Abstract
Fetal, infant, and toddler neuroimaging is commonly thought of as a development of modern times (last two decades). Yet, this field mobilized shortly after the discovery and implementation of MRI technology. Here, we provide a review of the parallel advancements in the fields of fetal, infant, and toddler neuroimaging, noting the shifts from clinical to research use, and the ongoing challenges in this fast-growing field. We chronicle the pioneering science of fetal, infant, and toddler neuroimaging, highlighting the early studies that set the stage for modern advances in imaging during this developmental period, and the large-scale multi-site efforts which ultimately led to the explosion of interest in the field today. Lastly, we consider the growing pains of the community and the need for an academic society that bridges expertise in developmental neuroscience, clinical science, as well as computational and biomedical engineering, to ensure special consideration of the vulnerable mother-offspring dyad (especially during pregnancy), data quality, and image processing tools that are created, rather than adapted, for the young brain.
Abbreviations: MRI, Magnetic Resonance Imaging; fMRI, functional MRI; FDA, Food and Drug Administration; FIT’NG, Fetal, Infant, Toddler Neuroimaging Group
Keywords: MRI, FMRI, FIT’NG, Infant neuroimaging, Fetal neuroimaging, Brain development, Longitudinal studies
1. Introduction
Research in fetal, infant, and toddler neuroimaging has steadily increased from an average of 160 publications per year during the 1990’s, to roughly 530 in 2021. In a period of heightened scientific interest and rapid technological advancements in neuroimaging in early life, it is timely to review how magnetic resonance imaging (MRI) became a tool to study the developing brain. Few may realize that the field of fetal, infant, and toddler neuroimaging first emerged in the 1980s – shortly after the development of MRI. Since that time, we have learned an enormous amount about brain development. This review chronicles the parallel developments in the fields of fetal, infant, and toddler neuroimaging (noting a shift from clinical to research use) and the resolved and ongoing challenges in this fast-growing interdisciplinary field. We highlight several key advances in the field to illustrate how early developments both in MRI technology and basic research laid the groundwork for modern advances in early childhood imaging. We also highlight the major large-scale multi-site studies, key findings, and technological advancements that ultimately led to the explosion of interest in the field today and propelled our understanding of the early development of the human brain. Lastly, we consider the growing pains of the community and the need for a society that bridges expertize in developmental neuroscience, clinical science, and computational and biomedical engineering to ensure special consideration of the vulnerable mother-offspring dyad, data quality, and image processing tools.
In doing so, we introduce a new academic society, the Fetal, Infant, and Toddler Neuroimaging Group (FIT’NG), which aims to bring researchers together whose work share the common goal of expanding our understanding of neurodevelopment during the first years of life. While the fetal period through toddlerhood is a large age range, cognitive development during this time is both formative and rapid. As such, an understanding of developmental milestones within this window is essential to advancing developmental science. FIT’NG is building a community for networking and collaborative endeavors and a platform for software and hardware developers (e.g., engineers, programmers, and physicists) and end users of the tools (e.g., psychologists, psychiatrists, neonatologists, neuroscientists) to engage in constructive discussions related to technological and methodological gaps in our knowledge. Such gaps are inherent to working with such young and vulnerable populations and require collaborative effort to be filled. Together, this manuscript reviews the history and accomplishments of the field of fetal, infant, and toddler neuroimaging (using MRI) during the past 40 years and the role that FIT’NG aims to play in its advancement in the upcoming years.
2. Historical context: A brief history of MRI
MRI is a non-invasive imaging modality that was invented in the 20th century (Lauterbur, 1973, Mansfield and Maudsley, 1977) and is widely used today for the study of the human body. At least six Nobel prizes between 1943 and 2003 were awarded to scientists for their groundbreaking work that led to the invention of MRI as we know it today. However, just as with any new area of research, many practical and technical challenges in the early years existed. The first MRI machine, named ‘Indomitable’ (Kleinfield, 2014), regularly leaked liquid helium and was too small to fit the first participant. It was only after several iterations of refinement that Indomitable produced the first image of a human chest; at this time, it took five hours to acquire an image (Damadian et al., 1977). In 1980, several publications demonstrated the feasibility of brain imaging in adults (Hawkes et al., 1980, Holland et al., 1980, Holland et al., 1980). The first infant and fetal brain scans would not be documented until 1982 and 1983, respectively (Levene et al., 1982, Smith et al., 1983). In 1984, following technological improvements, the Food and Drug Administration (FDA) approved the use of MRI for human imaging in the United States. Since then, MRI has become popular in both clinical and research settings. In 1995 there were 2785 MRI scanners in the United States (OECD, 2021). Today, there are approximately 13,278 MRI scanners, with approximately 42 million scans being performed annually in the United States alone (OECD, 2019, OECD, 2021).
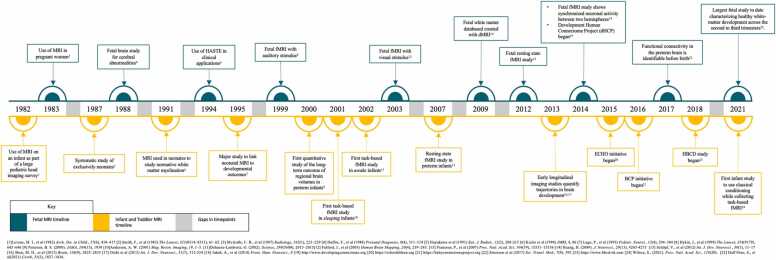
As accessibility to MRI increased, the images that were acquired also evolved. The earliest scans (in the 1980s) were largely structural and used to examine the anatomy and pathology of the brain. Soon, the advances in echo-planar imaging brought forth other neuroimaging modalities. Functional magnetic resonance imaging (fMRI) was developed in 1990 (Ogawa et al., 1990). fMRI measures changes in the spatiotemporal distribution of neural system physiology by measuring the blood oxygen level dependent (BOLD) signal (Anderson and Thomason, 2013). By 1992, it was clear that the BOLD signal could be used as an indirect measure of neural activity (Kwong et al., 1992). Also in the 1990’s, a new technique called diffusion weighted imaging (dMRI) emerged (Basser et al., 1994). Since water diffusion varies with the brain tissue microstructure, dMRI combined with tractography uses the anisotropic property of diffusion in a white matter axonal bundle to estimate the organization of connections (Huang, 2010, Kasprian et al., 2008). These advancements in MRI data acquisition laid the foundation for the field of fetal, infant, and toddler neuroimaging. (See Fig. 1 for timeline of key developments in fetal, infant and toddler neuroimaging).
Fig. 1.
Timeline of key events in the history of fetal, infant and toddler MRI imaging.
3. Fetal MRI
3.1. The early years (1980s)
Fetal MRI was driven in large part by a clinical demand. The first report documenting imaging of fetuses and pregnant women was in 1983 (Smith et al., 1983, Smith et al., 1984). At the time, there was insufficient information regarding the safety3 of this new technology for pregnant women and fetuses. Consequently, much of the research at this time was aimed at demonstrating the feasibility of this method and comparing the output of structural MRI to ultrasound. During most of the 1980s, MRI was used in pregnant women to evaluate maternal anatomy and pathology (McCarthy et al., 1985, Weinreb et al., 1985), and fetal anatomy (Daffos et al., 1988, McCarthy et al., 1985). Particularly there was a need for defining congenital anomaly of the brain and early brain injury in the fetal period (Menticoglou et al., 1989, Sims et al., 1985).
3.2. Maturation of sequences to improve structural MRI (1990s)
By the 1990s, the use of MRI during pregnancy had increased substantially and was used as a complement to ultrasound when findings were not definitive (Girard et al., 1993), demonstrating its utility in certain clinical cases (Angtuaco et al., 1992). While providing unprecedented visual access to the developing fetus, image quality was distorted by fetal movements and the mother's breathing movements. To reduce fetal movement, various pharmacological methods (e.g., neuromuscular blocking agents) were introduced to sedate either the mother and/or fetus (Daffos et al., 1988, Girard et al., 1993, Horvath and Seeds, 1989, Yuh et al., 1994). These techniques may pose risks for the fetus and pregnant women (e.g., Garel and Brisse, 1998), limiting them to clinically necessary scans, and necessitating the development of new data acquisition strategies to allow for research data collection in this vulnerable population.
In the 1990s, new sequences, such as the single-shot rapid acquisition sequence with refocused echoes (HASTE), were developed (Tsuchiya et al., 1996, Yamashita et al., 1997). These new faster sequences reduced acquisition times to less than a second and propelled fetal MRI research forward. With the ability to visualize fetal anomalies with high contrast and precision (Coakley et al., 1999, Garel and Brisse, 1998, Hubbard et al., 1999, Levine et al., 1997, Levine et al., 1998, Levine et al., 1999, Sonigo et al., 1998), and to estimate fetal brain volumes (Gong et al., 1998), MRI was recognized as having superior accuracy (relative to ultrasound) in identifying fetal anomalies (Bilaniuk, 1999, Sonigo et al., 1998). Nevertheless, even with the advent of more rapid sequences, fetal and maternal movement remains an ongoing challenge and a number of clinical investigators continued using invasive pharmacological methods to reduce fetal activity until the end of the 1990s4 (Resta et al., 1998).
3.3. Emergence of echo-planar imaging in fetuses (2000–2010s)
While structural MRI studies of fetuses flourished in the 1980s and 1990s, it would not be until the early 2000s that fMRI and dMRI studies in fetuses were published. In 1999, Hykin and colleagues performed the first fetal fMRI study (Hykin et al., 1999). This work provided evidence of fetal brain activity in utero, in response to an auditory stimulus, which was later replicated in a larger sample (Moore et al., 2001). Forthcoming task-based fMRI studies in fetuses assessed brain responses to visual stimuli (i.e., a light source shone at the maternal abdomen; Fulford et al., 2003) and vibroacoustic stimuli (Fulford et al., 2004). Similarly, maternal speech has shown evidence for cortical sensory activation at the beginning of the third trimester (Jardri et al., 2012) and maternal singing has shown activation of the fetal auditory network and Heschl’s gyrus (Goldberg et al., 2020). These study designs were later combined with exciting developments in the analysis capabilities with fetal fMRI data including methods for automatic brain extraction, segmentation, registration, and reconstruction of the moving fetal brain (Caldairou et al., 2011, Keraudren et al., 2014; Kim et al., 2010; Kuklisova-Murgasova et al., 2012; Rousseau et al., 2016; Seshamani et al., 2014, Seshamani et al., 2013; You et al., 2016). While challenges exist (see Dunn et al., 2015 for a review), these foundational studies have allowed for deeper interrogation of the intrinsic functional connectivity of the fetal brain.
After these early task fMRI studies in fetuses, many fMRI researchers shifted their focus to functional connectivity – functionally integrated association between the BOLD time courses of spatially distinct brain regions – collected in the absence of external structured stimuli (i.e., during “resting state”). While a variety of phenotypic information are thought to stem from patterns of functional connectivity (Liao et al., 2017), it was not until 2012 that researchers discovered that resting state networks are detectable in utero (Schöpf et al., 2012). Subsequent longitudinal studies demonstrated that the proximal and distal connections between different brain networks form over the second half of the pregnancy and peak between 27 and 30 weeks (Jakab et al., 2014). These foundational studies provided key insight into the organization and development of fetal brain networks.
The 2000s were also characterized by expansion of structural imaging as fetal dMRI began to emerge. This modality revealed new details about the microstructural changes that occur in fetal brain development (Huang, 2010, Jakab et al., 2017). Critically, this work was made possible by concurrent advancements in dMRI sequence development. dMRI studies are especially sensitive to motion (both from the mother and fetus). Thus, this work relied heavily on work that shortened dMRI scan times (Kim et al., 2008, Norris and Driesel, 2001; for review see Studholme, 2011).
3.4. Current trends
Several trends have emerged in fetal imaging that are currently shaping the focus of the field. Here we highlight a few of them.
3.4.1. Brain development from the second to third trimester
Recent work has continued to utilize fetal MRI to characterize brain development over the course of pregnancy (Dubois et al., 2014, Khan et al., 2019, Wilson et al., 2021), providing new insights into brain development prior to birth. Fetal MRI has also been used for gestational-age equivalent controls for preterm infants (Bouyssi-Kobar et al., 2016, De Asis-Cruz et al., 2020, Khan et al., 2019). In large part, this work has been made possible by new methodological advancements in MRI acquisition techniques and analysis pipelines (Fogtmann et al., 2014, Kim et al., 2010, Marami et al., 2017, Pontabry et al., 2017, Rutherford et al., 2021, Seshamani et al., 2013).
3.4.2. Predicting postnatal development
Recently, fetal MRI has been used to predict postnatal development (Turk et al., 2019, van den Heuvel and Thomason, 2016, Vasung et al., 2019). This includes pinpointing patterns of brain development in the fetus in association with both typical behaviors, such as motor development (Schöpf et al., 2014, Thomason et al., 2018) and atypical outcomes, such as autism spectrum disorder (Hulshof et al., 2021, Sanz-Cortes et al., 2014, Villa et al., 2021). For example, recent work has identified in utero markers of preterm birth (Story et al., 2021, Thomason et al., 2017) – including reduced connectivity in cortical regions associated with language and reduced cerebrospinal fluid and cerebral cortex volume. However, at present, predicting clinically relevant, long-term individual outcomes from fetal MRI data remains unreliable and requires further refinement (Hart et al., 2020).
3.4.3. Prenatal exposures/maternal factors
Several lines of work have also begun to explore how maternal factors and prenatal exposures (e.g., maternal anxiety, obesity, stress, and toxins such as alcohol) shape fetal brain development (De Asis-Cruz et al., 2020, Norr et al., 2021, van den Heuvel et al., 2021). This work has begun to pinpoint the factors that influence brain development before birth.
In sum, while the field of fetal MRI has faced several challenges such as lack of fetal-specific computational pipelines and hardware (Serai et al., 2013), and no uniform best practices for data acquisition, harmonization, and integration, it has provided us with unprecedented access to investigate the developing brain in utero and has demonstrated the potential for important clinical applications.
4. Infant and toddler MRI
4.1. The early years (1980s)
Like fetal MRI, much of the infant and toddler MRI, in the early 1980s, was driven by clinical need, often focused on individuals with brain injury (typically in preterm infants). Although studies during this time generally included participants between birth and 5 years of age in a single group, this work provided proof-of-concept that MRI was safe to use in infants and children (Smith, 1983) and could be used to measure many aspects of brain injury (Johnson et al., 1983, Levene et al., 1982, McArdle et al., 1987, McArdle et al., 1987). Thus, just as for fetal MRI, infant and toddler MRI research began in the 1980s with early work focused on the use of MRI in a clinical context and demonstrating safety of the technology.
4.2. Larger studies focusing on infancy (late 1980–1990s)
In the latter half of the 1980s and into the 1990s, with safety established and increased accessibility to scanners, the field shifted towards narrower age ranges and larger sample sizes (up to 160 infants in some cases). For example, with sample sizes of 90 newborns, scientists were able to document variation in white matter maturation (Barkovich et al., 1988). During this time, forerunner scientists also began to understand how early brain injury corresponded to later developmental outcomes (Barkovich et al., 1998, Lago et al., 1995, Mercuri et al., 1999, Mercuri et al., 1999, Robertson et al., 1999; Rutherford et al., 1991, Rutherford et al., 1998), more than twenty years before similar studies with fetal MRI. Again, several studies of preterm infants paved the way to understanding the development of white matter microstructure (Fujii et al., 1993, Hüppi et al., 1998, Hüppi et al., 1998, McArdle et al., 1987, Sie et al., 1997) and associations between the brain and cognitive outcomes (Hüppi et al., 1996, Pike et al., 1994).
4.3. Emergence of an independent research field (2000–2010s)
In the early 2000s, several notable research trends emerged that helped to establish the potential of this exciting field.
4.3.1. Beginning of longitudinal cohorts
Most infant and toddler neuroimaging work before the 2000s relied on cross-sectional imaging (Gilmore et al., 2007, Gilmore et al., 2007, Lin et al., 2008). However, the need for longitudinal cohorts with imaging at multiple time points to truly assess developmental trajectories soon became clear (Dyet et al., 2006). Some were successful at building large longitudinal cohort studies starting in infancy at a single site (Gilmore et al., 2006; Inder et al., 1999a, Inder et al., 1999b; Looney et al., 2007; Maalouf et al., 1999), while others combined their resources to build collaborative cohorts across institutes (e.g., the Newborn Individualized Developmental Care and Assessment Program (NIDCAP; Als et al., 2004; Mewes et al., 2006) and the Infant Brain Imaging Study (IBIS; https://autismbabybrain.com/infant/; Wolff et al., 2012). Together, these studies demonstrated robust growth of the human brain in the first two years of life (Garcia et al., 2018, Knickmeyer et al., 2008), and highlighted alterations in growth trajectories that are associated with neurodevelopmental risk (Gao et al., 2009, Gilmore et al., 2007, Hazlett et al., 2011, Hazlett et al., 2017, Kapellou et al., 2006, Shen et al., 2013).
These early collaborations set the stage for funding agencies, such as the National Institutes of Health (NIH) to initiate large-scale studies of early human brain development that assembled consortiums across research institutions. The NIH MRI Study of Normal Brain Development was, to our knowledge, the first large-scale longitudinal MRI study conducted with healthy infants and toddlers (Almli et al., 2007, Evans, 2006, Sanchez et al., 2012). This seven-year study enrolled over 500 children (across 6 U.S.-based institutions), including over 100 children between birth and 4 years of age. The goal was to establish a database of healthy MRI data from the first few years of life that could be used as a standard for identifying pathologies (Sanchez et al., 2012).
4.3.2. Infant MRI to identify risk for neurodevelopmental disorders
In the 2000s, researchers also began to use infant MRI to identify early markers of risk for neurodevelopmental disorders (e.g., autism spectrum disorder, developmental dyslexia). Early structural MRI studies compared brain morphometry of infants and toddlers with and without neurodevelopmental disorders (Courchesne et al., 2001, Hazlett et al., 2005, Sparks et al., 2002). However, in the mid- 2000s there was a shift toward measuring brain changes before behavioral symptoms of impairment emerged (Gilmore et al., 2010, Hüppi and Dubois, 2006, Krishnan et al., 2007, Langer et al., 2017, Peterson, 2000, Sylvester et al., 2018, Woodward et al., 2006).
4.3.3. Emergence of infant and toddler resting-state fMRI
Just as fetal fMRI experienced a rapid expansion in the 2000s, so too did infant and toddler fMRI; though, infant and toddler fMRI studies predated those in fetuses by approximately four years. Early resting state fMRI studies in preterm infants have been the long-time workhorse in infant imaging and have taught us a great deal about the brain. For example, some of the earliest studies indicating that the BOLD signal can be reliably identified in infants and is similar to that found in adults were originally conducted in preterm cohorts (Arichi et al., 2010, Fransson et al., 2007, Heep et al., 2009). These findings were replicated in healthy full-term infants (Fransson et al., 2009, Gao et al., 2009, Smyser and Neil, 2015). Pivotal findings identified that while most resting state networks are found in infancy, preterm infants exhibited immature forms of some adult resting state networks (Smyser et al., 2010); suggesting that the last trimester of gestation shapes network development (Damaraju et al., 2010, Doria et al., 2010). Disruption to brain networks in infants from the neonatal intensive care unit at term-equivalent age has been found to be predictive of developmental impairment (Linke et al., 2018). To date, studies on preterm infants make up roughly one third of all infant imaging research (Cabez et al., 2019, Hüppi et al., 1996, Krishnan et al., 2007, Peterson, 2000, Rogers et al., 2017, Woodward et al., 2006).
4.3.4. Emergence of infant and toddler dMRI
Over the course of the 2000s, there were parallel developments in dMRI mapping postnatal white matter development (Counsell et al., 2003, Dubois et al., 2006, Hüppi et al., 2001, Hüppi and Dubois, 2006, Krishnan et al., 2007). Early dMRI work focused on feasibility of acquisition and analysis—often concentrating on preterm samples (Berman et al., 2005, Maas et al., 2004, Partridge et al., 2005) and those with brain damage (Agid et al., 2006, Baldoli et al., 2002). This initial feasibility work led the way for research characterizing white matter maturation in typically developing samples (Bui et al., 2006, Dubois et al., 2006, Hüppi and Dubois, 2006, Kasprian et al., 2008). While some similarities exist in the methods for acquisition and post-processing analysis of infant versus toddler dMRI, differences in the tissue maturation, with low white matter myelination, have required several methodological advancements that are age-specific. The development of these tools has been essential to the growth of this area of research (Bastiani et al., 2019, Dubois et al., 2014, Hutter et al., 2018, Tournier et al., 2020).
4.4. Current trends
Currently, several research trends have received increased attention from researchers and funding agencies alike. These approaches aim to improve data acquisition and to establish developmentally sensitive markers for identifying those most at risk of developing illness.
4.4.1. Infant specific equipment
Historically, MRI technology was not designed for use in these populations and thus there were major challenges (and still are in many places) regarding access and acquisition quality. These challenges have historically made it difficult to engage manufacturers in discussions about improving these issues. However, recently several key advancements have been made from a technology standpoint. In 2017, the United States FDA cleared the first neonatal intensive care unit (NICU) MRI—installed at Brigham and Women’s Hospital in the United States (Partners Healthcare, 2018). This regulatory clearance facilitated growth in the development of infant specific hardware including the development of the Embrace ® MRI System (Rona et al., 2010), a neonatal MRI machine used directly in their NICU, and infant size-adaptive head coils (Ghotra et al., 2021, Hughes et al., 2017). These new technologies make conducting infant/toddler MRIs more accessible and improve data quality.
4.4.2. Task-based fMRI in infants and toddlers
Renewed interest in task-based fMRI, both in sleeping (Adam-Darque et al., 2018, Allievi et al., 2016, Dall’Orso et al., 2018, Dall’Orso et al., 2021, Graham et al., 2013, Sylvester et al., 2021, Wild et al., 2017) and awake infants and toddlers (Baxter et al., 2019, Baxter et al., 2021, Biagi et al., 2015, Deen et al., 2017, Ellis et al., 2021), has expanded the early work of Dehaene-Lambertz and others (Anderson et al., 2001, Arichi et al., 2012, Dehaene-Lambertz, 2002, Dehaene-Lambertz et al., 2006). This area of research has been one of the slowest to progress, with many investigators paving the way by spending years refining protocols to optimize infant comfort during MRI scans (Raschle et al., 2012).
4.4.3. Open-source datasets and tools
With an increasing interest in early intervention and prediction of future developmental outcomes, the need for larger sample sizes combined with longitudinal imaging through early childhood to identify age-specific versus persistent brain markers of emerging risk became clear. Two large-scale connectome projects, the Developing Human Connectome Project (dHCP; http://www.developingconnectome.org; Bastiani et al., 2019; Bozek et al., 2018; Fitzgibbon et al., 2020; Hughes et al., 2017; Makropoulos et al., 2018) and the Baby Connectome Project (BCP; https://babyconnectomeproject.org/; Howell et al., 2019) amassed datasets including longitudinal scans from over 2000 participants ranging from 20 to 45 weeks post-conception to age five years. This work stemmed from a growing interest in creating dynamic maps of brain connectivity during early life, and factors that impact their development (Eyre et al., 2021). Large-scale datasets have also allowed for normative modeling of brain development (Dimitrova et al., 2020, Dimitrova et al., 2021, Eyre et al., 2021, O’Muircheartaigh et al., 2020). In parallel, there has been tremendous growth in software dedicated to processing infant neuroimaging data including: Infant FreeSurfer (Zollei et al., 2020), Infant Brain Extraction and Analysis Toolbox (iBEAT; Dai et al., 2013), Melbourne Children’s Regional Infant Brain (M-CRIB; Adamson et al., 2020) and Automated Segmentation tool (AutoSeg; Wang et al., 2014) for structural data analysis, neonatal diffusion MRI (Bastiani et al., 2019) for diffusion tensor imaging data, and resting-state data processing pipelines (Fitzgibbon et al., 2020).
The NIH has recently expanded this line of work with the HEALthy Brain and Child Development Study (HBCD; https://heal.nih.gov/research/infants-and-children/healthy-brain; Jordan et al., 2020; Kohlasch et al., 2021), set to launched in fall 2021 (planning phase started in 2018). This project grew out of the acknowledgment that discovering causal links between early experiences (i.e., substance use, environmental exposures, and adversity) and future health outcomes is complex and requires large prospective studies with detailed assessments of brain, behavior, genetics, and environmental contexts. As such, the goal of this project is to track neurodevelopmental trajectories from 0 to age 10 to determine how early experiences shape brain development and health outcomes and to illuminate factors associated with risk and resilience. In many ways, the fact that eight institutes at the NIH have come together to fund this large, multi-site, multi-modal, longitudinal research project is reflective of a broader trend of collaborative common protocol endeavors.
5. The need for a community to mature the field
While impressive advances have been made in fetal, infant, and toddler imaging over the past 40 years, several key challenges remain related to data acquisition and analysis (Dubois et al., 2021, Raschle et al., 2012). These challenges necessitate novel approaches for the field to collectively resolve (Hughes et al., 2017). For example, the field has yet to establish best practices for key features of this work, including how to account for data acquisition during natural sleep versus awake (Smyser and Neil, 2015), improve the resolution of the data acquired from a machinery standpoint (Cusack et al., 2018), and maximize the possibility of acquiring data at different ages (Graham et al., 2015). Determining best practices requires transparency that many researchers want but lack the avenue to achieve. If such an avenue existed, it would allow experts in the field to come together to share what procedures have/have not worked for them, and to share associated software and data.
The need for greater collaboration has led to the creation of special interest groups and academic societies focused on neurodevelopment during the fetal and infant period. The International Perinatal Brain and Behavior Network (IPBBN), a special interest group of the International Society for Developmental Psychobiology (established in 2007; https://babybrain.isdp.org/), aims to advance research of prenatal, perinatal, and early postnatal human development, and to support new investigators in this area. In 2015, the Newborn Brain Society (NBS) was established (https://newbornbrainsociety.org) in response to several neonatal neurocritical care programs launching worldwide and questions about their efficacy. The NBS has since grown to have the broader goal of advancing newborn brain care through international collaboration. To date, NBS leadership is largely comprised of clinicians and individuals with research programs focused on brain injury. These initiatives opened channels of communication for either clinician scientists or developmental scientist with a foci on the perinatal to newborn periods. Still missing was a community forum for those interested in neurodevelopmental trajectories during the most rapid periods of brain growth—fetal through toddler age, and scientists with diverse backgrounds (e.g., clinicians, engineers) interested in early brain development. Critically, studying brain changes over this period has several unique challenges both in terms of acquisition and analysis. The challenges of longitudinal neuroimaging remained tangential to both the IPBBN and NBS’s primary aims.
6. Building a new community – Introduction to FIT’NG
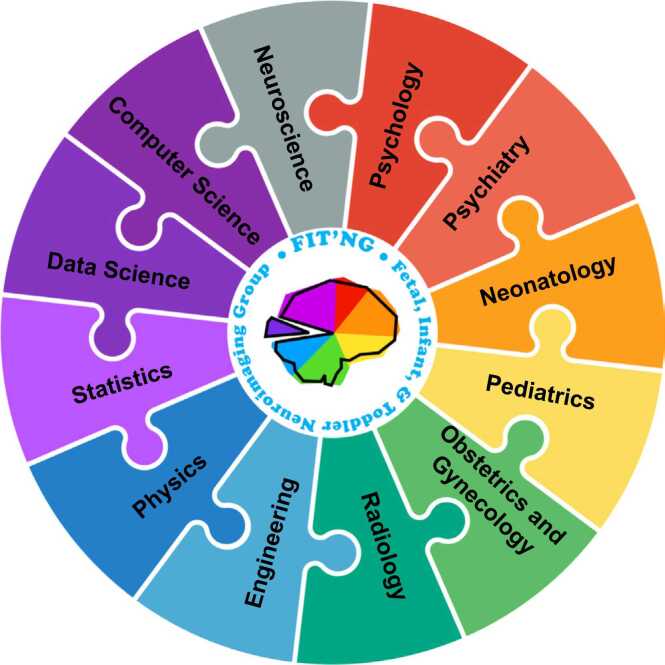
In response to this gap, the Fetal, Infant, Toddler Neuroimaging Group (FIT’NG) is an academic society founded in 2018 that aims to provide a forum for early childhood neuroimaging researchers, including those technical experts (“developers,” e.g., engineers, physicists, etc.) and applied researchers (“appliers,” e.g., psychologists, neuroscientists, psychiatrists, neonatologists, etc.) (Fig. 2). FIT’NG communication among developers and appliers is focused around three core areas: establishing best practices within the field (e.g., scan time, staffing, preparatory procedures for scanning, data harmonization); community exchange and collaboration (e.g., sharing processing and analysis tools, sharing data); and education (e.g., training across institutions at a range of levels).
Fig. 2.
FIT’NG aims to provide a forum for early childhood neuroimaging researchers, including those who have technical expertize (e.g., engineers, physicists) and applied researchers (e.g., psychologists, psychiatrists, neonatologists).
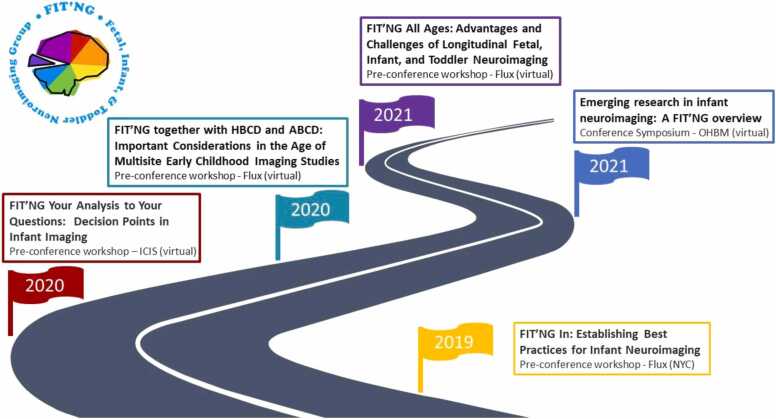
Members of FIT’NG work in diverse departments across medical and main university systems, and thus attend various scientific meetings. FIT’NG members are often a small subgroup of researchers at the conferences they attend, and consequently, they lack the community needed at these meetings to meaningfully advance the field. FIT’NG seeks to establish that community and to highlight the value and innovation in the fast-growing area of early childhood neuroimaging research. To facilitate interactions and provide a common space for connection, FIT’NG has organized annual pre-conference workshops and several conference symposia since its inception at international meetings focused on infant, developmental neuroscience, and neuroimaging research (Fig. 3). Since our first full-day pre-conference workshop in 2019 entitled “FIT’NG In: Establishing Best Practices for Infant Neuroimaging” at the Flux Congress, our annual workshops have expanded from 60 to over 250 participants. In addition to pre-conference workshops at FLUX and the International Congress on Infant Studies (ICIS), FIT’NG has hosted symposiums, some in collaboration with the National Institutes of Health partners and social gatherings at other societal meetings that different pockets of scientists in our field attend to further strengthen our sense of community. These formal and informal dialogs and gatherings are essential to the advancement of the field, as they provide a starting point to move toward consensus of best practice standards. Individuals interested in FIT’NG can join our listserv, follow us on Twitter (@FIT_NGIn), and/or become members via our website https://fitng.org/. Members have on-demand web access to materials created to facilitate training in FIT imaging including tutorials on how to use infant-specific software, discussions of recent papers that are of broad interest, and interviews with leaders in the field among other benefits detailed on our website. These materials are curated and maintained by our trainee-led committee, FIT’NG Together, and offer an opportunity for trainees to identify key topics that they believe would advance their training.
Fig. 3.
Scientific events and activities organized by FIT’NG.
In 2020, we incorporated – becoming an official non-profit society. Unfortunately, the COVID-19 pandemic has limited the initiatives that FIT’NG has been able to launch to be virtual and has limited research on the developing brain because pregnant women and infants/toddlers are particularly vulnerable to COVID-19. For this reason, we have postponed our inaugural meeting until 2022. In 2021, we held a one-day virtual workshop entitled “FIT’NG All Ages: Advantages and Challenges of Longitudinal Fetal, Infant, and Toddler Neuroimaging.” This as well as our other prior events sets the stage for an annual meeting in perpetuity and a connected community of multidisciplinary scientists who will advance the field. FIT’NG is currently in the process of expanding to include scientists using modalities other than MRI (including EEG, fNIRS, MEG, and ultrasound) to facilitate new directions in the study of brain development.
7. Conclusion
Over the course of 40 years, fetal, infant, and toddler neuroimaging has seen a rapid maturation as a research field from small safety and proof of concept studies to massive, large-scale NIH and international initiatives. To celebrate the pioneering science of the field and appreciate just how far we have come, this review documents a brief highlights’ reel of this maturation. Nevertheless, many challenges continue to exist in fetal, infant, and toddler neuroimaging that hinder its growth and that cannot be solved in silos. FIT’NG provides a forum for community building, scientific collaboration, and communication of new advancements in the field.
Funding sources
This work was supported by the National Institute of Mental Health grant numbers R01MH117983 and K24MH127381, the National Institute of Child Health and Development (NICHD) grant number K23HD9258901, and the Nathaniel Wharton Fund (Dr. Spann). Also, the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR001873 (Dr. Spann) and TL1TR001875 (Dr. Pollatou), NIMH through grant number T32MH018268 (Dr. Dufford), NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (#28024; Dr. Filippi) and the Intramural Research Program (ZIAMH002782; Dr. Filippi) supported this work. Dr. Howell is an iTHRIV Scholar. The iTHRIV Scholars Program is supported in part by the National Center for Advancing Translational Sciences, National Institutes of Health through grant numbers UL1TR003015 and KL2TR003016. Dr Zöllei has been supported by NICHD grant numbers R01HD65762, R03EB022754, R21HD095338-01, R01HD093578, R01HD099846. This work was also supported by the NICHD R01HD100560 (Dr. Vaughn). Dr. Thompson has been supported by the Australian National Health and Medical Research Council's (NHMRC) Career Development Fellowship (1085754 & 1160003) and Early Career Fellowship (1012236); and the Victorian Government's Operational Infrastructure Support Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Arline Pierre-Louis, Kiarra Alleyne, Antonette Davids, Misha Jaramillo, and Hannah Hardiman for their helpful comments, and Stephanie Noble for creating our beautiful logo. We also thank the pioneers of this field who made studying the developing brain possible. Most of all, we owe an infinite amount of gratitude to the thousands of study participants without whom none of this would be possible.
Footnotes
An expanded list of the FIT’NG organization members who contributed to the manuscript appears in the Supplementary Information
For further discussion of MRI safety see: Garel and Brisse (1998); Tocchio et al. (2015); Welsh et al. (2011)
Although not the majority, some groups continue to use sedation today.
Appendix A. . FIT’NG membership (in alphabetical order)
-
•
Zeena M. Ammar
Neuroscience Graduate Program Emory University Atlanta, GA, USA
-
•
James Barkovich
Radiology Department
University of California, San Francisco
San Francisco, CA, USA
-
•
Johanna Bick
Psychology Department University of Houston Houston, TX, USA
-
•
M. Catalina Camacho
Division of Biology and Biomedical Sciences
Washington University in St. Louis
St. Louis, MO, USA
-
•
Shruthi Chakrapani
Cognitive Neuroimaging Center
Boston University
Boston, MA, USA
-
•
Rhodri Cusack
Trinity College Institute of Neuroscience, Trinity College Dublin
-
•
Kelsey E. Davison
Boston University College of Health and Rehabilitation Sciences: Sargent College Boston, MA, USA
-
•
Adriana Di Martino
Child Mind Institute
New York, New York, USA
-
•
Jessica Dubois
Université de Paris, NeuroDiderot, Inserm, Paris, France Université Paris-Saclay, NeuroSpin-UNIACT, CEA, Gif-sur-Yvette, France
-
•
Aidan Ford
Neuroscience Graduate Program Emory University Atlanta, GA, USA
-
•
Nadine Gaab
Harvard Graduate School of Education, Cambridge, MA, USA Harvard Medical School, Boston, MA, USA
-
•
Ghislaine Dehaene-Lambertz
Cognitive Neuroimaging Unit
CNRS
Paris, France
-
•
Kathryn L. Humphreys
Department of Psychology and Human Development Vanderbilt University Nashville, TN, USA
-
•
Terrie Inder
Department of Pediatric Newborn Medicine
Harvard Medical School
Boston, MA, USA
-
•
Roxane Licandro
Medical University of Vienna
Department of Biomedical Imaging and Image-guided Therapy
Computational Imaging Research
Vienna, Austria
-
•
Kathrine Skak Madsen
Danish Research Center for Magnetic Resonance, Center for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital - Amager and Hvidovre Radiography, Department of Technology, University College Copenhagen Copenhagen, Denmark
-
•
Joseph Piven
Departments of Psychiatry and Pediatrics
University of North Carolina
School of Medicine
Chapel Hill, NC, USA
-
•
Cynthia Rogers Departments of Psychiatry and Pediatrics Washington University School of Medicine St. Louis, MO, USA
-
•
Magdalena Sanz-Cortes
Department of Obstetrics and Gynecology
Baylor College of Medicine
Texas Children’s Hospital
Houston, TX, USA
-
•
Sarah Shultz
Marcus Autism Center
Children’s Healthcare of Atlanta
Atlanta, GA, USA
&
Department of Pediatrics
Emory School of Medicine
Atlanta, GA, USA
-
•
Cristina Simon-Martinez
Institute of Information Systems, School of Management, HES-SO Valais-Wallis University of Applied Sciences and Arts Western Switzerland, Switzerland
-
•
Christopher D. Smyser
Departments of Neurology, Pediatrics, and Radiology Washington University in St. Louis St. Louis, MO, USA
-
•
Chad M. Sylvester
Department of Psychiatry Washington University in St. Louis St. Louis, MO, USA
-
•
Elina Thomas
Department of Psychiatry University of Vermont Burlington, VT, USA
-
•
Ted K. Turesky Harvard Graduate School of Education Cambridge, MA, USA
-
•
Sylia Wilson
Institute of Child Development University of Minnesota Minneapolis, MN, USA
-
•
Elizabeth Yen
Tufts University School of Medicine Mother Infant Research Institute at Tufts Medical Center Boston, MA, USA
-
•
Xi Yu
State Key Laboratory of Cognitive Neuroscience and Learning Beijing Normal University Beijing, China
-
•
Jennifer Zuk
Department of Speech, Language & Hearing Sciences Boston University Boston, MA, USA.
Data availability
No data was used for the research described in the article.
References
- Adam-Darque A., Grouiller F., Vasung L., Ha-Vinh Leuchter R., Pollien P., Lazeyras F., Hüppi P.S. FMRI-based neuronal response to new odorants in the newborn brain. Cereb. Cortex. 2018;28(8):2901–2907. doi: 10.1093/cercor/bhx167. [DOI] [PubMed] [Google Scholar]
- Adamson C., Alexander B., Ball G., Beare R., Cheong J., Spittle A., Doyle L., Anderson P., Seal M., Thompson D. Parcellation of the neonatal cortex using Surface-based Melbourne Children’s Regional Infant Brain atlases (M-CRIB-S) Scientific Reports. 2020;10 doi: 10.1038/s41598-020-61326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agid R., Lieberman S., Nadjari M., Gomori J.M. Prenatal MR diffusion-weighted imaging in a fetus with hemimegalencephaly. Pediatr. Radiol. 2006;36(2):138–140. doi: 10.1007/s00247-005-0003-3. [DOI] [PubMed] [Google Scholar]
- Allievi A.G., Arichi T., Tusor N., Kimpton J., Arulkumaran S., Counsell S.J., Edwards A.D., Burdet E. Maturation of sensori-motor functional responses in the preterm brain. Cereb. Cortex. 2016;26(1):402–413. doi: 10.1093/cercor/bhv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almli C.R., Rivkin M.J., McKinstry R.C. The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. NeuroImage. 2007;35(1):308–325. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Als H., Duffy F.H., McAnulty G.B., Rivkin M.J., Vajapeyam S., Mulkern R.V., Warfield S.K., Huppi P.S., Butler S.C., Conneman N., Fischer C., Eichenwald E.C. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- Anderson A.L., Thomason M.E. Functional plasticity before the cradle: a review of neural functional imaging in the human fetus. Neurosci. Biobehav. Rev. 2013;37(9):2220–2232. doi: 10.1016/j.neubiorev.2013.03.013. [DOI] [PubMed] [Google Scholar]
- Anderson A.W., Marois R., Colson E.R., Peterson B.S., Duncan C.C., Ehrenkranz R.A., Schneider K.C., Gore J.C., Ment L.R. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn. Reson. Imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Angtuaco T.L., Shah H.R., Mattison D.R., Quirk J.G., Jr. MR Imaging in high-risk obstetric patients: a aluable complement to US. RadioGraphics. 1992;12(1):91–109. doi: 10.1148/radiographics.12.1.1734485. [DOI] [PubMed] [Google Scholar]
- Arichi T., Moraux A., Melendez A., Doria V., Groppo M., Merchant N., Combs S., Burdet E., Larkman D.J., Counsell S.J., Beckmann C.F., Edwards A.D. Somatosensory cortical activation identified by functional MRI in preterm and term infants. NeuroImage. 2010;49(3):2063–2071. doi: 10.1016/j.neuroimage.2009.10.038. [DOI] [PubMed] [Google Scholar]
- Arichi T., Fagiolo G., Varela M., Melendez-Calderon A., Allievi A., Merchant N., Tusor N., Counsell S.J., Burdet E., Beckmann C.F., Edwards A.D. Development of BOLD signal hemodynamic responses in the human brain. NeuroImage. 2012;63(2):663–673. doi: 10.1016/j.neuroimage.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldoli C., Righini A., Parazzini C., Scotti G., Triulzi F. Demonstration of acute ischemic lesions in the fetal brain by diffusion magnetic resonance imaging. Ann. Neurol. 2002;52 doi: 10.1002/ana.10255. [DOI] [PubMed] [Google Scholar]
- Barkovich A.J., Kjos B.O., Jackson D.E., Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T’. Neuroradiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Barkovich A.J., Hajnal B.L., Vigneron D., Sola A., Partridge J.C., Allen F., Ferriero D.M. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR AM J. Neuroradiol. 1998;19:143–149. [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., Lebihan D. Estimation of effective self-diffusion tensor from the NMR spin echo. J. Magn. Reson. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Bastiani M., Andersson J.L.R., Cordero-Grande L., Murgasova M., Hutter J., Price A.N., Makropoulos A., Fitzgibbon S.P., Hughes E., Rueckert D., Victor S., Rutherford M., Edwards A.D., Smith S.M., Tournier J.-D., Hajnal J.V., Jbabdi S., Sotiropoulos S.N. Automated processing pipeline for neonatal diffusion MRI in the developing human connectome project. NeuroImage. 2019;185:750–763. doi: 10.1016/j.neuroimage.2018.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter L., Fitzgibbon S., Moultrie F., Goksan S., Jenkinson M., Smith S., Andersson J., Duff E., Slater R. Optimising neonatal fMRI data analysis: design and validation of an extended dHCP preprocessing pipeline to characterise noxious-evoked brain activity in infants. NeuroImage. 2019;186:286–300. doi: 10.1016/j.neuroimage.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter L., Moultrie F., Fitzgibbon S., Aspbury M., Mansfield R., Bastiani M., Rogers R., Jbabdi S., Duff E., Slater R. Functional and diffusion MRI reveal the neurophysiological basis of neonates’ noxious-stimulus evoked brain activity. Nat. Commun. 2021;12(1):2744. doi: 10.1038/s41467-021-22960-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J.I., Mukherjee P., Partridge S.C., Miller S.P., Ferriero D.M., Barkovich A.J., Vigneron D.B., Henry R.G. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. NeuroImage. 2005;27(4):862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Biagi L., Crespi S.A., Tosetti M., Morrone M.C. BOLD response selective to flow-motion in very young infants. PLOS Biol. 2015;13(9) doi: 10.1371/journal.pbio.1002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilaniuk L.T. Magnetic resonance imaging of the fetal brain. MRI Fetal Brain. 1999;34:14–61. doi: 10.1016/s0037-198x(99)80020-0. [DOI] [PubMed] [Google Scholar]
- Bouyssi-Kobar M., du Plessis A.J., McCarter R., Brossard-Racine M., Murnick J., Tinkleman L., Robertson R.L., Limperopoulos C. Third trimester brain growth in preterm infants compared with in utero healthy fetuses. Pediatrics. 2016;138(5) doi: 10.1542/peds.2016-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozek J., Makropoulos A., Schuh A., Fitzgibbon S., Wright R., Glasser M.F., Coalson T.S., O’Muircheartaigh J., Hutter J., Price A.N., Cordero-Grande L., Teixeira R.P.A.G., Hughes E., Tusor N., Baruteau K.P., Rutherford M.A., Edwards A.D., Hajnal J.V., Smith S.M., Robinson E.C. Construction of a neonatal cortical surface atlas using multimodal surface matching in the developing human connectome project. NeuroImage. 2018;179:11–29. doi: 10.1016/j.neuroimage.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui T., Daire J.-L., Chalard F., Zaccaria I., Alberti C., Elmaleh M., Garel C., Luton D., Blanc N., Sebag G. Microstructural development of human brain assessed in utero by diffusion tensor imaging. Pediatr. Radiol. 2006;36(11):1133–1140. doi: 10.1007/s00247-006-0266-3. [DOI] [PubMed] [Google Scholar]
- Cabez M.B., Sullivan G., Anblagan D., Telford E.J., Quigley A.J., Sparrow S.A., Serag A., Semple S.I., Bastin M.E., Boardman J.P. Early breast milk exposure modifies brain connectivity in preterm infants. NeuroImage. 2019;184:431–439. doi: 10.1016/j.neuroimage.2018.09.045. [DOI] [PubMed] [Google Scholar]
- Caldairou B., Passat N., Habas P., Studholme C., Koob M., Dietemann J.-L., Rousseau F. In: Computer Analysis of Images and Patterns. Real P., Diaz-Pernil D., Molina-Abril H., Berciano A., Kropatsch W., editors. vol. 6854. Springer Berlin, Heidelberg; 2011. Data-driven cortex segmentation in reconstructed fetal mri by using structural constraints; pp. 503–511. [DOI] [Google Scholar]
- Coakley F.V., Hricak H., Filly R.A., Barkovich A.J., Harrison M.R. Complex fetal disorders: effect of MR imaging on management – preliminary clinical experience. Radiology. 1999;213(3):691–696. doi: 10.1148/radiology.213.3.r99dc39691. [DOI] [PubMed] [Google Scholar]
- Counsell S.J., Allsop J.M., Harrison M.C., Larkman D.J., Kennea N.L., Kapellou O., Cowan F.M., Hajnal J.V., Edwards A.D., Rutherford M.A. Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics. 2003;112(1):1–7. doi: 10.1542/peds.112.1.1. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Karns C.M., Davis H.R., Ziccardi R., Carper R.A., Tigue Z.D., Chisum H.J., Moses P., Pierce K., Lord C., Lincoln A.J., Pizzo S., Schreibman L., Haas R.H., Akshoomoff N.A., Courchesne R.Y. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57(2):245–254. doi: 10.1212/WNL.57.2.245. [DOI] [PubMed] [Google Scholar]
- Cusack R., McCuaig O., Linke A.C. Methodological challenges in the comparison of infant fMRI across age groups. Dev. Cogn. Neurosci. 2018;33:194–205. doi: 10.1016/j.dcn.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffos F., Forestier F., Mac Aleese J., Aufrant C., Mandelbrot L., Cabanis E.A., Iba-Zizen M.T., Alfonso J.M., Tamraz J. Fetal curarization for prenatal magnetic resonance imaging. Prenat. Diagn. 1988;8(4):311–314. doi: 10.1002/pd.1970080412. [DOI] [PubMed] [Google Scholar]
- Dall’Orso S., Steinweg J., Allievi A.G., Edwards A.D., Burdet E., Arichi T. Somatotopic mapping of the developing sensorimotor cortex in the preterm human brain. Cereb. Cortex. 2018;28(7):2507–2515. doi: 10.1093/cercor/bhy050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Shi F., Wang L., Wu G., Shen D. iBEAT: A toolbox for infant brain magnetic resonance image processing. Neuroinformatics. 2013;11:211–225. doi: 10.1007/s12021-012-9164-z. [DOI] [PubMed] [Google Scholar]
- Dall’Orso S., Fifer W.P., Balsam P.D., Brandon J., O’Keefe C., Poppe T., Vecchiato K., Edwards A.D., Burdet E., Arichi T. Cortical processing of multimodal sensory learning in human neonates. Cereb. Cortex. 2021;31(3):1827–1836. doi: 10.1093/cercor/bhaa340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R., Goldsmith M., Minkoff L. NMR in cancer: XVI. Fonar Image of the live human body. Physiol. Chem. Phys. 1977;9(1):97–100. [PubMed] [Google Scholar]
- Damaraju E., Phillips J., Lowe J.R., Ohls R., Calhoun V.D., Caprihan A. Resting-state functional connectivity differences in premature children. Front. Syst. Neurosci. 2010;0:1–13. doi: 10.3389/fnsys.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Asis-Cruz J., Krishnamurthy D., Zhao L., Kapse K., Vezina G., Andescavage N., Quistorff J., Lopez C., Limperopoulos C. Association of prenatal maternal anxiety with fetal regional brain connectivity. JAMA Netw. Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.22349. [DOI] [PubMed] [Google Scholar]
- De Asis-Cruz J., Kapse K., Basu S.K., Said M., Scheinost D., Murnick J., Chang T., du Plessis A., Limperopoulos C. Functional brain connectivity in ex utero premature infants compared to in utero fetuses. NeuroImage. 2020;219 doi: 10.1016/j.neuroimage.2020.117043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen B., Richardson H., Dilks D.D., Takahashi A., Keil B., Wald L.L., Kanwisher N., Saxe R. Organization of high-level visual cortex in human infants. Nat. Commun. 2017;8(1):13995. doi: 10.1038/ncomms13995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G. Functional neuroimaging of speech perception in infants. Science. 2002;298(5600):2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G., Hertz-Pannier L., Dubois J., Mériaux S., Roche A., Sigman M., Dehaene S. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc. Natl. Acad. Sci. USA. 2006;103(38):14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova R., Pietsch M., Christiaens D., Ciarrusta J., Wolfers T., Batalle D., Hughes E., Hutter J., Cordero-Grande L., Price A.N., Chew A., Falconer S., Vecchiato K., Steinweg J.K., Carney O., Rutherford M.A., Tournier J.-D., Counsell S.J., Marquand A.F., O’Muircheartaigh J. Heterogeneity in brain microstructural development following preterm birth. Cereb. Cortex. 2020;30(9):4800–4810. doi: 10.1093/cercor/bhaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova R., Pietsch M., Ciarrusta J., Fitzgibbon S.P., Williams L.Z.J., Christiaens D., Cordero-Grande L., Batalle D., Makropoulos A., Schuh A., Price A.N., Hutter J., Teixeira R.P., Hughes E., Chew A., Falconer S., Carney O., Egloff A., Tournier J.-D., O’Muircheartaigh J. Preterm birth alters the development of cortical microstructure and morphology at term-equivalent age. BioRxiv. 2021;2021(06.03) doi: 10.1101/2021.06.03.446550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V., Beckmann C.F., Arichi T., Merchant N., Groppo M., Turkheimer F.E., Counsell S.J., Murgasova M., Aljabar P., Nunes R.G., Larkman D.J., Rees G., Edwards A.D. Emergence of resting state networks in the preterm human brain. Proc. Natl. Acad. Sci. USA. 2010;107(46):20015–20020. doi: 10.1073/pnas.1007921107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Hertz-Pannier L., Dehaene-Lambertz G., Cointepas Y., Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. NeuroImage. 2006;30(4):1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Dubois J., Kulikova S., Hertz-Pannier L., Mangin J.-F., Dehaene-Lambertz G., Poupon C. Correction strategy for diffusion-weighted images corrupted with motion: application to the DTI evaluation of infants’ white matter. Magn. Reson. Imaging. 2014;32(8):981–992. doi: 10.1016/j.mri.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Dubois J., Dehaene-Lambertz G., Kulikova S., Poupon C., Hüppi P.S., Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Dubois J., Alison M., Counsell S.J., Hertz-Pannier L., Hüppi P.S., Benders M.J.N.L. MRI of the neonatal brain: a review of methodological challenges and neuroscientific advances. J. Magn. Reson. Imaging.: JMRI. 2021;53(5):1318–1343. doi: 10.1002/jmri.27192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn K., Reissland N., Reid V.M. The functional foetal brain: a systematic preview of methodological factors in reporting foetal visual and auditory capacity. Dev. Cogn. Neurosci. 2015;13:43–52. doi: 10.1016/j.dcn.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyet L.E., Kennea N., Counsell S.J., Maalouf E.F., Ajayi-Obe M., Duggan P.J., Harrison M., Allsop J.M., Hajnal J., Herlihy A.H., Edwards B., Laroche S., Cowan F.M., Rutherford M.A., Edwards A.D. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118(2):536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- Ellis C.T., Skalaban L.J., Yates T.S., Turk-Browne N.B. Attention recruits frontal cortex in human infants. Proc. Natl. Acad. Sci. USA. 2021;118(12) doi: 10.1073/pnas.2021474118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans A.C. The NIH MRI study of normal brain development. NeuroImage. 2006;30(1):184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Eyre M., Fitzgibbon S.P., Ciarrusta J., Cordero-Grande L., Price A.N., Poppe T., Schuh A., Hughes E., O’Keeffe C., Brandon J., Cromb D., Vecchiato K., Andersson J., Duff E.P., Counsell S.J., Smith S.M., Rueckert D., Hajnal J.V., Arichi T., Edwards A.D. The developing human connectome project: typical and disrupted perinatal functional connectivity. Brain. 2021;118 doi: 10.1093/brain/awab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon S.P., Harrison S.J., Jenkinson M., Baxter L., Robinson E.C., Bastiani M., Bozek J., Karolis V., Cordero Grande L., Price A.N., Hughes E., Makropoulos A., Passerat-Palmbach J., Schuh A., Gao J., Farahibozorg S.-R., O’Muircheartaigh J., Ciarrusta J., O’Keeffe C., Brandon J., Arichi T., Rueckert D., Hajnal J.V., Edwards A.D., Smith S.M., Duff E., Andersson J. The developing Human Connectome Project (dHCP) automated resting-state functional processing framework for newborn infants. NeuroImage. 2020;223 doi: 10.1016/j.neuroimage.2020.117303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogtmann M., Seshamani S., Kroenke C., Cheng X., Chapman T., Wilm J., Rousseau F., Studholme C. A unified approach to diffusion direction sensitive slice registration and 3-D DTI reconstruction from moving fetal brain anatomy. IEEE Trans. Med. Imaging. 2014;33(2):272–289. doi: 10.1109/TMI.2013.2284014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Skiold B., Horsch S., Nordell A., Blennow M., Lagercrantz H., Aden U. Resting-state networks in the infant brain. Proc. Natl. Acad. Sci. USA. 2007;104(39):15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Skiöld B., Engström M., Hallberg B., Mosskin M., Åden U., Lagercrantz H., Blennow M. Spontaneous brain activity in the newborn brain during natural sleep – an fMRI study in infants born at full term. Pediatr. Res. 2009;66(3):301–305. doi: 10.1203/PDR.0b013e3181b1bd84. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Konishi Y., Kuriyama M., Maeda M., Saito M., Ishii Y., Sudo M. MRI assessment of myelination patterns in high-risk infants. Pediatr. Neurol. 1993;9(3):194–197. doi: 10.1016/0887-8994(93)90083-O. [DOI] [PubMed] [Google Scholar]
- Fulford J., Vadeyar S.H., Dodampahala S.H., Moore R.J., Young P., Baker P.N., James D.K., Gowland P.A. Fetal brain activity in response to a visual stimulus. Hum. Brain Mapp. 2003;20(4):239–245. doi: 10.1002/hbm.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulford J., Vadeyar S.H., Dodampahala S.H., Ong S., Moore R.J., Baker P.N., James D.K., Gowland P. Fetal brain activity and hemodynamic response to a vibroacoustic stimulus. Hum. Brain Mapp. 2004;22(2):116–121. doi: 10.1002/hbm.20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. USA. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia K.E., Robinson E.C., Alexopoulos D., Dierker D.L., Glasser M.F., Coalson T.S., Ortinau C.M., Rueckert D., Taber L.A., Essen D.C.V., Rogers C.E., Smyser C.D., Bayly P.V. Dynamic patterns of cortical expansion during folding of the preterm human brain. Proc. Natl. Acad. Sci. USA. 2018;115(12):3156–3161. doi: 10.1073/pnas.1715451115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel C., Brisse H. Magnetic resonance imaging of the fetus. Pediatr. Radiol. 1998;28:201–211. doi: 10.1007/s002470050334. [DOI] [PubMed] [Google Scholar]
- Ghotra A., Kosakowski H.L., Takahashi A., Etzel R., May M.W., Scholz A., Jansen A., Wald L.L., Kanwisher N., Saxe R., Keil B. A size-adaptive 32-channel array coil for awake infant neuroimaging at 3 Tesla MRI. Magn. Reson. Med. 2021;86(3):1773–1785. doi: 10.1002/mrm.28791. [DOI] [PubMed] [Google Scholar]
- Gilmore J.H., Lin W., Gerig G. Fetal and neonatal brain development. Am. J. Psychiatry. 2006;163(12):2046. doi: 10.1176/ajp.2006.163.12.2046. [DOI] [PubMed] [Google Scholar]
- Gilmore J.H., Lin W., Corouge I., Vetsa Y.S.K., Smith J.K., Kang C., Gu H., Hamer R.M., Lieberman J.A., Gerig G. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. Am. J. Neuroradiol. 2007;28(9):1789–1795. doi: 10.3174/ajnr.A0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Lin W., Prastawa M.W., Looney C.B., Vetsa Y.S.K., Knickmeyer R.C., Evans D.D., Smith J.K., Hamer R.M., Lieberman J.A., Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J. Neurosci. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J.H., Kang C., Evans D.D., Wolfe H.M., Smith J.K., Lieberman J.A., Lin W., Hamer R.M., Styner M., Gerig G. Prenatal and neonatal brain structure and white matter maturation in children at high risk for Schizophrenia. Am. J. Psychiatry. 2010;167(9):1083–1091. doi: 10.1176/appi.ajp.2010.09101492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard N., Raybaud C., Dercole C., Boubli L., Chau C., Cahen S., Potier A., Gamerre M. In vivo MRI of the fetal brain. Neuroradiology. 1993;35(6):431–436. doi: 10.1007/BF00602823. [DOI] [PubMed] [Google Scholar]
- Goldberg E., McKenzie C.A., Vrijer B., Eagleson R., Ribaupierre S. Fetal response to a maternal internal auditory stimulus. J. Magn. Reson. Imaging. 2020;52(1):139–145. doi: 10.1002/jmri.27033. [DOI] [PubMed] [Google Scholar]
- Gong Q.Y., Roberts N., Garden A.S., Whitehouse G.H. Fetal and fetal brain volume estimation in the third trimester of human pregnancy using gradient echo MR Imaging. Magn. Reson. Imaging. 1998;16(3):235–240. doi: 10.1016/S0730-725X(97)00281-6. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Fisher P.A., Pfeifer J.H. What sleeping babies hear: a functional MRI study of interparental conflict and infants’ emotion processing. Psychol. Sci. 2013;24(5):782–789. doi: 10.1177/0956797612458803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Pfeifer J.H., Fisher P.A., Lin W., Gao W., Fair D.A. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev. Cogn. Neurosci. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A.R., Embleton N.D., Bradburn M., Connolly D.J.A., Mandefield L., Mooney C., Griffiths P.D. Accuracy of in-utero MRI to detect fetal brain abnormalities and prognosticate developmental outcome: Postnatal follow-up of the MERIDIAN cohort. Lancet Child Adolesc. Health. 2020;4(2):131–140. doi: 10.1016/S2352-4642(19)30349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R.C., Holland G.N., Moore W.S., Worthington B.S. Nuclear magnetic resonance (NMR) tomography of the brain: a preliminary clinical assesment with demonstration of pathology. J. Comput. Assist. Tomogr. 1980;4(5):577–586. doi: 10.1097/00004728-198010000-00001. [DOI] [PubMed] [Google Scholar]
- Hazlett H.C., Poe M., Gerig G., Smith R.G., Provenzale J., Ross A., Gilmore J., Piven J. Magnetic resonance imaging and head circumference study of brain size in Autism: birth through age 2 years. Arch. Gen. Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hazlett H.C., Poe M.D., Gerig G., Styner M., Chappell C., Smith R.G., Vachet C., Piven J. Early brain overgrowth in Autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry. 2011;68(5):467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett H.C., Gu H., Munsell B.C., Kim S.H., Styner M., Wolff J.J., Elison J.T., Swanson M.R., Zhu H., Botteron K.N., Collins D.L., Constantino J.N., Dager S.R., Estes A.M., Evans A.C., Fonov V.S., Gerig G., Kostopoulos P., McKinstry R.C., Piven J. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heep A., Scheef L., Jankowski J., Born M., Zimmermann N., Sival D., Bos A., Gieseke J., Bartmann P., Schild H., Boecker H. Functional magnetic resonance imaging of the sensorimotor system in preterm infants. Pediatrics. 2009;123(1):294–300. doi: 10.1542/peds.2007-3475. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M.I., Thomason M.E. Functional connectivity of the human brain in utero. Trends Cogn. Sci. 2016;20(12):931–939. doi: 10.1016/j.tics.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.I., Hect J.L., Smarr B.L., Qawasmeh T., Kriegsfeld L.J., Barcelona J., Hijazi K.E., Thomason M.E. Maternal stress during pregnancy alters fetal cortico-cerebellar connectivity in utero and increases child sleep problems after birth. Sci. Rep. 2021;11(1):2228. doi: 10.1038/s41598-021-81681-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland G.N., Hawkes R.C., Moore W.S. Nuclear magnetic resonance (NMR) tomography of the brain: coronal and sagittal. J. Comput. Assist. Tomogr. 1980;4(4):429–433. doi: 10.1097/00004728-198008000-00002. [DOI] [PubMed] [Google Scholar]
- Holland G.N., Moore W.S., Hawkes R.C. Nuclear magentic resonance tomography. J. Comput. Assist. Tomogr. 1980;4(1):1–3. doi: 10.1097/00004728-198002000-00001. [DOI] [PubMed] [Google Scholar]
- Horvath L., Seeds J.W. Temporary arrest of fetal movement with pancuronium bromide to enable antenatal magentic resonance imaging of holoprosencephaly. Am. J. Perinatol. 1989;6(4):418–420. doi: 10.1055/s-2007-999629. [DOI] [PubMed] [Google Scholar]
- Howell B.R., Styner M.A., Gao W., Yap P.-T., Wang L., Baluyot K., Yacoub E., Chen G., Potts T., Salzwedel A., Li G., Gilmore J.H., Piven J., Smith J.K., Shen D., Ugurbil K., Zhu H., Lin W., Elison J.T. The UNC/UMN baby connectome project (BCP): an overview of the study design and protocol development. NeuroImage. 2019;185:891–905. doi: 10.1016/j.neuroimage.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. Structure of the fetal brain: what we are learning from diffusion tensor imaging. Neuroscientist. 2010;16(6):634–639. doi: 10.1177/1073858409356711. [DOI] [PubMed] [Google Scholar]
- Hubbard A.M., Harty M.P., States L.J. A new tool for prenatal diagnosis: ultrafast fetal MRI. Semin. Perinatol. 1999;23(6):437–447. doi: 10.1016/S0146-0005(99)80023-8. [DOI] [PubMed] [Google Scholar]
- Hughes E.J., Winchman T., Padormo F., Teixeira R., Wurie J., Sharma M., Fox M., Hutter J., Cordero-Grande L., Price A.N., Allsop J., Bueno-Conde J., Tusor N., Arichi T., Edwards A.D., Rutherford M.A., Counsell S.J., Hajnal J.V. A dedicated neonatal brain imaging system. Magn. Reson. Med. 2017;78(2):794–804. doi: 10.1002/mrm.26462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulshof H.M., Slot E.M.H., Lequin M., Breuillard D., Boddaert N., Jozwiak S., Kotulska K., Riney K., Feucht M., Samueli S., Scholl T., Krsek P., Benova B., Braun K.P.J., Jansen F.E., Nabbout R., Curatolo P., Lagae L., Jansen A., Urbańska M. Fetal brain magnetic resonance imaging findings predict neurodevelopment in children with tuberous sclerosis complex. J. Pediatr. 2021;233:156–162. doi: 10.1016/j.jpeds.2021.02.060. [DOI] [PubMed] [Google Scholar]
- Hüppi P.S., Dubois J. Diffusion tensor imaging of brain development. Semin. Fetal Neonatal Med. 2006;11(6):489–497. doi: 10.1016/j.siny.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Hüppi P.S., Schuknecht B., Boesch C., Bossi E., Felblinger J., Fusch C., Herschkowitz N. Structural and neurobehavioral delay in postnatal brain development of preterm infants. Pediatr. Res. 1996;39(5):895–901. doi: 10.1203/00006450-199605000-00026. [DOI] [PubMed] [Google Scholar]
- Hüppi P.S., Maier S.E., Peled S., Zientara G.P., Barnes P.D., Jolesz F.A., Volpe J.J. Microstructural development of human newborn cerebral white matter assessed in Vivo by diffusion tensor magnetic resonance imaging. Pediatr. Res. 1998;44(4):584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Hüppi P.S., Warfield S., Kikinis R., Barnes P.D., Zientara G.P., Jolesz F.A., Tsuji M.K., Volpe J.J. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 1998;43:224–235. doi: 10.1002/ana.410430213. [DOI] [PubMed] [Google Scholar]
- Hüppi P.S., Murphy B., Maier S.E., Zientara G.P., Inder T.E., Barnes P.D., Kikinis R., Jolesz F.A., Volpe J.J. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107(3):455–460. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- Hutter J., Tournier J.D., Price A.N., Cordero-Grande L., Hughes E.J., Malik S., Steinweg J., Bastiani M., Sotiropoulos S.N., Jbabdi S., Andersson J., Edwards A.D., Hajnal J.V. Time-efficient and flexible design of optimized multishell HARDI diffusion. Magn. Reson. Med. 2018;79(3):1276–1292. doi: 10.1002/mrm.26765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hykin J., Moore R., Duncan K., Clare S., Baker P., Johnson I., Bowtell R., Mansfield P., Gowland P. Fetal brain activity demonstrated by functional magnetic resonance imaging. Lancet. 1999;354(9179):645–646. doi: 10.1016/S0140-6736(99)02901-3. [DOI] [PubMed] [Google Scholar]
- Inder T., Huppi P.S., Zientara G.P., Maier S.E., Jolesz F.A., Salvo D., di, Robertson R., Barnes P.D., Volpe J.J. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J. Pediatr. 1999;134(5):631–634. doi: 10.1016/S0022-3476(99)70251-9. [DOI] [PubMed] [Google Scholar]
- Inder T.E., Huppi P.S., Zientara G.P., Jolesz F.A., Holling E.E., Robertson R., Barnes P.D., Volpe J.J. The postmigrational development of polymicrogyria documented by magnetic resonance imaging from 31 weeks’ postconceptional age. Ann. Neurol. 1999;45(6):798–801. doi: 10.1002/1531-8249(199906)45:6<798::aid-ana16>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Jakab A., Schwartz E., Kasprian G., Gruber G.M., Prayer D., SchÃpf V., Langs G. Fetal functional imaging portrays heterogeneous development of emerging human brain networks. Front. Hum. Neurosci. 2014;8:1–17. doi: 10.3389/fnhum.2014.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab A., Tuura R., Kellenberger C., Scheer I. In utero diffusion tensor imaging of the fetal brain: a reproducibility study. NeuroImage: Clin. 2017;15:601–612. doi: 10.1016/j.nicl.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardri R., Houfflin‐Debarge V., Delion P., Pruvo J., Thomas P., Pins D. Assessing fetal response to maternal speech using a noninvasive functional brain imaging technique. Int. J. Dev. Neurosci. 2012;30(2):159–161. doi: 10.1016/j.ijdevneu.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Johnson M., Pennock J., Bydder G., Steiner R., Thomas D., Hayward R., Bryant D., Payne J., Levene M., Whitelaw A. Clinical NMR imaging of the brain in children: normal and neurologic disease. AJR Am. J. Roentgenol. 1983;141:1005–1018. doi: 10.2214/ajr.141.5.1005. [DOI] [PubMed] [Google Scholar]
- Jordan C.J., Weiss S.R.B., Howlett K.D., Freund M.P. Introduction to the special issue on “Informing longitudinal studies on the effects of maternal stress and substance use on child development: planning for the healthy brain and child development (HBCD) study. Advers. Resil. Sci. 2020;1(4):217–221. doi: 10.1007/s42844-020-00022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapellou O., Counsell S.J., Kennea N., Dyet L., Saeed N., Stark J., Maalouf E., Duggan P., Ajayi-Obe M., Hajnal J., Allsop J.M., Boardman J., Rutherford M.A., Cowan F., Edwards A.D. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLOS Med. 2006;3(8) doi: 10.1371/journal.pmed.0030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprian G., Brugger P.C., Weber M., Krssak M., Krampi E., Herold C., Prayer D. In utero tractography of fetal white matter development. NeuroImage. 2008;43(2):213–224. doi: 10.1016/j.neuroimage.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Keraudren K., Kuklisova-Murgasova M., Kyriakopoulou V., Malamateniou C., Rutherford M.A., Kainz B., Hajnal J.V., Rueckert D. Automated fetal brain segmentation from 2D MRI slices for motion correction. NeuroImage. 2014;101:633–643. doi: 10.1016/j.neuroimage.2014.07.023. [DOI] [PubMed] [Google Scholar]
- Khan S., Vasung L., Marami B., Rollins C.K., Afacan O., Ortinau C.M., Yang E., Warfield S.K., Gholipour A. Fetal brain growth portrayed by a spatiotemporal diffusion tensor MRI atlas computed from in utero images. NeuroImage. 2019;185:593–608. doi: 10.1016/j.neuroimage.2018.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-H., Chung S., Vigneron D.B., Barkovich A.J., Glenn O.A. Diffusion-weighted imaging of the fetal brain in vivo. Magn. Reson. Med. 2008;59(1):216–220. doi: 10.1002/mrm.21459. [DOI] [PubMed] [Google Scholar]
- Kim K., Habas P.A., Rousseau F., Glenn O.A., Barkovich A.J., Studholme C. Intersection based motion correction of multi-slice MRI for 3D in utero fetal brain image formation. IEEE Trans. Med. Imaging. 2010;29(1):146–158. doi: 10.1109/TMI.2009.2030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Habas P.A., Rousseau F., Glenn O.A., Barkovich A.J., Koob M., Dietemann J.-L., Robinson A.J., Poskitt K.J., Miller S.P., Studholme C. Reconstruction of a geometrically correct diffusion tensor image of a moving human fetal brain. Proc. SPIE. 2010:76231I. doi: 10.1117/12.844542. [DOI] [Google Scholar]
- Zollei L., Iglesias J.E., Ou Y., Grant E., Fischl B. Infant FreeSurfer: An automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0-2 years. Neuroimage. 2020;218 doi: 10.1016/j.neuroimage.2020.116946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinfield, S. (2014). Kleinfield, Sonny. A machine called indomitable: The remarkable story of a scientist’s inspiration, invention, and medical breakthrough. Open Road Media.
- Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlasch K.L., Cioffredi L.-A., Lenninger C., Stewart E., Vatalaro T., Garavan H., Graham A., Heil S.H., Krans E.E., Robakis T., Rommel A., Sullivan E.L., Thomason M., Potter A. Factors associated with parent views about participation in infant MRI research provide guidance for the design of the healthy brain and child development (HBCD) study. Dev. Cogn. Neurosci. 2021;50 doi: 10.1016/j.dcn.2021.100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan M.L., Dyet L.E., Boardman J.P., Kapellou O., Allsop J.M., Cowan F., Edwards A.D., Rutherford M.A., Counsell S.J. Relationship between white matter apparent diffusion coefficients in preterm infants at term-equivalent age and developmental outcome at 2 years. Pediatrics. 2007;120(3):e604–e609. doi: 10.1542/peds.2006-3054. [DOI] [PubMed] [Google Scholar]
- Kuklisova-Murgasova M., Quaghebeur G., Rutherford M.A., Hajnal J.V., Schnabel J.A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med. Image Anal. 2012;16(8):1550–1564. doi: 10.1016/j.media.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K.K., Belliveau J.W., Chesler D.A., Goldberg I.E., Weisskoff R.M., Poncelet B.P., Kennedy D.N., Hoppel B.E., Cohen M.S., Turner R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. USA. 1992;89(12):5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lago P., Rebsamen S., Clancy R.R., Pinto-Martin J., Kessler A., Zimmerman R., Schmelling D., Bernbaum J., Gerdes M., D’Agostino J.A., Baumgart S. MRI, MRA, and neurodevelopmental outcome following neonatal ECMO. Pediatr. Neurol. 1995;12(4):294–304. doi: 10.1016/0887-8994(95)00047-J. [DOI] [PubMed] [Google Scholar]
- Langer N., Peysakhovich B., Zuk J., Drottar M., Sliva D.D., Smith S., Becker B.L.C., Grant P.E., Gaab N. White matter alterations in infants at risk for developmental dyslexia. Cereb. Cortex. 2017;27(2):1027–1036. doi: 10.1093/cercor/bhv281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbur P.C. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature. 1973;242:190–191. doi: 10.1038/242190a0. [DOI] [PubMed] [Google Scholar]
- Levene M.I., Fawer C.L., Lamont R.F. Risk factors in the development of intraventricular haemorrhage in the preterm neonate. Arch. Dis. Child. 1982;57(6):410–417. doi: 10.1136/adc.57.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine D., Barnes P.D., Madsen J.R., Lei W., Edelman R.R. Fetal central nervous system anomalies: MR imaging augments sonographic diagnosis. Radiology. 1997;204(3):635–642. doi: 10.1148/radiology.204.3.9280237. [DOI] [PubMed] [Google Scholar]
- Levine D., Barnes P.D., Sher S., Li W., McArdle C.R., Worawattanakul S., Edelman R.R. Fetal fast MR imaging: reproducibility, technical quality, and conspicuity of anatomy. Radiology. 1998;206(2):549–554. doi: 10.1148/radiology.206.2.9457211. [DOI] [PubMed] [Google Scholar]
- Levine D., Barnes P.D., Edelman R.R. Obstetric MR imaging. Radiology. 1999;211(3):609–617. doi: 10.1148/radiology.211.3.r99jn20609. [DOI] [PubMed] [Google Scholar]
- Liao X., Vasilakos A.V., He Y. Small-world human brain networks: perspectives and challenges. Neurosci. Biobehav. Rev. 2017;77:286–300. doi: 10.1016/j.neubiorev.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Lin W., Zhu Q., Gao W., Chen Y., Toh C.-H., Styner M., Gerig G., Smith J.K., Biswal B., Gilmore J.H. Functional connectivity MR Imaging reveals cortical functional connectivity in the developing brain. Am. J. Neuroradiol. 2008;29(10):1883–1889. doi: 10.3174/ajnr.A1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke A.C., Wild C., Zubiaurre-Elorza L., Herzmann C., Duffy H., Han V.K., Lee D.S.C., Cusack R. Disruption to functional networks in neonates with perinatal brain injury predicts motor skills at 8 months. NeuroImage Clin. 2018;18:399–406. doi: 10.1016/j.nicl.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney C.B., Smith J.K., Merck L.H., Wolfe H.M., Chescheir N.C., Hamer R.M., Gilmore J.H. Intracranial hemorrhage in asymptomatic neonates: prevalence on MR Images and relationship to obstetric and neonatal risk factors. Radiology. 2007;242(2):535–541. doi: 10.1148/radiol.2422060133. [DOI] [PubMed] [Google Scholar]
- Maalouf E.F., Duggan P.J., Rutherford M.A., Counsell S.J., Fletcher A.M., Battin M., Cowan F., Edwards A.D. Magnetic resonance imaging of the brain in a cohort of extremely preterm infants. J. Pediatr. 1999;135(3):351–357. doi: 10.1016/S0022-3476(99)70133-2. [DOI] [PubMed] [Google Scholar]
- Maas L.C., Mukherjee P., Carballido-Gamio J., Veeraraghavan S., Miller S.P., Partridge S.C., Henry R.G., Barkovich A.J., Vigneron D.B. Early laminar organization of the human cerebrum demonstrated with diffusion tensor imaging in extremely premature infants. NeuroImage. 2004;22(3):1134–1140. doi: 10.1016/j.neuroimage.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Makropoulos A., Robinson E.C., Schuh A., Wright R., Fitzgibbon S., Bozek J., Counsell S.J., Steinweg J., Vecchiato K., Passerat-Palmbach J., Lenz G., Mortari F., Tenev T., Duff E.P., Bastiani M., Cordero-Grande L., Hughes E., Tusor N., Tournier J.-D., Rueckert D. The developing human connectome project: a minimal processing pipeline for neonatal cortical surface reconstruction. NeuroImage. 2018;173:88–112. doi: 10.1016/j.neuroimage.2018.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield P., Maudsley A.A. Medical imaging by NMR. Br. J. Radiol. 1977;50(591):188–194. doi: 10.1259/0007-1285-50-591-188. [DOI] [PubMed] [Google Scholar]
- Marami B., Mohseni Salehi S.S., Afacan O., Scherrer B., Rollins C.K., Yang E., Estroff J.A., Warfield S.K., Gholipour A. Temporal slice registration and robust diffusion-tensor reconstruction for improved fetal brain structural connectivity analysis. NeuroImage. 2017;156:475–488. doi: 10.1016/j.neuroimage.2017.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle C.B., Richardson C.J., Hayden C.K., Nicholas D.A., Crofford M.J., Amparo E.G. Abnormalities of the neonatal brain: MR imaging. Part I. Intracranial hemorrhage. Radiology. 1987;163(2):387–394. doi: 10.1148/radiology.163.2.3550881. [DOI] [PubMed] [Google Scholar]
- McArdle C.B., Richardson C.J., Nicholas D.A., Mirfakhraee M., Hayden C.K., Amparo E.G. Developmental features of the neonatal brain: MR imaging. Part I. Gray-white matter differentiation and myelination. Radiology. 1987;162(1):223–229. doi: 10.1148/radiology.162.1.3786767. [DOI] [PubMed] [Google Scholar]
- McCarthy S.M., Filly R.A., Stark D.D., Hricak H., Brant-Zawadzki M.N., Callen P.W., Higgins C.B. Obstetrical magnetic resonance imaging: fetal anatomy. Radiology. 1985;154(2):427–432. doi: 10.1148/radiology.154.2.3966129. [DOI] [PubMed] [Google Scholar]
- McCarthy S.M., Stark D.D., Filly R.A., Callen P.W., Hricak H., Higgins C.B. Obstetrical magnetic resonance imaging: maternal anatomy. Radiology. 1985;154(2):421–425. doi: 10.1148/radiology.154.2.3966128. [DOI] [PubMed] [Google Scholar]
- Menticoglou S.M., Manning F.A., Harman C.R., Morrison I. Severe fetal brain injury without evident intrapartum asphyxia or trauma. Obstet. Gynecol. 1989;74(3 Pt 2):457–461. [PubMed] [Google Scholar]
- Mercuri E., Rutherford M., Cowan F., Pennock J., Counsell S., Papadimitriou M., Azzopardi D., Bydder G., Dubowitz L. Early prognostic indicators of outcome in infants with neonatal cerebral infarction: a clinical, electroencephalogram, and magnetic resonance imaging study. Pediatrics. 1999;103(1):39–46. doi: 10.1542/peds.103.1.39. [DOI] [PubMed] [Google Scholar]
- Mercuri E., Guzzetta A., Haataja L., Cowan F., Rutherford M., Counsell S., Papadimitriou M., Cioni G., Dubowitz L. Neonatal neurological examination in infants with hypoxic ischaemic encephalopathy: correlation with MRI findings. Neuropediatrics. 1999;30(2):83–89. doi: 10.1055/s-2007-973465. [DOI] [PubMed] [Google Scholar]
- Mewes A.U.J., Huppi P.S., Als H., Rybicki F.J., Inder T.E., McAnulty G.B., Mulkern R.V., Robertson R.L., Rivkin M.J., Warfield S.K. Regional brain development in serial magnetic resonance imaging of low-risk preterm infants. Pediatrics. 2006;118(1):23–33. doi: 10.1542/peds.2005-2675. [DOI] [PubMed] [Google Scholar]
- Moore R.J., Vadeyar S., Fulford J., Tyler D.J., Gribben C., Baker P.N., James D., Gowland P.A. Antenatal determination of fetal brain activity in response to an acoustic stimulus using functional magnetic resonance imaging. Hum. Brain Mapp. 2001;12:94–99. doi: 10.1002/1097-0193(200102)12:2<94::AID-HBM1006>3.0.CO;2-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norr M.E., Hect J.L., Lenniger C.J., Van den Heuvel M., Thomason M.E. An examination of maternal prenatal BMI and human fetal brain development. J. Child Psychol. Psychiatry, Allied Discip. 2021;62(4):458–469. doi: 10.1111/jcpp.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris D.G., Driesel W. Online motion correction for diffusion-weighted imaging using navigator echoes: application to RARE imaging without sensitivity loss. Magn. Reson. Med. 2001;45(5):729–733. doi: 10.1002/mrm.1099. [DOI] [PubMed] [Google Scholar]
- O’Muircheartaigh J., Robinson E.C., Pietsch M., Wolfers T., Aljabar P., Grande L.C., Teixeira R.P.A.G., Bozek J., Schuh A., Makropoulos A., Batalle D., Hutter J., Vecchiato K., Steinweg J.K., Fitzgibbon S., Hughes E., Price A.N., Marquand A., Reuckert D., Edwards A.D. Modelling brain development to detect white matter injury in term and preterm born neonates. Brain. 2020;143(2):467–479. doi: 10.1093/brain/awz412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD , 2019. Health at a Glance 2019: OECD Indicators. OECD. doi: 10.1787/4dd50c09-en.
- OECD, 2021. Magnetic resonance imaging (MRI) units. doi: 10.1787/1a72e7d1-en.
- Ogawa S., Lee T.-M., Nayak A.S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 1990;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Partners Healthcare Annual report 2018: What’s next? Brigh. Women’s Hosp. Mass. Gen. Hosp. 2018:1–60. https://www.partners.org/Assets/Documents/About-Us/Annual-Report-2018.pdf [Google Scholar]
- Partridge S.C., Mukherjee P., Berman J.I., Henry R.G., Miller S.P., Lu Y., Glenn O.A., Ferriero D.M., Barkovich A.J., Vigneron D.B. Tractography-based quantitation of diffusion tensor imaging parameters in white matter tracts of preterm newborns. J. Magn. Reson. Imaging. 2005;22(4):467–474. doi: 10.1002/jmri.20410. [DOI] [PubMed] [Google Scholar]
- Peterson B.S. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Pike M.G., Holmstrom G., Vries L.S., de, Pennock J.M., Drew K.J., Sonksen P.M., Dubowitz L.M.S. Patterns Of visual impairment associated with lesions of the preterm infant brain. Dev. Med. Child Neurol. 1994;36(10):849–862. doi: 10.1111/j.1469-8749.1994.tb11776.x. [DOI] [PubMed] [Google Scholar]
- Pontabry J., Rousseau F., Studholme C., Koob M., Dietemann J.-L. A discriminative feature selection approach for shape analysis: application to fetal brain cortical folding. Med. Image Anal. 2017;35:313–326. doi: 10.1016/j.media.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschle N., Zuk J., Ortiz-Mantilla S., Sliva D.D., Franceschi A., Grant P.E., Benasich A.A., Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Ann. N. Y. Acad. Sci. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta M., Burdi N., Medicamento N. Magnetic resonance imaging of normal and pathologic fetal brain. Child’s Nerv. Syst. 1998;14(4–5):151–154. doi: 10.1007/s003810050201. [DOI] [PubMed] [Google Scholar]
- Robertson N.J., Cox I.J., Cowan F.M., Counsell S.J., Azzopardi D., Edwards A.D. Cerebral intracellular lactic alkalosis persisting months after neonatal encephalopathy measured by magnetic resonance spectroscopy. Pediatr. Res. 1999;46(3):287–296. doi: 10.1203/00006450-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Rogers C.E., Sylvester C.M., Mintz C., Kenley J.K., Shimony J.S., Barch D.M., Smyser C.D. Neonatal amygdala functional connectivity at rest in healthy and preterm infants and early internalizing symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(2):157–166. doi: 10.1016/j.jaac.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rona Z., Klebermass K., Cardona F., Czaba C.D., Brugger P.C., Weninger M., Pollak A., Prayer D. Comparison of neonatal MRI examinations with and without an MR-compatible incubator: advantages in examination feasibility and clinical decision-making. Eur. J. Paediatr. Neurol. 2010;14(5):410–417. doi: 10.1016/j.ejpn.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Studholme C., Jardri R., Thomason M.E. In: Fetal Development. Reissland N., Kisilevsky B.S., editors. Springer International Publishing; 2016. In vivo human fetal brain analysis using MR imaging; pp. 407–427. [DOI] [Google Scholar]
- Rutherford M.A., Cowan F.M., Manzur A.Y., Dubowitz L.M., Pennock J.M., Hajnal J.V., Young I.R., Bydder G.M. MR imaging of anisotropically restricted diffusion in the brain of neonates and infants. J. Comput. Assist. Tomogr. 1991;15(2):188–198. doi: 10.1097/00004728-199103000-00002. [DOI] [PubMed] [Google Scholar]
- Rutherford M.A., Pennock J.M., Counsell S.J., Mercuri E., Cowan F.M., Dubowitz L.M.S., Edwards A.D. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics. 1998;102(2):323–328. doi: 10.1542/peds.102.2.323. [DOI] [PubMed] [Google Scholar]
- Rutherford S., Sturmfels P., Angstadt M., Hect J., Wiens J., van den Heuvel M.I., Scheinost D., Sripada C., Thomason M. Automated brain masking of fetal functional MRI with open data. Neuroinformatics. 2021 doi: 10.1007/s12021-021-09528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C.E., Richards J.E., Almli C.R. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev. Psychobiol. 2012;54(1):77–91. doi: 10.1002/dev.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Cortes M., Egaña-Ugrinovic G., Zupan R., Figueras F., Gratacos E. Brainstem and cerebellar differences and their association with neurobehavior in term small-for-gestational-age fetuses assessed by fetal MRI. Am. J. Obstet. Gynecol. 2014;210(5):452. doi: 10.1016/j.ajog.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Schöpf V., Kasprian G., Brugger P.C., Prayer D. Watching the fetal brain at ‘rest. Int. J. Dev. Neurosci. 2012;30(1):11–17. doi: 10.1016/j.ijdevneu.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Schöpf V., Schlegl T., Jakab A., Kasprian G., Woitek R., Prayer D., Langs G. The relationship between eye movement and vision develops before birth. Front. Hum. Neurosci. 2014;8:1–6. doi: 10.3389/fnhum.2014.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serai S.D., Merrow A.C., Kline-Fath B.M. Fetal MRI on a multi-element digital coil platform. Pediatr. Radiol. 2013;43(9):1213–1217. doi: 10.1007/s00247-013-2695-0. [DOI] [PubMed] [Google Scholar]
- Seshamani S., Cheng X., Fogtmann M., Thomason M.E., Studholme C. A method for handling intensity inhomogenieties in fMRI sequences of moving anatomy of the early developing brain. Med. Image Anal. 2014;18(2):285–300. doi: 10.1016/j.media.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshamani, S., Fogtmann, M., Cheng, X., Thomason, M., Gatenby, C., Studholme, C., 2013. Cascaded slice to volume registration for moving fetal FMRI. In: Proceedings of the 2013 IEEE 10th International Symposium on Biomedical Imaging, pp. 796–799. doi: 10.1109/ISBI.2013.6556595.
- Shen M.D., Nordahl C.W., Young G.S., Wootton-Gorges S.L., Lee A., Liston S.E., Harrington K.R., Ozonoff S., Amaral D.G. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 2013;136(9):2825–2835. doi: 10.1093/brain/awt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sie L.T., van der Knaap M.S., van Wezel-Meijler G., Valk J. MRI assessment of myelination of motor and sensory pathways in the brain of preterm and term-born infants. Neuropediatrics. 1997;28(2):97–105. doi: 10.1055/s-2007-973680. [DOI] [PubMed] [Google Scholar]
- Sims M.E., Turkel S.B., Halterman G., Paul R.H. Brain injury and intrauterine death. Am. J. Obstet. Gynecol. 1985;151(6):721–723. doi: 10.1016/0002-9378(85)90503-4. [DOI] [PubMed] [Google Scholar]
- Smith F., Adam A.H., Phillips W.D.P. NMR imaging in pregnancy. Lancet. 1983;321(8314–8315):61–62. doi: 10.1016/S0140-6736(83)91588-X. [DOI] [PubMed] [Google Scholar]
- Smith F.W. The value of NMR imaging in pediatric practice: a preliminary report. Pediatr. Radiol. 1983;13(3):141–147. doi: 10.1007/BF01624398. [DOI] [PubMed] [Google Scholar]
- Smith F.W., MacLennan F., Abramovich D.R., MacGilivray I., Hutchison J.M.S. NMR imaging in human pregnancy: a preliminary study. Magn. Reson. Imaging. 1984;2(1):57–64. doi: 10.1016/0730-725X(84)90126-7. [DOI] [PubMed] [Google Scholar]
- Smyser C.D., Neil J.J. Use of resting-state functional MRI to study brain development and injury in neonates. Semin. Perinatol. 2015;39(2):130–140. doi: 10.1053/j.semperi.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Inder T.E., Shimony J.S., Hill J.E., Degnan A.J., Snyder A.Z., Neil J.J. Longitudinal analysis of neural network development in preterm infants. Cereb. Cortex. 2010;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P.C., Rypens F.F., Carteret M., Delezoide A.-L., Brunelle F.O. MR imaging of fetal cerebral anomalies. Pediatr. Radiol. 1998;28(4):212–222. doi: 10.1007/s002470050335. [DOI] [PubMed] [Google Scholar]
- Sparks B.F., Friedman S.D., Shaw D.W., Aylward E.H., Echelard D., Artru A.A., Maravilla K.R., Giedd J.N., Munson J., Dawson G., Dager S.R. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/WNL.59.2.184. [DOI] [PubMed] [Google Scholar]
- Story L., Davidson A., Patkee P., Fleiss B., Kyriakopoulou V., Colford K., Sankaran S., Seed P., Jones A., Hutter J., Shennan A., Rutherford M. Brain volumetry in fetuses that deliver very preterm: an MRI pilot study. NeuroImage: Clin. 2021;30 doi: 10.1016/j.nicl.2021.102650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C. Mapping fetal brain development in utero using magnetic resonance imaging: the Big bang of brain mapping. Annu. Rev. Biomed. Eng. 2011;13:345–368. doi: 10.1146/annurev-bioeng-071910-124654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Smyser C.D., Smyser T., Kenley J., Ackerman J.J., Shimony J.S., Petersen S.E., Rogers C.E. Cortical functional connectivity evident after birth and behavioral inhibition at age 2. Am. J. Psychiatry. 2018;175(2):180–187. doi: 10.1176/appi.ajp.2017.17010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester C.M., Myers M.J., Perino M.T., Kaplan S., Kenley J.K., Smyser T.A., Warner B.B., Barch D.M., Pine D.S., Luby J.L., Rogers C.E., Smyser C.D. Neonatal brain response to deviant auditory stimuli and relation to maternal trait anxiety. Am. J. Psychiatry. 2021;178:1–9. doi: 10.1176/appi.ajp.2020.20050672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Scheinost D., Manning J.H., Grove L.E., Hect J., Marshall N., Hernandez-Andrade E., Berman S., Pappas A., Yeo L., Hassan S.S., Constable R.T., Ment L.R., Romero R. Weak functional connectivity in the human fetal brain prior to preterm birth. Sci. Rep. 2017;7(1):39286. doi: 10.1038/srep39286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Hect J., Waller R., Manning J.H., Stacks A.M., Beeghly M., Boeve J.L., Wong K., van den Huevel M.I., Hernandez-Andrade E., Hassan S.S., Romero R. Prenatal neural origins of infant motor development: associations between fetal brain and infant motor development. Dev. Psychopathol. 2018;30(3):763–772. doi: 10.1017/S095457941800072X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchio S., Kline-Fath B., Kanal E., Schmithorst V.J., Panigrahy A. MRI evaluation and safety in the developing brain. Semin. Perinatol. 2015;39(2):73–104. doi: 10.1053/j.semperi.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J.-D., Christiaens D., Hutter J., Price A.N., Cordero-Grande L., Hughes E., Bastiani M., Sotiropoulos S.N., Smith S.M., Rueckert D., Counsell S.J., Edwards A.D., Hajnal J.V. A data-driven approach to optimising the encoding for multi-shell diffusion MRI with application to neonatal imaging. NMR Biomed. 2020;33(9) doi: 10.1002/nbm.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya K., Katase S., Mizutani Y., Hachiya J. MR imaging of fetal brain abnormalities using a HASTE sequence. Br. J. Radiol. 1996;69(823):668–670. doi: 10.1259/0007-1285-69-823-668. [DOI] [PubMed] [Google Scholar]
- Turk E., van den Heuvel M.I., Benders M.J., de Heus R., Franx A., Manning J.H., Hect J.L., Hernandez-Andrade E., Hassan S.S., Romero R., Kahn R.S., Thomason M.E., van den Heuvel M.P. Functional connectome of the fetal brain. J. Neurosci. 2019;39(49):9716–9724. doi: 10.1523/JNEUROSCI.2891-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasung L., Abaci Turk E., Ferradal S.L., Sutin J., Stout J.N., Ahtam B., Lin P.-Y., Grant P.E. Exploring early human brain development with structural and physiological neuroimaging. NeuroImage. 2019;187:226–254. doi: 10.1016/j.neuroimage.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa L.M., Hampton S., Aydin E., Tait R., Leming M.J., Tsompanidis A., Patterson I., Allison C., Austin T., Suckling J., Baron-Cohen S., Holt R.J. Sex differences in brain development in fetuses and infants who are at low or high likelihood for autism. Preprint. 2021 doi: 10.1101/2021.03.08.21251862. [DOI] [Google Scholar]
- Wang J., et al. Multi-atlas segmentation of subcortical brain structures via the AutoSeg software pipeline. Frontiers in Neuroinformatics. 2014;8 doi: 10.3389/fninf.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreb J.C., Lowe T.W., Santos-Ramos R., Cunningham F.G., Parkey R. Magnetic resonance Imagiing in obstetric diagnosis. Radiology. 1985;154:157–161. doi: 10.1148/radiology.154.1.3880601. [DOI] [PubMed] [Google Scholar]
- Welsh R.C., Nemec U., Thomason M.E. Fetal magnetic resonance imaging at 3.0 T. Top. Magn. Reson. Imaging. 2011;22(3):119–131. doi: 10.1097/RMR.0b013e318267f932. [DOI] [PubMed] [Google Scholar]
- Wild C.J., Linke A.C., Zubiaurre-Elorza L., Herzmann C., Duffy H., Han V.K., Lee D.S.C., Cusack R. Adult-like processing of naturalistic sounds in auditory cortex by 3- and 9-month old infants. NeuroImage. 2017;157:623–634. doi: 10.1016/j.neuroimage.2017.06.038. [DOI] [PubMed] [Google Scholar]
- Wilson S., Pietsch M., Cordero-Grande L., Price A.N., Hutter J., Xiao J., McCabe L., Rutherford M.A., Hughes E.J., Counsell S.J., Tournier J.-D., Arichi T., Hajnal J.V., Edwards A.D., Christiaens D., O’Muircheartaigh J. Development of human white matter pathways in utero over the second and third trimester. Proc. Natl. Acad. Sci. USA. 2021;118(20) doi: 10.1073/pnas.2023598118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.J., Gu H., Gerig G., Elison J.T., Styner M., Gouttard S., Botteron K.N., Dager S.R., Dawson G., Estes A.M., Evans A.C., Hazlett H.C., Kostopoulos P., McKinstry R.C., Paterson S.J., Schultz R.T., Zwaigenbaum L., Piven J. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry. 2012;169(6):589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward L.J., Anderson P.J., Austin N.C., Howard K., Inder T.E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Namimoto T., Abe Y., Takahashi M., Iwamasa J., Miyazaki K., Okamura H. MR imaging of the fetus by a HASTE sequence. Am. J. Roentgenol. 1997;168(2):513–519. doi: 10.2214/ajr.168.2.9016238. [DOI] [PubMed] [Google Scholar]
- You W., Evangelou I.E., Zun Z., Andescavage N., Limperopoulos C. Robust preprocessing for stimulus-based functional MRI of the moving fetus. J. Med. Imaging. 2016;3(2) doi: 10.1117/1.JMI.3.2.026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh W.T.C., Nguyen H.D., Fisher D.J., Tali E.T., Gao F., Simonson T.M., Kao S.C.S., Weiner C.P. MR of fetal central nervous system abnormalities. Am. J. Neuroradiol. 1994;15(3):459–464. http://www.ajnr.org/content/15/3/459/tab-article-info [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.