Abstract
Here we describe four isolations of Erysipelothrix rhusiopathiae associated with polyarthralgia and renal failure, septic arthritis, classic erysipeloid, and peritonitis. Although the biochemical identification was straightforward in each case, recognition presented a challenge to the clinical microbiologist, since in three cases E. rhusiopathiae was not initially considered due to unusual clinical presentations, in two cases the significance might not have been appreciated because growth was in broth only, and in one case the infection was thought to be polymicrobic. Because the Gram stain can be confusing, abbreviated identification schemes that do not include testing for H2S production could allow E. rhusiopathiae isolates to be misidentified as Lactobacillus spp. or Enterococcus spp. in atypical infections.
Erysipelothrix rhusiopathiae is a gram-positive, nonsporulating, rod-shaped bacterium that is widely distributed in nature and has primarily been seen as a veterinary pathogen (2, 3, 6, 7). Historically it has been associated with erysipeloid, a cutaneous inflammatory disease often affecting the hands and fingers, resulting from occupational exposure of persons who handle animals or animal products. More recently, it has been reported to be associated with more diverse clinical syndromes (1, 5). In this study, we isolated E. rhusiopathiae from various sites; in three cases, E. rhusiopathiae was not initially considered as a possible causative agent. Because the Gram stain, colony morphology, and negative catalase results might have suggested Lactobacillus, Actinomyces, Streptococcus, or even Enterococcus spp., which are common laboratory isolates that are not always fully identified, recognition presented a challenge to the clinical microbiologist.
Case 1.
A 61-year-old male was admitted with anemia, polyarthralgia, and renal failure. The patient was a carpenter who had worked on a farm for 3 years and had extensive contact with cattle, horses, and swine. Four months prior to admission, he experienced migratory arthralgias of the joints, with decreased range of motion, and was treated symptomatically with ibuprofen and heat with no improvement. One month prior to admission, prednisone was administered for suspected polymyalgia rheumatica. An evaluation 1 day prior to admission revealed a hematocrit of 30%, a white blood cell count of 21,000/mm with 85% polymorphonuclear cells (PMN), 4+ proteinuria, and 4+ hematuria.
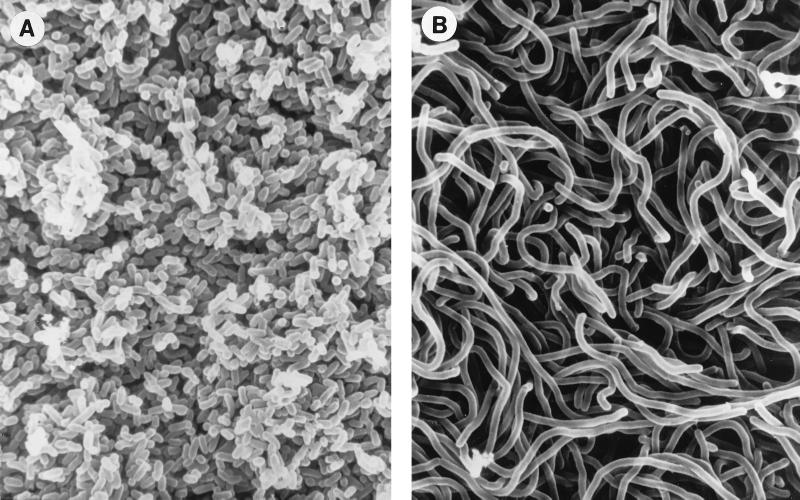
Blood cultures drawn on the third and fifth days of hospitalization were positive for multiple gram-positive forms, suggestive of a polymicrobial infection (2). After the positive blood cultures were subcultured to routine blood and chocolate agar media, two distinct colony types could be distinguished at 48 h. Type 1 colonies were smaller, moist, and convex, with an entire margin, and type 2 colonies were larger, with a flattened, serrated edge. Scanning electron microscopy of these colonies (Fig. 1) showed their very different morphologies, which were retained on subculture. Correspondingly, by Gram stain, organisms in type 1 colonies were short, almost coccoid, gram-positive rods while type 2 colonies were composed of long gram-positive and overdecolorized gram-negative filamentous forms. Both were subsequently identified as E. rhusiopathiae. We include these findings as we could not find documentation in the recent literature that both morphologic types can be retained in pure culture from a single specimen.
FIG. 1.
Scanning electron micrographs of the short forms seen in colonial type 1 (A) and the filamentous forms seen in colonial type 2 (B) E. rhusiopathiae. A Millipore filter with 72-h growth of E. rhusiopathiae was placed on a 5% sheep blood agar plate. The filter was lifted off and placed in 2% buffered glutaraldehyde. The width of the organism is 0.5 μm.
The patient was treated with nafcillin (6 g/day) for 13 days followed by cefazolin (1 g/6 h) for 4 weeks. The patient became afebrile, and blood cultures remained negative.
Case 2.
A 67-year-old male with chronic lymphocytic leukemia was admitted with complaints of swelling and pain in the right elbow for 3 months. The patient's past medical history was significant, including chronic lymphocytic leukemia (CLL) for 8 years and diabetes mellitus for 6 months. The patient had multiple rounds of chemotherapy and cryptococcal pneumonia. Aspirates of the elbow, obtained 3 months as well as 1 week prior to admission, showed many PMN but no bacteria or crystals on microscopic examination, and all cultures were negative. On the day of admission, 5 ml of turbid purulent fluid was obtained by aspiration; many PMN but no organisms were seen on the Gram stain, and the culture was negative.
The patient underwent right-elbow arthroscopy on hospitalization day 2, and both tissue and synovial fluid were sent to the microbiology laboratory for Gram stain and culture. The Gram stain showed much debris, few PMN, and no organisms. Culture of the synovial fluid grew E. rhusiopathiae, but only in thioglycollate broth, on culture day 4. The patient remained afebrile postoperatively, with clean wounds and full range of motion of the elbow. He was discharged with no antibiotic or systemic treatment and had no further complaints.
Case 3.
A 69-year-old male outpatient employed as a butcher was seen in the dermatology clinic for a localized lesion on the index finger that was associated with pain and swelling. The lesion was well defined and slightly elevated, with a peripheral zone of discoloration, but was not edematous. A tissue sample was obtained and submitted to the microbiology laboratory for Gram stain and culture. Microscopic examination of the Gram stain revealed moderate PMN but no organisms. E. rhusiopathiae was isolated from the thioglycollate broth on culture day 7. Treatment with a topical bacitracin ointment resulted in resolution of symptoms.
Case 4.
A 45-year-old male with a history of end-stage renal disease, on home peritoneal dialysis for 1 year, was seen 1 week prior to admission for complaints of fever to 101°F (38.3°C). His dialysis tubing was changed, and he was treated empirically with vancomycin for presumed peritonitis. Three days prior to admission, the patient again spiked fevers and was treated with gentamicin. One day prior to admission, the patient developed abdominal pain and was subsequently admitted for complicated peritonitis and suspected abscess due to treatment failure.
Peritoneal fluid was submitted to the microbiology laboratory for Gram stain and culture. Microscopic examination of the direct smear showed many PMN but no organisms. After 48 h of incubation, two alpha-hemolytic colonies were visible on 5% sheep blood agar. Since the colonies were smooth, the catalase reaction was negative, and the Gram stain of the colonies revealed predominantly short forms, all of which are suggestive of Enterococcus spp., a disc test for l-pyrrolidonyl-β-naphthylamide hydrolysis (PYR) was performed and found to be positive. The isolate would have been reported as Enterococcus sp. except that a few long pleomorphic gram-positive rods were seen on Gram stain for both colonies and the broth. Interestingly, the thioglycollate broth showed no visible turbidity. Both isolates were identified as E. rhusiopathiae. The patient's therapy was changed to penicillin (500,000 U/6 h), and he became afebrile on hospitalization day 7, with negative cultures on day 8. These clinical data have also been presented elsewhere (1).
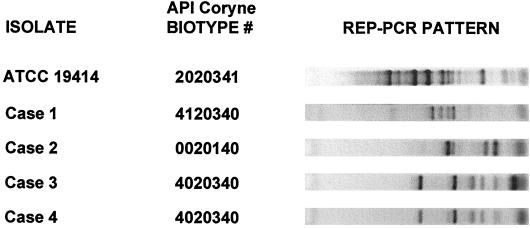
The features of these four cases of E. rhusiopathiae are summarized in Table 1. However, in none of the cases did the laboratory personnel know the patient's exposure history before isolation of E. rhusiopathiae. Because of the unexpected clinical presentations, we performed a detailed characterization of these isolates, thinking that they might represent atypical strains. However, all isolates were reproducibly identified as E. rhusiopathiae by the API Coryne method (biotype numbers are shown in Fig. 2) and Vitek systems, with a greater than 99% probability. However, the American Type Culture Collection (ATCC) type strain (from a swine spleen) was distinct biochemically since it was positive for sucrose. It is difficult to interpret this result since it has been reported that Erysipelothrix tonsillorum isolates are positive for sucrose and E. rhusiopathiae isolates are negative (2). All isolates were similar in that they were catalase negative, positive for H2S production on triple-sugar iron medium, positive for PYR hydrolysis, resistant to optochin, vancomycin resistant with no zone of inhibition, and penicillin susceptible with zone diameters of 28 to 34 mm (using the National Committee for Clinical Laboratory Standards criteria for Enterococcus spp.). Cellular fatty acid analysis by gas-liquid chromatography (performed by the MIDI system, Newark, Del., as described in reference 3) revealed a single cluster, with all isolates designated as E. rhusiopathiae and a similarity index of greater than 0.6. Repetitive extragenic palindromic PCR (REP-PCR) analysis (3) showed that isolates 3 and 4 exhibited similar REP-PCR patterns and that the ATCC strain was the most divergent (Fig. 2).
TABLE 1.
Characteristics of four cases of infection caused by E. rhusiopathiae
| Case no. | Patient's agea (yrs) | Occupation/source | Specimen type | Clinical data | Microbiological concern |
|---|---|---|---|---|---|
| 1 | 61 | Farm worker/cattle, horses, and swine | Blood | Polyarthralgias, bacteremia, renal involvement | Both colony and cell morphologies suggested polymicrobial infection |
| 2 | 67 | None known | Synovial fluid, right elbow | CLL, septic arthritis | Grew in broth only, on day 4 |
| 3 | 69 | Butcher/meat | Tissue, index finger | Classic erysipeloid | Grew in broth only, on day 7 |
| 4 | 45 | Cattle rancher/cattle | Peritoneal fluid | CAPD,b peritonitis | Only two colonies on solid medium; resembled Enterococcus spp. |
All patients were male.
CAPD, continuous ambulatory peritoneal dialysis.
FIG. 2.
Characterization of E. rhusiopathiae isolates and comparison to the type strain (ATCC 19414). When subjected to biochemical testing (API Coryne), the clinical strains differed only in the pyrrolidonyl arylamidase, alkaline phosphatase, and ribose tests. On the right are the REP-PCR patterns of the isolates. The ATCC strain was the only strain positive for sucrose.
It has been reported that the type 1 colony morphology is associated with septicemia and that the type 2 colony is associated with chronic conditions such as arthritis and endocarditis (5, 6). Interestingly, most of our isolates were of type 1; our case 1 showed the largest proportion of long type 2 forms, and case 3, the classical erysipeloid, showed the fewest. This is worth noting since most clinical microbiologists expect to see the filamentous forms.
Although E. rhusiopathiae is usually reported as an occupational pathogen and typically causes a cutaneous infection, our recent cases demonstrate a wider spectrum of disease (polyarthralgia with renal failure, septic arthritis, and peritonitis). Despite our isolates being biochemically typical, since the index of suspicion was low, confusion arose because the colonial appearance, negative catalase reaction, vancomycin resistance, and Gram stain suggested Enterococcus spp. (PYR hydrolysis was also consistent with Enterococcus) or Lactobacillus spp. We emphasize the importance of careful interpretation of Gram stains, since colonial appearance can be misleading, and of confirming the identity of catalase-negative gram-positive rods isolated in the presence of PMN from a normally sterile site by performing an H2S test. The importance of inoculating sterile fluids and tissues into an enrichment broth is evidenced by the fact that two of our isolates grew only in broth.
REFERENCES
- 1.Carlini M E, Clarridge J E, Rodrigues-Barradas M C. Erysipelothrix rhusiopathiae peritonitis in a patient on continuous ambulatory peritoneal dialysis. Infect Dis Clin Pract. 1998;7:53–55. [Google Scholar]
- 2.Clarridge J E, Spiegel C A. Corynebacterium and miscellaneous irregular gram-positive rods, Erysipelothrix, and Gardnerella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: ASM Press; 1995. pp. 357–378. [Google Scholar]
- 3.Clarridge J E, III, Raich T J, Sjösted A, Sandström G, Darouiche R O, Shawar R M, Georghiou P R, Osting C, Vo L. Characterization of two unusual clinically significant Francisella strains. J Clin Microbiol. 1996;34:1995–2000. doi: 10.1128/jcm.34.8.1995-2000.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones D. Genus Erysipelothrix. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams and Wilkins; 1986. pp. 1245–1249. [Google Scholar]
- 5.Jones N, Khoosal M. Erysipelothrix rhusiopathiae septicemia in a neonate. Clin Infect Dis. 1997;24:511. doi: 10.1093/clinids/24.3.511. [DOI] [PubMed] [Google Scholar]
- 6.Reboli A C, Farrar W E. Erysipelothrix rhusiopathiae: an occupational pathogen. Clin Microbiol Rev. 1989;2:354–359. doi: 10.1128/cmr.2.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodbine M. Erysipelothrix rhusiopathiae. Bacteriology and chemotherapy. Bacteriol Rev. 1950;14:161–178. doi: 10.1128/br.14.2.161-178.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]