Abstract
Aims
A diet with modified components, such as a ketogenic low-carbohydrate (LC) diet, potentially extends longevity and healthspan. However, how an LC diet impacts on cardiac pathology during haemodynamic stress remains elusive. This study evaluated the effects of an LC diet high in either fat (Fat-LC) or protein (Pro-LC) in a mouse model of chronic hypertensive cardiac remodelling.
Methods and results
Wild-type mice were subjected to transverse aortic constriction, followed by feeding with the Fat-LC, the Pro-LC, or a high-carbohydrate control diet. After 4 weeks, echocardiographic, haemodynamic, histological, and biochemical analyses were performed. LC diet consumption after pressure overload inhibited the development of pathological hypertrophy and systolic dysfunction compared to the control diet. An anti-hypertrophic serine/threonine kinase, GSK-3β, was re-activated by both LC diets; however, the Fat-LC, but not the Pro-LC, diet exerted cardioprotection in GSK-3β cardiac-specific knockout mice. β-hydroxybutyrate, a major ketone body in mammals, was increased in the hearts of mice fed the Fat-LC, but not the Pro-LC, diet. In cardiomyocytes, ketone body supplementation inhibited phenylephrine-induced hypertrophy, in part by suppressing mTOR signalling.
Conclusion
Strict carbohydrate restriction suppresses pathological cardiac growth and heart failure after pressure overload through distinct anti-hypertrophic mechanisms elicited by supplemented macronutrients.
Keywords: Low-carbohydrate diet, Cardiac hypertrophy, Heart failure, Ketone body
Graphical Abstract
1. Introduction
Dietary nutrients alter the metabolites in the circulating blood, which, in turn, affect cellular metabolism. Increasing lines of evidence suggest that modulating the components of a diet affects organ functions, contributing to the health and pathology of organisms. For example, high-carbohydrate intake is associated with a higher risk of total mortality in the general population.1,2 Strict restriction of carbohydrates in conjunction with high-fat (HF) intake improves midlife survival, memory, and healthspan in aged mice.3,4 Low-carbohydrate (LC)/HF consumption attenuates cardiac hypertrophy in response to high-salt in Dahl salt-sensitive rats.5 Since investigations of the effect of a very low percentage of carbohydrates on health showed mixed results in general populations,1,2 we hypothesized that the percentage of energy from other dietary macronutrients might also be critical for regulating organ function, rather than a low level of carbohydrates alone. For instance, an LC diet supplemented with HF increases circulating levels of ketone bodies through enhanced ketogenesis in the liver,6,7 which may affect cardiac function.8 Thus, the current study aimed to determine whether strict restriction of carbohydrates supplemented with high levels of either fat or protein differentially affects the development and progression of cardiac pathology in the context of haemodynamic stress.
The heart and individual cardiomyocytes undergo hypertrophy and contractile dysfunction in response to increased workload.9 Although pathological cardiac hypertrophy is associated with changes in metabolism,10–12 whether and how modification of dietary components, such as an LC diet, regulates pathological cardiomyocyte cell growth during stress remains elusive. We here demonstrate that an LC diet with either high-protein (Pro-LC) or high-fat (Fat-LC) supplementation inhibits pressure overload-induced pathological hypertrophy through distinct signalling mechanisms.
2. Methods
2.1. Animal procedures
Male 8- to 10-week-old mice were subjected to transverse aortic constriction (TAC) or sham surgery. Mice were fed a custom LC diet [Fat-LC (D16102003) or Pro-LC (D16102004) diet, purchased from Research Diets] or a control high-carbohydrate diet (Research Diets, D12450K) ad libitum. All protocols concerning the use of animals were approved by the Institutional Animal Care and Use Committee at Rutgers New Jersey Medical School and all procedures conformed to the NIH guidelines (Guide for the Care and Use of Laboratory Animals).
2.2. Transverse aortic constriction
Mice were anaesthetized with pentobarbital (60–70 mg/kg, intraperitoneal injection) and mechanically ventilated with a tidal volume of 0.2 mL and a respiratory rate of 110 breaths per minute. The mice were kept warm with heat lamps. It took around 5 min to establish full anaesthesia. The left chest was opened at the second intercostal space. Aortic constriction was achieved by tying around the transverse thoracic aorta against a 28-gauge needle using a 7-0 prolene suture and then removing the needle. Sham operation was performed without constricting the aorta. The TAC procedure was completed within 20–30 min per mouse. When recovered from anaesthesia 1–2 h after the closure of the chest, the mice were extubated and returned to their cages. Upon completion of all experimental procedures, mice were euthanized by cervical dislocation followed by harvest of the hearts for biochemical studies, including signalling pathways and metabolism. Detailed methods and the scientific justification for the selection of anaesthetics are described in the Supplementary material online.
2.3. Primary rat neonatal cardiomyocytes
Primary cultures of ventricular cardiomyocytes were prepared from 1-day-old Crl:(WI)BR-Wistar rats (Envigo, Somerville) and maintained in culture as described previously.11 The neonatal rats were deeply anaesthetized by inhaling isoflurane in a sealed, quart-size jar at room temperature. The chest was opened and the heart was harvested. A cardiomyocyte-rich fraction was obtained by centrifugation through a discontinuous Percoll gradient.
2.4. Immunoblotting
Cardiomyocyte lysates and heart homogenates were prepared in RIPA buffer containing protease and phosphatase inhibitors (Sigma-Aldrich) as described previously.13 Lysates were centrifuged at 13 200 r.p.m. at 4°C for 15 min. Total protein lysates (10–30 μg) were incubated with SDS sample buffer at 95°C for 5 min. The denatured protein samples were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes by wet electrotransfer, and probed with primary antibodies.
2.5. Adenovirus constructs
Recombinant adenovirus vector for overexpression was constructed, propagated, and tittered as previously described.14 pBHGloxΔE1,3Cre (Microbix), including the ΔE adenoviral genome, was co-transfected with the pDC shuttle vector containing the gene of interest into 293 cells. Replication-defective human adenovirus type 5 (devoid of E1) harbouring full-length wild-type Rheb cDNA (Ad-Rheb) was generated by homologous recombination in 293 cells. Adenovirus harbouring beta-galactosidase (Ad-LacZ) was used as a control.
2.6. Immunohistochemistry
The heart tissue was washed with PBS, fixed in 4% paraformaldehyde overnight, embedded in paraffin, and sectioned at 10-µm thickness onto a glass slide. After de-paraffinization, sections were stained with Picric acid Sirius red (PASR) for evaluation of fibrosis or wheat germ agglutinin (WGA) for evaluation of the cross-sectional area of cardiomyocytes. The outline of 100–200 myocytes was traced in each section, using ImageJ software (NIH).
2.7. Cardiomyocyte cell size
Rat neonatal cardiomyocytes were cultured on coverslips, washed with PBS twice, fixed with 3.7% paraformaldehyde for 15 min, and washed with PBS three times. Samples were permeabilized with PBST (0.5% Triton-X in PBS) for 15 min, and blocked in 5% bovine serum albumin, 5% goat serum in PBST for 30 min at 37°C. Cardiomyocytes were stained with Alexa Fluor 555 phalloidin (Thermo Fisher Scientific, A34055). Samples were washed with PBS and mounted on glass slides with mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI) to stain nuclei (VECTASHIELD, Vector Laboratories). Cells were observed under a fluorescence microscope. Cardiomyocyte cell size was evaluated from 25 to 30 myocytes in each myocyte culture preparation and the mean value was obtained. The measurement was conducted with five independent culture preparations and the mean value was reported.
2.8. Statistical analysis
All values are expressed as mean ± SEM. Statistical analyses were carried out by two-tailed unpaired Student’s t-test for two groups or one-way analysis of variance (ANOVA) followed by the Tukey post hoc analysis for three groups or more unless otherwise stated. If the data distribution failed normality by the Shapiro–Wilk test or Kolmogorov–Smirnov test, the Mann–Whitney U test for two groups or Kruskal–Wallis test with the Dunn’s multiple comparison test for three groups or more was performed. Survival curves were plotted by the Kaplan–Meier method, with statistical significance analysed by log-rank test. A P-value of <0.05 was considered significant.
Detailed methods are available in the Supplementary material online.
3. Results
3.1 LC diets attenuate cardiac hypertrophy and failure in response to pressure overload
We tested whether restricted carbohydrate intake affects the development of cardiac hypertrophy and failure in response to pressure overload. In the traditional 4:1 ratio of the ketogenic diet (4 g of fats to 1 g of proteins plus carbohydrates), 90% of the total calories originate from fats, 7–8% from proteins, and 2–3% from carbohydrates.15 Since it would be unclear whether restriction of carbohydrates per se or the other components in the diet, namely fats or proteins, impact on cardiac pathology, we used two different LC diets (3% from carbohydrates): supplemented with HF (90% from fats, Fat-LC) or supplemented with high-protein (90% from proteins, Pro-LC). An iso-caloric high-carbohydrate diet (70% from carbohydrates, Con) was used as a control (Supplementary material online, Table S1). Mice fed the Fat-LC diet gained slightly in body weight, at a level similar to those fed the Con diet, although the increase was not statistically significant. Those fed the Pro-LC diet exhibited a slightly but significantly reduced body weight at 3 days and thereafter (Supplementary material online, Figure S1A and B). The mice fed the Pro-LC diet exhibited a lower calorie intake than the other groups due to lower food intake in ad libitum feeding (Supplementary material online, Figure S1C).
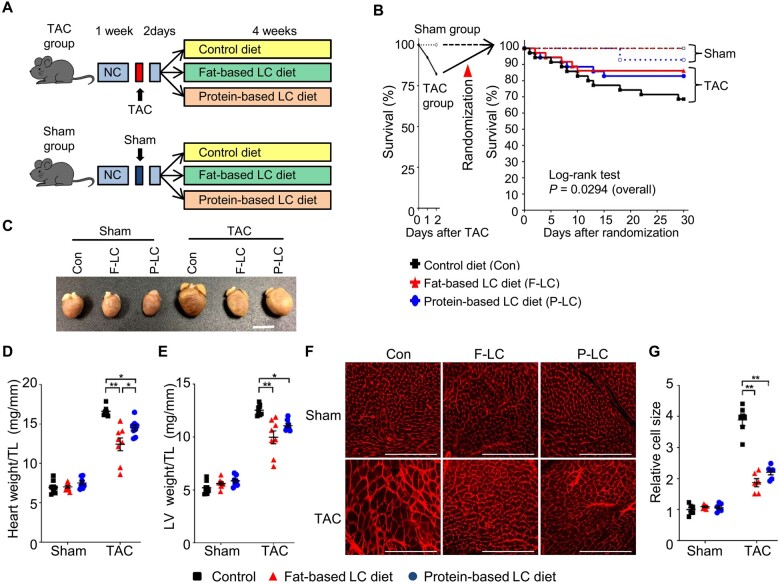
TAC transiently induces left ventricular (LV) dysfunction during the first few days, followed by a recovery to baseline before the heart develops cardiac hypertrophy and heart failure.9,16 To evaluate the effects of the Fat-LC and Pro-LC diets on the development of hypertrophy and heart failure, while minimizing the potential effect on transient heart failure right after the initiation of TAC, mice were fed Fat-LC, Pro-LC, or Con diet beginning 2 days after TAC or sham surgery (Figure 1A). There was no significant difference in the pressure gradient (Supplementary material online, Figure S1D), suggesting that similar levels of pressure overload were applied to the Fat-LC, Pro-LC, and Con groups. Mice fed the Fat-LC or Pro-LC diet tended to exhibit better survival after TAC than those fed the Con diet (Figure 1B). Although Fat-LC or Pro-LC diet intake for 4 weeks had no impact on the morphology of the heart or the cardiomyocytes therein at baseline (Figure 1C–G), both Fat-LC and Pro-LC diets significantly attenuated cardiac hypertrophy in response to pressure overload, as evidenced by smaller gross morphology of the heart, heart, and LV weights normalized by tibia length, and individual cardiomyocyte size, compared to the Con diet (Figure 1C–G). Intake of the Fat-LC diet tended to exert a more prominent anti-hypertrophic effect than the Pro-LC diet as evaluated with heart weight/tibial length, but not LV weight/tibial length or the myocyte cross-sectional area (Figure 1C–G).
Figure 1.
LC diets attenuate pressure overload-induced cardiac hypertrophy. (A) Schematic representation of the study design. After a 1-week running period of NC, 8- to 10-week-old C57BL/6J wild-type (WT) mice were subjected to TAC or sham operation, followed by a 2-day observation period with NC. The surviving mice were randomly assigned to three groups: LC diet supplemented with high fats, LC diet supplemented with high proteins, or isocaloric high-carbohydrates control diet. (B) Kaplan–Meier survival curves after TAC or sham surgery. Overall statistical differences after the surgeries were analysed by log-rank test (n = 14 for sham and n = 35–36 for TAC groups). (C–G) The mouse hearts were harvested 4 weeks after the randomization into the indicated diet. (C) Representative gross appearance of the indicated hearts. Scale bar, 5 mm. (D) Heart weight normalized by tibial length (TL) (n = 7–8). (E) LV weight normalized by TL (n = 7–8). (F) Representative WGA staining. Scale bar, 200 μm. (G) Quantification of relative cardiomyocyte size (n = 6). One-way ANOVA followed by the Tukey’s post hoc analysis was used. Error bars indicate SEM. *P < 0.05, **P < 0.001.
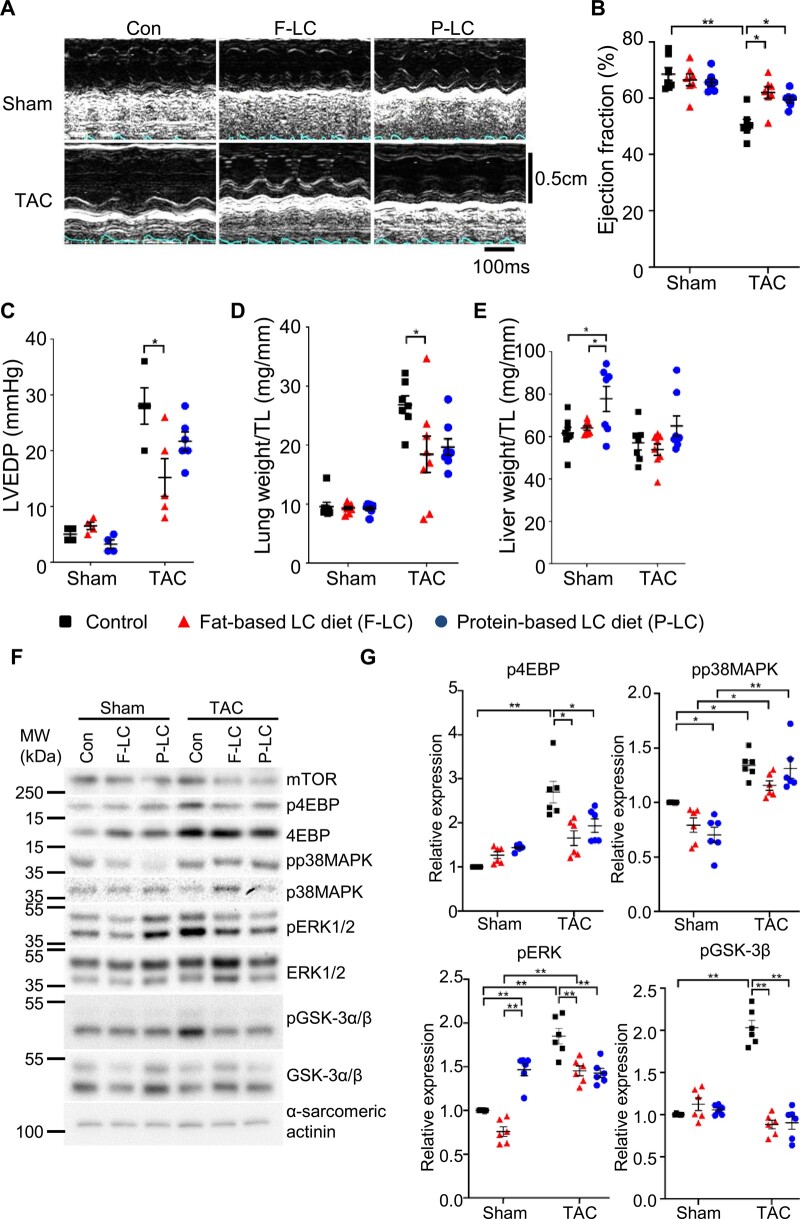
In mice fed the Con diet, TAC for 4 weeks induced cardiac dysfunction, as evidenced by decreases in LV ejection fraction (LVEF) and increases in LV end-diastolic pressure (LVEDP), and heart failure, indicated by an increase in lung weight/tibia length (TL), an index of lung congestion. The TAC-induced decrease in LVEF was alleviated in mice fed either the Fat-LC or Pro-LC diet (Figure 2A and B and Supplementary material online, Table S2). Fat-LC, but not Pro-LC, significantly reduced TAC-induced elevation in LVEDP and lung weight/TL, an index of heart failure (Figure 2C and D). Neither LC diet reduced the level of fibrosis in the heart (Supplementary material online, Figure S1E). Liver weight/TL was increased in mice fed the Pro-LC diet (Figure 2E), probably due to the high burden of amino acid metabolism on the liver. These results suggest that Fat-LC and Pro-LC diet consumption prevents pathological cardiac hypertrophy and LV systolic dysfunction in response to pressure overload.
Figure 2.
LC diets mitigate pressure overload-induced heart failure. Cardiac function and heart failure were evaluated 4 weeks after the randomization into the indicated diet. (A) Representative pictures of M-mode echocardiography. Transverse scale bar, 100 ms. Vertical scale bar, 0.5 cm. (B) Ejection fraction, as evaluated by M-mode echocardiography (n = 7–8). (C) LVEDP, as evaluated by haemodynamic study (n = 4–6). (D) Lung weight normalized by TL (n = 7–8). (E) Liver weight normalized by TL (n = 7–8). Kruskal–Wallis test with the Dunn’s multiple comparison test was performed. (F–G) LC diet attenuates hypertrophic signalling pathways in response to pressure overload. Representative immunoblots showing the hypertrophic signalling pathways (F) and associated densitometry analyses, normalized by α-sarcomeric actinin (n = 6) (G). One-way ANOVA followed by the Tukey’s post hoc analysis was used unless otherwise stated. Error bars indicate SEM. *P < 0.05, **P < 0.001.
3.2 A Pro-LC, but not Fat-LC, diet inhibits hypertrophy and heart failure through up-regulation of GSK-3β
We evaluated how known signalling mechanisms of cardiac hypertrophy are affected. In mice fed the Con diet, TAC increased phosphorylation of 4EBP-1, ERK, and p38MAPK in the heart, suggesting that mTORC1, ERK, and p38MAPK are activated (Figure 2F and G). TAC also increased Ser9 phosphorylation of GSK-3β, suggesting that GSK-3β is inactivated in mice fed the Con diet. TAC-induced increases in phosphorylation of 4EBP-1 and ERK, but not p38MAPK, were significantly attenuated in mice fed the Fat-LC or Pro-LC diet, suggesting that TAC-induced activation of mTOR and ERK was inhibited by Fat-LC and Pro-LC. TAC-induced increases in Ser9 phosphorylation of GSK-3β were abolished, suggesting that TAC-induced inactivation of GSK-3β is abolished, leaving GSK-3β active in the presence of pressure overload, in mice fed the Fat-LC or Pro-LC diet (Figure 2F and G).
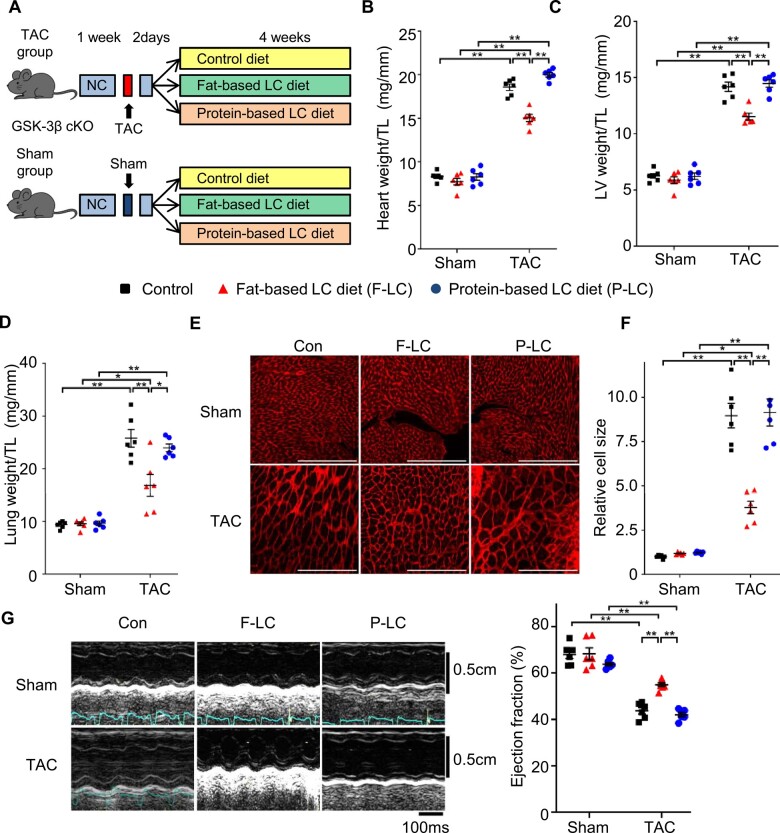
Since constitutive activation of GSK-3β inhibits pressure overload-induced hypertrophy and heart failure,17 we investigated whether the retention of GSK-3β activity in the presence of Fat-LC and Pro-LC confers cardioprotection against pressure overload, using cardiac-specific GSK-3β knockout (GSK-3β cKO) mice. GSK-3β cKO mice fed Con, Fat-LC, or Pro-LC diet exhibited a normal cardiac phenotype in the presence of sham operation. However, the anti-hypertrophic effect of the Pro-LC, but not Fat-LC, diet in response to pressure overload was abolished in GSK-3β cKO mice (Figure 3A–F). Similarly, although the attenuation of pressure overload-induced cardiac dysfunction observed in mice fed the Fat-LC diet was preserved in GSK-3β cKO mice, it was abolished in mice fed the Pro-LC diet (Figure 3G and Supplementary material online, Table S3). These results suggest that the Pro-LC diet exerts its anti-hypertrophic effect in part through GSK-3β.
Figure 3.
Deletion of GSK-3β disrupts the cardioprotection exerted by the high-protein, but not high-fat, LC diet during pressure overload. GSK-3β cKO mice were subjected to TAC or sham surgery. (A) Schematic representation of the study design. After an 1-week running period of NC, 8- to 10-week-old GSK-3β cKO mice were subjected to TAC or sham operation, followed by a two-day observation period with NC. The surviving mice were randomly assigned into three groups: LC diet supplemented with high fats, LC diet supplemented with high proteins, or isocaloric high-carbohydrate control diet. (B) Heart weight normalized by TL (n = 6). (C) LV weight normalized by TL (n = 6). (D) Lung weight normalized by TL (n = 6). (E) Representative WGA staining of the indicated mouse hearts. Scale bar, 200 μm. (F) Quantification of relative cardiomyocyte size (n = 6). (G) Representative pictures of M-mode echocardiography. Transverse scale bar, 100 ms. Vertical scale bar, 0.5 cm (left). Ejection fraction, as evaluated by M-mode echocardiography (right, n = 6–7). One-way ANOVA followed by the Tukey’s post hoc analysis was used. Error bars indicate SEM. *P < 0.05, **P < 0.001.
3.3 Fat-LC, but not Pro-LC, diet increases the level of ketone bodies in the heart
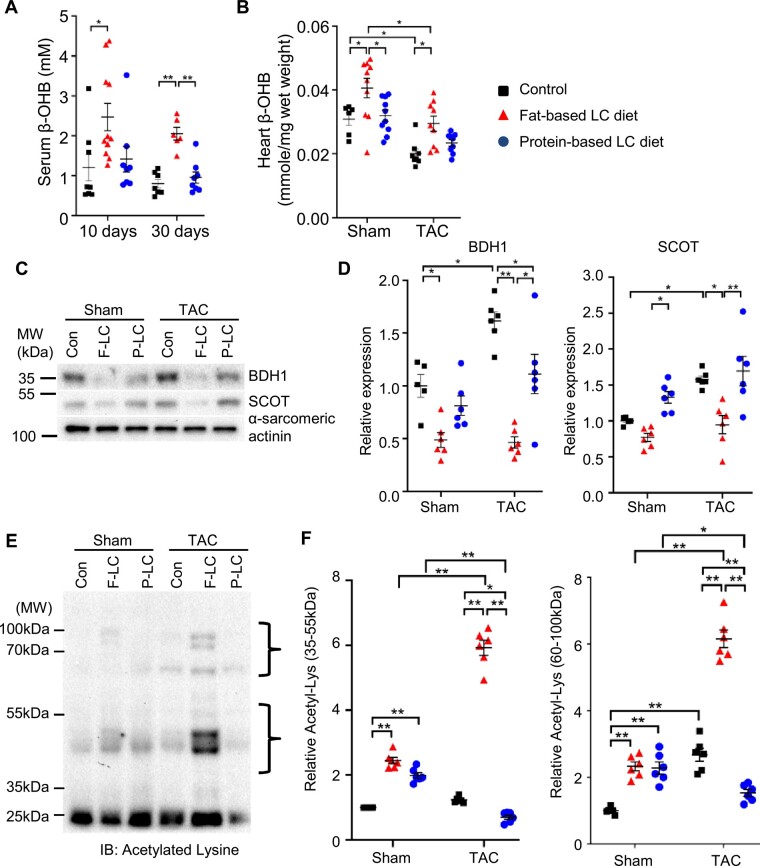
We investigated the underlying mechanism of cardioprotection mediated by the Fat-LC diet. It has been shown that ketone body metabolism is up-regulated in hypertrophied or failing hearts in mice and humans.18 An LC diet stimulates ketogenesis in the liver as a vital and alternative metabolic fuel source and ketolysis is subsequently up-regulated in the heart.7 Therefore, we investigated the role of ketone bodies in regulating cardiac hypertrophy in mice fed the Fat-LC or Pro-LC diet. Fat-LC, but not Pro-LC, diet feeding increased the serum and heart levels of β-hydroxybutyrate (β-OHB), a major ketone body in mammals (Figure 4A and Supplementary material online, Figure S2). In line with a previous report,18 the level of β-OHB was significantly decreased in the hypertrophied and failing heart after 4 weeks of TAC, but it was normalized in the hearts of mice fed the Fat-LC diet (Figure 4B). The Pro-LC diet had no impact on the level of β-OHB in the heart (Figure 4B). In line with previous reports,18–20 the levels of BDH1 and SCOT, key ketolysis enzymes in the heart, were increased in response to TAC in the hearts of mice fed the Con diet. On the other hand, unexpectedly, Fat-LC diet feeding reduced the protein levels of both BDH1 and SCOT, possibly due to negative feedback mechanisms caused by a sufficient amount of ketone bodies in the serum and heart, while Pro-LC diet feeding reduced the level of BDH1 alone (Figure 4C and D).
Figure 4.
A LC diet supplemented with high-fat increases ketone bodies. (A) Serum concentration of β-OHB in mice fed Fat-LC, Pro-LC, or Con diet for the indicated period at baseline (n = 8–11 for 10 days and n = 6–8 for 30 days). Kruskal–Wallis test with the Dunn’s multiple comparison test was performed for 10 days. (B) The level of β-OHB in the hearts of mice subjected to sham or TAC surgery and fed the indicated diet for 4 weeks (n = 6–10 for sham and n = 8–9 for TAC groups). (C and D) Representative immunoblots showing the expression of BDH1 and SCOT (C) and the densitometry analyses (n = 5–6) (D). (E and F) Representative immunoblots showing lysine acetylation (E) and the densitometry analyses (n = 6) (F). One-way ANOVA followed by the Tukey’s post hoc analysis was used unless otherwise stated. Error bars indicate SEM. *P < 0.05, **P < 0.001.
Since ketone bodies contribute to compartmentalized pools of acetyl-CoA that serve as a substrate for acetylation, we evaluated the level of lysine-acetylation in the heart. Although the Pro-LC diet contributed poorly to the β-OHB concentration (Figure 4B), both the Fat-LC and Pro-LC diets increased the level of lysine-acetylation in the hearts of mice subjected to sham operation (Figure 4E and F). Pressure overload enhanced global lysine-acetylation in the heart, consistent with a previous report.21 Lysine-acetylation in the whole heart, as well as in subcellular fractions, including mitochondria, nuclei, and cytosol, was further increased by the Fat-LC diet and decreased by the Pro-LC diet (Figure 4E and F and Supplementary material online, Figure S3A). These results further support the notion that the Fat-LC, but not Pro-LC, diet enhances the level of ketone bodies in the heart.
3.4 Ketone bodies suppress phenylephrine-induced cardiomyocyte hypertrophy
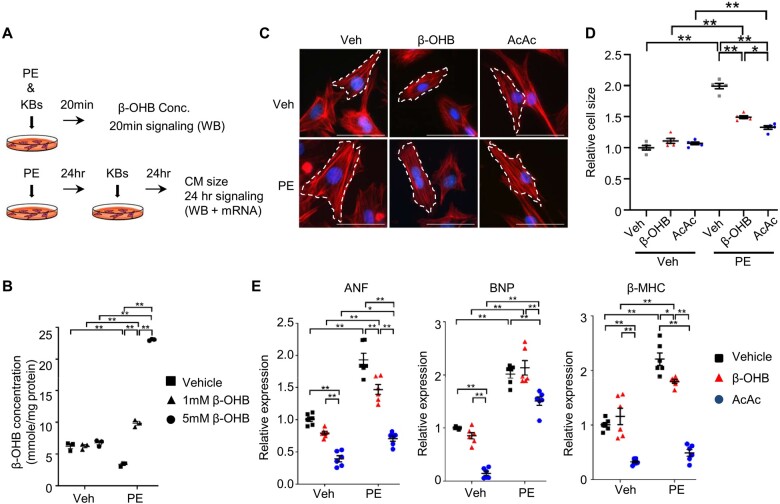
We then asked how ketone bodies regulate cell growth. We treated primary rat neonatal cardiomyocytes with ketone body and phenylephrine (PE), an α-1 adrenergic receptor agonist, to induce cardiomyocyte hypertrophy in vitro (Figure 5A). The concentration of β-OHB in cardiomyocytes was significantly increased within 20 min after administration of β-OHB in the presence of PE (Figure 5A and B). PE induced hypertrophy in cardiomyocytes treated with vehicle, whereas both β-OHB and acetoacetate (AcAc), another major ketone body, inhibited PE-induced hypertrophy, as evaluated by measuring cardiomyocyte size using phalloidin staining (Figure 5C and D). Consistent with this result, PE-induced expression of foetal-type genes, including atrial natriuretic factor (ANF), B-type natriuretic peptide (BNP), and myosin heavy chain beta (β-MHC), was suppressed in cardiomyocytes in the presence of β-OHB or AcAc (Figure 5E). AcAc exerted a more prominent anti-hypertrophic effect than β-OHB in cardiomyocytes. Lysine-acetylation of proteins in mitochondria, nuclei, and cytosol was increased in the presence of PE by treatment with β-OHB and/or a cocktail of β-OHB and AcAc (Supplementary material online, Figure S3B).
Figure 5.
Ketone bodies inhibit cardiomyocyte growth in response to phenylephrine. (A) Schematic representation of the in vitro experiments in CM treated with PE and KBs, including β-OHB (1 mM) and acetoacetate (AcAc, 500 μM). Hypertrophic signalling was evaluated by WB and mRNA expression. (B) β-OHB concentrations in cardiomyocytes treated with phenylephrine or vehicle for 24 h, followed by incubation with 1 mM or 5 mM of β-OHB or vehicle for 20 minutes (n = 3). (C and D) Cardiomyocyte size was evaluated by phalloidin staining. Representative images (C) and quantification analyses (D) are shown. Scale bar, 50 µm. n = 5. (E) Expression of foetal genes, including ANF, BNP, and β-MHC (n = 6). One-way ANOVA followed by the Tukey’s post hoc analysis was used. Error bars indicate SEM. *P < 0.05, **P < 0.001.
3.5 mTOR is involved in the ketone body-mediated anti-hypertrophic effect
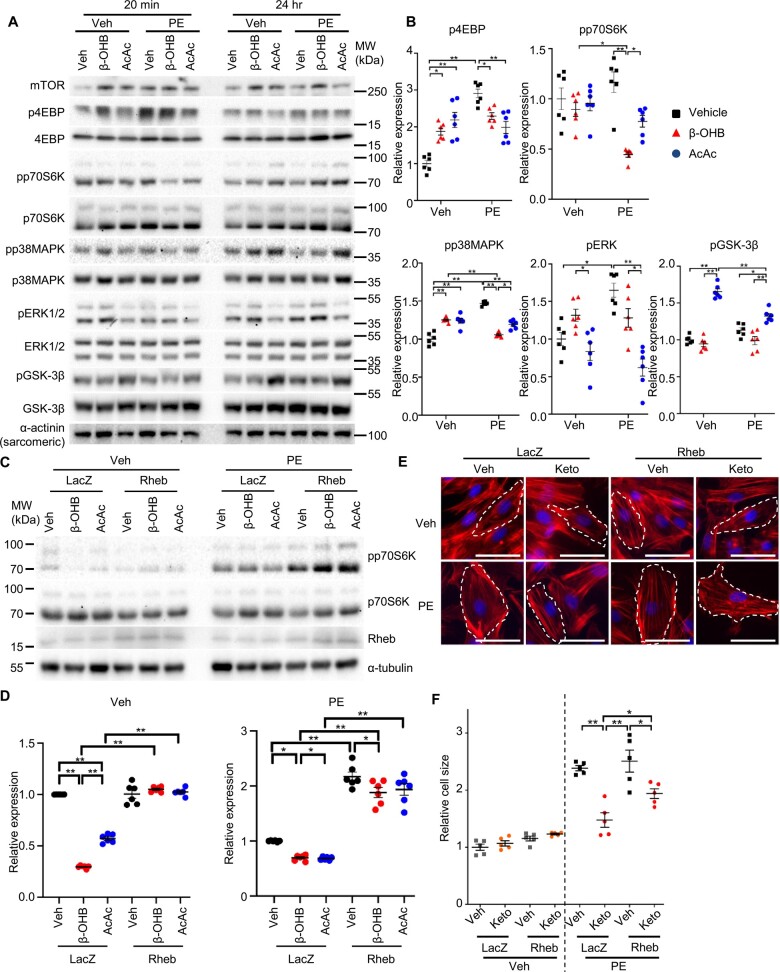
We investigated the mechanisms by which supplementation of ketone bodies inhibits cardiomyocyte hypertrophy. The 4EBP, but not the S6K, branch of the mTOR and MAPK signalling pathways was stimulated within 20 min after PE administration in cardiomyocytes, whereas ketone bodies suppressed PE-induced activation of the mTOR and MAPK pathways (Figure 6A and B). While β-OHB did not alter the activity of GSK-3β in cardiomyocytes in either the presence or absence of PE, AcAc reduced the activity of GSK-3β independently of PE stimulation (Figure 6A and B).
Figure 6.
Ketone bodies suppress phenylephrine-induced cardiomyocyte growth through inhibition of mTOR. (A and B) Representative immunoblots of cardiomyocyte lysates (A) and the densitometry analyses, normalized by α-sarcomeric actinin (n = 6) (B). (C and D) Representative immunoblots of phosphorylated p70S6K in cardiomyocytes transduced with either Rheb-expressing adenovirus or LacZ-expressing adenovirus (C) and the densitometry analyses relative to total p70S6K expression (n = 6) (D). (E and F) Phalloidin staining of cardiomyocytes treated with the indicated adenovirus, followed by incubation with PE or vehicle in the presence or absence of ketone bodies for 24 h (Keto, cocktail of β-OHB and AcAc, 1 mM and 500 µM each). One-way ANOVA with Tukey post hoc test was performed (n = 5). Error bars indicate SEM. *P < 0.05, **P < 0.001.
In order to demonstrate the causality between mTOR suppression and the anti-hypertrophic effect of ketone bodies, cardiomyocytes were transduced with adenovirus harbouring Rheb, a GTPase that stimulates mTORC1 signalling (Figure 6C and D). Rheb partially but significantly reversed ketone body-mediated suppression of cardiomyocyte hypertrophy in response to PE (Figure 6E and F). These results suggest that ketone bodies negatively affect cardiomyocyte hypertrophy in part through suppression of mTOR signalling. Thus, Fat-LC diet feeding increases the level of ketone bodies in the heart, which in turn attenuates pathological cardiomyocyte hypertrophy in part by suppressing mTOR.
4. Discussion
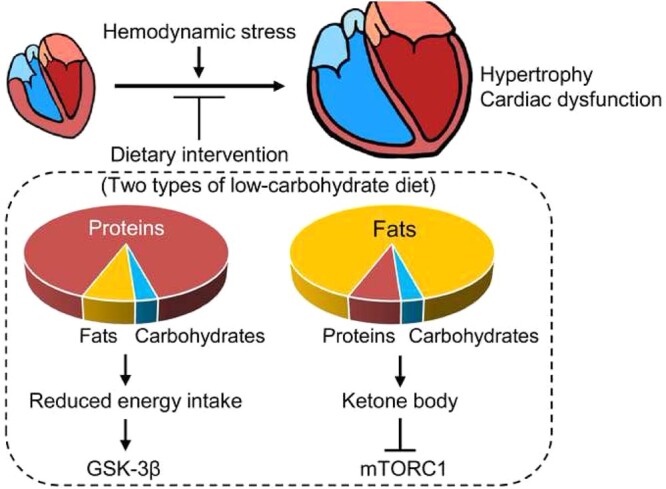
We here show that two types of LC diets, namely Fat-LC and Pro-LC, both alleviate the progression of pathological hypertrophy through distinct underlying signalling mechanisms. Up-regulation of ketone bodies and consequent inhibition of mTOR signalling plays an essential role in mediating the cardioprotective effect of Fat-LC. In contrast, activation of GSK-3β primarily mediates the cardioprotective effect of Pro-LC (Supplementary material online, Figure S5).
4.1 GSK-3β mediates the protective effect of the Pro-LC diet
GSK-3β is active at baseline but inactivated through phosphorylation at Serine 9 in response to stress. Upregulation or activation of GSK-3β inhibits pathological cell growth in the heart.17 However, a clinically achievable intervention to stimulate GSK-3β has remained elusive. The current finding that LC diet intake stimulates GSK-3β activity appears useful clinically for the treatment of cardiac hypertrophy and heart failure. Although both Pro-LC and Fat-LC equally prevented TAC-induced inactivation of GSK-3β, the effect upon GSK-3β was only critical in mediating the protective effect of Pro-LC, not Fat-LC. We speculate that Fat-LC has an additional effect upon ketone bodies and, thus, it can exert cardioprotective effects even in the absence of GSK-3β.
Clinical studies suggest that the increased risk of type 2 diabetes or coronary heart disease associated with animal-derived proteins is reduced by shifting to plant-derived proteins.22 Casein, an animal-derived protein, was used as the main source of proteins in the current study. Thus, it would be interesting to investigate whether LC diets supplemented with plant-derived proteins would provide better cardioprotection against pressure overload, and, if so, whether the protection is also mediated through GSK-3β.
In line with a previous report showing the association between a high-protein-content diet and reduced energy intake,23 mice fed the Pro-LC diet underwent caloric restriction, and gained less body weight in the current study. Since caloric restriction alone can protect the heart,24 it is possible that the protective effect of the Pro-LC diet during TAC may be mediated through caloric restriction. It should be noted, however, that short-term caloric restriction per se inhibits GSK-3β in the mouse heart.25 Thus, the GSK-3β activation and consequent cardioprotection observed in mice fed Pro-LC during TAC most likely occurs through the effect of LC rather than caloric restriction. Furthermore, a previous study showed that a higher (30%) protein diet (56% from carbohydrate and 14% from fat) did not alter the degree of hypertrophy and cardiac dysfunction in response to pressure overload in rats compared to a standard (18%) protein diet,26 suggesting that LC, rather than the high-protein content, in the Pro-LC diet mediates cardioprotection.
4.2 Ketone bodies mediate the protective effect of Fat-LC
Pathological hypertrophy and heart failure shift cardiac substrate utilization from fatty acids and glucose to glycolysis and oxidation of other substrates, including ketone bodies. Loss-of-function studies suggest an adaptive role of increased ketone metabolism against pressure overload-induced cardiac pathology in mice.20,27 β-OHB administration also showed beneficial haemodynamic effects in patients with heart failure with reduced ejection fraction without impairing myocardial energy efficiency8 and provided additional energy in the failing mouse heart.6,28 We show here that a Fat-LC diet inhibits pathological hypertrophy through an increase in ketone body levels in the heart.
Ketone bodies suppressed cardiomyocyte hypertrophy in part through suppression of mTOR signalling. The ketone body-mediated cardioprotective mechanisms were observed only when reduced energy intake from carbohydrates is compensated for with HFs, but not high-proteins. Ketosis is induced by a fat-enriched LC diet (0–10% kcal from carbohydrate and ≥ 65% kcal from fat),19,20,29 but not an HF diet,30 which promotes transcriptional suppression of OXCT1, the gene encoding SCOT, in the intact heart.31 The effect of a ketogenic diet on cardiac levels of ketone bodies remains largely unexplored. Ketone bodies stimulate the endothelial nitric oxide synthase pathway,7,32 which might activate protein kinase G (PKG) through cGMP. A recent study showed that PKG phosphorylates tuberin (TSC2) at S1365/1366, which activates TSC2, thereby inhibiting mTORC1 activity.33 Thus, the PKG-TSC2 pathway might be involved in ketone body-mediated inhibition of mTORC1. Ketone bodies also increased the activity of AMPK (Supplementary material online, Figure S4), which might in turn inhibit mTOR signalling. Since overexpression of Rheb alone could not completely reverse the anti-hypertrophic effect of ketone bodies, several pathways might be involved in ketone body-mediated inhibition of mTORC1.
β-OHB, but not AcAc, negatively affects inflammation through inhibition of NLRP3 inflammasome.34 In our study, however, neither type of LC-diet decreased inflammation. A recent study showed that NLRP3 inflammasome activation is induced within a few hours and no later than 1 day after pressure overload.35 Thus, starting an LC diet 2 days after the initiation of pressure overload may be too late to inhibit the NLRP3 inflammasome and induction of inflammation in the heart. It would be interesting to evaluate the effect of a Fat-LC diet when started earlier. Ketone bodies also induced hyperacetylation of nuclear proteins (Supplementary material online, Figure S3), which might have epigenetic effects. Thus, it would be interesting to investigate whether ketone bodies play a role in epigenetic regulation of cardiac function and morphology.
Although lard was a main source of fats (40% from saturated fats and 45% from monounsaturated fats) in the Fat-LC diet in this study, the diet nevertheless exerted cardioprotection against pressure overload, suggesting that the benefits of enhanced ketone body metabolism may exceed the detrimental effect of saturated fats.36,37 It would be interesting to examine whether an LC diet supplemented with polyunsaturated fats could mitigate hypertrophy and heart failure to a greater degree than the current regime of the Fat-LC diet.
Increased calorie intake during the acute phase of heart failure or critical illness is associated with a higher re-admission rate or slower recovery and more complications, respectively, compared to lower calorie intake.38 Mice fed the Fat-LC diet exhibited attenuated hypertrophy and better cardiac function in response to pressure overload despite high caloric intake in our study; thus, we speculate that ketone body-mediated inhibition of cardiac hypertrophy may outweigh the detrimental aspect of high-calorie intake in the presence of pressure overload.
Altogether, our study suggests that modulating a diet towards LC with supplementation with either high-protein or -fat during existing heart failure could improve cardiovascular outcomes through distinct mechanisms.
4.2 Study limitations
Although the current study addresses the effect of LC diets on cardiac pathology during the early phase of haemodynamic stress, whether LC diets also prevent or reverse the progression of heart failure long-term remains to be clarified. Although Fat-LC induced more prominent reduction in heart weight/TL than Pro-LC (Figure 1D), generally modest differences in the effect of Fat-LC and Pro-LC upon other parameters of cardiac function and heart failure were noted. Thus, despite the difference in the underlying molecular mechanisms, Fat-LC, and Pro-LC are almost equally protective. Alternatively, either more severe pressure overload or a longer follow up may be required for manifestation of the functional or survival benefit in the Fat-LC diet group. Diets with very high-protein contents may be potentially harmful for humans. It is therefore essential to optimize the level of proteins used to substitute for carbohydrates. Since the effects of a diet on the development of cardiac hypertrophy and metabolism may differ between male and female mice,39 whether the mechanisms proposed in this study, in which only male mice were used, hold true in female mice as well should be addressed in a separate study. Finally, since Pro-LC diet consumption under ad libitum conditions is accompanied by reduced caloric intake, the contribution of caloric restriction to the overall salutary effect of Pro-LC remains to be clarified.
5. Conclusions
Strict restriction of dietary carbohydrates and supplementation with either protein or fat inhibits pathological cardiac hypertrophy and heart failure in response to pressure overload in mice through ketone bodies or inhibition of GSK-3β inactivation. LC-based diets may provide a unique therapeutic option for cardiac diseases and heart failure.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
We thank Daniela Zablocki for critical reading of the manuscript.
Conflict of interest: none declared.
Funding
This work was supported in part by U.S. Public Health Service grants HL067724, HL091469, HL112330, HL138720, HL144626, HL150881, and AG023039, the Leducq Foundation Transatlantic Network of Excellence 15CBD04 (to J.S.) and American Heart Association Scientist Development Grant 17SDG33660358 (to M.N.) and Merit Award 20 Merit 35120374 (to J.S.).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Contributor Information
Michinari Nakamura, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Natalija Odanovic, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Yasuki Nakada, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Satomi Dohi, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Peiyong Zhai, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Andreas Ivessa, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Zhi Yang, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Maha Abdellatif, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Junichi Sadoshima, Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, 185 South Orange Ave, MSB G-609, Newark, NJ 07103, USA.
Translational perspective
Haemodynamic stress, such as hypertension, induces pathological cardiac hypertrophy, leading to heart failure. There is growing evidence that modulating components of diet affects cardiac function in humans, although the causality and underlying mechanisms are poorly understood. Our study demonstrates that strict restriction of dietary carbohydrates supplemented with either fat or proteins during acute haemodynamic stress attenuates the development and progression of cardiac hypertrophy and heart failure by activating distinct anti-hypertrophic and cardioprotective signalling mechanisms. The study suggests that it would be useful to investigate the therapeutic benefit of carbohydrate restriction in patients with hypertension and cardiac hypertrophy in clinical studies.
References
- 1. Seidelmann SB, Claggett B, Cheng S, Henglin M, Shah A, Steffen LM, Folsom AR, Rimm EB, Willett WC, Solomon SD. Dietary carbohydrate intake and mortality: a prospective cohort study and meta-analysis. Lancet Public Health 2018;3:e419–e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dehghan M, Mente A, Zhang X, Swaminathan S, Li W, Mohan V, Iqbal R, Kumar R, Wentzel-Viljoen E, Rosengren A, Amma LI, Avezum A, Chifamba J, Diaz R, Khatib R, Lear S, Lopez-Jaramillo P, Liu X, Gupta R, Mohammadifard N, Gao N, Oguz A, Ramli AS, Seron P, Sun Y, Szuba A, Tsolekile L, Wielgosz A, Yusuf R, Hussein Yusufali A, Teo KK, Rangarajan S, Dagenais G, Bangdiwala SI, Islam S, Anand SS, Yusuf S; Prospective Urban Rural Epidemiology (PURE) study investigators. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050–2062. [DOI] [PubMed] [Google Scholar]
- 3. Newman JC, Covarrubias AJ, Zhao M, Yu X, Gut P, Ng CP, Huang Y, Haldar S, Verdin E. Ketogenic diet reduces midlife mortality and improves memory in aging mice. Cell Metab 2017;26:547–557.e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, Perez G, Gutierrez-Casado E, Koike S, Knotts TA, Imai DM, Griffey SM, Kim K, Hagopian K, McMackin MZ, Haj FG, Baar K, Cortopassi GA, Ramsey JJ, Lopez-Dominguez JA. A ketogenic diet extends longevity and healthspan in adult mice. Cell Metab 2017;26:539–546.e535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension 2006;48:1116–1123. [DOI] [PubMed] [Google Scholar]
- 6. Nakamura M, Sadoshima J. Ketone body can be a fuel substrate for failing heart. Cardiovasc Res 2019;115:1567–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab 2017;25:262–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, Harms HJ, Frøkiær J, Eiskjaer H, Jespersen NR, Mellemkjaer S, Lassen TR, Pryds K, Bøtker HE, Wiggers H. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation 2019;139:2129–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 2018;15:387–407. [DOI] [PubMed] [Google Scholar]
- 10. Nakamura M, Sadoshima J. Cardiomyopathy in obesity, insulin resistance and diabetes. J Physiol 2020;598:2977–2993. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura M, Liu T, Husain S, Zhai P, Warren JS, Hsu CP, Matsuda T, Phiel CJ, Cox JE, Tian B, Li H, Sadoshima J. Glycogen synthase kinase-3alpha promotes fatty acid uptake and lipotoxic cardiomyopathy. Cell Metab 2019;29:1119–1134.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakamura M, Bhatnagar A, Sadoshima J. Overview of pyridine nucleotides review series. Circ Res 2012;111:604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakamura M, Zhai P, Del Re DP, Maejima Y, Sadoshima J. Mst1-mediated phosphorylation of Bcl-xL is required for myocardial reperfusion injury. JCI Insight 2016;1:e86217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 2012; 125:1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kossoff EH, Zupec-Kania BA, Amark PE, Ballaban-Gil KR, Christina Bergqvist AG, Blackford R, Buchhalter JR, Caraballo RH, Helen Cross J, Dahlin MG, Donner EJ, Klepper J, Jehle RS, Kim HD, Christiana Liu YM, Nation J, Nordli DR Jr, Pfeifer HH, Rho JM, Stafstrom CE, Thiele EA, Turner Z, Wirrell EC, Wheless JW, Veggiotti P, Vining EP; Charlie Foundation, Practice Committee of the Child Neurology Society; Practice Committee of the Child Neurology Society; International Ketogenic Diet Study Group. Optimal clinical management of children receiving the ketogenic diet: recommendations of the International Ketogenic Diet Study Group. Epilepsia 2009;50:304–317. [DOI] [PubMed] [Google Scholar]
- 16. Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J. Drp1-dependent mitochondrial autophagy plays a protective role against pressure overload-induced mitochondrial dysfunction and heart failure. Circulation 2016;133:1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S, Sadoshima J. Distinct roles of GSK-3alpha and GSK-3beta phosphorylation in the heart under pressure overload. Proc Natl Acad Sci USA 2008;105:20900–20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bedi KC Jr, Snyder NW, Brandimarto J, Aziz M, Mesaros C, Worth AJ, Wang LL, Javaheri A, Blair IA, Margulies KB, Rame JE. Evidence for intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 2016;133:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, Koves T, Gardell SJ, Kruger M, Hoppel CL, Lewandowski ED, Crawford PA, Muoio DM, Kelly DP. The failing heart relies on ketone bodies as a fuel. Circulation 2016;133:698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Horton JL, Davidson MT, Kurishima C, Vega RB, Powers JC, Matsuura TR, Petucci C, Lewandowski ED, Crawford PA, Muoio DM, Recchia FA, Kelly DP. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight 2019;4:e124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC Jr, Margulies KB, Coon JJ, Kelly DP. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 2016;1:e84897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010;122:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 2014;19:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation 2007;116:2809–2817. [DOI] [PubMed] [Google Scholar]
- 25. Noyan H, El-Mounayri O, Isserlin R, Arab S, Momen A, Cheng HS, Wu J, Afroze T, Li RK, Fish JE, Bader GD, Husain M. Cardioprotective signature of short-term caloric restriction. PLoS One 2015;10:e0130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ribeiro RF, Dabkowski ER, O’Connell KA, Xu W, Galvao T. D F, Hecker PA, Shekar KC, Stefanon I, Stanley WC. Effect of a high-protein diet on development of heart failure in response to pressure overload. Appl Physiol Nutr Metab 2014;39:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schugar RC, Moll AR, André d’Avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab 2014;3:754–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ho KL, Zhang L, Wagg C, Al Batran R, Gopal K, Levasseur J, Leone T, Dyck JRB, Ussher JR, Muoio DM, Kelly DP, Lopaschuk GD. Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency. Cardiovasc Res 2019;115:1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luukkonen PK, Dufour S, Lyu K, Zhang X-M, Hakkarainen A, Lehtimäki TE, Cline GW, Petersen KF, Shulman GI, Yki-Järvinen H. Effect of a ketogenic diet on hepatic steatosis and hepatic mitochondrial metabolism in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA 2020;117:7347–7354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ang QY, Alexander M, Newman JC, Tian Y, Cai J, Upadhyay V, Turnbaugh JA, Verdin E, Hall KD, Leibel RL, Ravussin E, Rosenbaum M, Patterson AD, Turnbaugh PJ. Ketogenic diets alter the gut microbiome resulting in decreased intestinal Th17 cells. Cell 2020;181:1263–1275.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wentz AE, d'Avignon DA, Weber ML, Cotter DG, Doherty JM, Kerns R, Nagarajan R, Reddy N, Sambandam N, Crawford PA. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem 2010;285:24447–24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, Murphy MP, Sokola BS, Bauer B, Hartz AMS, Lin AL. Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice. Sci Rep 2018;8:6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ranek MJ, Kokkonen-Simon KM, Chen A, Dunkerly-Eyring BL, Vera MP, Oeing CU, Patel CH, Nakamura T, Zhu G, Bedja D, Sasaki M, Holewinski RJ, Van Eyk JE, Powell JD, Lee DI, Kass DA. PKG1-modified TSC2 regulates mTORC1 activity to counter adverse cardiac stress. Nature 2019;566:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D'Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 2015;21:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Willeford A, Suetomi T, Nickle A, Hoffman HM, Miyamoto S, Heller Brown J. CaMKIIdelta-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight 2018;3:e97054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, Stone NJ, Van Horn LV., Van Horn LV; American Heart Association. Dietary fats and cardiovascular disease: a presidential advisory from the American heart association. Circulation 2017;136:e1–e23. [DOI] [PubMed] [Google Scholar]
- 37. Ikeda S, Mukai R, Mizushima W, Zhai P, Oka SI, Nakamura M, Del Re DP, Sciarretta S, Hsu CP, Shimokawa H, Sadoshima J. Yes-associated protein (YAP) facilitates pressure overload-induced dysfunction in the diabetic heart. JACC Basic Transl Sci 2019;4:611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G. Early versus late parenteral nutrition in critically ill adults. N Engl J Med 2011;365:506–517. [DOI] [PubMed] [Google Scholar]
- 39. Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 2017;97:1–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.