Abstract
Molecular hydrogen exerts biological effects on nearly all organs. It has anti-oxidative, anti-inflammatory, and anti-aging effects and contributes to the regulation of autophagy and cell death. As the primary organ for gas exchange, the lungs are constantly exposed to various harmful environmental irritants. Short- or long-term exposure to these harmful substances often results in lung injury, causing respiratory and lung diseases. Acute and chronic respiratory diseases have high rates of morbidity and mortality and have become a major public health concern worldwide. For example, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic. An increasing number of studies have revealed that hydrogen may protect the lungs from diverse diseases, including acute lung injury, chronic obstructive pulmonary disease, asthma, lung cancer, pulmonary arterial hypertension, and pulmonary fibrosis. In this review, we highlight the multiple functions of hydrogen and the mechanisms underlying its protective effects in various lung diseases, with a focus on its roles in disease pathogenesis and clinical significance.
Keywords: Molecular hydrogen, Pulmonary disease, Reactive oxygen species (ROS), Oxidative stress, Inflammation
1. Introduction
Molecular hydrogen is a colorless, odorless, and tasteless gas molecule with poor water solubility. It is considered inert in mammalian cells under physiological conditions. Molecular hydrogen can be broken down by some bacteria via enzymatic catalysis to provide energy and electrons. In addition, bacteria produce molecular hydrogen by anaerobic metabolism. Genes encoding the iron- or nickel-containing enzymes necessary to catalyze these reactions, such as hydrogenase, are lacking in mammals (Fritsch et al., 2013). However, molecular hydrogen is now recognized as a novel medically relevant gas with therapeutic potential. Ohsawa et al. (2007) reported that the inhalation of 2% molecular hydrogen results in the selective scavenging of hydroxyl free radical (·OH) and peroxynitrite anion (ONOO-), significantly improving oxidative stress injury caused by cerebral ischemia/reperfusion (I/R). This study prompted substantial interest in the medical value of molecular hydrogen, and many cellular, animal, and clinical trials and studies have since investigated its preventive and therapeutic effects. Molecular hydrogen can exert biological effects on almost all organs, including the brain, heart, lung, liver, and pancreas. It has a variety of biological functions, including roles in the regulation of oxidative stress and anti-inflammatory and anti-apoptotic effects (Iketani and Ohsawa, 2017; Kura et al., 2019; Hu et al., 2020; Kawamura T et al., 2020).
The lungs are the primary organ for gas exchange between the surrounding environment and the circulatory system. During respiration, they are constantly exposed to various environmental irritants, such as bacteria, viruses, and other pathogens, and to other harmful external stimuli, such as tobacco smoke and airborne particulate matter. Short- or long-term exposure to these harmful substances often results in lung injury, causing respiratory and lung diseases (Bhattacharya and Matthay, 2013). Acute and chronic respiratory diseases have high rates of morbidity and mortality, and the efficacy of treatment strategies is insufficient, making these diseases a major public health concern worldwide (Bhattacharya and Matthay, 2013). For example, coronavirus disease 2019 (COVID-19), a form of pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has spread widely, resulting in an ongoing pandemic.
This review is based on publications up to May 2021 which were retrieved by a selective search in the PubMed database, and includes clinical trials, animal studies, and reviews. The search terms included "hydrogen," "acute lung injury (ALI)," "chronic obstructive pulmonary disease (COPD)," "asthma," "cancer," "pulmonary arterial hypertension (PAH)," "pulmonary fibrosis (PF)," and "hydrogen." We briefly discuss the mechanism of action of molecular hydrogen and its effects on biological functions and cellular processes. We then describe the status of research on its contributions to various lung diseases. We expect molecular hydrogen to have an increasing role in the treatment and prevention of lung diseases. This review provides support for the development of future treatments based on molecular hydrogen.
2. Mechanism of action of hydrogen
2.1. Anti-oxidation
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are by-products of energy metabolism during daily activities. ROS/RNS include superoxide anion (O2 -), ·OH, peroxyl (RO2·), alkoxyl (RO·), and nitric oxide (NO·) radicals. They play critical roles under normal conditions in immune defense, signaling processes, and the extraction of energy from organic molecules (Genestra, 2007). However, if ROS and RNS production exceeds the antioxidant capacity of the body or if the antioxidant capacity of the body is decreased, oxidative stress occurs. Acute oxidative stress often occurs during inflammation and I/R (e.g., cardiac arrest, myocardial and cerebral infarction, organ transplantation, and intraoperative hemostasis) (Ferrari et al., 1991; Vaziri and Rodríguez-Iturbe, 2006; Reuter et al., 2010). Chronic ROS injury can occur in a variety of pathological conditions, such as malignant cancer, diabetes, chronic inflammatory diseases, atherosclerosis, and neurodegeneration, as well as in the process of aging (Stump et al., 2005; Kim and Byzova, 2014; Guzik and Touyz, 2017). Humans have antioxidant defense systems to protect against free radical toxicity (Birben et al., 2012). Antioxidants are divided into enzymatic and non-enzymatic types. Enzymatic types include superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), and non-enzymatic types include bilirubin, α-tocopherol (vitamin E), β-carotene, and uric acid (Wu et al., 2013).
The antioxidant effects of molecular hydrogen are primarily mediated by the following mechanisms. (1) Molecular hydrogen has a lower molecular weight than other common antioxidants (e.g., SOD, CAT, and α-tocopherol). It can selectively react with strong oxidants and can easily penetrate biological membranes, such as nuclear and mitochondrial membranes, without affecting the metabolic redox reaction (Ohta, 2012, 2015). (2) By stimulating nuclear factor erythroid 2-related factor 2 (Nrf2), which regulates the basal and induces expression of many antioxidant enzymes and the proteasome (Zhang HQ et al., 2015), hydrogen can increase the expression of heme oxygenase-1 (HO-1) (Kawamura et al., 2013; Xie et al., 2020; Yu et al., 2020). It also decreases ·ONOO--related gene expression and production (Shinbo et al., 2013) and increases the activity of the antioxidant enzymes SOD, CAT, and myeloperoxidase (MPO) (Cai et al., 2013). (3) Molecular hydrogen can block the apoptosis signal-regulating kinase 1 (ASK1) signaling pathway and the downstream signaling molecule p38 mitogen-activated protein kinase (p38MAPK), thereby inhibiting nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and decreasing free radical production. Through these antioxidant effects, molecular hydrogen protects cells from lipid and fatty acid peroxidation.
2.2. Anti-inflammatory effects
The anti-inflammatory effects of molecular hydrogen were first identified in a study of Gharib et al. (2001) in which parasite-induced liver inflammation was treated by the inhalation of high-pressure hydrogen. Subsequent studies of a variety of injury models have confirmed that molecular hydrogen has anti-inflammatory effects. In the lungs, acute and persistent chronic inflammation underlies the pathogenesis of numerous pulmonary diseases, such as ALI, COPD, asthma, and PF. The protective effect of molecular hydrogen on lung tissues is mediated by its anti-inflammatory effects.
The major mechanisms underlying the anti-inflammatory effects of molecular hydrogen are as follows. (1) It inhibits the synthesis and release of the pro-inflammatory factors tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, nuclear factor-κB (NF-κB), and high-mobility group box 1 (HMGB-1); increases the expression of the anti-inflammatory factor IL-10 (Buchholz et al., 2008; Shao et al., 2016; Fu et al., 2020); inhibits the release of chemokines, including keratinocyte-derived chemokine, macrophage inflammatory protein (MIP)-1α, MIP-2, and monocyte chemoattractant protein 1 (Xie et al., 2012); and inhibits the release of intercellular cell adhesion molecule-1 (ICAM-1), granulocyte-macrophage colony-stimulating factor (GMCSF), and granulocyte colony-stimulating factor (G-CSF) (Xie et al., 2012; Chen MH et al., 2018). (2) It promotes macrophage phagocytosis at lesion sites (Huang et al., 2019) and inhibits the recruitment of neutrophils and M1 macrophages to lesions. (3) The anti-inflammatory effects of molecular hydrogen involve multiple signaling pathways. For example, the stimulation of the Nrf2/HO-1/HMGB-1 pathway mitigates endothelial dysfunction and lung injury caused by polymicrobial sepsis (Chen et al., 2015; Yang et al., 2019). The inhibition of p38MAPK and c-Jun N-terminal kinase (JNK) alleviates lipopolysaccharide (LPS)-induced ALI (Tao et al., 2016). The modulation of autophagy-related pathways, such as the mammalian target of rapamycin (mTOR)/transcription factor EB (TFEB) and phosphatase and tensin homolog (PTEN)-induced kinase 1 (PINK1)/Parkin pathways, mitigates ALI endothelial dysfunction and myocardial I/R injury (Chen et al., 2020; Fu et al., 2020).
2.3. Regulation of autophagy
In eukaryotic cells, catabolism is the degradation of intracellular components by the ubiquitin-proteasome system and lysosomes. Autophagy (particularly macroautophagy) is defined as a lysosome-dependent catabolic process for maintaining cellular homeostasis (Yang and Klionsky, 2010). However, when stress exceeds a critical duration or intensity threshold, it may have maladaptive effects (Maiuri et al., 2007), causing cell damage or even death. Emerging evidence suggests that hydrogen has dual roles in the modulation of autophagy (i.e., roles in both its promotion and inhibition).
2.3.1. Promotion of autophagy
Hydrogen can promote autophagy-mediated nucleotide-binding domain and leucine-rich repeat protein 3 (NLRP3) inactivation in macrophages and alleviate inflammatory reactions and subsequent organ damage and mitochondrial dysfunction (Chen HG et al., 2019). In sepsis, endoplasmic reticulum (ER) stress is triggered and is often accompanied by impaired autophagy. Hydrogen alleviates ER stress by activating autophagy pathways, thereby attenuating inflammation and organ injury (Chen et al., 2020). These observations suggest that under pathological conditions, as a key regulator of innate and adaptive immunity, autophagy is activated, but is not sufficient to overcome stress. Hydrogen exerts protective effects by promoting autophagy for the maintenance of homeostasis and attenuation of the effects of stress.
2.3.2. Inhibition of autophagy
Basal autophagy is essential for the maintenance of normal cellular functions and homeostasis. However, dysregulated or excessive autophagy may disrupt intracellular homeostasis, predisposing individuals to pathological conditions. Under these conditions, appropriate inhibition of autophagy serves a protective function. We have demonstrated that hydrogen alleviates LPS-induced ALI by inhibiting excessive autophagy (Zhang Y et al., 2015; Liu and Zhang, 2017). Similarly, in traumatic brain injury, by inhibiting autophagy, hydrogen improves the viability of microvascular endothelial cells (ECs) (Wang YF et al., 2020).
The mechanisms underlying the dual effects of hydrogen on autophagy are yet to be fully clarified and might depend on disease stage or severity, as well as cell types and locations. Nevertheless, these findings suggest that hydrogen is a candidate therapeutic agent for the modulation of autophagy pathways and therefore, disease outcomes.
2.4. Effects of hydrogen on cell death
2.4.1. Regulation of apoptosis
Apoptosis is a canonical form of programmed cell death that does not stimulate inflammatory responses (Elmore, 2007). It is an evolutionarily conserved type of cell death with major effects on biological processes (Singh et al., 2019). The molecular mechanism underlying apoptosis involves the sequential activation of cysteine proteases, called caspases (Shalini et al., 2015), and a series of pro-apoptotic and anti-apoptotic B-cell lymphoma-2 (Bcl-2) family proteins (Westphal et al., 2014). In various disease models and organs, molecular hydrogen plays a protective role by regulating apoptosis. It can inhibit apoptosis by regulating apoptosis signaling pathways and apoptosis-related proteins, such as the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)/glycogen synthase kinase-3β (GSK3β) (Chen et al., 2017), ASK1/JNK (Liu YQ et al., 2015), rat sarcoma (Ras)-extracellular signal-related kinase 1/2 (ERK1/2)-mitogen-activated protein kinase kinase 1/2 (MEK1/2), and Akt pathways (Chen et al., 2013), and by suppressing the activation of caspase-3, -8, and -9 and the Bcl-2/Bcl-2-associated X (Bax) ratio (Huo et al., 2014; Liu YQ et al., 2015; Mo et al., 2019). Molecular hydrogen also reduces the rate of apoptosis by reducing inflammation and oxidative damage (Guo SX et al., 2015; Li et al., 2016), and protects mitochondrial function (Cui et al., 2014; Jiao et al., 2020). The inhibition of autophagy improves cell survival and inhibits apoptosis. For example, in an I/R myocardial injury model, PINK-mediated autophagy alleviates inflammation and apoptosis (Yao et al., 2019). Accordingly, there is crosstalk among molecular hydrogen, apoptosis, and autophagy.
Although hydrogen has anti-apoptotic effects in the above conditions, it promotes apoptosis under various other conditions. For example, hydrogen can increase rates of early and late apoptosis in lung cancer (Jiang et al., 2018; Wang et al., 2018). It facilitates the scavenging of carcinoma cells in body, reduces cell proliferation, and promotes cancer cell apoptosis. The mechanisms underlying the contrasting effects of hydrogen on apoptosis are not completely understood. However, they may be explained by the ability of hydrogen to modulate cell death via different pathways to protect against injury and other harmful attacks.
2.4.2. Regulation of pyroptosis
Pyroptosis is a novel form of programmed cell death associated with inflammation. The activation of pattern-recognition receptors (PRRs) in inflammasomes can trigger pyroptosis by stimulating inflammatory responses (Guo HT et al., 2015; Shi et al., 2017). Pyroptosis is a protective mechanism, but excessive pyroptosis can have serious consequences. ROS and the activation of caspase-1 and inflammasomes play important roles in promoting pyroptosis (Lamkanfi and Dixit, 2014; Zha et al., 2016). Molecular hydrogen plays an important regulatory role in resistance to inflammation and oxidation. Thus, molecular hydrogen might affect pyroptosis in inflammatory diseases, such as sepsis and I/R injury. In a rat model of myocardial I/R injury, hydrogen inhibited oxidative stress and NLRP3-mediated pyroptosis and significantly improved cardiac function, microstructure, and mitochondrial morphology (Nie et al., 2021).
2.5. Regulation of aging
Aging is a progressive loss of physiological function and is an unavoidable process ending in death (Golden and Melov, 2007; Newgard and Sharpless, 2013). It is now considered a major factor underlying the development and progression of various diseases, such as COPD and idiopathic PF. Molecular hydrogen can decrease the expression of the aging-related proteins β-galactosidase, p53, and p21 (Han et al., 2017; Zhang WB et al., 2018), suppress downregulation of sirtuin 3 (Sirt3) expression, and reduce oxidative stress damage, thereby extending cell survival (Li et al., 2019; Zhang et al., 2020). Research has shown that molecular hydrogen produced by intestinal bacteria in the body suppresses increased hydrogen peroxide (H2O2) by suppressing intracellular ·OH-mediated lipid peroxide formation and cellular senescence, thus contributing to the suppression of aging (Sakai et al., 2019). Genomic instability is one of the primary hallmarks of the aging process (Li et al., 2021). By reducing oxidative DNA damage, hydrogen can help maintain genomic stability. For example, in cigarette smoke (CS)-induced emphysema, hydrogen significantly decreased phosphorylated histone H2AX and 8-hydroxy-2'-deoxyguanosine (8-OHdG), which are markers of oxidative DNA damage (Suzuki et al., 2017). As a "philosophical molecule," hydrogen may be used for the treatment of intractable diseases and aging (Hirano et al., 2020).
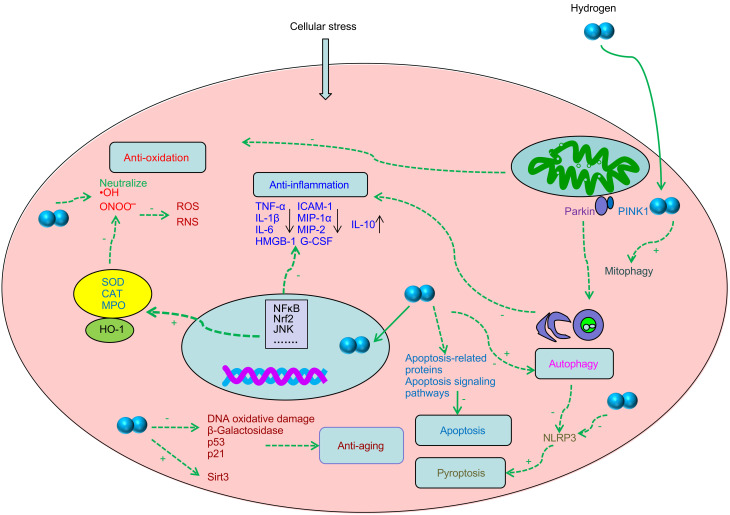
Some possible mechanisms underlying the biological effects of hydrogen are summarized in Fig. 1.
Fig. 1. Illustration of the possible biological effects of hydrogen. Hydrogen exerts antioxidant activity by directly neutralizing ·OH, upregulating Nrf2, HO-1, SOD, CAT, and MPO, and scavenging ONOO - ; hydrogen exerts anti-inflammatory activity by inhibiting NF-κB and the pro-inflammatory factors (TNF-α, IL-1β, IL-6, and HMGB-1), inhibiting MIP-1α, MIP-2, G-CSF, and ICAM-1, and increasing the expression of the anti-inflammatory factor IL-10; hydrogen modulates autophagy including Parkin/PINK1-mediated mitophagy, alleviates inflammation and NLRP3-mediated pyroptosis; hydrogen inhibits apoptosis by modulating apoptosis-related proteins and signaling pathways, but it promotes apoptosis in cancer cells; hydrogen has anti-aging effects by reducing oxidative DNA damage, decreasing the expression of the aging-related proteins β-galactosidase, p53, and p21, and upregulating Sirt3 expression. + refers to activate; - refers to inhibit. RNS: reactive nitrogen species; ROS: reactive oxygen species; NF-κB: nuclear factor-κB; JNK: c-Jun N-terminal kinase; ·OH: hydroxyl free radical; ONOO - : peroxynitrite anion; HO-1: heme oxygenase-1; SOD: superoxide dismutase; CAT: catalase; MPO: myeloperoxidase; NLRP3: nucleotide-binding domain and leucine-rich repeat protein 3; PINK: phosphatase and tensin homolog (PTEN)-induced kinase; TNF-α: tumor necrosis factor-α; G-CSF: granulocyte colony-stimulating factor; ICAM-1: intercellular cell adhesion molecule-1; IL: interleukin; HMGB-1: high-mobility group box 1; MIP: macrophage inflammatory protein; Sirt3: sirtuins 3; Nrf2: nuclear factor erythroid 2-related factor 2.
3. Protective function of molecular hydrogen in lung diseases
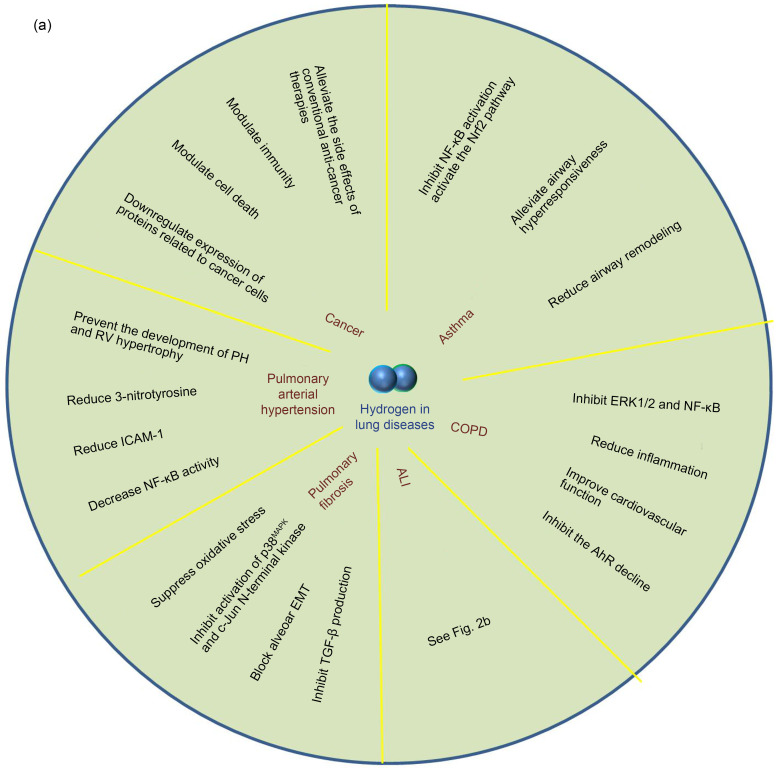
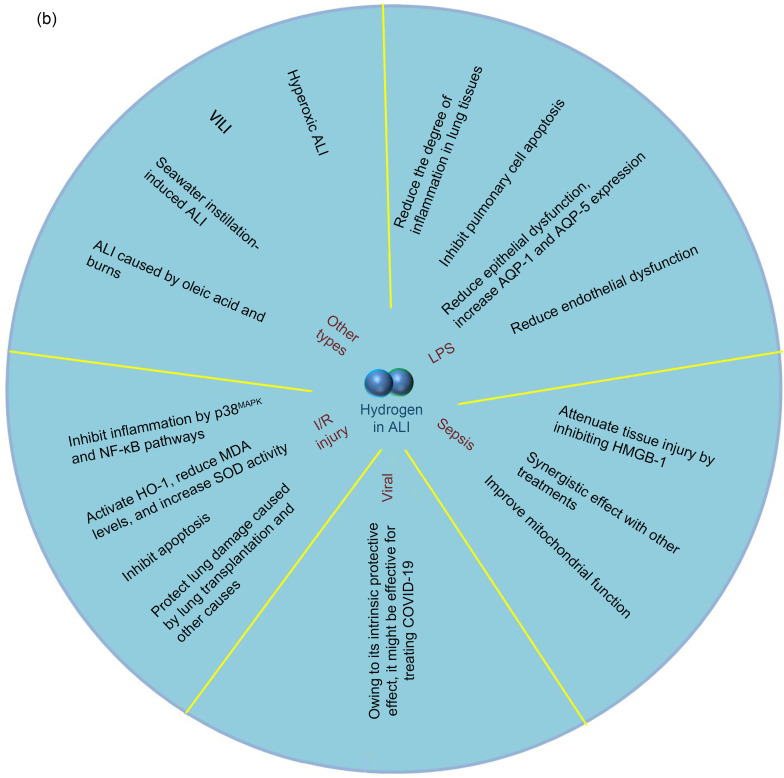
To understand the following text easily and fully explain the preventive and therapeutic effects of hydrogen in various lung diseases, we first summarize the extensive therapeutic spectrum of hydrogen in lung diseases in Fig. 2a. We describe the specific therapeutic effects of hydrogen in ALI in detail in Fig. 2b.
Fig. 2. Effects of hydrogen on various lung diseases. (a) Hydrogen has protective and therapeutic effects on various lung diseases. In COPD, hydrogen inhibits ERK1/2 and NF-κB, reduces inflammation, inhibits AhR decline, which is associated with pathogenesis of COPD, and improves cardiovascular function. In asthma, hydrogen inhibits NF-κB, activates the Nrf2 pathway, and reduces airway remodeling. In cancer, hydrogen alleviates side effects of other anti-cancer therapies, modulates immunity and cell death, and inhibits cancer cell-related protein expression. In PAH, hydrogen reduces 3-nitrotyrosine, ICAM-1, and NF-κB, and prevents development of PH and RV hypertrophy. In PF, hydrogen inhibits oxidative stress and inflammation and blocks alveolar EMT and TGF-β production. (b) Effects of hydrogen on ALI. In LPS-induced ALI, hydrogen inhibits inflammation and apoptosis in lung tissue, and reduces epithelial and endothelial dysfunction. In sepsis-induced ALI, hydrogen inhibits HMGB-1, reduces mitochondrial dysfunction, and exerts a synergistic effect with other treatments. In virus-induced ALI, hydrogen might be effective for treating COVID-19. In I/R-induced ALI, hydrogen inhibits inflammation and oxidative stress, inhibits apoptosis of lung tissue, and alleviates lung injury caused by lung transplantation and other causes. Hydrogen can also alleviate ALI caused by various other factors. COPD: chronic obstructive pulmonary disease; ERK1/2: extracellular signal-regulated kinase 1/2; NF-κB: nuclear factor-κB; AhR: aryl hydrocarbon receptor; Nrf2: nuclear factor erythroid 2-related factor 2; PAH: pulmonary arterial hypertension; ICAM-1: intercellular cell adhesion molecule-1; PH: pulmonary hypertension; RV: right ventricular; PF: pulmaonary fibrosis; EMT: epithelial-mesenchymal transition; TGF-β: transforming growth factor-β; ALI: acute lung injury; LPS: lipopolysaccharide; HMGB-1: high-mobility group box 1; COVID-19: coronavirus disease 2019; I/R: ischemia/reperfusion; VILI: ventilator-induced lung injury; HO-1: heme oxygenase-1; MDA: malonaldehyde; SOD: superoxide dismutase; AQP: aquaporin.
3.1. Role of hydrogen in ALI
ALI and its more severe form, acute respiratory distress syndrome (ARDS), are characterized by an excessive inflammatory response in the lungs and severe gas exchange dysfunction due to disruption of the alveolar–capillary barrier and pulmonary edema. Although supportive critical care medicine has resulted in an increase in survival rates of ALI/ARDS in the past two decades, mortality rates remain high (Goss et al., 2003; Maybauer et al., 2006). A variety of anti-inflammatory drugs have been studied to treat ALI. Palrnatine can inhibit the activation of the Akt/NF-κB signaling pathway, and relieve LPS-induced ALI in mice and in LPS-induced RAW264.7 cells (Kan et al., 2021). Recent studies of animal models by our group and others have indicated that hydrogen has the potential to alleviate the pathogenesis of ALI caused by LPS, sepsis, and other pathogenic factors.
3.1.1. Effect of hydrogen on ALI caused by LPS
LPS, also known as endotoxin, is a component of the outer membrane of Gram-negative bacteria. Toll-like receptors (TLRs) are representative PRRs, which induce pro-inflammatory responses to pathogen invasion (Fitzgerald and Kagan, 2020). PRRs recognize evolutionarily conserved components in microorganisms, collectively called pathogen-associated molecular patterns (PAMPs). LPS is a PAMP and is recognized by TLR4, stimulating TLR4-dependent inflammatory responses and triggering the host innate immune system (Akira and Hemmi, 2003; Tang et al., 2012). Many studies have shown that hydrogen can alleviate ALI caused by LPS, serving a protective function by the following major mechanisms.
Hydrogen treatment can reduce the degree of inflammation in lung tissues, inhibit early and late NF-κB activation-mediated inflammation and pulmonary cell apoptosis, and markedly attenuate neutrophil recruitment caused by LPS. These protective effects are mediated by the downregulation of lung MPO activity and proinflammatory cytokines and chemokines (Xie et al., 2012). Hydrogen significantly reduces p38MAPK expression and activation (Liang et al., 2012) and p38MAPK-dependent ROS (Shi et al., 2016; Tao et al., 2016). In addition to its good efficacy when used alone, hydrogen in combination with NO can provide enhanced therapeutic efficacy for LPS-induced ALI. The underlying mechanism may involve the interaction between these two gases, whereby the combination of NO with hydrogen eliminates nitrotyrosine caused by NO inhalation alone (Liu HY et al., 2015).
Hydrogen can reduce LPS-induced lung epithelial barrier dysfunction. Under normal conditions, the alveolar epithelium serves as an important barrier to prevent pulmonary edema. In mammalian airways, aquaporins (AQPs), a family of cell membrane transport proteins, play critical roles in airway fluid transport, humidification, and surface liquid hydration, and modulate water permeability in the lungs (Bai et al., 1999; Song et al., 2000, 2001). We have demonstrated that LPS significantly decreases AQP-1 and AQP-5 expression and causes barrier dysfunction in the lungs. Hydrogen-rich saline (HRS) significantly increases AQP-1 and AQP-5 expression and alleviates pulmonary epithelial cell damage, thereby protecting the pulmonary epithelial barrier function (Liu et al., 2016; Tao et al., 2016).
Hydrogen reduces LPS-induced endothelial dysfunction. Pulmonary microvascular endothelial cells (PMVECs) are primary targets in LPS-induced ALI. LPS causes PMVEC dysfunction, resulting in increased permeability of EC monolayers as well as EC shrinkage and death (Lee and Slutsky, 2010). Interendothelial junctions of ECs, including tight junctions (TJs) and adherens junctions (AJs), are critical components of the physiological barrier. Occludin and vascular endothelial (VE)-cadherin are major representative TJ and AJ components (Giannotta et al., 2013). LPS reduces the expression levels of occludin and VE-cadherin in PMVECs. Hydrogen-rich medium significantly increases the down-regulation and redistribution of occludin and VE-cadherin induced by LPS in PMVECs and Caco-2 cells (Yang et al., 2016; Li Y et al., 2020). In addition, LPS facilitates the formation of contractile stress fibers in the EC cytoskeleton (Marcos-Ramiro et al., 2014). This is mediated by RhoA activation of Rho kinase (ROCK) and myosin light chain kinase, accompanied by inhibition of myosin light chain phosphatase (Schnittler, 2016). Hydrogen significantly ameliorates the excessive expression of ROCK, inhibits the activity of RhoA, prevents LPS-induced junctional injury and cell death, improves LPS-induced hyperpermeability of the endothelial barrier, and reduces the disruption of TJ and AJ (Yang et al., 2016; Li Y et al., 2020). HRS inhibits autophagy and has anti-apoptotic effects, alleviating LPS-induced ALI (Wang et al., 2019). In mice, its effects are mediated by the ROS/adenosine 5'-monophosphate (AMP)-activated protein kinase (AMPK)/mTOR pathway. However, hydrogen can also inhibit the mTOR/TFEB signaling pathway, thereby increasing autophagy and alleviating LPS-induced endothelial dysfunction and apoptosis in ALI (Fu et al., 2020). Despite these conflicting effects of hydrogen on autophagy, it clearly has a cytoprotective role in LPS-induced lung injury by modulating autophagy and apoptosis.
3.1.2. Protective function of hydrogen in ALI due to sepsis
Sepsis is a major cause of morbidity and mortality worldwide. It causes life-threatening organ dysfunction due to a dysfunctional host response to infection (Singer et al., 2016). The lungs are highly vulnerable during sepsis (Park et al., 2019). Emerging evidence suggests that hydrogen can prevent sepsis, providing a novel treatment strategy for sepsis-induced ALI. The precise protective effects of hydrogen are summarized as follows.
Hydrogen attenuates tissue injury and dysfunction by inhibiting HMGB-1. HMGB-1 often contributes to inflammatory disorders (Andersson et al., 2018). Hydrogen activates the Nrf2/HO-1 pathway, reduces HMGB-1 release in lung tissues of mice with sepsis, inhibits pro-inflammatory cytokines, increases the expression of antioxidant enzymes and anti-inflammatory cytokines, and increases mouse survival rates (Xie et al., 2010; Li et al., 2015; Yu et al., 2019).
The protective effects of hydrogen are enhanced by its combination with other treatments. In zymosan-induced sepsis, the combination of inhalation of 2% hydrogen and hyperoxia had obvious beneficial effects on organs, including the lungs, liver, and kidneys (Hong et al., 2016). Similarly, hydrogen inhalation combined with early fluid resuscitation significantly reduced increases in malonaldehyde (MDA) and MPO levels due to septic shock, and significantly increased SOD activity in lung tissue, thereby decreasing the degree of inflammation (Liu et al., 2013). The combination of hydrogen with propofol improves the survival rate of mice with sepsis and reduces tissue damage and the release of cytokines (Hong et al., 2017). In addition, the anti-oxidative effect of hydrogen can offset the side effects of other therapies. For example, hyperoxia may be beneficial in sepsis, but is accompanied by an increase in free radical formation and the exacerbation of organ injury in cecal ligation and puncture-induced ALI. Combination treatment with hydrogen and hyperoxia increases the therapeutic efficacy over that of monotherapy via both antioxidant and anti-inflammatory mechanisms, and markedly reduces the degree of ALI (Xie et al., 2010).
Mitochondrial dysfunction is involved in the progression of septic lung injury. Hydrogen blocks the opening of mitochondrial permeability transition pores and restores mitochondrial structure and function in sepsis-induced ALI (Chen X et al., 2018). Hydrogen can also activate autophagy, decreasing NLRP3 inflammasome activity, mitochondrial dysfunction, and cytokine release (Chen HG et al., 2019). In addition, hydrogen improves mitochondrial quality control by enhancing mitophagy via the activation of the FUN14 domain-containing protein 1 (FUNDC1) pathway, thereby alleviating organ injury (Yan et al., 2019). These results suggest that hydrogen targets mitochondria to improve energy metabolism in sepsis, alleviating mitochondrial dysfunction in the lungs.
To fully understand the molecular mechanisms underlying the protective effect of hydrogen against sepsis, an isobaric tags for relative and absolute quantitation (iTRAQ)-based proteomic analysis of the murine lung with polymicrobial sepsis has been performed (Bian et al., 2018). This study showed that 192 of 4472 differentially expressed proteins were related to the protective effects of hydrogen against sepsis. These proteins are associated with muscle contraction, oxygen transport, protein synthesis, collagen barrier membranes, cell adhesion, and coagulation function. Hydrogen also exerted protective effects via the downregulation of the expression of proteins which are involved in the regulation of immune function, cell death, and inflammation, and the upregulation of the expression of transferrin, which is an iron-binding protein with cytoprotective activity (Bian et al., 2018). These results may facilitate the clinical application of hydrogen in patients with sepsis.
3.1.3. Role of hydrogen in viral pneumonia injury
Viral pneumonia has received substantial attention recently owing to the novel coronavirus. COVID-19 control remains a challenging task, and the treatments and prognoses of COVID-19 are different from influenza (Bai and Tao, 2021). To date, specific antiviral drugs for the treatment of COVID-19 are lacking. Concentrations of inflammatory factors, such as IL-2, IL-7, IL-10, and TNF-α, in the plasma are higher in patients with severe or critical disease than in other patients (Huang et al., 2005; Chien et al., 2006; Tang et al., 2020; Xu et al., 2020). Owing to its established antioxidant, anti-inflammatory, and anti-apoptotic effects, it is reasonable to use hydrogen in the early stage of COVID-19 to reduce the destructive cytokine storm and lung injury (Yang FX et al., 2020). In rats treated with hydrogen-rich water (HRW), airway damage was alleviated, and mucin 5AC (MUC5AC) expression and mucus secretion in smoke-induced COPD models were decreased (Ning et al., 2013). Hydrogen might be an effective agent for decreasing COVID-19-related viscous secretions.
Although the direct use of hydrogen for the treatment of COVID-19 is a promising approach, additional experimental data are needed. In China, in the seventh edition of Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment issued by the China National Health Commission (2020), the inhalation of oxygen mixed with hydrogen gas (33.3% O2 and 66.6% H2) is recommended.
3.1.4. Role of hydrogen in I/R injury of the lungs
I/R injury is associated with serious clinical manifestations, posing a serious therapeutic challenge for physicians (Wu MY et al., 2018). The total tissue injury induced by I/R is divided into ischemia injury and reperfusion injury (Gottlieb, 2011). The pathophysiology of I/R injury involves ROS, aseptic inflammation, and cell death pathways.
I/R injury in the lungs often develops during lung transplantation. Severe I/R injury leads to primary graft dysfunction. This is the major cause of short- and long-term morbidity and mortality after lung transplantation (den Hengst et al., 2010; Porteous et al., 2015). Currently, there is no effective method for the prevention of I/R injury, and treatment strategies are focused on supportive care. Molecular hydrogen can reduce inflammation, oxidative stress, and apoptosis, and alleviate I/R injury.
Inflammation induced by lung ischemia causes the release of the proinflammatory cytokines IL-8 and TNF-α and the activation of neutrophils during perfusion, resulting in EC damage, ultimately leading to alterations in permeability and lung edema. Hydrogen treatment decreased levels of IL-8, IL-1β, and TNF-α in the recipient lungs and serum, and decreased MPO levels in lung grafts (Liu RF et al., 2015; Saito et al., 2020). Hydrogen also attenuated inflammatory response pathways, particularly the p38MAPK and NF-κB pathways (Zhang GC et al., 2018).
·OH is the most detrimental ROS in I/R injury. Hydrogen can selectively scavenge ·OH and ONOO- (Ohsawa et al., 2007). Moreover, hydrogen inhalation during mechanical ventilation activates HO-1 (Kawamura et al., 2011), reduces MDA level, and increases SOD activity in rabbit lungs (Kawamura et al., 2010; Li et al., 2012).
Apoptosis is also elevated after lung transplantation. Hydrogen significantly inhibits the proapoptotic proteins caspase-3 and caspase-8 in lung grafts and activates the Bcl-2 and B-cell lymphoma-xL (Bcl-xL), which inhibit apoptosis by stabilizing the mitochondrial outer membrane, and stop the release of cytochrome C into the cytosol via the intrinsic apoptotic pathway (Kawamura et al., 2010; Liu RF et al., 2015).
Hydrogen not only has protective effects in lung damage caused by lung transplantation itself, but also plays a role in I/R injury due to other causes. For example, in lung injury induced by intestinal or limb I/R due to clamping the bilateral femoral arteries for 3 h, compared to the control group, HRS treatment significantly decreased neutrophil infiltration, NF-κB activation, and levels of IL-1β, TNF-α, MPO, and MDA in the lung tissues, and downregulated chemerin and NLRP3, thereby attenuating apoptosis (Mao et al., 2009; Zou et al., 2019).
These findings suggest that hydrogen is a potential novel therapy to resolve the difficult clinical issue of lung I/R injury and may improve outcomes after lung transplantation and lung I/R resulting from other causes.
3.1.5. Effect of hydrogen on other types of lung injury
In hyperoxic ALI, by activating the PI3K/Akt/forkhead box O3a (FoxO3a) signaling pathway, hydrogen upregulates Sirt1, protects type II alveolar epithelial cells against hyperoxia-induced apoptosis, and alleviates ER stress (Sun et al., 2017; Wu D et al., 2018). In ventilator-induced lung injury (VILI), hydrogen gas inhalation effectively reduces VILI-associated inflammatory responses, including both local and systemic inflammation. Interestingly, hydrogen modulates the NF-κB signaling pathway by increasing NF-κB activation at an early stage of ventilation then decreasing it after 2 h of ventilation. The early activation of NF-κB was correlated with elevated levels of the antiapoptotic protein Bcl-2 and decreased levels of Bax. This may contribute to the cytoprotective effects of hydrogen against apoptotic and inflammatory signaling pathway activation, improve gas exchange, and reduce VILI-induced apoptosis (Huang et al., 2010, 2011). These findings may provide guidance for single-lung ventilation anesthesia in thoracic surgery. In seawater instillation-induced ALI, 2% hydrogen gas inhalation attenuates lung injury by the activation of Nrf2 and HO-1 expression and inhibition of caspase-3 expression (Diao et al., 2016). In addition, in ALI caused by oleic acid and burns (early stages of severe burn and extensive burn), hydrogen gas inhalation has a similar protective role (Fang et al., 2011; Qin et al., 2017; Ying et al., 2017). These findings show that hydrogen has a broad range of potential applications for the treatment of diseases associated with lung injury.
3.2. Effects of hydrogen on COPD
COPD includes chronic bronchitis and pulmonary emphysema. It often leads to breathlessness and a reduction in exercise tolerance, thereby decreasing quality of life and increasing mortality (Liu et al., 2011). Oxidative stress and inflammation play important roles in the pathogenesis of COPD (Pinamonti et al., 1996; Ricciardolo et al., 2006).
By reducing CS exposure-induced COPD and inhibiting ERK1/2 and NF-κB in the lungs (Liu et al., 2017; Lu et al., 2018), hydrogen can reduce CS-induced lung function decline, emphysema, inflammation, small-airway remodeling, and goblet-cell hyperplasia in the tracheal epithelium. It significantly reduces inflammatory cells in the bronchoalveolar lavage fluid, improves cardiovascular function, and reduces the right ventricular (RV) hypertrophy index.
In addition to CS, other environmental risk factors, such as exposure to ambient inhaled toxicants (DeVries et al., 2016; Busch et al., 2017), can exacerbate COPD. The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor involved in mediating responses to human-made environmental toxicants (Guerrina et al., 2018; Feng et al., 2019). A decline in AhR contributes to the pro-oxidative and pro-inflammatory effects of fine ambient particulate matter (PM2.5) (Yue et al., 2017). Hydrogen can effectively inhibit the AhR decline induced by concentrated ambient particles, and alleviate lung injury induced by exposure to concentrated ambient PM2.5, which is involved in the pathogenesis of COPD (Feng et al., 2019). A clinical trial showed that 2.4% hydrogen gas inhaled for 45 min in patients with asthma or COPD decreases the levels of pro-inflammatory factors in the peripheral blood of patients and in exhaled breath condensates (Wang ST et al., 2020).
Hydrogen can reach lung cells easily and quickly, and act directly on the lungs, leading to good therapeutic efficacy (Liu et al., 2011). Hydrogen is a novel candidate preventive and therapeutic strategy for COPD (Liu et al., 2011).
3.3. Effects of hydrogen on asthma
Asthma is a chronic complex lung disease associated with airway inflammation, and leads to hyperreactivity, airflow limitation, and airway remodeling (Haahtela, 1997; Comhair et al., 2000). Endogenous and exogenous ROS play a major role in airway inflammation and are determinants of asthma severity (Comhair et al., 2000, 2001).
Hydrogen can attenuate inflammation and oxidative stress in asthma. In ovalbumin-induced allergic airway inflammation, hydrogen gas inhalation reduces eosinophils and lymphocytes in bronchial alveolar lavage fluid (BALF), markedly decreases pro-inflammatory factors in the serum, upregulates the activity of SOD, and attenuates the increases in MDA and MPO (Zhang N et al., 2018). Hydrogen significantly alleviates airway hyperresponsiveness and goblet cell hyperplasia, diminishes the T helper type 2 (TH2) response, and decreases IL-4 as well as immunoglobulin E (IgE) levels by inhibiting NF-κB activation and activating the Nrf2 pathway (Huang et al., 2019).
Normally, alveolar macrophages in the lung function to maintain an airway clear of bacteria, foreign particles, and apoptotic cells. However, in asthma, the phagocytic ability of alveolar macrophages is significantly reduced. This defective phagocytosis is reversed by hydrogen gas inhalation (Huang et al., 2019). In addition, hydrogen reduces airway remodeling which causes persistent alterations in airway wall structures and function, ultimately resulting in the progressive loss of lung function (Fehrenbach et al., 2017). In ovalbumin-induced asthma, HRS inhibits the NF-κB pathway and significantly decreases the mucus index, the expression of MUC5AC, collagen III, and vascular endothelium growth factor (VEGF), and collagen deposition (Xiao et al., 2013), effectively alleviating airway remodeling.
These results suggest that hydrogen alleviates allergic airway inflammation and improves airway function in asthma. Although research has been limited mainly to animal models, which imperfectly recapitulate asthma, these studies have improved our understanding of the benefits of hydrogen as a therapeutic intervention in asthma. Further studies are necessary to establish the clinical value of hydrogen.
3.4. Effects of hydrogen on lung cancer
Lung cancer is one of the most common lethal malignancies, with a poor prognosis owing to its high metastatic potential and drug resistance. An increasing number of animal experiments and clinical trials have established the efficacy of hydrogen against cancers, including lung cancer. The mechanisms underlying the anti-cancer effects of hydrogen are summarized as follows.
Hydrogen downregulates the expression of proteins involved in stemness, proliferation, and angiogenesis and upregulates the expression of proteins that promote differentiation (Liu et al., 2019). Hydrogen can also downregulate the chromosome condensation regulator structural maintenance of chromosomes 3 (SMC3), as well as the migration and invasion of A549 and H1975 cells, thereby inhibiting lung cancer progression (Wang et al., 2018).
Hydrogen modulates cell death. The dysregulation of apoptosis is a hallmark of cancer. The alteration of apoptosis contributes not only to tumor development and progression, but also to tumor resistance to therapies (Pistritto et al., 2016). Hydrogen promotes apoptosis, including early and late apoptosis, in lung cancer (Jiang et al., 2018; Wang et al., 2018). The PI3K/Akt pathway is an important signaling pathway involved in extracellular signaling events and cellular processes, including cell proliferation, apoptosis, and survival. In non-small cell lung cancer, when combined with the PI3K inhibitor LY294002, hydrogen reduced cell proliferation and promoted apoptosis by downregulating Akt phosphorylation and inhibiting the PI3K pathway. Recently, it has been shown that hydrogen pretreatment enhances ROS and the expression of pyroptosis-related proteins, stimulates NLRP3 inflammasome/gasdermin D (GSDMD) activation, and inhibits endometrial cancer (Yang Y et al., 2020). Further studies are needed to determine whether hydrogen can induce pyroptosis in lung cancer and to clarify the underlying molecular mechanism.
Hydrogen modulates immunity by reducing the proportion of terminal programmed cell death 1 positive (PD-1+) cluster of differentiation 8 positive (CD8+) T cells, which are associated with a poor prognosis in cancer (Akagi, 2018). In patients with lung cancer, hydrogen can activate coenzyme Q10, restore exhausted CD8+ T cells (especially PD-1+Tim3+ terminal CD8+ T cells; Tim3+, T cell immunoglobulin and mucin domaincontaining protein 3 positive), enhance the clinical efficacy of nivolumab, and increase overall survival time (Akagi and Baba, 2020).
Hydrogen can alleviate the side effects of conventional anti-cancer therapies, such as chemotherapy and radiotherapy, and improve quality of life. By reducing oxidative stress and inflammation, hydrogen protects cultured cells and mice against radiation-induced injury (Qian et al., 2010; Qiu et al., 2020). It also attenuates the gefitinib-induced exacerbation of naphthalene-induced ALI without impairing anti-tumor activity (Terasaki et al., 2019b).
Hydrogen is well-suited for the treatment of advanced cancer. It significantly improves the physical status of patients, reduces fatigue, insomnia, anorexia, and pain, and decreases elevated tumor markers. These effects are particularly evident in patients with lung cancer (Chen JB et al., 2019). As a simple, low-cost treatment with few adverse reactions, hydrogen therapy in lung cancer is very promising.
3.5. Effects of hydrogen on PAH
Pulmonary hypertension (PH) is a progressive complication of chronic lung disease (resting mean pulmonary artery pressure ≥25 mmHg (1 mmHg=133.3 Pa) (Simonneau et al., 2019). PAH is one of most common forms of PH. Although therapies have improved the prognosis of patients with PAH, morbidity and mortality rates are still high (Barst et al., 2012; Benza et al., 2012). PAH is a progressive vasculopathy involving an imbalance of vasodilators and vasoconstrictors, ROS, and inflammation (Perez-Vizcaino et al., 2010; Price et al., 2012).
The oral administration and injection of hydrogen have similar preventive effects against the development of PH and RV hypertrophy in monocrotaline (MCT)-induced PAH in rats (Paulin et al., 2011; Wang Y et al., 2011; He et al., 2013). Via its antioxidant and anti-inflammatory effects, hydrogen treatment effectively alleviates smooth muscle cell proliferation, increases vascular density, and alleviates PH and RV hypertrophy. Hydrogen can reduce 3-nitrotyrosine, which is a specific metabolic product of ONOO- and may participate in PH formation, decrease levels of MDA and 8-OHdG, and decrease SOD activity in PH (Wang Y et al., 2011). Hydrogen could reduce ICAM-1, which is associated with the infiltration of chronic inflammatory cells in local lung tissues (He et al., 2013).
NF-κB plays a critical role in the progression of PAH, causing fibroblast growth factor 2 (FGF2)-induced inflammation in a rat model of MCT-induced PAH. Selectively inhibiting NF-κB can prevent the increase in RV pressure, suppress proliferation, and induce apoptosis in pulmonary arterial smooth muscle cells (Hosokawa et al., 2013). Hydrogen treatment effectively reduces inflammation and apoptosis by decreasing NF-κB activity (Wang C et al., 2011; Xie et al., 2012). These findings highlight the ability of hydrogen to modulate NF-κB-mediated inflammation in the pathogenesis of PAH.
3.6. Effect of hydrogen on PF
PF is a lung disease caused by aberrant wound healing due to repeated alveolar injury in genetically susceptible individuals. It is one of the major causes of morbidity, and effective treatments are lacking (Osborn-Heaford et al., 2015). The pathology includes chronic inflammation, excess extracellular matrix (ECM) deposition, and scarring of lung tissue.
The epithelial-mesenchymal transition (EMT) in ALI can cause the activation of myofibroblasts, which play a central role in the pathogenesis of PF via the synthesis and deposition of ECM proteins (Cottin, 2013; Zhang YQ et al., 2015). In addition, ROS and inflammation in ALI can induce a fibrotic response to lung injury by modulating ECM deposition (Murthy et al., 2009; He et al., 2011). HRS effectively suppresses oxidative stress, inhibits the LPS-induced activation of p38MAPK and JNK, significantly decreases the LPS-induced loss of E-cadherin (an epithelial marker), and decreases α-smooth muscle actin (a myofibroblast marker) in lung tissues, thereby blocking alveolar EMT and PF. Additionally, hydrogen inhibits pulmonary transforming growth factor-β (TGF-β) production, which also takes part in the reprogramming of gene expression during EMT (Tao et al., 2016).
Hydrogen also alleviates PF caused by other factors, such as paraquat-induced lung fibroblast injury and rheumatoid arthritis‐associated interstitial lung disease (Terasaki et al., 2019a; Li T et al., 2020). Laboratory experiments have not verified the effects of hydrogen on idiopathic PF, which accounts for 25%–30% of cases of interstitial lung disease. There is convincing evidence that the EMT has important roles in organ fibrosis. TGF-β is a major EMT inducer, with a profibrotic effect in the lung epithelium during the pathological process of idiopathic PF (Thiery et al., 2009; Hewlett et al., 2018). We speculate that hydrogen can interfere with the process of idiopathic PF via related pathways, particularly given the great deal of overlap between mechanisms underlying idiopathic PF and other types of PF. However, further analyses are needed to evaluate this hypothesis.
4. Route of hydrogen administration
Hydrogen gas has some special characteristics, such as its nonpolarity, small size, and low solubility (1.6 ppm (part per million, ×10-6)) under physiological conditions. Typical administration routes include the inhalation of hydrogen gas, oral intake of HRW, and injection of HRS. Inhalation is a simple method in which a ventilator circuit, facemask, or nasal cannula is used to administer hydrogen gas. The effects are rapid, and it can be used to treat acute oxidative stress (Ohta, 2014). The oral intake of HRS, which is hydrogen dissolved in water, is a portable, safe, and convenient method for administration (Sun et al., 2012; Koyama et al., 2014). Under normal atmospheric pressure and at room temperature, hydrogen can be dissolved in water up to 0.8 mmol/L (1.6 mg/L) (Asada et al., 2020). The injection of HRS can provide highly accurate hydrogen doses. Either peritoneal or intravenous injection can be applied to provide a protective effect (Yan et al., 2017; Ying et al., 2017; Xu et al., 2018). In addition, intrathecal injection can produce neuro-protective effects (Ge et al., 2014). Its lack of polarity and easy cell penetration make hydrogen promising for the treatment of skin and eye diseases with topical formulations and eye drops (Asada et al., 2019; Cejka et al., 2020).
However, these applications of hydrogen often do not achieve highly targeted effects. Targeted delivery and controlled release of hydrogen can address this issue (Kawamura M et al., 2020). Recently, investigators have designed palladium (Pd) hydride nanocrystals as a multifunctional hydrogen carrier for tumor-targeted delivery and the controlled release of bio-reductive hydrogen. This method not only has anticancer effects, but also protects normal cells from hyperthermia damage (Zhao et al., 2018). A similar nanomedicine strategy has been used to alleviate oxidative stress and I/R injury (Kou et al., 2019; Kawamura T et al., 2020). These applications of molecular hydrogen provide new avenues for the treatment of lung diseases, especially for lung cancer. The routes of hydrogen administration are summarized in Table 1.
Table 1.
Routes of hydrogen administration and their characteristics
| Dosage form | Administration route | Characteristics |
|---|---|---|
| Hydrogen gas | Inhalation | Simple method; rapid action |
| Hydrogen water | Oral intake | Portable, safe, and convenient |
| Bath | ||
| Hydrogen saline | Peritoneal injection | Provides highly accurate hydrogen doses; produces neuro-protective effects; treats eye diseases |
| Intravenous injection | ||
| Intrathecal injection | ||
| Eye drops | ||
| Hydrogen nanocrystals | Oral intake | Highly targeted effects |
| Injection |
There are many routes for hydrogen administration, such as hydrogen gas inhalation, oral intake or a hydrogen water bath, injection of hydrogen saline (including peritoneal, intravenous and intratheral injection), and local eye drops. To achieve highly targeted effects, hydrogen can also be designed as nanocrystals, providing a new avenue for the treatment of lung diseases such as lung cancer.
5. Conclusions and perspectives
There is accumulating evidence for the broad biological effects of hydrogen. It regulates oxidative stress, inflammation, autophagy, programmed cell death, and the aging process. Animal experiments and clinical trials have clearly demonstrated the protective effects of hydrogen on many organs and systems. In particular, there is increasing evidence that hydrogen exerts a protective effect in various lung diseases. From a systemic perspective, in addition to the direct protective effects of hydrogen on the lungs, it indirectly protects lung tissues via protective effects on other tissues and organs. However, the detailed molecular mechanisms underlying the protective effects of hydrogen remain to be determined, and our current understanding of its effects is based mainly on animal experiments. Applicability to humans is yet to be tested. Therefore, a better understanding of protective pathways mediating the effects of hydrogen may facilitate the design of specific therapies for the treatment of pulmonary diseases. We expect large-scale clinical trials to confirm the therapeutic efficacy and safety of hydrogen. Owing to their broad potential applications, safety, convenience, and simple properties, hydrogen products are promising candidates for the treatment of diverse pulmonary diseases.
Acknowledgments
This study was supported by the Technology Bureau of Liaoning Province (No. 17-230-9-45), China.
Author contributions
Zhiling FU analyzed the literature and prepared the first draft of the manuscript. Jin ZHANG revised, edited, and checked the final version. Both authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Zhiling FU and Jin ZHANG declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by either of the authors.
References
- Akagi J, 2018. Immunological effect of hydrogen gas—hydrogen gas improves clinical outcomes of cancer patients. Gan To Kagaku Ryoho, 45(10): 1475-1478 (in Japanese). [PubMed] [Google Scholar]
- Akagi J, Baba H, 2020. Hydrogen gas activates coenzyme Q10 to restore exhausted CD8+ T cells, especially PD-1+Tim3+terminal CD8+ T cells, leading to better nivolumab outcomes in patients with lung cancer. Oncol Lett, 20: 258. 10.3892/ol.2020.12121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Hemmi H, 2003. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett, 85(2): 85-95. 10.1016/S0165-2478(02)00228-6 [DOI] [PubMed] [Google Scholar]
- Andersson U, Yang H, Harris H, 2018. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Exp Opin Ther Targets, 22(3): 263-277. 10.1080/14728222.2018.1439924 [DOI] [PubMed] [Google Scholar]
- Asada R, Saitoh Y, Miwa N, 2019. Effects of hydrogen-rich water bath on visceral fat and skin blotch, with boiling-resistant hydrogen bubbles. Med Gas Res, 9(2): 68-73. 10.4103/2045-9912.260647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada R, Tazawa K, Sato S, et al. , 2020. Effects of hydrogen-rich water prepared by alternating-current-electrolysis on antioxidant activity, DNA oxidative injuries, and diabetes-related markers. Med Gas Res, 10(3): 114-121. 10.4103/2045-9912.296041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai CX, Fukuda N, Song YL, et al. , 1999. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J Clin Invest, 103(4): 555-561. 10.1172/JCI4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Tao XN, 2021. Comparison of COVID-19 and influenza characteristics. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(2): 87-98. 10.1631/jzus.B2000479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barst RJ, McGoon MD, Elliott CG, et al. , 2012. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation, 125(1): 113-122. 10.1161/CIRCULATIONAHA.111.026591 [DOI] [PubMed] [Google Scholar]
- Benza RL, Miller DP, Barst RJ, et al. , 2012. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest, 142(2): 448-456. 10.1378/chest.11-1460 [DOI] [PubMed] [Google Scholar]
- Bhattacharya J, Matthay MA, 2013. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Phys, 75: 593-615. 10.1146/annurev-physiol-030212-183756 [DOI] [PubMed] [Google Scholar]
- Bian YX, Qin C, Xin Y, et al. , 2018. iTRAQ-based quantitative proteomic analysis of lungs in murine polymicrobial sepsis with hydrogen gas treatment. Shock, 49(2): 187-195. 10.1097/SHK.0000000000000927 [DOI] [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, et al. , 2012. Oxidative stress and antioxidant defense. World Allergy Organ J, 5(1): 9-19. 10.1097/WOX.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz BM, Kaczorowski DJ, Sugimoto R, et al. , 2008. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am J Transplant, 8(10): 2015-2024. 10.1111/j.1600-6143.2008.02359.x [DOI] [PubMed] [Google Scholar]
- Busch R, Hobbs BD, Zhou J, et al. , 2017. Genetic association and risk scores in a chronic obstructive pulmonary disease meta-analysis of 16, 707 subjects. Am J Respir Cell Mol Biol, 57(1): 35-46. 10.1165/rcmb.2016-0331OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai WW, Zhang MH, Yu YS, et al. , 2013. Treatment with hydrogen molecule alleviates TNFα-induced cell injury in osteoblast. Mol Cell Biochem, 373(1-2): 1-9. 10.1007/s11010-012-1450-4 [DOI] [PubMed] [Google Scholar]
- Cejka C, Kossl J, Holan V, et al. , 2020. An immunohistochemical study of the increase in antioxidant capacity of corneal epithelial cells by molecular hydrogen, leading to the suppression of alkali-induced oxidative stress. Oxid Med Cell Longev, 2020: 7435260. 10.1155/2020/7435260 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chen HG, Xie KL, Han HZ, et al. , 2015. Molecular hydrogen protects mice against polymicrobial sepsis by ameliorating endothelial dysfunction via an Nrf2/HO-1 signaling pathway. Int Immunopharmacol, 28(1): 643-654. 10.1016/j.intimp.2015.07.034 [DOI] [PubMed] [Google Scholar]
- Chen HG, Mao X, Meng XY, et al. , 2019. Hydrogen alleviates mitochondrial dysfunction and organ damage via autophagy-mediated NLRP3 inflammasome inactivation in sepsis. Int J Mol Med, 44(4): 1309-1324. 10.3892/IJMM.2019.4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HG, Han HZ, Li Y, et al. , 2020. Hydrogen alleviated organ injury and dysfunction in sepsis: the role of cross-talk between autophagy and endoplasmic reticulum stress: experimental research. Int Immunopharmacol, 78: 106049. 10.1016/j.intimp.2019.106049 [DOI] [PubMed] [Google Scholar]
- Chen JB, Kong XF, Lv YY, et al. , 2019. “Real world survey” of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients. Med Gas Res, 9(3): 115-121. 10.4103/2045-9912.266985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Wang N, Diao Y, et al. , 2017. Hydrogen-rich saline attenuates brain injury induced by cardiopulmonary bypass and inhibits microvascular endothelial cell apoptosis via the PI3K/Akt/GSK3β signaling pathway in rats. Cell Phys Biochem, 43(4): 1634-1647. 10.1159/000484024 [DOI] [PubMed] [Google Scholar]
- Chen MH, Zhang J, Chen Y, et al. , 2018. Hydrogen protects lung from hypoxia/re-oxygenation injury by reducing hydroxyl radical production and inhibiting inflammatory responses. Sci Rep, 8: 8004. 10.1038/s41598-018-26335-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Cui J, Zhai X, et al. , 2018. Inhalation of hydrogen of different concentrations ameliorates spinal cord injury in mice by protecting spinal cord neurons from apoptosis, oxidative injury and mitochondrial structure damages. Cell Phys Biochem, 47: 176-190. 10.1159/000489764 [DOI] [PubMed] [Google Scholar]
- Chen YL, Jiang JY, Miao HB, et al. , 2013. Hydrogen-rich saline attenuates vascular smooth muscle cell proliferation and neointimal hyperplasia by inhibiting reactive oxygen species production and inactivating the Ras-ERK1/2-MEK1/2 and Akt pathways. Int J Mol Med, 31(3): 597-606. 10.3892/ijmm.2013.1256 [DOI] [PubMed] [Google Scholar]
- Chien JY, Hsueh PR, Cheng WC, et al. , 2006. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology, 11(6): 715-722. 10.1111/j.1440-1843.2006.00942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- China National Health Commission , 2020. Chinese Clinical Guidance for COVID-19 pneumonia diagnosis and treatment (7th Edition). http://kjfy.meetingchina.org/msite/news/show/cn/3337.html?from=singlemessage&isappinstalled=0 [Accessed on May 10, 2021]. [Google Scholar]
- Comhair SAA, Bhathena PR, Dweik RA, et al. , 2000. Rapid loss of superoxide dismutase activity during antigen-induced asthmatic response. Lancet, 355(9204): 624. 10.1016/S0140-6736(99)04736-4 [DOI] [PubMed] [Google Scholar]
- Comhair SAA, Bhathena PR, Farver C, et al. , 2001. Extracellular glutathione peroxidase induction in asthmatic lungs: evidence for redox regulation of expression in human airway epithelial cells. FASEB J, 15(1): 70-78. 10.1096/fj.00-0085com [DOI] [PubMed] [Google Scholar]
- Cottin V, 2013. Interstitial lung disease. Eur Respir Rev, 22(127): 26-32. 10.1183/09059180.00006812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YM, Zhang H, Ji MH, et al. , 2014. Hydrogen-rich saline attenuates neuronal ischemia-reperfusion injury by protecting mitochondrial function in rats. J Surg Res, 192(2): 564-572. 10.1016/j.jss.2014.05.060 [DOI] [PubMed] [Google Scholar]
- Den Hengst WA, Gielis JF, Lin JY, et al. , 2010. Lung ischemia-reperfusion injury: a molecular and clinical view on a complex pathophysiological process. Am J Phys Heart Circ Phys, 299(5): H1283-H1299. 10.1152/ajpheart.00251.2010 [DOI] [PubMed] [Google Scholar]
- DeVries R, Kriebel D, Sama S, 2016. Low level air pollution and exacerbation of existing copd: a case crossover analysis. Environ Health, 15: 98. 10.1186/s12940-016-0179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao MY, Zhang S, Wu LF, et al. , 2016. Hydrogen gas inhalation attenuates seawater instillation-induced acute lung injury via the Nrf2 pathway in rabbits. Inflammation, 39(6): 2029-2039. 10.1007/s10753-016-0440-1 [DOI] [PubMed] [Google Scholar]
- Elmore S, 2007. Apoptosis: a review of programmed cell death. Toxicol Pathol, 35(4): 495-516. 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Fu XJ, Gu C, et al. , 2011. Hydrogen-rich saline protects against acute lung injury induced by extensive burn in rat model. J Burn Care Res, 32(3): e82-e91. 10.1097/BCR.0b013e318217f84f [DOI] [PubMed] [Google Scholar]
- Fehrenbach H, Wagner C, Wegmann M, 2017. Airway remodeling in asthma: what really matters. Cell Tissue Res, 367(3): 551-569. 10.1007/s00441-016-2566-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Duan EH, Shi XJ, et al. , 2019. Hydrogen ameliorates lung injury in a rat model of subacute exposure to concentrated ambient PM2.5 via Aryl hydrocarbon receptor. Int Immunopharmacol, 77: 105939. 10.1016/j.intimp.2019.105939 [DOI] [PubMed] [Google Scholar]
- Ferrari R, Ceconi C, Curello S, et al. , 1991. The occurrence of oxidative stress during reperfusion in experimental animals and men. Cardiovasc Drugs Ther, 5(S2): 277-287. 10.1007/BF00054749 [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Kagan JC, 2020. Toll-like receptors and the control of immunity. Cell, 180(6): 1044-1066. 10.1016/j.cell.2020.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch J, Lenz O, Friedrich B, 2013. Structure, function and biosynthesis of O2-tolerant hydrogenases. Nat Rev Microbiol, 11(2): 106-114. 10.1038/nrmicro2940 [DOI] [PubMed] [Google Scholar]
- Fu ZL, Zhang Z, Wu XY, et al. , 2020. Hydrogen-rich saline inhibits lipopolysaccharide-induced acute lung injury and endothelial dysfunction by regulating autophagy through mTOR/TFEB signaling pathway. Biomed Res Int, 2020: 9121894. 10.1155/2020/9121894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge YH, Wu FX, Sun XJ, et al. , 2014. Intrathecal infusion of hydrogen-rich normal saline attenuates neuropathic pain via inhibition of activation of spinal astrocytes and microglia in rats. PLoS ONE, 9(5): e97436. 10.1371/journal.pone.0097436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestra M, 2007. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal, 19(9): 1807-1819. 10.1016/j.cellsig.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Gharib B, Hanna S, Abdallahi OM, et al. , 2001. Anti-inflammatory properties of molecular hydrogen: investigation on parasite-induced liver inflammation. C R Acad Sci III, 324(8): 719-724. 10.1016/S0764-4469(01)01350-6 [DOI] [PubMed] [Google Scholar]
- Giannotta M, Trani M, Dejana E, 2013. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell, 26(5): 441-454. 10.1016/j.devcel.2013.08.020 [DOI] [PubMed] [Google Scholar]
- Golden TR, Melov S, 2007. Gene expression changes associated with aging in C. elegans. In: WormBook (Ed.), The C. elegans Research Community. WormBook, p.1-12. 10.1895/wormbook.1.127.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss CH, Brower RG, Hudson LD, et al. , 2003. Incidence of acute lung injury in the United States. Crit Care Med, 31(6): 1607-1611. 10.1097/01.CCM.0000063475.65751.1D [DOI] [PubMed] [Google Scholar]
- Gottlieb RA, 2011. Cell death pathways in acute ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther, 16(3-4): 233-238. 10.1177/1074248411409581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrina N, Traboulsi H, Eidelman DH, et al. , 2018. The Aryl Hydrocarbon Receptor and the maintenance of lung health. Int J Mol Sci, 19(12): 3882. 10.3390/ijms19123882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo HT, Callaway JB, Ting JPY, 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med, 21(7): 677-687. 10.1038/nm.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SX, Fang Q, You CG, et al. , 2015. Effects of hydrogen-rich saline on early acute kidney injury in severely burned rats by suppressing oxidative stress induced apoptosis and inflammation. J Trans Med, 13: 183. 10.1186/s12967-015-0548-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzik TJ, Touyz RM, 2017. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension, 70(4): 660-667. 10.1161/HYPERTENSIONAHA.117.07802 [DOI] [PubMed] [Google Scholar]
- Haahtela T, 1997. Airway remodelling takes place in asthma—what are the clinical implications? Clin Exp Allergy, 27(4): 351-353. 10.1111/j.1365-2222.1997.tb00717.x [DOI] [PubMed] [Google Scholar]
- Han AL, Park SH, Park MS, 2017. Hydrogen treatment protects against cell death and senescence induced by oxidative damage. J Microbiol Biotechnol, 27(2): 365-371. 10.4014/jmb.1608.08011 [DOI] [PubMed] [Google Scholar]
- He B, Zhang YF, Kang B, et al. , 2013. Protection of oral hydrogen water as an antioxidant on pulmonary hypertension. Mol Biol Rep, 40(9): 5513-5521. 10.1007/s11033-013-2653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Murthy S, McCormick ML, et al. , 2011. Mitochondrial Cu, Zn-superoxide dismutase mediates pulmonary fibrosis by augmenting H2O2 generation. J Biol Chem, 286(17): 15597-15607. 10.1074/jbc.M110.187377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett JC, Kropski JA, Blackwell TS, 2018. Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol, 71-72: 112-127. 10.1016/j.matbio.2018.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano SI, Ichikawa Y, Kurokawa R, et al. , 2020. A “philosophical molecule, ” hydrogen may overcome senescence and intractable diseases. Med Gas Res, 10(1): 47-49. 10.4103/2045-9912.279983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YC, Sun L, Sun RQ, et al. , 2016. Combination therapy of molecular hydrogen and hyperoxia improves survival rate and organ damage in a zymosan-induced generalized inflammation model. Exp Ther Med, 11(6): 2590-2596. 10.3892/etm.2016.3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YC, Chen HG, Yu YH, et al. , 2017. Effect of combination therapy with propofol and hydrogen-rich saline on organ damage and cytokines in a murine model of sepsis. Chin Crit Care Med, 29(4): 316-320 (in Chinese). 10.3760/cma.j.issn.2095-4352.2017.04.006 [DOI] [PubMed] [Google Scholar]
- Hosokawa S, Haraguchi G, Sasaki A, et al. , 2013. Pathophysiological roles of nuclear factor kappaB (NF-κB) in pulmonary arterial hypertension: effects of synthetic selective NF-κB inhibitor IMD-0354. Cardiovasc Res, 99(1): 35-43. 10.1093/cvr/cvt105 [DOI] [PubMed] [Google Scholar]
- Hu QG, Zhou YX, Wu SJ, et al. , 2020. Molecular hydrogen: a potential radioprotective agent. Biomed Pharmacother, 130: 110589. 10.1016/j.biopha.2020.110589 [DOI] [PubMed] [Google Scholar]
- Huang CS, Kawamura T, Lee S, et al. , 2010. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit Care, 14: R234. 10.1186/cc9389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Kawamura T, Peng XM, et al. , 2011. Hydrogen inhalation reduced epithelial apoptosis in ventilator-induced lung injury via a mechanism involving nuclear factor-kappa B activation. Biochem Biophys Res Commun, 408(2): 253-258. 10.1016/j.bbrc.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Huang KJ, Su IJ, Theron M, et al. , 2005. An interferon-γ-related cytokine storm in SARS patients. J Med Virol, 75(2): 185-194. 10.1002/jmv.20255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PK, Wei SS, Huang WH, et al. , 2019. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int Immunopharmacol, 74: 105646. 10.1016/j.intimp.2019.05.031 [DOI] [PubMed] [Google Scholar]
- Huo TT, Zeng Y, Liu XN, et al. , 2014. Hydrogen-rich saline improves survival and neurological outcome after cardiac arrest and cardiopulmonary resuscitation in rats. Anesth Analg, 119(2): 368-380. 10.1213/ANE.0000000000000303 [DOI] [PubMed] [Google Scholar]
- Iketani M, Ohsawa I, 2017. Molecular hydrogen as a neuroprotective agent. Curr Neuropharmacol, 15(2): 324-331. 10.2174/1570159X14666160607205417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu G, Zhang L, et al. , 2018. Therapeutic efficacy of hydrogen-rich saline alone and in combination with PI3K inhibitor in non-small cell lung cancer. Mol Med Rep, 18(2): 2182-2190. 10.3892/MMR.2018.9168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Yu Y, Li B, et al. , 2020. Protective effects of hydrogen-rich saline against experimental diabetic peripheral neuropathy via activation of the mitochondrial ATP-sensitive potassium channel channels in rats. Mol Med Rep, 21(1): 282-290. 10.3892/mmr.2019.10795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan XC, Chen YS, Huang BX, et al. , 2021. Effect of Palrnatine on lipopolysaccharide-induced acute lung injury by inhibiting activation of the Akt/NF-κB pathway. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 22(11): 929-940. 10.1631/jzus.B2000583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Imamura R, Kobayashi Y, et al. , 2020. Oral administration of Si-based agent attenuates oxidative stress and ischemia-reperfusion injury in a rat model: a novel hydrogen administration method. Front Med, 7: 95. 10.3389/FMED.2020.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Huang CS, Tochigi N, et al. , 2010. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation, 90(12): 1344-1351. 10.1097/TP.0b013e3181fe1357 [DOI] [PubMed] [Google Scholar]
- Kawamura T, Huang CS, Peng XM, et al. , 2011. The effect of donor treatment with hydrogen on lung allograft function in rats. Surgery, 150(2): 240-249. 10.1016/j.surg.2011.05.019 [DOI] [PubMed] [Google Scholar]
- Kawamura T, Wakabayashi N, Shigemura N, et al. , 2013. Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway in vivo. Am J Phys Lung Cell Mol Phys, 304(10): L646-L656. 10.1152/ajplung.00164.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Higashida K, Muraoka I, 2020. Application of molecular hydrogen as a novel antioxidant in sports science. Oxid Med Cell Longev, 2020: 2328768. 10.1155/2020/2328768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YW, Byzova TV, 2014. Oxidative stress in angiogenesis and vascular disease. Blood, 123(5): 625-631. 10.1182/blood-2013-09-512749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Z, Zhao PH, Wang ZH, et al. , 2019. Acid-responsive H2-releasing Fe nanoparticles for safe and effective cancer therapy. J Mater Chem B, 7(17): 2759-2765. 10.1039/c9tb00338j [DOI] [PubMed] [Google Scholar]
- Koyama Y, Taura K, Hatano E, et al. , 2014. Effects of oral intake of hydrogen water on liver fibrogenesis in mice. Hepatol Res, 44(6): 663-677. 10.1111/hepr.12165 [DOI] [PubMed] [Google Scholar]
- Kura B, Bagchi AK, Singal PK, et al. , 2019. Molecular hydrogen: potential in mitigating oxidative-stress-induced radiation injury. Can J Phys Pharmacol, 97(4): 287-292. 10.1139/cjpp-2018-0604 [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM, 2014. Mechanisms and functions of inflammasomes. Cell, 157(5): 1013-1022. 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Lee WL, Slutsky AS, 2010. Sepsis and endothelial permeability. N Engl J Med, 363(7): 689-691. 10.1056/NEJMcibr1007320 [DOI] [PubMed] [Google Scholar]
- Li H, Zhou RH, Liu J, et al. , 2012. Hydrogen-rich saline attenuates lung ischemia-reperfusion injury in rabbits. J Surg Res, 174(1): e11-e16. 10.1016/j.jss.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Li H, Yin YR, Liu J, et al. , 2021. Hydrogen-rich water attenuates the radiotoxicity induced by tritium exposure in vitro and in vivo. J Radiat Res, 62(1): 34-45. 10.1093/jrr/rraa104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hong ZJ, Liu H, et al. , 2016. Hydrogen-rich saline promotes the recovery of renal function after ischemia/reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front Pharmacol, 7: 106. 10.3389/fphar.2016.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RC, Liu YL, Xie J, et al. , 2019. Sirt3 mediates the protective effect of hydrogen in inhibiting ROS-induced retinal senescence. Free Radic Biol Med, 135: 116-124. 10.1016/j.freeradbiomed.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Li T, Deng SH, Lei W, et al. , 2020. Hydrogen water alleviates paraquat-induced lung fibroblast injury in vitro by enhancing Nrf2 expression. J Southern Med Univ, 40(2): 233-239 (in Chinese). 10.12122/j.issn.1673-4254.2020.02.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xie KL, Chen HG, et al. , 2015. Hydrogen gas inhibits high-mobility group box 1 release in septic mice by upregulation of heme oxygenase 1. J Surg Res, 196(1): 136-148. 10.1016/j.jss.2015.02.042 [DOI] [PubMed] [Google Scholar]
- Li Y, Chen HG, Shu RC, et al. , 2020. Hydrogen treatment prevents lipopolysaccharide-induced pulmonary endothelial cell dysfunction through RhoA inhibition. Biochem Biophys Res Commun, 522(2): 499-505. 10.1016/j.bbrc.2019.11.101 [DOI] [PubMed] [Google Scholar]
- Liang CX, Liu XW, Liu L, et al. , 2012. Effect of hydrogen inhalation on p38 MAPK activation in rats with lipopolysaccharide-induced acute lung injury. J Southern Med Univ, 32(8): 1211-1213, 1217. 10.3969/j.issn.1673-4254.2012.08.32 [DOI] [PubMed] [Google Scholar]
- Liu HY, Liang XJ, Wang DD, et al. , 2015. Combination therapy with nitric oxide and molecular hydrogen in a murine model of acute lung injury. Shock, 43(5): 504-511. 10.1097/SHK.0000000000000316 [DOI] [PubMed] [Google Scholar]
- Liu LD, Wu XY, Tao BD, et al. , 2016. Protective effect and mechanism of hydrogen treatment on lung epithelial barrier dysfunction in rats with sepsis. Genet Mol Res, 15(1): gmr.15016050.. 10.4238/gmr.15016050 [DOI] [PubMed] [Google Scholar]
- Liu MY, Xie F, Zhang Y, et al. , 2019. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res Ther, 10: 145. 10.1186/s13287-019-1241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RF, Fang XH, Meng C, et al. , 2015. Lung inflation with hydrogen during the cold ischemia phase decreases lung graft injury in rats. Exp Biol Med, 240(9): 1214-1222. 10.1177/1535370214563895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Liu K, Sun Q, et al. , 2011. Hydrogen therapy may be a novel and effective treatment for COPD. Front Pharmacol, 2: 19. 10.3389/fphar.2011.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Shan LP, Dong XS, et al. , 2013. Combined early fluid resuscitation and hydrogen inhalation attenuates lung and intestine injury. World J Gastroenterol, 19(4): 492-502. 10.3748/wjg.v19.i4.492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma C, Wang X, et al. , 2017. Hydrogen coadministration slows the development of COPD-like lung disease in a cigarette smoke-induced rat model. Int J Chron Obstruct Pulmon Dis, 12: 1309-1324. 10.2147/COPD.S124547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YM, Zhang J, 2017. Saturated hydrogen saline ameliorates lipopolysaccharide-induced acute lung injury by reducing excessive autophagy (Review). Exp Ther Med, 13(6): 2609-2615. 10.3892/etm.2017.4353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YQ, Liu YF, Ma XM, et al. , 2015. Hydrogen-rich saline attenuates skin ischemia/reperfusion induced apoptosis via regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway. J Plast Reconstr Aesthet Surg, 68(7): e147-e156. 10.1016/j.bjps.2015.03.001 [DOI] [PubMed] [Google Scholar]
- Lu WJ, Li DF, Hu JY, et al. , 2018. Hydrogen gas inhalation protects against cigarette smoke-induced COPD development in mice. J Thorac Dis, 10(6): 3232-3243. 10.21037/jtd.2018.05.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, et al. , 2007. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol, 8(9): 741-752. 10.1038/nrm2239 [DOI] [PubMed] [Google Scholar]
- Mao YF, Zheng XF, Cai JM, et al. , 2009. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochem Biophys Res Commun, 381(4): 602-605. 10.1016/j.bbrc.2009.02.105 [DOI] [PubMed] [Google Scholar]
- Marcos-Ramiro B, García-Weber D, Millán J, 2014. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost, 112(6): 1088-1102. 10.1160/th14-04-0299 [DOI] [PubMed] [Google Scholar]
- Maybauer MO, Maybauer DM, Herndon DN, 2006. Incidence and outcomes of acute lung injury. N Engl J Med, 354(4): 416-417. 10.1056/NEJMc053159 [DOI] [PubMed] [Google Scholar]
- Mo XY, Li XM, She CS, et al. , 2019. Hydrogen-rich saline protects rat from oxygen glucose deprivation and reperusion-induced apoptosis through VDAC1 via Bcl-2. Brain Res, 1706: 110-115. 10.1016/j.brainres.2018.09.037 [DOI] [PubMed] [Google Scholar]
- Murthy S, Adamcakova-Dodd A, Perry SS, et al. , 2009. Modulation of reactive oxygen species by Rac1 or catalase prevents asbestos-induced pulmonary fibrosis. Am J Phys Lung Cell Mol Phys, 297(5): L846-L855. 10.1152/ajplung.90590.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, Sharpless NE, 2013. Coming of age: molecular drivers of aging and therapeutic opportunities. J Clin Invest, 123(3): 946-950. 10.1172/JCI68833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie CQ, Ding X, A R, et al. , 2021. Hydrogen gas inhalation alleviates myocardial ischemia-reperfusion injury by the inhibition of oxidative stress and NLRP3-mediated pyroptosis in rats. Life Sci, 272: 119248. 10.1016/j.lfs.2021.119248 [DOI] [PubMed] [Google Scholar]
- Ning YY, Shang Y, Huang HD, et al. , 2013. Attenuation of cigarette smoke-induced airway mucus production by hydrogen-rich saline in rats. PLoS ONE, 8(12): e83429. 10.1371/journal.pone.0083429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, et al. , 2007. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med, 13(6): 688-694. 10.1038/nm1577 [DOI] [PubMed] [Google Scholar]
- Ohta S, 2012. Molecular hydrogen is a novel antioxidant to efficiently reduce oxidative stress with potential for the improvement of mitochondrial diseases. Biochim Biophys Acta Gener Subj, 1820(5): 586-594. 10.1016/j.bbagen.2011.05.006 [DOI] [PubMed] [Google Scholar]
- Ohta S, 2014. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol Ther, 144(1): 1-11. 10.1016/j.pharmthera.2014.04.006 [DOI] [PubMed] [Google Scholar]
- Ohta S, 2015. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol, 555: 289-317. 10.1016/BS.MIE.2014.11.038 [DOI] [PubMed] [Google Scholar]
- Osborn-Heaford HL, Murthy S, Gu LL, et al. , 2015. Targeting the isoprenoid pathway to abrogate progression of pulmonary fibrosis. Free Radic Biol Med, 86: 47-56. 10.1016/j.freeradbiomed.2015.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I, Kim M, Choe K, et al. , 2019. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur Respir J, 53(3): 1800786. 10.1183/13993003.00786-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin R, Courboulin A, Meloche J, et al. , 2011. Signal transducers and activators of transcription-3/Pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation, 123(11): 1205-1215. 10.1161/CIRCULATIONAHA.110.963314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vizcaino F, Cogolludo A, Moreno L, 2010. Reactive oxygen species signaling in pulmonary vascular smooth muscle. Respir Phys Neurobiol, 174(3): 212-220. 10.1016/j.resp.2010.08.009 [DOI] [PubMed] [Google Scholar]
- Pinamonti S, Muzzoli M, Chicca MC, et al. , 1996. Xanthine oxidase activity in bronchoalveolar lavage fluid from patients with chronic obstructive pulmonary disease. Free Radic Biol Med, 21(2): 147-155. 10.1016/0891-5849(96)00030-5 [DOI] [PubMed] [Google Scholar]
- Pistritto G, Trisciuoglio D, Ceci C, et al. , 2016. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging, 8(4): 603-619. 10.18632/aging.100934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteous MK, Diamond JM, Christie JD, 2015. Primary graft dysfunction: lessons learned about the first 72 h after lung transplantation. Curr Opin Organ Trans, 20(5): 506-514. 10.1097/MOT.0000000000000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LC, Wort SJ, Perros F, et al. , 2012. Inflammation in pulmonary arterial hypertension. Chest, 141(1): 210-221. 10.1378/chest.11-0793 [DOI] [PubMed] [Google Scholar]
- Qian LR, Cao F, Cui JG, et al. , 2010. Radioprotective effect of hydrogen in cultured cells and mice. Free Radic Res, 44(3): 275-282. 10.3109/10715760903468758 [DOI] [PubMed] [Google Scholar]
- Qin C, Bian YX, Feng TT, et al. , 2017. Effects of hydrogen on the lung damage of mice at early stage of severe burn. Chin J Burns, 33(11): 682-687 (in Chinese). 10.3760/cma.j.issn.1009-2587.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Qiu XC, Dong KS, Guan JZ, et al. , 2020. Hydrogen attenuates radiation-induced intestinal damage by reducing oxidative stress and inflammatory response. Int Immunopharmacol, 84: 106517. 10.1016/j.intimp.2020.106517 [DOI] [PubMed] [Google Scholar]
- Reuter S, Gupta SC, Chaturvedi MM, et al. , 2010. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med, 49(11): 1603-1616. 10.1016/j.freeradbiomed.2010.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardolo FLM, di Stefano A, Sabatini F, et al. , 2006. Reactive nitrogen species in the respiratory tract. Eur J Pharmacol, 533(1-3): 240-252. 10.1016/j.ejphar.2005.12.057 [DOI] [PubMed] [Google Scholar]
- Saito M, Chen-Yoshikawa TF, Takahashi M, et al. , 2020. Protective effects of a hydrogen-rich solution during cold ischemia in rat lung transplantation. J Thorac Cardiovasc Surg, 159(5): 2110-2118. 10.1016/j.jtcvs.2019.09.175 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kurokawa R, Hirano SI, et al. , 2019. Hydrogen indirectly suppresses increases in hydrogen peroxide in cytoplasmic hydroxyl radical-induced cells and suppresses cellular senescence. Int J Mol Sci, 20(2): 456. 10.3390/ijms20020456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittler H, 2016. Contraction of endothelial cells: 40 years of research, but the debate still lives. Histochem Cell Biol, 146(6): 651-656. 10.1007/s00418-016-1501-0 [DOI] [PubMed] [Google Scholar]
- Shalini S, Dorstyn L, Dawar S, et al. , 2015. Old, new and emerging functions of caspases. Cell Death Differ, 22(4): 526-539. 10.1038/cdd.2014.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao AW, Wu HJ, Hong Y, et al. , 2016. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-κB pathway and NLRP3 inflammasome. Mol Neurobiol, 53(5): 3462-3476. 10.1007/s12035-015-9242-y [DOI] [PubMed] [Google Scholar]
- Shi JJ, Gao WQ, Shao F, 2017. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci, 42(4): 245-254. 10.1016/j.tibs.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Shi Q, Chen C, Deng WH, et al. , 2016. Hydrogen-rich saline attenuates acute hepatic injury in acute necrotizing pancreatitis by inhibiting inflammation and apoptosis, involving JNK and p38 mitogen-activated protein kinase-dependent reactive oxygen species. Pancreas, 45(10): 1424-1431. 10.1097/MPA.0000000000000678 [DOI] [PubMed] [Google Scholar]
- Shinbo T, Kokubo K, Sato Y, et al. , 2013. Breathing nitric oxide plus hydrogen gas reduces ischemia-reperfusion injury and nitrotyrosine production in murine heart. Am J Phys Heart Circ Phys, 305(4): H542-H550. 10.1152/AJPHEART.00844.2012 [DOI] [PubMed] [Google Scholar]
- Simonneau G, Montani D, Celermajer DS, et al. , 2019. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J, 53(1): 1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M, Deutschman CS, Seymour CW, et al. , 2016. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA, 315(8): 801-810. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Letai A, Sarosiek K, 2019. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol, 20(3): 175-193. 10.1038/s41580-018-0089-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YL, Ma TH, Matthay MA, et al. , 2000. Role of aquaporin-4 in airspace-to-capillary water permeability in intact mouse lung measured by a novel gravimetric method. J Gen Phys, 115(1): 17-27. 10.1085/jgp.115.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YL, Jayaraman S, Yang BX, et al. , 2001. Role of aquaporin water channels in airway fluid transport, humidification, and surface liquid hydration. J Gen Phys, 117(6): 573-582. 10.1085/jgp.117.6.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump CS, Clark SE, Sowers JR, 2005. Oxidative stress in insulin-resistant conditions: cardiovascular implications. Treat Endocrinol, 4(6): 343-351. 10.2165/00024677-200504060-00003 [DOI] [PubMed] [Google Scholar]
- Sun Q, Kawamura T, Masutani K, et al. , 2012. Oral intake of hydrogen-rich water inhibits intimal hyperplasia in arterialized vein grafts in rats. Cardiovasc Res, 94(1): 144-153. 10.1093/cvr/cvs024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Han WJ, Hu HJ, et al. , 2017. Hydrogen alleviates hyperoxic acute lung injury related endoplasmic reticulum stress in rats through upregulation of SIRT1. Free Radic Res, 51(6): 622-632. 10.1080/10715762.2017.1351027 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Sato T, Sugimoto M, et al. , 2017. Hydrogen-rich pure water prevents cigarette smoke-induced pulmonary emphysema in SMP30 knockout mice. Biochem Biophys Res Commun, 492(1): 74-81. 10.1016/j.bbrc.2017.08.035 [DOI] [PubMed] [Google Scholar]
- Tang DL, Kang R, Coyne CB, et al. , 2012. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev, 249(1): 158-175. 10.1111/j.1600-065X.2012.01146.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YJ, Liu JJ, Zhang DY, et al. , 2020. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol, 11: 1708. 10.3389/fimmu.2020.01708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao BD, Liu LD, Wang N, et al. , 2016. Effects of hydrogen-rich saline on aquaporin 1, 5 in septic rat lungs. J Surg Res, 202(2): 291-298. 10.1016/j.jss.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Terasaki Y, Terasaki M, Kanazawa S, et al. , 2019a. Effect of H2 treatment in a mouse model of rheumatoid arthritis-associated interstitial lung disease. J Cell Mol Med, 23(10): 7043-7053. 10.1111/JCMM.14603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki Y, Suzuki T, Tonaki K, et al. , 2019b. Molecular hydrogen attenuates gefitinib-induced exacerbation of naphthalene-evoked acute lung injury through a reduction in oxidative stress and inflammation. Lab Invest, 99(6): 793-806. 10.1038/s41374-019-0187-z [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RYJ, et al. , 2009. Epithelial-mesenchymal transitions in development and disease. Cell, 139(5): 871-890. 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Rodríguez-Iturbe B, 2006. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol, 2(10): 582-593. 10.1038/ncpneph0283 [DOI] [PubMed] [Google Scholar]
- Wang C, Li J, Liu Q, et al. , 2011. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-κB activation in a rat model of amyloid-beta-induced Alzheimer's disease. Neurosci Lett, 491(2): 127-132. 10.1016/j.neulet.2011.01.022 [DOI] [PubMed] [Google Scholar]
- Wang DC, Wang LF, Zhang Y, et al. , 2018. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother, 104: 788-797. 10.1016/j.biopha.2018.05.055 [DOI] [PubMed] [Google Scholar]
- Wang ST, Bao C, He Y, et al. , 2020. Hydrogen gas (XEN) inhalation ameliorates airway inflammation in asthma and COPD patients. QJM Int J Med, 113(12): 870-875. 10.1093/qjmed/hcaa164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jing L, Zhao XM, et al. , 2011. Protective effects of hydrogen-rich saline on monocrotaline-induced pulmonary hypertension in a rat model. Respir Res, 12: 26. 10.1186/1465-9921-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang JH, Bo JS, et al. , 2019. Hydrogen-rich saline ameliorated LPS-induced acute lung injury via autophagy inhibition through the ROS/AMPK/mTOR pathway in mice. Exp Biol Med, 244(9): 721-727. 10.1177/1535370219847941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Wang L, Hu TP, et al. , 2020. Hydrogen improves cell viability partly through inhibition of autophagy and activation of PI3K/Akt/GSK3β signal pathway in a microvascular endothelial cell model of traumatic brain injury. Neurol Res, 42(6): 487-496. 10.1080/01616412.2020.1747717 [DOI] [PubMed] [Google Scholar]
- Westphal D, Kluck RM, Dewson G, 2014. Building blocks of the apoptotic pore: how Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ, 21(2): 196-205. 10.1038/cdd.2013.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Liang ML, Dang HX, et al. , 2018. Hydrogen protects against hyperoxia-induced apoptosis in type II alveolar epithelial cells via activation of PI3K/Akt/Foxo3a signaling pathway. Biochem Biophys Res Commun, 495(2): 1620-1627. 10.1016/j.bbrc.2017.11.193 [DOI] [PubMed] [Google Scholar]
- Wu JQ, Kosten TR, Zhang XY, 2013. Free radicals, antioxidant defense systems, and schizophrenia. Prog Neuro-Psychopharmacol Biol Psych, 46: 200-206. 10.1016/j.pnpbp.2013.02.015 [DOI] [PubMed] [Google Scholar]
- Wu MY, Yiang GT, Liao WT, et al. , 2018. Current mechanistic concepts in ischemia and reperfusion injury. Cell Phys Biochem, 46(4): 1650-1667. 10.1159/000489241 [DOI] [PubMed] [Google Scholar]
- Xiao M, Zhu T, Wang T, et al. , 2013. Hydrogen-rich saline reduces airway remodeling via inactivation of NF-κB in a murine model of asthma. Eur Rev Med Pharmacol Sci, 17(8): 1033-1043. [PubMed] [Google Scholar]
- Xie KL, Yu YH, Pei YP, et al. , 2010. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock, 34(1): 90-97. 10.1097/SHK.0b013e3181cdc4ae [DOI] [PubMed] [Google Scholar]
- Xie KL, Yu YH, Huang Y, et al. , 2012. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock, 37(5): 548-555. 10.1097/SHK.0b013e31824ddc81 [DOI] [PubMed] [Google Scholar]
- Xie KL, Zhang Y, Wang YQ, et al. , 2020. Hydrogen attenuates sepsis-associated encephalopathy by NRF2 mediated NLRP3 pathway inactivation. Inflamm Res, 69(7): 697-710. 10.1007/s00011-020-01347-9 [DOI] [PubMed] [Google Scholar]
- Xu FF, Yu SQ, Qin ML, et al. , 2018. Hydrogen-rich saline ameliorates allergic rhinitis by reversing the imbalance of Th1/Th2 and up-regulation of CD4+CD25+Foxp3+regulatory T Cells, interleukin-10, and membrane-bound transforming growth factor-β in guinea pigs. Inflammation, 41(1): 81-92. 10.1007/s10753-017-0666-6 [DOI] [PubMed] [Google Scholar]
- Xu Z, Shi L, Wang YJ, et al. , 2020. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med, 8(4): 420-422. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan MY, Yu Y, Mao X, et al. , 2019. Hydrogen gas inhalation attenuates sepsis-induced liver injury in a FUNDC1-dependent manner. Int Immunopharmacol, 71: 61-67. 10.1016/j.intimp.2019.03.021 [DOI] [PubMed] [Google Scholar]
- Yan WM, Zhang L, Chen T, et al. , 2017. Effects of hydrogen-rich saline on endotoxin-induced uveitis. Med Gas Res, 7(1): 9-18. 10.4103/2045-9912.202905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FX, Yue RM, Luo XX, et al. , 2020. Hydrogen: a potential new adjuvant therapy for COVID-19 patients. Front Pharmacol, 11: 543718. 10.3389/fphar.2020.543718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Yu Y, Xie KL, et al. , 2019. Effects of hydrogen on lung injury in wild-type and Nrf2 gene knockout mice: relationship with Nrf2/HO-1/HMGB1 pathway. Chin Crit Care Med, 31(7): 862-866 (in Chinese). 10.3760/cma.j.issn.2095-4352.2019.07.013 [DOI] [PubMed] [Google Scholar]
- Yang T, Wang L, Sun RQ, et al. , 2016. Hydrogen-rich medium ameliorates lipopolysaccharide-induced barrier dysfunction via RhoA-mDia1 signaling in Caco-2 cells. Shock, 45(2): 228-237. 10.1097/SHK.0000000000000503 [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu PY, Bao W, et al. , 2020. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer, 20: 28. 10.1186/s12885-019-6491-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZF, Klionsky DJ, 2010. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol, 22(2): 124-131. 10.1016/j.ceb.2009.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Chen HG, Wu QH, et al. , 2019. Hydrogen-rich saline alleviates inflammation and apoptosis in myocardial I/R injury via PINK-mediated autophagy. Int J Mol Med, 44(3): 1048-1062. 10.3892/IJMM.2019.4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying YG, Xu HZ, Yao M, et al. , 2017. Protective effect of hydrogen-saturated saline on acute lung injury induced by oleic acid in rats. J Orthop Surg Res, 12: 134. 10.1186/s13018-017-0633-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Yang YY, Yang M, et al. , 2019. Hydrogen gas reduces HMGB1 release in lung tissues of septic mice in an Nrf2/HO-1-dependent pathway. Int Immunopharmacol, 69: 11-18. 10.1016/j.intimp.2019.01.022 [DOI] [PubMed] [Google Scholar]
- Yu Y, Feng JC, Lian NQ, et al. , 2020. Hydrogen gas alleviates blood-brain barrier impairment and cognitive dysfunction of septic mice in an Nrf2-dependent pathway. Int Immunopharmacol, 85: 106585. 10.1016/j.intimp.2020.106585 [DOI] [PubMed] [Google Scholar]
- Yue C, Ji C, Zhang H, et al. , 2017. Protective effects of folic acid on PM2.5-induced cardiac developmental toxicity in zebrafish embryos by targeting AhR and Wnt/β-catenin signal pathways. Environ Toxicol, 32(10): 2316-2322. 10.1002/tox.22448 [DOI] [PubMed] [Google Scholar]
- Zha QB, Wei HX, Li CG, et al. , 2016. ATP-induced inflammasome activation and pyroptosis is regulated by AMP-activated protein kinase in macrophages. Front Immunol, 7: 597. 10.3389/FIMMU.2016.00597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GC, Li Z, Meng C, et al. , 2018. The anti-inflammatory effect of hydrogen on lung transplantation model of pulmonary microvascular endothelial cells during cold storage period. Transplantation, 102(8): 1253-1261. 10.1097/TP.0000000000002276 [DOI] [PubMed] [Google Scholar]
- Zhang HQ, Davies KJA, Forman HJ, 2015. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med, 88: 314-336. 10.1016/j.freeradbiomed.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Li ZH, Gao DW, et al. , 2020. Hydrogen extends Caenorhabditis elegans longevity by reducing reactive oxygen species. PLoS ONE, 15: e0231972. 10.1371/JOURNAL.PONE.0231972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Deng CW, Zhang XX, et al. , 2018. Inhalation of hydrogen gas attenuates airway inflammation and oxidative stress in allergic asthmatic mice. Asthma Res Pract, 4: 3. 10.1186/s40733-018-0040-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WB, Huang C, Sun AJ, et al. , 2018. Hydrogen alleviates cellular senescence via regulation of ROS/p53/p21 pathway in bone marrow-derived mesenchymal stem cells in vivo. Biomed Pharmacother, 106: 1126-1134. 10.1016/j.biopha.2018.07.020 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu YM, Zhang J, 2015. Saturated hydrogen saline attenuates endotoxin-induced lung dysfunction. J Surg Res, 198(1): 41-49. 10.1016/j.jss.2015.04.055 [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Liu YJ, Mao YF, et al. , 2015. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-β1 signaling. Clin Nutr, 34(4): 752-760. 10.1016/j.clnu.2014.08.014 [DOI] [PubMed] [Google Scholar]
- Zhao PH, Jin ZK, Chen Q, et al. , 2018. Local generation of hydrogen for enhanced photothermal therapy. Nat Commun, 9: 4241. 10.1038/s41467-018-06630-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R, Wang MH, Chen Y, et al. , 2019. Hydrogen-rich saline attenuates acute lung injury induced by limb ischemia/reperfusion via down-regulating chemerin and NLRP3 in rats. Shock, 52(1): 134-141. 10.1097/SHK.0000000000001194 [DOI] [PubMed] [Google Scholar]