Heat stroke is a potentially life-threatening condition characterized by a core temperature that exceeds the external temperature and is accompanied by central nervous system (CNS) abnormalities.[1] It has two forms, classical heat stroke and exertional heat stroke (EHS). EHS is usually experienced by athletes, military personnel, and outdoor laborers in hot and humid environments.[2]
Given the need for physical training and some intrinsic and extrinsic risk factors, military personnel have a high chance of having exertional heat illness (EHI), which is an occupational hazard. The incidence of EHI ranges from 0.2 to 10.5 cases per 1,000 person-years among military personnel, and severe EHI is classified as EHS.[3] In the United States Armed Forces, 4,188 cases of EHS were reported between 2008 and 2018,[4] and the overall crude incidence rate of EHS was 0.36 cases per 1,000 person-years in 2020; furthermore, male military personnel was more prone to EHS than female.[5]
EHS can lead to acute kidney injury (AKI), rhabdomyolysis, liver injury, and coagulation dysfunction and cause multiple organ dysfunction syndrome (MODS).[1,6] Its pre-hospital treatment involves a timely escape from the thermal environment and rapid cooling therapy. Its common feature is mild to moderate liver injury, and liver function usually return to normal in 2–16 d.[6] However, a small number of patients experience extensive hepatocyte damage leading to severe acute liver injury (SALI) and acute liver failure (ALF), which might be fatal.[7,8] The clinical course of ALF is initiated by a SALI, which is characterized by the development of coagulopathy and impaired liver function but does not have any level of clinical hepatic encephalopathy (HE).[9] As a new definition, SALI can be considered the prophase of ALF, affecting the prognosis of patients with acute hepatitis.[10] However, solid clinical research on the role of SALI in EHS remains lacking. In this study, we aimed to evaluate the clinical characteristics and 30-day outcomes of military personnel with SALI due to EHS.
METHODS
Study population
Patients who had EHS and were admitted to the First Medical Center of Chinese PLA General Hospital from January 2011 to December 2020 were retrospectively analyzed. This study was conducted following the Helsinki Declaration and approved by the Ethics Committee of Chinese PLA General Hospital. Inclusion criteria: military personnel and diagnosed with EHS. They were excluded if their length of stay was <24 h, they had incomplete laboratory results, or had uncertain prognosis on the 30th day of onset.
Fifty-two patients were included. Death on the 30th day of onset was considered the primary outcome. Information on prognosis was confirmed through telephone follow-up and medical records. All patients were administered cooling treatment at the onset through pre-hospital emergency care. After admission, they were given fluid resuscitation, airway protection, sedation, brain protection, coagulation factor supplement, and platelet transfusion. In addition, some patients received mechanical ventilation, renal replacement therapy, artificial liver support, and anti-infection and anticoagulation therapy accordingly. No patients needed organ transplantation during hospitalization.
Definitions
EHS was defined as a rapid increase in the body core temperature to higher than 40.5 °C and CNS dysfunction (such as delirium, convulsions, or coma) associated with strenuous exercise.[11, 12] Liver injury was defined as alanine aminotransferase (ALT), aspartate aminotransferase (AST), or total bilirubin (TBIL) higher than the upper limit of the normal (ULN). SALI was diagnosed based on the international normalized ratio (INR) > 1.5, ALT ≥ 10 × ULN, TBIL ≥ 3.0 mg/dL (51.3 μmol/L) within four weeks and without HE or chronic liver disease (CLD).[9,10] ALF was diagnosed on the basis of INR > 1.5, impaired liver function, obvious HE within four weeks, and without CLD.[9] Sequential organ failure assessment (SOFA) score was assessed based on the severity of respiratory, coagulation, liver, circulation, consciousness, and renal function.[13] MODS was identified as the SOFA score of >5, affecting more than one system.[14] HE was defined following the West Haven criteria.[15] AKI was defined using the criteria of kidney disease improving global guidelines.[16] Bacterial infections (BIs) were diagnosed through clinical features, laboratory tests, and imaging findings. Disseminated intravascular coagulopathy (DIC) was diagnosed on the basis of the International Society for Thrombosis and Haemostasis criteria.[17] Rhabdomyolysis was identified as creatine kinase exceeding five times the ULN.[18] The state of consciousness was determined by Glasgow coma scale (GCS) score, and a GCS score of <9 was considered a CNS disorder. Myocardial injury was defined as cardiac troponin I (cTnI) > 200 ng/mL. Respiratory failure was defined as the arterial partial pressure of oxygen lower than 60 mmHg (1 mmHg=0.133 kPa) or accompanied by the partial pressure of carbon dioxide higher than 50 mmHg under breathing room air conditions.
Statistical analysis
Statistical analyses were performed using SPSS 23.0 (IBM, USA). Categorical variables were expressed using count and proportion, and continuous variables were described as mean and standard deviation for data with normal distribution or median and interquartile range (IQR) for skewed data. Continuous variables were compared via Student’s t-test. A Mann-Whitney U-test was also performed to compare the skewed parameters. Categorical data were compared using the Chi-square test or Fisher’s exact test. Cumulative survival probability curves were assessed with the Kaplan-Meier method and compared through a log-rank test. A P-value of <0.05 was considered statistically significant.
RESULTS
Baseline clinical characteristics of all patients
All 52 patients were male Chinese military personnel. Of these patients, 45 were in the Army, six were in the Air Force, and one was in the Navy. These patients, with a mean age of 21.0 years (19.0–24.0 years), had no underlying disease before. All patients developed EHS during vigorous military training. They were hospitalized from April to September, with one case in April, 13 cases in May, nine cases in June, 17 cases in July, 10 cases in August, and two cases in September. Furthermore, 21 patients were transferred to our tertiary medical center from other military treatment facilities, and the average transfer time was 3 d (2–4 d) after onset. In addition, 31 patients were admitted to our hospital on the day of onset. These patients had a SOFA score of 2.5 (1.0–7.0). Of these patients, 14 (26.9%) had MODS, 40 (76.9%) had the liver injury, 33 (63.5%) had AKI, 25 (48.1%) had rhabdomyolysis, and 12 (23.1%) had CNS disorder. They were the most common complications upon admission (Table 1).
Table 1.
Clinical characteristic in the SALI and non-SALI groups at admission
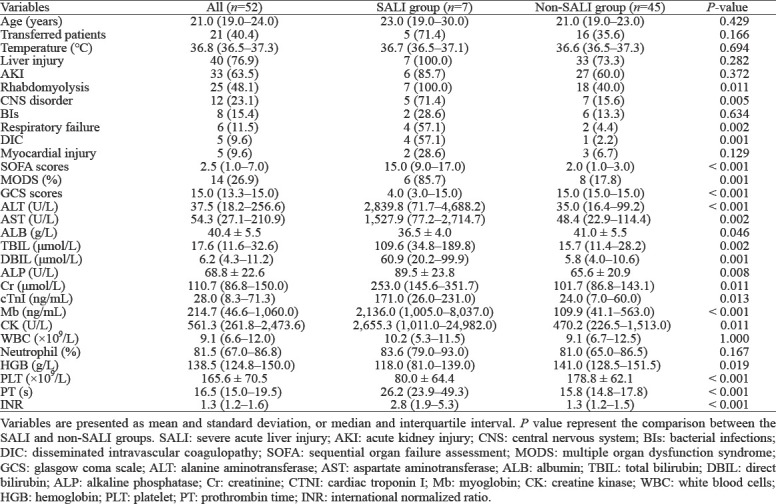
Clinical characteristics of patients with SALI
Seven (13.5%) patients were diagnosed with SALI in the course of EHS, while the remaining patients (86.5%) did not develop SALI. The SOFA score was 15.0 (9.0–17.0) in the SALI group. Of these patients, 100% had the liver injury and rhabdomyolysis, 85.7% had AKI, 71.4% had CNS disorder, 57.1% had respiratory failure, and 57.1% had DIC upon admission. Moreover, 85.7% of these patients developed MODS (Table 1). The incidence rates of rhabdomyolysis, CNS disorder, respiratory failure, DIC, and MODS of the SALI group were higher than those of the non-SALI group (P<0.05). In addition, the SOFA scores, ALT, AST, TBIL, direct bilirubin (DBIL), alkaline phosphatase (ALP), creatinine (Cr), cTnI, myoglobin (Mb), creatine kinase (CK), prothrombin time (PT), and INR of the SALI group were higher than those of the non-SALI group (P<0.05). By contrast, the GCS scores, albumin (ALB), hemoglobin (HGB), and platelet (PLT) in the SALI group were lower than those in the non-SALI group (P<0.05; Table 1).
The patients developed SALI with an average occurrence time of 4 d (4–6 d) after onset. When SALI occurred, the laboratary test indices showed that ALT was 3,995.2 U/L (2,839.8–4,688.2 U/L), AST was 2,710.0 U/L (1,527.9–4,773.2 U/L), TBIL was 131.4 μmol/L (98.1–189.8 μmol/L), DBIL was 60.9 μmol/L (41.1–99.9 μmol/L), INR was 2.9 (2.8–7.9), and PT was 35.0 s (26.2–64.5 s). In the SALI group, two patients (3.8%) developed HE and progressed to ALF within four weeks of onset. Furthermore, one patient had grade 2 HE, which was controlled after one week of the treatment. The other case developed grades 3–4 HE, which was progressed after the treatment.
Patients’ 30-day outcomes
On the 30th day of onset, the overall survival rate was 92.3%. Four patients died 16.0 ± 3.0 d after onset (11.5 ± 3.9 d after SALI). At the same time, no deaths were noted in the non-SALI group. When SALI occurred, all the patients who died suffered from AKI, rhabdomyolysis, CNS disorder, myocardial injury, and MODS, with SOFA scores ranging from 16 to 19. The results of the Kaplan-Meier method indicated that the 30-day survival rates in the non-SALI group were significantly higher than those in the SALI group (100.0% vs. 42.9%; χ2 = 33.008, P<0.001; Figure 1).
Figure 1.
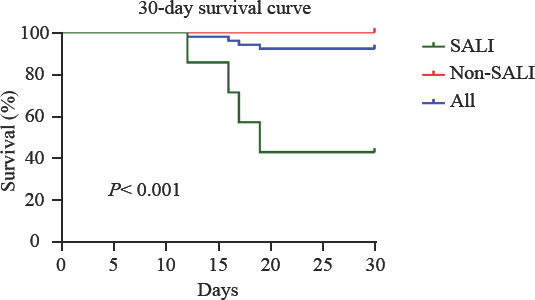
Comparison of the 30-day survival rates between the SALI and non-SALI groups. The 30-day survival rate in the non-SALI group was significantly higher than that in the SALI group (P< 0.001). SALI: severe acute liver injury.
DISCUSSION
This study on the clinical manifestations and prognosis of Chinese military personnel with SALI due to EHS in the past decade had three main findings. First, 13.5% of patients with EHS developed SALI, the average occurrence time was on the 4th day after onset, and 3.8% of patients with EHS had ALF. Second, the clinical conditions of patients with SALI were more severe than those of non-SALI patients, and the incidence of MODS of the former was higher than that of the latter. Third, the 30-day survival rates of the SALI patients significantly decreased compared with those without SALI.
The pathogenesis of liver injury caused by EHS may be related to multiple factors: direct thermal damage to vascular endothelium and hepatocytes, ischemic hepatitis caused by hypoperfusion, microthrombosis, and apoptosis and necrosis of hepatocytes caused by systemic inflammatory response syndrome (SIRS).[19, 20] Liver injury in most patients with EHS is usually asymptomatic, and their aminotransferase levels exhibit mild to moderate reversible elevation.[21] A large-sample study showed that most military patients with EHS have a liver injury. ALT peaked at 3.62 × ULN in 3–4 d and persisted for 1–14 d. AST (4.41 × ULN) peaked for 2 d and was on the constant rise for 0–8 d.[6] However, a small number of cases of severe liver damage and ALF are noted among military and civilian patients with EHS.[8, 22, 23] Our study showed that mild liver damage was common, whereas severe liver damage was rare. Furthermore, 76.5% of the patients had liver injury upon admission, 13.5% had SALI, and only 3.8% developed ALF. However, the description of EHS with ALF may still be controversial. First, some patients do not exhibit obvious HE, and their liver function does not sharply deteriorate, which is inconsistent with the current definition of ALF. Second, the coma caused by HE may overlap with the initial and constant CNS disorder of EHS; as such, differential diagnoses may be difficult. Nevertheless, the two diseases may be distinguished by determining blood ammonia and electroencephalogram monitoring, which are rarely performed in primary hospitals and pre-hospital emergency care. Third, the existence of coagulation dysfunction (especially DIC) caused by EHS may affect ALF diagnosis, and prognosis may be worse when these two diseases coincide. As the prophase of ALF, SALI mainly focuses on the acute deterioration of liver function rather than HE, which is more easily recognized by clinicians. Our study also showed that SALI could be detected in the early stages of EHS (4th day of onset), and these SALI patients have poor short-term prognoses. Therefore, emergency physicians should pay more attention to SALI in EHS.
The most common injury among patients with EHS is manifested as blood coagulation and observed in the muscle, CNS, liver, myocardium, kidney, and respiratory system. These patients are more likely to develop MODS and have worse prognoses.[24-26] Our study also showed that the liver, kidneys, muscles, and CNS were the most commonly injured organs of patients with EHS. In addition, some patients developed the respiratory injury, blood coagulation, and myocardial injury. The condition of patients with SALI was more severe than that of patients without SALI in the course of EHS because the organ damage degree in SALI patients was more serious, and they had more incidences of MODS and worse prognosis. The incidence rates of rhabdomyolysis, CNS disorder, respiratory failure, and DIC of patients with SALI were higher than those of non-SALI patients. All the SALI patients’ 30-day survival rate (42.9%) was significantly lower than that of non-SALI patients (100.0%). These results suggested that SALI is a symptom of MODS and an indicator of worsen prognosis of EHS. These findings might be related to the pathophysiological mechanism and pathological changes caused by SALI and ALF in EHS. The course of severe heat-related illnesses combined with SALI and ALF involves protein denaturation, endotoxin release, thermoregulatory disorder, SIRS, interleukin-1beta (IL-1β) and high-mobility group box 1 (HMGB-1)-induced pyroptosis, leading to massive or submassive hepatocyte necrosis, which ultimately aggravates the conditions of such patients.[20, 27, 28]
The 10%–30% mortality rate of EHS in developing countries is still relatively high,[26, 29, 30] whereas its mortality rate in developed countries is less than 5%.[20, 31] An Indian study has demonstrated that the mortality rate reaches 86% when heat stroke is accompanied by MODS.[25] This gap may be related to the attention and early identification of EHS, highly efficient pre-hospital cooling treatment, and sufficient medical resources.[31] Our study revealed that the overall mortality rate of patients with EHS was 7.7%, patients without SALI was 0.0%, and the mortality rate of patients with SALI was more than 50%. However, studies have yet to establish standard international criteria and determine whether liver transplantation (LT) is necessary for patients with SALI or ALF caused by EHS. Some case reports have indicated that LT can benefit patients with fulminant liver failure caused by EHS.[32] Another study has demonstrated that among 24 patients with EHS complicated with SALI, nine patients entered the list of emergency LT, only four patients received this surgery, and 20 patients recovered after medical treatment. It suggested that patients with a prothrombin activity of <10% after 3 d of medical therapy should consider LT.[20] Our study showed that the patients died 11.5 d after SALI without LT, and had a high SOFA score. Therefore, patients with EHS complicated with SALI should receive medical treatment immediately. Those with high SOFA scores and no significant improvement after therapy should receive urgent LT.
Given the physical hazards and risk of death of EHS, military personnel should undergo scientific training methods, receive proper education, and make behavioral adaptations in hot and humid environments. Medical and cooling treatments in prehospital emergency care are also needed.[33] When patients develop EHS combined with SALI and MODS, they should be immediately transferred to a tertiary hospital.
This study has several limitations. First, it is a single-center retrospective study with a small sample size. As such, additional prospective studies with multicenter and large sample size should be conducted. Second, some patients were transferred from other primary military medical facilities. This condition ensured the objectivity of this study, but the specific medical information and prehospital cooling strategies were not provided in detail.
CONCLUSIONS
Liver injury is a common complication of EHS. More than 10% of patients may experience SALI, and a small number of patients may develop ALF. Patients with SALI have a higher incidence of rhabdomyolysis, AKI, CNS disorder, respiratory failure, and DIC and are more likely to develop MODS than those without SALI. In addition, the 30-day prognoses of patients with SALI are worse than those without SALI. Therefore, SALI may play a more critical role in assessing patients with EHS than ALF.
Footnotes
Funding: This work was supported by the Capital’s Funds for Health Improvement and Research of China (2020-1-5031) and the Military Medical Innovation Research Project of Chinese PLA General Hospital (CX19014).
Ethical approval: This study followed the Helsinki Declaration and was approved by the Ethics Committee of Chinese PLA General Hospital. Informed consent was waived because of the retrospective nature of this study.
Conflicts of interests: All authors declare that they have no competing interests.
Contributors: CL, HBS, QS and JHH designed this study. HL, XL and HMW collected the data of patients. CL wrote the first draft with assistance from QS, JHH and HBS. QS and JHH edited the final manuscript. All authors read and approved the final manuscript.
REFERENCES
- 1.Becker JA, Stewart LK. Heat-related illness. Am Fam Physician. 2011;83(11):1325–30. [PubMed] [Google Scholar]
- 2.Sawka MN, Leon LR, Montain SJ, Sonna LA. Integrated physiological mechanisms of exercise performance, adaptation, and maladaptation to heat stress. Compr Physiol. 2011;1(4):1883–928. doi: 10.1002/cphy.c100082. [DOI] [PubMed] [Google Scholar]
- 3.Alele FO, Malau-Aduli BS, Malau-Aduli AEO, Crowe MJ. Epidemiology of exertional heat illness in the military:a systematic review of observational studies. Int J Environ Res Public Heal. 2020;17(19):7037. doi: 10.3390/ijerph17197037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filep EM, Murata Y, Endres BD, Kim G, Stearns RL, Casa DJ. Exertional heat stroke, modality cooling rate, and survival outcomes:a systematic review. Medicina (Kaunas) 2020;56(11):589. doi: 10.3390/medicina56110589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Update:Heat illness, active component, U.S. Armed Forces, 2020. MSMR. 2021;28(4):10–5. [PubMed] [Google Scholar]
- 6.Ward MD, King MA, Gabrial C, Kenefick RW, Leon LR. Biochemical recovery from exertional heat stroke follows a 16-day time course. PLoS One. 2020;15(3):e0229616. doi: 10.1371/journal.pone.0229616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro F, Bibi M, Pereira M, Ferreira S, Pessegueiro H, Araújo R. Severe acute liver injury related to heat stroke. Eur J Case Rep Intern Med. 2020;7(2):001382. doi: 10.12890/2020_001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis BC, Tillman H, Chung RT, Stravitz RT, Reddy R, Fontana RJ, et al. Heat stroke leading to acute liver injury &failure:a case series from the Acute Liver Failure Study Group. Liver Int. 2017;37(4):509–13. doi: 10.1111/liv.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver. Clinical practice guidelines panel. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–81. doi: 10.1016/j.jhep.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Koch DG, Speiser JL, Durkalski V, Fontana RJ, Davern T, McGuire B, et al. The natural history of severe acute liver injury. Am J Gastroenterol. 2017;112(9):1389–96. doi: 10.1038/ajg.2017.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casa DJ, DeMartini JK, Bergeron MF, Csillan D, Eichner ER, Lopez RM, et al. National athletic trainers'association position statement:exertional heat illnesses. J Athl Train. 2015;50(9):986–1000. doi: 10.4085/1062-6050-50.9.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belval LN, Casa DJ, Adams WM, Chiampas GT, Holschen JC, Hosokawa Y, et al. Consensus statement- prehospital care of exertional heat stroke. Prehosp Emerg Care. 2018;22(3):392–7. doi: 10.1080/10903127.2017.1392666. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli M, Moreno R, Vincent JL, Sprung CL, Mendoça A, Passariello M, et al. Application of SOFA score to trauma patients. Sequential Organ Failure Assessment. Intensive Care Med. 1999;25(4):389–94. doi: 10.1007/s001340050863. [DOI] [PubMed] [Google Scholar]
- 14.Cole E, Gillespie S, Vulliamy P, Brohi K. Organ Dysfunction in Trauma (ORDIT) study collaborators. Multiple organ dysfunction after trauma. Br J Surg. 2020;107(4):402–12. doi: 10.1002/bjs.11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337(7):473–9. doi: 10.1056/NEJM199708143370707. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 17.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–30. [PubMed] [Google Scholar]
- 18.Nance JR, Mammen AL. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve. 2015;51(6):793–810. doi: 10.1002/mus.24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leon LR, Helwig BG. Heat stroke:role of the systemic inflammatory response. J Appl Physiol (1985) 2010;109(6):1980–8. doi: 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- 20.Ichai P, Laurent-Bellue A, Camus C, Moreau D, Boutonnet M, Saliba F, et al. Liver transplantation in patients with liver failure related to exertional heatstroke. J Hepatol. 2019;70(3):431–9. doi: 10.1016/j.jhep.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 21.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–88. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 22.Wijerathne BT, Pilapitiya SD, Vijitharan V, Farah MM, Wimalasooriya YV, Siribaddana SH. Exertional heat stroke in a young military trainee:is it preventable? Mil Med Res. 2016;3:8. doi: 10.1186/s40779-016-0078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Q, Chen EZ, Jiang J, Lu YM. Acute hepatic failure as a leading manifestation in exertional heat stroke. Case Rep Crit Care. 2012;2012:295867. doi: 10.1155/2012/295867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abriat A, Brosset C, Brégigeon M, Sagui E. Report of 182 cases of exertional heatstroke in the French armed forces. Mil Med. 2014;179(3):309–14. doi: 10.7205/MILMED-D-13-00315. [DOI] [PubMed] [Google Scholar]
- 25.Varghese GM, John G, Thomas K, Abraham OC, Mathai D. Predictors of multi-organ dysfunction in heatstroke. Emerg Med J. 2005;22(3):185–7. doi: 10.1136/emj.2003.009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong HS, Liu YS, Wen Q, Tang YQ, Yuan FF, Su L. Serum procalcitonin predicting mortality in exertional heatstroke. Emerg Med J. 2012;29(2):113–7. doi: 10.1136/emj.2010.107680. [DOI] [PubMed] [Google Scholar]
- 27.Pryor RR, Bennett BL, OʼConnor FG, Young JMJ, Asplund CA. Medical evaluation for exposure extremes:heat. Clin J Sport Med. 2015;25(5):437–42. doi: 10.1097/JSM.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 28.Geng Y, Ma Q, Liu YN, Peng N, Yuan FF, Li XG, et al. Heatstroke induces liver injury via IL-1βand HMGB1-induced pyroptosis. J Hepatol. 2015;63(3):622–33. doi: 10.1016/j.jhep.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Yang MM, Li Z, Zhao Y, Zhou FH, Zhang Y, Gao JL, et al. Outcome and risk factors associated with extent of central nervous system injury due to exertional heat stroke. Medicine (Baltimore) 2017;96(44):e8417. doi: 10.1097/MD.0000000000008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhong L, Ji JJ, Wang CL, Liu ZF. Clinical characteristics and risk factors of male exertional heatstroke in patients with myocardial injury:an over 10-year retrospective cohort study. Int J Hyperthermia. 2021;38(1):970–5. doi: 10.1080/02656736.2021.1941312. [DOI] [PubMed] [Google Scholar]
- 31.Donham BP, Frankfurt SB, Cartier RA, O'Hara SM, Sieg VC. Low incidence of death and renal failure in United States military service members hospitalized with exertional heat stroke:a retrospective cohort study. Mil Med. 2020;185(Suppl 1):362–7. doi: 10.1093/milmed/usz214. [DOI] [PubMed] [Google Scholar]
- 32.Figiel W, Morawski M, Grąt M, Kornasiewicz O, Niewiński G, Raszeja-Wyszomirska J, et al. Fulminant liver failure following a marathon:five case reports and review of literature. World J Clin Cases. 2019;7(12):1467–74. doi: 10.12998/wjcc.v7.i12.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein Y, Druyan A, Heled Y. Heat injury prevention--a military perspective. J Strength Cond Res. 2012;26(Suppl 2):S82–6. doi: 10.1519/JSC.0b013e31825cec4a. [DOI] [PubMed] [Google Scholar]


