 to IL-1β to IL-6 to C-reactive protein (CRP) pathway of innate immunity is also a potent target for atheroprotection.1 This proof-of-principle derives from the 10 000 participant multi-national CANTOS trial presented in 2017 in which a monoclonal antibody targeting IL-1β significantly reduced recurrent major adverse cardiovascular events by 15–17%2 with the benefit directly related to the magnitude of reduction in downstream IL-6 and CRP.3,4 Of considerable importance, the clinical benefit observed within CANTOS was of the same magnitude as that observed in trials of PCSK9 inhibition, yet was achieved in the absence of any reduction in LDL cholesterol.
Directly targeting IL-6 itself has thus rapidly become a priority for the atherosclerosis research community.5 This hypothesis has garnered considerable support from Mendelian randomization studies which suggest that genetic variants in the IL-6 signalling pathway associate with lifelong coronary risk,6,7 from phenome-wide association studies implicating IL-6 in multiple forms of atherosclerotic disease,8 and from murine models in which genetically modified mice with high IL-6 levels show increased susceptibility to atherogenesis yet also demonstrate fewer plaque lesions when pre-treated with an anti-mouse IL-6 receptor antagonist.9 All of these findings, and others, support the likelihood that lowering IL-6 activity will reduce incidence rates not just of myocardial infarction and stroke, but also of abdominal aortic aneurysm, post-PCI complications, and perhaps peripheral arterial disease. Other data implicate the IL-1β to IL-6 pathway in congestive heart failure10 and acute coronary ischaemia.11
Despite this promise, the safety and efficacy of IL-6 inhibition among individuals at high atherosclerotic risk have been uncertain. The recent phase II RESCUE trial addressed this issue with ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand and that is being specifically developed for atherothrombotic disease.12 RESCUE focused on individuals with elevated levels of high-sensitivity C-reactive protein (hsCRP) and chronic kidney disease (CKD), a group with substantial cardiovascular risk and unmet clinical need for whom alternative proven anti-inflammatory agents such as renally excreted colchicine are contraindicated. IL-6 levels are also a powerful predictor of incident vascular disease in the setting of CKD, more so in fact than LDL cholesterol.13
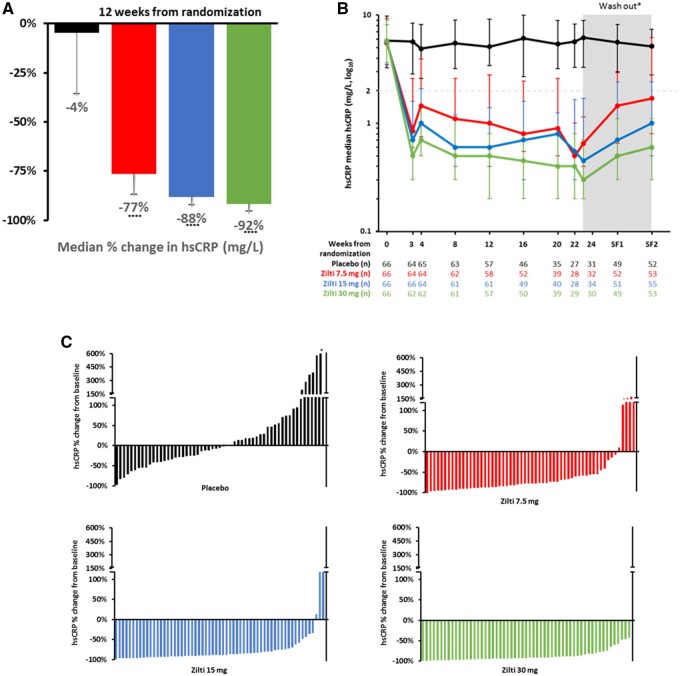
In brief, the RESCUE investigators randomly allocated 264 patients with residual inflammatory risk (hsCRP >2 mg/L) and Stages 3–5 CKD to subcutaneous administration of placebo or to ziltivekimab 7.5 mg, 15 mg, of 30 mg once every 4 weeks over a 24-week period. Overall, the trial primary endpoint of median hsCRP change over time were −77%, −88%, and −92% across the ascending ziltivekimab dose range as compared to −4% on placebo (all P-values <0.0001). (Figure 1) Thus, the magnitude of hsCRP reduction in RESCUE was roughly twice as large as that achieved within CANTOS using an upstream IL-1β inhibitor. Moreover, compared to placebo, waterfall plots demonstrate highly efficient inhibition of IL-6 and subsequent hsCRP reductions with ziltivekimab for virtually all randomized participants. These findings suggest that direct IL-6 inhibition may reduce the wide individual variation previously seen with IL-1β inhibition. In parallel with the primary hsCRP data, comparable dose-dependent reductions for fibrinogen, serum amyloid A, haptoglobin, secretory phospholipase A2, and lipoprotein(a) were also observed. The observation that ziltivekimab reduced lipoprotein(a) is consistent with evidence that IL-6 induces LPA mRNA expression in human hepatocytes, possibly providing another mechanism for benefit.14 Finally, ziltivekimab did not increase atherogenic lipid levels and was without serious injection site reactions, sustained thrombocytopenia or neutropenia, at least over the 24-week trial period. These safety data, if confirmed in larger long-term trials, raise the possibility that ziltivekimab may be unique among commercially available IL-6 inhibitors.
Figure 1.
Primary results of the RESCUE Trial of IL-6 inhibition with ziltivekimab on hsCRP. (A) Median percent change in hsCRP with ascending doses of ziltivekimab; (B) Time dependent effects of ziltivekimab over the 24 week treatment period followed by an 8 week washout period; (C) Waterfall plots for individual trial participants showing minimal variability for hsCRP reduction particularly at the 15 and 30 mg doses of ziltivekimab. Data are shown in black for placebo, red for ziltivekimab 7.5 mg, blue for ziltivekimab 15 mg, and green for ziltivekimab 30 mg, all given subcutaneously once monthly.
Although the total sample size and duration of therapy in RESCUE was modest, the presented data have allowed ziltivekimab to be advanced into a formal long-term endpoint trial know as ZEUS, the Zlitivekimab Cardiovascular Outcomes Study (5). With plans to initiate worldwide enrolment in late 2021, ZEUS will compare ziltivekimab to placebo among 6200 patients with Stages 3–4 CKD and elevated hsCRP to formally test whether reducing circulating IL-6 reduces cardiovascular event rates. As such, incident major adverse cardiovascular events inclusive of myocardial infarction, stroke, and cardiovascular death comprise the trial primary endpoint. Given the emerging role of IL-6 in renal disease, an important secondary aim of ZEUS will address whether chronic IL-6 inhibition slows the progression of renal disease as documented by changes in glomerular filtration rate and the urinary to albumin creatinine ratio over time, as well as progression to Stage 5 CKD and dialysis. With regard to this latter hypothesis, it should be noted that within CANTOS, IL-1β inhibition markedly reduced vascular event rates among the subgroup with baseline CKD but had only modest benefits on indices of renal function.15 In addition, baseline measures of left ventricular ejection fraction will be obtained in ZEUS so that participants can further be stratified on the basis of heart failure with reduced or preserved ejection fraction.
In sum, based on the recent RESCUE data, the opportunity has arisen to move past CANTOS and IL-1β inhibition to address in ZEUS whether targeting IL-6 can provide even larger reductions in cardiovascular event rates. Focusing initially among those with CKD and elevated hsCRP, ZEUS represents the next major step in our understanding of how, as we continue to move beyond lipids, residual inflammatory risk can be directly impacted upon.
Conflict of interest: P.M.R. has received research grant support from Novartis, Kowa, Amarin, Pfizer, and the NHLBI; and has served as a consultant to Corvidia, Novartis, Flame, Agepha, Inflazome, AstraZeneca, Jannsen, Civi Biopharm, SOCAR, Novo Nordisk, Uptton, and Omeicos, and Boehringer-Ingelheim.
Authors

Biography: Dr. Paul M Ridker is the Eugene Braunwald Professor of Medicine at the Harvard Medical School and directs the Center for Cardiovascular Disease Prevention, a translational research unit at the Brigham and Women’s Hospital in Boston. Dr. Ridker’s research focuses on the design and conduct of multi-national randomized trials, the development of inflammatory biomarkers for clinical and research use, the molecular and genetic epidemiology of cardiovascular diseases, and on novel strategies for cardiovascular disease detection and prevention.
References
- 1. Ridker PM. Anticytokine agents: targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ Res 2019;124:437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Kastelein J, Koenig W, Genest J, Lorenzatti A, Varigos J, Siostrzonek P, Sinnaeve P, Fonseca F, Nicolau J, Gotcheva N, Yong H, Urina-Triana M, Milicic D, Cifkova R, Vettus R, Anker SD, Manolis AJ, Wyss F, Forster T, Sigurdsson A, Pais P, Fucili A, Ogawa H, Shimokawa H, Veze I, Petrauskiene B, Salvador L, Cornel JH, Klemsdal TO, Medina F, Budaj A, Vida-Simiti L, Kobalava Z, Otasevic P, Pella D, Lainscak M, Seung K-B, Commerford P, Dellborg M, Donath M, Hwang J-J, Kultursay H, Flather M, Ballantyne C, Bilazarian S, Chang W, East C, Forgosh L, Harris B, Ligueros M; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 2018;391:319–328. [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM, Libby P, MacFadyen JG, Thuren T, Ballantyne C, Fonseca F, Koenig W, Shimokawa H, Everett BM, Glynn RJ. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur Heart J 2018;39:3499–3507. [DOI] [PubMed] [Google Scholar]
- 5. Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res 2021;128:1728–1746. [DOI] [PubMed] [Google Scholar]
- 6.IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet 2012;379:1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet 2012;379:1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cai T, Zhang Y, Ho YL, Link N, Sun J, Huang J, Cai TA, Damrauer S, Ahuja Y, Honerlaw J, Huang J, Costa L, Schubert P, Hong C, Gagnon D, Sun YV, Gaziano JM, Wilson P, Cho K, Tsao P, O'Donnell CJ, Liao KP; VA Million Veteran Program. Association of interleukin 6 receptor variant with cardiovascular disease effects of interleukin 6 receptor blocking therapy: a phenome-wide association study. JAMA Cardiol 2018;3:849–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akita K, Isoda K, Sato-Okabayashi Y, Kadoguchi T, Kitamura K, Ohtomo F, Shimada K, Daida H. An interleukin-6 receptor antibody suppresses atherosclerosis in atherogenic mice. Front Cardiovasc Med 2017;4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation 2019;139:1289–1299. [DOI] [PubMed] [Google Scholar]
- 11. Broch K, Anstensrud AK, Woxholt S, Sharma K, Tøllefsen IM, Bendz B, Aakhus S, Ueland T, Amundsen BH, Damås JK, Berg ES, Bjørkelund E, Bendz C, Hopp E, Kleveland O, Stensæth KH, Opdahl A, Kløw N-E, Seljeflot I, Andersen GØ, Wiseth R, Aukrust P, Gullestad L. Randomized trial of interleukin-6 receptor inhibition in patients with acute ST-segment elevation Myocardial infarction. J Am Coll Cardiol 2021;77:1845–1855. [DOI] [PubMed] [Google Scholar]
- 12. Ridker PM, Devalaraja M, Baeres FMM, Engelmann MDM, Hovingh GK, Ivkovic M, Lo L, Kling D, Pergola P, Raj D, Libby P, Davidson M, On B, Of The Rescue I. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 2021;397:2060–2069. [DOI] [PubMed] [Google Scholar]
- 13. Amdur RL, Feldman HI, Dominic EA, Anderson AH, Beddhu S, Rahman M, Wolf M, Reilly M, Ojo A, Townsend RR, Go AS, He J, Xie D, Thompson S, Budoff M, Kasner S, Kimmel PL, Kusek JW, Raj DS, Fink J, Appel LJ, Lash JP, Raj DS; CRIC Study Investigators. Use of measures of inflammation and kidney function for prediction of atherosclerotic vascular disease events and death in patients with CKD: findings from the CRIC study. Am J Kidney Dis 2019;73:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muller N, Schulte DM, Turk K, Freitag-Wolf S, Hampe J, Zeuner R, Schroder JO, Gouni-Berthold I, Berthold HK, Krone W, Rose-John S, Schreiber S, Laudes M. IL-6 blockade by monoconal antibodies inhibits apolipoprotein(a) expression and lipoprotein(a) synthesis in humans. J Lipid Res 2015;56:1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ridker PM, MacFadyen JG, Glynn RJ, Koenig W, Libby P, Everett BM, Lefkowitz M, Thuren T, Cornel JH. Inhibition of interleukin-1β by canakinumab and cardiovascular outcomes in patients with chronic kidney disease. J Am Coll Cardiol 2018;71:2405–2414. [DOI] [PubMed] [Google Scholar]