Abstract
A 43-year-old healthy female with no significant medical problems except for recently diagnosed pelvic inflammatory disease presented to our hospital with acute onset, severe head and neck pain. Brain imaging revealed a rim-enhancing lesion consistent with an abscess. The patient underwent successful surgical removal of the abscess and its capsule. Intraoperative cultures grew Streptococcus intermedius and she was discharged with a plan for four weeks of intravenous ceftriaxone.
Keywords: Cerebral abscess, Pelvic inflammatory disease, Intrauterine device
Introduction
Streptococcus intermedius is a gram-positive B-hemolytic pathogen, belonging to the larger Streptococcus anginosus group [1]. This organism is part of the normal oral, gastrointestinal, and urogenital flora of the human body and is considered to be a low-virulence organism in immunocompetent patients but can be associated with significant morbidity and mortality [2]. Reports have demonstrated a predilection for this organism to cause pyogenic infections, particularly brain and liver abscesses [1], [3].
Brain abscesses caused by S. intermedius have been well-described in the literature. However, brain abscesses in association with intrauterine device use and pelvic inflammatory disease (PID) are very rare. We describe the case of a healthy young female who was treated with guideline-based therapy for pelvic inflammatory disease yet developed a metastatic focus of infection to the brain, likely because of the pathogen causing her pelvic infection.
Case presentation
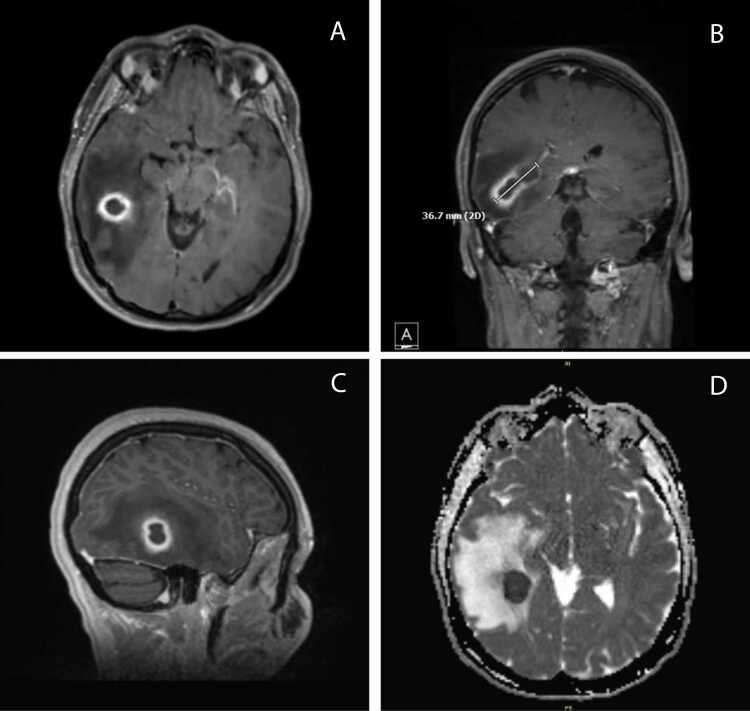
A 43-year-old Caucasian female with recently treated PID presented to the emergency department (ED) with severe neck pain and headache for six days. She denied fever, chills, shortness of breath, paresthesias, or focal neurological deficits. She had no recent upper respiratory infections, dental infections or procedures. On presentation she was hemodynamically stable and had a mildly elevated white blood cell count of 10,400/μL. Computed tomography (CT) imaging of the brain without contrast revealed a 16 × 17 mm right temporal lobe mass with substantial edema. CT imaging of the chest, abdomen, and pelvis were negative for a source of infection. The patient subsequently underwent Magnetic Resonance Imaging (MRI) of the brain with and without contrast which revealed the known right temporal mass (3.7 ×2.2 cm) with imaging features most consistent with abscess (Fig. 1).
Fig. 1.
MRI brain T1 (a–c) and diffusion-weighted (d) imaging depicting ovoid-shaped intra-axial mass in the posterior right temporal lobe measuring approximately 3.7 cm by 2.2 cm. The lesion demonstrates peripheral enhancement which is more thick-walled inferiorly and laterally and more thin-walled near the deformed atrium of the right lateral ventricle. The lesion has central restricted diffusion. There is a large amount of surrounding vasogenic edema. Imaging features are most consistent with abscess.
The patient had a long-standing history of intrauterine device (IUD) use. One month prior to admission she had a new IUD placed. Two weeks later, she presented to an outside ED with abdominal pain, nausea, and vomiting. CT of the abdomen and pelvis revealed a small amount of pelvic fluid and an appropriately positioned IUD. Pelvic exam was notable for yellow discharge, motion tenderness at the cervix as well as right adnexal tenderness. A wet mount was positive for clue cells, without evidence of trichomonads. Gonorrhea and chlamydia nucleic acid amplification testing of the discharge were negative and urine culture showed< 10,000 colony forming units (CFUs) gram positive flora. She received a dose of ceftriaxone and was discharged with a 1-week course of metronidazole and 2-week course of doxycycline for PID. She continued to have symptoms despite antimicrobial therapy and so her IUD was removed three days later, with resolution of symptoms. Repeat vaginal exam at that time was normal. Antimicrobial therapy with metronidazole was extended by an extra week and she was instructed to complete the 2 weeks of doxycycline. When she began to develop headache, however, she stopped doxycycline after only a week, having heard that this medication could cause headaches.
On admission to our hospital the patient was empirically started on vancomycin, ceftriaxone, and metronidazole. She underwent an uncomplicated right sided temporal craniotomy with evacuation of the abscess and its capsule. Intra-operative cultures grew S. intermedius, resistant only to clindamycin. Her pain improved significantly after surgery and subsequent imaging showed regression of the surrounding edema. She was discharged on a 4-week course of ceftriaxone. Follow-up imaging one month after discharge showed mild persistent enhancement in the right posterior lateral temporal lobe so the patient’s antimicrobial therapy was extended for an additional three weeks.
Discussion
Pelvic inflammatory disease is the general term for infection of the female upper genital tract including the endometrium, fallopian tubes, ovaries, and pelvic peritoneum [4]. It is typically caused by ascending infection from the vagina and is secondary to sexual activity and/or instrumentation. Symptoms of PID can include abnormal vaginal discharge, mild to severe lower abdominal pain, dyspareunia, and dysuria. Risk factors for PID include multiple sexual partners, younger age, previous history of PID, and bacterial vaginosis (BV). Usual pathogens in acute PID are Chlamydia trachomatis and Neisseria gonorrhoeae; less common pathogens include respiratory/enteric pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, Escherichia coli and Bacteroides fragilis. Mycobacterium tuberculosis and Actinomyces spp are known pathogens of chronic PID [4].
Recommended treatment for outpatient cases of PID is doxycycline for two weeks with metronidazole, plus a third-generation cephalosporin [5]. There has been a recent push to add metronidazole to the PID treatment regimen for broader anaerobic as well as BV coverage [5], [6]. Guidelines also discuss possible Mycoplasma genitalium coverage with moxifloxacin, though the importance of providing coverage for this pathogen is unknown [5]. An initial episode of PID is not an indication to remove an IUD.
Pelvic abscess in the setting of IUD use is a well-described, albeit rare, phenomenon. Persistent inflammation and invasion of the tubal epithelium by bacterial pathogens result in the breakdown of the normal structure of the fallopian tubes and ovaries, leading to a leukocyte response and abscess formation [7], [8]. Studies suggest that approximately one-third of patients with PID who are admitted to the hospital may have tubo-ovarian abscesses (TOAs). Metastatic foci to the brain are much rarer. Presumably they occur secondary to hematogenous spread from a pelvic site of infection. Only three cases of brain abscesses associated with IUD use have been reported in the literature [9], [10], [11]. Causative pathogens were Actinomyces, Fusobacterium nucleatum, and Bacteroides fragilis. The authors postulated that in these cases, uncontrolled or incompletely treated pelvic infection led to hematogenous spread of pathogens to the central nervous system, resulting in brain abscesses.
To the best of our knowledge, this is the first reported case of a cerebral abscess caused by S. intermedius as a complication of IUD-associated PID. S. intermedius is known to be a normal part of the genitourinary (GU) microbiome, and we hypothesize that our patient had chronic pelvic and IUD colonization by this pathogen. The patient was treated appropriately for PID based on guidelines, and though she did receive ceftriaxone which is active against S. intermedius, one dose may not be sufficient for treatment. Metronidazole likely treated BV that may have been present, but doxycycline does not have good Streptococcus coverage and it is possible this pathogen escaped due to lack of targeted therapy. Despite the fact that our patient underwent IUD removal, she may have had continued ascending infection due to S. intermedius, which subsequently spread hematogenously to the central nervous system.
Conclusion
Cerebral abscesses following PID are rare and may be the result of hematogenous spread of organisms following acute infection or long-term colonization of the GU tract. Clinicians should be aware of this possible complication of PID in the setting of IUD use even in patients who received guideline-based treatment.
Ethical approval
Ethical approval was not required for this case report.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Financial disclosure
The authors have no financial disclosures.
CRediT authorship contribution statement
Simran Gupta: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Sabirah N. Kasule: Writing – original draft, Writing – review & editing. Maria Teresa Seville: Writing – review & editing, Supervision.
Conflict of interest
The authors have no conflicts of interest.
References
- 1.Issa E., Salloum T., Tokajian S. From normal flora to brain abscesses: a review of Streptococcus intermedius. Front Microbiol. 2020;11:826. doi: 10.3389/fmicb.2020.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doern C.D., Burnham C.A. It’s not easy being green: the viridans group streptococci, with a focus on pediatric clinical manifestations. J Clin Microbiol. 2010;48:3829–3835. doi: 10.1128/JCM.01563-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darlow C.A., McGlashan N., Kerr R., Oakley S., Pretorius P., Jones N., et al. Microbial aetiology of brain abscess in a UK cohort: prominent role of Streptococcus intermedius. J Infect. 2020;80(6):623–629. doi: 10.1016/j.jinf.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunham R.C., Gottlieb S.L., Paavonen J. Pelvic inflammatory disease. N Engl J Med. 2015;372(21):2039–2048. doi: 10.1056/NEJMra1411426. [DOI] [PubMed] [Google Scholar]
- 5.Workowski K.A., Bachmann L.H., Chan P.A., Johnston C.M., Muzny C.A., Park I., et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(No. RR-4):1–187. doi: 10.15585/mmwr.rr7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiesenfeld H.C., Meyn L.A., Darville T., Macio I.S., Hillier S.L. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis. 2021;72(7):1181–1189. doi: 10.1093/cid/ciaa101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osborne N.G. Tubo-ovarian abscess: pathogenesis and management. J Natl Med Assoc. 1986;78(10):937–951. [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S.H., Kim S.H., Yang D.M., Kim K.A. Unusual causes of tubo-ovarian abscess: CT and MR imaging findings. Radiographics. 2004;24(6):1575–1589. doi: 10.1148/rg.246045016. [DOI] [PubMed] [Google Scholar]
- 9.Kum N., Charles D. Cerebral abscess associated with an intrauterine contraceptive device. Obstet Gynecol. 1979;54(3):375–378. [PubMed] [Google Scholar]
- 10.Rachdi K., Legros V., Mestrallet S., Just B., Mateu P. Multiple brain abscesses of gynecologic origin caused by Fusobacterium nucleatum. Réanimation. 2014;3(3):327–328. [Google Scholar]
- 11.de la Monte S.M., Gupta P.K., White C.L., 3rd Systemic actinomyces infection. A potential complication of intrauterine contraceptive devices. JAMA. 1982;248(15):1876–1877. doi: 10.1001/jama.248.15.1876. [DOI] [PubMed] [Google Scholar]