Abstract
BACKGROUND
Brain metastases (BM) are the most common type of brain tumor malignancy in the US. They are also the most common indication for stereotactic radiosurgery (SRS). However, the incidence of both local recurrence and radiation necrosis (RN) is increasing as treatments improve. MRI imagery often fails to differentiate BM from RN; thus, patients must often undergo surgical biopsy or resection to obtain a definitive diagnosis.
OBJECTIVE
To hypothesize that a marker of immunosuppression might serve as a surrogate marker to differentiate patients with active vs inactive cancer—including RN.
METHODS
We thus purified and quantified Monocytic Myeloid-Derived Suppressor Cells (Mo-MDSC) by flow cytometry in patients proven by biopsy to represent BM or RN.
RESULTS
We report the utility of the previously reported HLA-Dr-Vnn2 Index or DVI to discriminate recurrent BM from RN using peripheral blood. The presence of CD14+ HLA-DRneg/low Mo-MDSC is significantly increased in the peripheral blood of patients with brain metastasis recurrence compared to RN (Average 61.5% vs 7%, n = 10 and n = 12, respectively, P < .0001). In contrast, expression of VNN2 on circulating CD14+ monocytes is decreased in BM patients compared to patients with RN (5.5% vs 26.5%, n = 10 and n = 12, respectively, P = .0008). In patients with biopsy confirmed recurrence of brain metastasis, the average DVI was 11.65, whereas the average DVI for RN patients was consistently <1 (Avg. of 0.17).
CONCLUSION
These results suggest that DVI could be a useful diagnostic tool to differentiate recurrent BM from RN using a minimally invasive blood sample.
Keywords: Brain tumors, Brain metastasis, MDSC, Liquid biopsy, Radiation necrosis, VNN2
ABBREVIATIONS
- BM
brain metastases
- CT
computed tomography
- FLAIR
fluid-attenuated inversion-recovery
- GBM
glioblastoma multiforme
- IRB
Institutional Review Board
- LITT
laser interstitial thermotherapy
- Mo-MDSC
Monocytic Myeloid-Derived Suppressor Cells
- MRI
magnetic resonance imaging
- PBMC
peripheral blood mononuclear cells
- PE
pulmonary embolism
- RN
radiation necrosis
- SRS
stereotactic radiosurgery
- WBRT
whole brain radiotherapy
Brain metastases (BM), which result most commonly from hematogenous spread of cancer from the systemic circulation, is found in patients suffering from primary lung, breast, colon, and renal cancers and as well as melanoma.1 The prevalence of BM is the highest among lung tumor patients, with approximately 40% to 50% of patients developing BM during the course of their disease.1 The incidence of brain metastasis is expected to increase as immunotherapy and other new treatment modalities improve survival time for primary cancer patients.2 While treatment for brain metastasis has traditionally consisted of surgery and whole brain radiotherapy (WBRT), lesions are increasingly found when they are small and asymptomatic with minimal mass effect. Such lesions are increasingly treated with stereotactic radiosurgery (SRS), a minimally invasive surgical technique. However, 1 possible consequence of SRS is the increasing occurrence of radiation necrosis (RN), an inflammatory response thought to reflect radiation damage to surrounding normal parenchyma, which occurs in 3% to 5% of patients undergoing SRS.3
While SRS is highly effective, with a response rate of 85% to 90% for tumors <3 cm in diameter, some lesions do not respond and others recur after initial response. Post-op scarring may also occur after open surgical resection. In this setting, both true recurrence and RN must be considered.
Unfortunately, clinicians cannot differentiate true brain metastasis from RN based on current imaging technology. This represents a significant clinical challenge as the treatments are different despite their similar appearance on magnetic resonance imaging (MRI) as enhancing lesions. RN, an inflammatory response thought to reflect radiation damage to surrounding normal parenchyma, which occurs in 3% to 5% of patients undergoing SRS.3 At present, the only definitive way to differentiate recurrent BM from RN is by brain biopsy. This is critical, as these etiologies are treated differently despite similar MRI appearance. For example, recurrent tumor could be treated with SRS, laser interstitial thermotherapy (LITT), or salvage WBRT,4 while radiation is not used for treatment of RN, which is typically treated medically or with LITT.5 A minimally invasive test that could reliably differentiate the 2 would enable patients to avoid the expense and risk of invasive biopsy while receiving the appropriate treatment.
We previously demonstrated that a liquid biopsy could differentiate recurrent glioblastoma multiforme (GBM) from RN6 by quantification of 2 Monocytic Myeloid-Derived Suppressor Cell (Mo-MDSC) surface biomarkers on CD14+ monocytes isolated from peripheral blood mononuclear cells (PBMC): traditional HLA-DR, and vascular noninflammatory molecule 2 (VNN2+, aka, Glycosylphosphatidyl inositol-anchored protein, GPI-80). We hypothesized that this approach could also differentiate RN from BM. As in our previous studies,6 we took advantage of a hallmark of cancer, namely, the increase in immunosuppression7 mediated by MDSCs in patients with BM relative to healthy controls.8,9 Here, we assess the ability of DVI to distinguish new BM from RN, and to complement existing imaging approaches while obviating the risk and expense of surgical biopsy.
METHODS
Human Subjects
All studies of human subjects were approved by the Institutional Review Board (IRB). Peripheral blood samples were obtained from 22 patients with stage IV cancer with brain metastasis previously treated with SRS undergoing resection of new or recurrent enhancing intra-axial lesions consistent with either newly (at another site) or recurrent BM or RN, in accordance with Declaration of Helsinki principles. Tissue samples were acquired in the operating room prior to skin incision under IRB-approved protocols. Resected lesions that had <5% active tumor were defined as RN. Both lesions that represented local recurrence after SRS had ≥60% active tumor, as did all new lesions. All patients had imaging confirming complete or near-complete resection, and pathological diagnosis of BM or RN as defined above.
Inclusion and Exclusion Criteria
For participation in this study, patients were required to meet the following inclusion criteria: Adults (≥18 yr of age) who had previously undergone radiosurgery for brain metastasis and were presenting with lesions felt to represent either new brain metastasis (at a different site) or recurrent lesions previously treated with SRS felt to represent either locally recurrent brain metastasis or RN and were undergoing surgical resection. All patients had restaging computed tomography (CT) of the chest, abdomen, and pelvis and were found to have no evidence of active systemic extracranial metastasis. Candidates were excluded from participating in the study if any of the following criteria applied: inability to provide informed consent, immunocompromised state, or a history of prior malignancy other than the index neoplasm (patients with adequately treated basal cell or squamous cell carcinoma, or any other cancer from which the patient had been disease-free for 5 or more years were also eligible6).
Study Design
This study was a clinical observational cohort study designed to evaluate and validate Mo-MDSC biomarkers in conjunction with BM to identify unique predictive biomarkers capable of distinguishing BM from RN. The primary outcome was the calculation of a composite measure of HLA-DRneg/low and VNN2 expression on CD14+ monocytes.6 In order to obtain the DVI, the percentage of circulating HLA-DRneg/low was divided by the percentage of circulating VNN2+ cells among CD14+ cells. Because DVI is as yet an unvalidated biomarker, DVI calculations were not presented at clinical conference nor revealed to clinicians until after management had been instituted based on purely clinical criterion.
Cell Isolation
Up to 8 ml of peripheral blood was freshly obtained from patients with either BM or RN and separation by density gradient centrifugation using Histopaque (Sigma-Aldrich, St Louis, Missouri) to obtain PBMC, as described previously.10 CD14+ monocytes were positively selected from PBMC using magnetic CD14 MicroBeads according to the manufacturer's instructions (Miltenyi Biotec, San Diego, California). CD14+ cell isolation was done within 6 h of obtaining fresh blood.
Surface Staining and Flow Cytometric Analysis
Mo-MDSC surface biomarkers on CD14+ monocytes were identified using analytic flow cytometry. Antibodies used included monoclonal mouse-anti-human, CD14-conjugated to APC (Invitrogen, Carlsbad, California), HLA-DR-conjugated to FITC (BD Biosciences, San Jose, California), and VNN2 conjugated to pulmonary embolism (PE) (MBL, Woburn, Massachusetts). VNN2 antibodies were diluted 1 to 10 and 2 μl was then used to stain 5 × 104 cells. Anti-CD14 and -HLA-DR antibodies were used at 100 μg/ml concentrations, respectively. Cells were stained for 30 min at room temperature and staining was analyzed using WinList software V7.0 (Verity, Topsham, Maine). Isotype-matched control antibodies were used with all the samples.6 The scientist performing the DVI studies was not involved in clinical care and communicated the DVI results at regular intervals in batch format. DVI results were not available to clinicians until after treatment had been instituted.
Statistical Analysis
All biomarker data were summarized using medians. Probability values of P ≤ .05 were considered significant. Wilcoxon test was used for nonparametric analysis and P ≤ .05 was considered significant.
RESULTS
Clinical Cohort
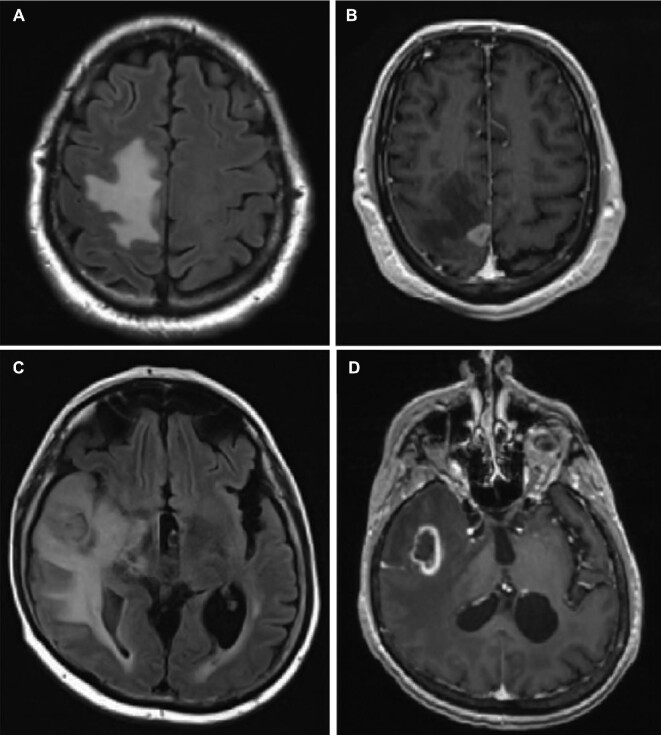
This study was approved by the IRB. Patients were identified by the Brain Tumor & Neuro-Oncology Center. To date, we have performed an observational study on a total of 32 subjects: 10 BM, 12 RN, and 10 control subjects (Table: Patient description). Representative images of patient with local tumor recurrence vs RN after previous SRS are illustrated in Figure 1.
TABLE.
Patient Description
| Case number | Age | Gender | Diagnosis on brain pathology | Status of systemic disease |
|---|---|---|---|---|
| 1 | 54 | M | New brain metastasis | Lung adenocarcinoma, spine metastases |
| 2 | 58 | M | Brain metastasis | Neuro-endocrine carcinoma |
| 3 | 27 | M | New brain metastasis | Melanoma, spine metastases |
| 4 | 60 | M | Brain metastasis | Renal cell carcinoma |
| 5 | 58 | F | New brain metastasis | Melanoma |
| 6 | 74 | M | New brain metastasis | Metastatic urothelial carcinoma, lung metastases |
| 7 | 47 | M | New brain metastasis | Breast adenocarcinoma |
| 8 | 61 | M | New brain metastasis | Breast adenocarcinoma, bone metastases |
| 9 | 59 | M | New brain metastasis | Large B cell lymphoma |
| 10 | 79 | M | New brain metastasis | Lung adenocarcinoma |
| 11 | 64 | F | RN | Lung adenocarcinoma |
| 12 | 34 | M | RN | Basal cell adenocarcinoma of parotid gland |
| 13 | 87 | M | RN | History of adenocarcinoma; systemic disease stable |
| 14 | 64 | F | RN | Small cell carcinoma of the lung |
| 15 | 54 | F | RN | Breast adenocarcinoma |
| 16 | 67 | F | RN | Adenocarcinoma of the lung |
| 17 | 75 | F | RN | Breast adenocarcinoma |
| 18 | 63 | F | RN | Breast adenocarcinoma, lung metastases |
| 19 | 37 | F | RN | Breast adenocarcinoma |
| 20 | 70 | M | RN | Lung adenocarcinoma |
| 21 | 73 | F | RN | Breast adenocarcinoma |
| 22 | 54 | F | RN | Breast adenocarcinoma |
FIGURE 1.
MRIs of representative patients. A, B, Represent recurrent symptomatic brain lesion 6 mo s/p SRS in patient 4 on fluid-attenuated inversion-recovery (FLAIR), A, and T1 weighted MRI + Gd, B. This proved to be recurrent renal cell carcinoma. C, D, Represent recurrent lesion 8 mo s/p SRS in patient 11 on FLAIR and T1 weighted MRI + Gd, which proved to be consistent with RN.
Separation and Staining of Mo-MDSCs
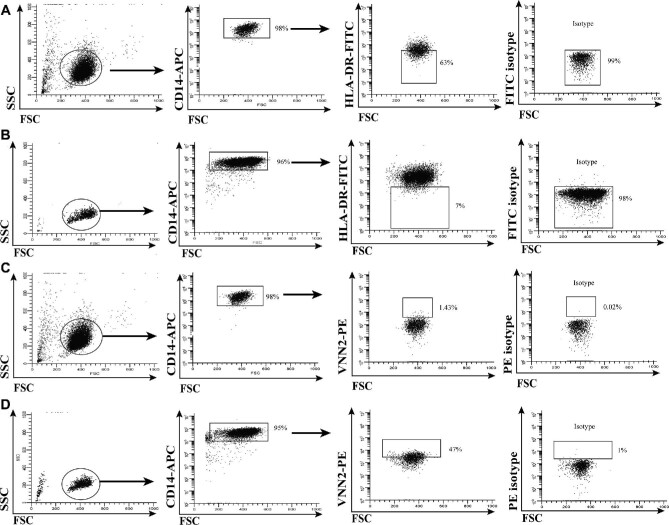
CD14+ monocytes were isolated from PBMCs using magnetic bead separation. Following purification, CD14+ monocytes were stained with either FITC-conjugated anti-HLA-DR (BD Biosciences, San Jose, California) or PE-conjugated anti-VNN2 (MBL, Woburn, Massachusetts), and their respective isotype antibody controls.6 The populations of interest were the percentage of circulating CD14+ cells expressing low levels (below isotype) of HLA-DR, as shown in Figure 2A (BM) and Figure 2B (RN), and the percentage of CD14+ cells expressing positive VNN2 staining (above isotype), as shown in Figure 2C (BM) and Figure 2D (RN). We previously determined that monocytic MDSCs (Mo-MDSC) identified by these parameters were also CD33+ CD11b+ and CD3neg, CD15neg, CD16neg, CD19neg, CD20neg, and CD56neg (Linneg).6 These MDSCs were capable of suppressing activated T cell expansion as observed in T-cell functional suppression assays (data not shown); thus, they functioned as MDSCs.6 Determination of the percentage of HLA-DRneg/low and VNN2+ cells among CD14+ monocytes was calculated using a BD C6 Flow Cytometer (BD Biosciences, San Jose, California) and WinList V7 software (Verity, Topsham, Maine) analysis.6 The ratio of the percentage of circulating HLA-DRneg/low CD14+ monocytes to VNN2+ CD14+ monocytes was then calculated. We define the ratio of HLA-DR expression to VNN2 expression on CD14+ cells as the DR-VNN Index or DVI.6
FIGURE 2.
Flow cytometry quantification of Mo-MDSC and CD14+ VNN2+ cells. A, B, Show representative flow cytometry images for staining Mo-MDSC in BM and RN patients, respectively; C, D, show staining for CD14+ VNN2+ cells in BM and RN patients, respectively.
DVI Index Calculations
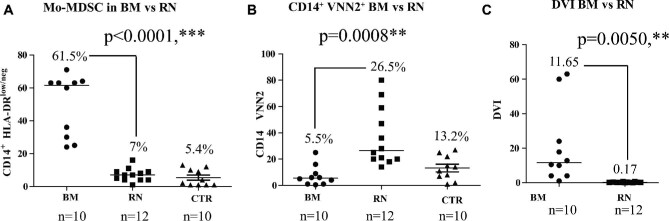
The combination of the percentages of HLA-DRneg/low and VNN2+ expressing cells among CD14+ monocytes from PBMC was calculated for BM and for RN patients. As shown in Figure 3A, the median expression of CD14+ HLA-DRneg/low Mo-MDSC cells was 61.5% (range 25-71) for BM, whereas the median expression of CD14+ HLA-DRneg/low cells was 7.0% (range 1-16) in RN patients. For VNN2 expression, BM patients exhibited a median expression of CD14+ VNN2+ -Mo-MDSC of 5.5% (range 0.5-25), and RN patients exhibited a median expression of CD14+ VNN2+ Mo-MDSC of 26.5% (range 14-80), Figure 3B. The level of expression for VNN2 and HLA-DR in RN patients was similar to previously published healthy control subjects.6 In patients diagnosed histologically with BM, the average DVI was 11.65 (range 1-63), whereas the average DVI for RN patients was 0.17 (range 0.05-0.643), which was also statistically significant (P = .0050), as shown in Figure 3C.
FIGURE 3.
HLA-DR and VNN2 expression on CD14+ cells distinguishes BM from RN. A, In patients with BM, a higher number of CD14+ cells that express low levels of HLA-DR (HLA-DRlow/neg) in the periphery are observed (ie, Mo-MDSCs), compared with patients experiencing RN (Median 65.5%, n = 10 vs Median 7%, n = 12, respectively, P < .0001) and control (CTR) patient (5.4%) with, B, a reciprocal proportion of myeloid CD14+ expressing cells exhibiting lower VNN2 on BM patients vs patients undergoing RN (Median 5.5%, n = 10 and Median 26.5%, n = 12, respectively, P = .0008) and CTR patients (13.2%). C, The DVI index for each patient was calculated using the HLA-DRneg: VNN2+ CD14+ ratio of MDSCs. A ratio of equal to or greater than 1 was considered indicative of BM, while a ratio less than 1 indicates RN. BM demonstrated a median DVI of 11.65, while RN patients had a median DVI of 0.17, P < .0050.
DISCUSSION
BM are often the cause of increased mortality and death among patients with advanced cancer and lead to other comorbidities, such as pulmonary emboli, sepsis, and systemic diseases. As cancer treatment regimens improve, the incidence of BM are expected to rise. Although new ways to protect healthy tissue from SRS may lead to better patient outcomes, the difficulty in distinguishing brain metastasis from SRS-induced RN using routinely available studies will likely continue to represent a significant clinical challenge. Currently, the differential diagnosis is generated solely from imaging techniques, which are expensive and often difficult to interpret. Conversely, advanced techniques are being actively developed, such as using machine learning and radiomics or MR perfusion and spectroscopy,11,12 but they are typically expensive, operator-dependent, non-FDA-approved (thus un-reimbursable), and not widely available. Conversely, the current diagnostic gold standard of brain biopsy is expensive, invasive, imperfect, and carries risks for the patients. The ability to obtain a liquid biopsy derived from peripheral blood would largely eliminate these issues improving both patient experience and the value of care.
It is well known that advanced stages of cancer produce a high degree of immunosuppression, as demonstrated by increased levels of circulating MDSCs.13 Therefore, we hypothesized that a biomarker of systemic immunosuppression may correlate with the presence of stage IV BM compared to RN, where tumor recurrence has not occurred.14 Although elevated levels of circulating MDSCs are observed in nearly all oncogenic diseases,15 these undifferentiated immature myeloid cells have not been characterized in BM. With the concomitant increase of MDSCs in brain metastasis patients, we have also found a significant decrease of VNN2, the same surface ectoenzyme described in our previous work on GBM6 in monocytes of patients with brain metastasis. Although the pathological significance for this decrease is presently unclear, results suggest its potential to differentiate BM from RN from peripheral blood.
CONCLUSION
Our findings suggest that DVI, a biomarker derived from expression of markers on circulating peripheral blood cells and obtained by minimally invasive liquid biopsy offers an alternative to invasive biopsies and resections. If this inexpensive blood biopsy, which costs less than $200 per study to perform, is validated in larger studies, it has the potential to improve both access and quality of clinical care by providing safer, less expensive, and more reliable diagnostic alternatives and would likely face few barriers to implementation.
Disclosures
Dr Soler, Dr Cooper, Dr McCormick, and Dr Sloan have a patent involving the work presented herein. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Barnholtz-Sloan was supported by CCCC. Dr Sloan was partially supported by the Peter D. Cristal Chair, the Kimble Family Foundation, the Gerry Kaufman Jr Foundation, and the Ferry Family Foundation.
Contributor Information
David C Soler, Department of Neurosurgery, Case Western Reserve University School of Medicine, Cleveland, Ohio; Brain Tumor and Neuro-Oncology Center, Case Western Reserve University School of Medicine, Cleveland, Ohio; University Hospitals-Cleveland Medical Center and the Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Amber Kerstetter-Fogle, Department of Neurosurgery, Case Western Reserve University School of Medicine, Cleveland, Ohio; Brain Tumor and Neuro-Oncology Center, Case Western Reserve University School of Medicine, Cleveland, Ohio; University Hospitals-Cleveland Medical Center and the Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Theresa Elder, Department of Neurosurgery, Case Western Reserve University School of Medicine, Cleveland, Ohio; Brain Tumor and Neuro-Oncology Center, Case Western Reserve University School of Medicine, Cleveland, Ohio; University Hospitals-Cleveland Medical Center and the Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Alankrita Raghavan, Department of Neurosurgery, Case Western Reserve University School of Medicine, Cleveland, Ohio; Brain Tumor and Neuro-Oncology Center, Case Western Reserve University School of Medicine, Cleveland, Ohio; University Hospitals-Cleveland Medical Center and the Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Jill S Barnholtz-Sloan, Department of Neurosurgery, Case Western Reserve University School of Medicine, Cleveland, Ohio; Brain Tumor and Neuro-Oncology Center, Case Western Reserve University School of Medicine, Cleveland, Ohio; University Hospitals-Cleveland Medical Center and the Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Kevin D Cooper, Department of Dermatology, University Hospitals-Cleveland Medical Center and the Case Western University School of Medicine, Cleveland, Ohio; The Murdough Family Center for Psoriasis, University Hospitals-Cleveland Medical Center and the Case Western University School of Medicine, Cleveland, Ohio.
Thomas S McCormick, Department of Dermatology, University Hospitals-Cleveland Medical Center and the Case Western University School of Medicine, Cleveland, Ohio; The Murdough Family Center for Psoriasis, University Hospitals-Cleveland Medical Center and the Case Western University School of Medicine, Cleveland, Ohio.
Andrew E Sloan, Department of Neurosurgery, Case Western Reserve University School of Medicine, Cleveland, Ohio; Brain Tumor and Neuro-Oncology Center, Case Western Reserve University School of Medicine, Cleveland, Ohio; University Hospitals-Cleveland Medical Center and the Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, Ohio.
REFERENCES
- 1. Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872. [DOI] [PubMed] [Google Scholar]
- 2. Yamanaka R. Medical management of brain metastases from lung cancer (review). Oncol Rep. 2009;22(06):1269-1276. [DOI] [PubMed] [Google Scholar]
- 3. Soo TM, Bernstein M, Provias J, Tasker R, Lozano A, Guha A. Failed stereotactic biopsy in a series of 518 cases. Stereotact Funct Neurosurg. 1995;64(4):183-196. [DOI] [PubMed] [Google Scholar]
- 4. Levin KJ, Youssef EF, Sloan AE, Patel R, Zabad RK, Zamorano L. Gamma knife radiosurgery in patients with advanced breast cancer undergoing bone marrow transplant. J Neurosurg. 2002;97(5 Suppl):663-665. [DOI] [PubMed] [Google Scholar]
- 5. Hong CS, Deng D, Vera A, Chiang VL. Laser-interstitial thermal therapy compared to craniotomy for treatment of radiation necrosis or recurrent tumor in brain metastases failing radiosurgery. J Neurooncol. 2019;142(2):309-317. [DOI] [PubMed] [Google Scholar]
- 6. Soler DC, Young AB, Cooper KD et al. The ratio of HLA-DR and VNN2+ expression on CD14+ myeloid derived suppressor cells can distinguish glioblastoma from radiation necrosis patients. J Neurooncol. 2017;134(1):189-196. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674. [DOI] [PubMed] [Google Scholar]
- 8. Kitano S, Postow MA, Ziegler CG et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol Res. 2014;2(8):812-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boire A, Brandsma D, Brastianos PK et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol. 2019;21(5):571-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Soler DC, Sugiyama H, Young AB, Massari JV, McCormick TS, Cooper KD. Psoriasis patients exhibit impairment of the high potency CCR5+ t regulatory cell subset. Clin Immunol. 2013;149(1):111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng L, Parekh V, Huang P et al. Distinguishing true progression from radionecrosis after stereotactic radiation therapy for brain metastases with machine learning and radiomics. Int J Radiat Oncol Biol Phys. 2018;102(4):1236-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chuang MT, Liu YS, Tsai YS, Chen YC, Wang CK. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: a meta-analysis. PLoS One. 2016;11(1):e0141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martins AN, Johnston JS, Henry JM, Stoffel TJ, Di Chiro G. Delayed radiation necrosis of the brain. J Neurosurg. 1977;47(3):336-345. [DOI] [PubMed] [Google Scholar]
- 15. Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol. 2011;11(7):802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]