Abstract
Neurofibromatosis type 1 (NF-1) is associated with fatal vascular complications. A 40-year-old woman with NF-1 who had previously undergone left iliac artery ligation and femorofemoral bypass grafting for internal iliac artery (IIA) aneurysm rupture was transported to our hospital for the treatment of a newly developed IIA aneurysm. Although endovascular therapy was difficult owing to the previous surgery, we successfully performed embolization of the aneurysm and its feeding vessels via direct percutaneous puncture under ultrasound guidance. Aneurysm enhancement had completely disappeared at 2 months postoperatively. We have reported a novel approach of direct percutaneous puncture for IIA aneurysm embolization in a patient with NF-1.
Keywords: Endovascular treatment, Neurofibromatosis type 1, Pelvic bleeding
In neurofibromatosis type 1 (NF-1; von Recklinghausen disease), vascular complications resulting from vascular fragility, such as spontaneous arterial bleeding, aneurysm formation, and arteriovenous fistulas, are well known.1, 2, 3, 4 Open surgical repair will not always be the best approach for the treatment of this condition, and endovascular therapy (EVT) could be a better approach considering the vascular fragility owing to the development of endovascular devices and techniques. In the present report, we have described a case of NF-1–related spontaneous internal iliac artery (IIA) aneurysm formation treated by embolization via direct percutaneous puncture. The patient provided written informed consent for the report of her case and related imaging studies.
Case report
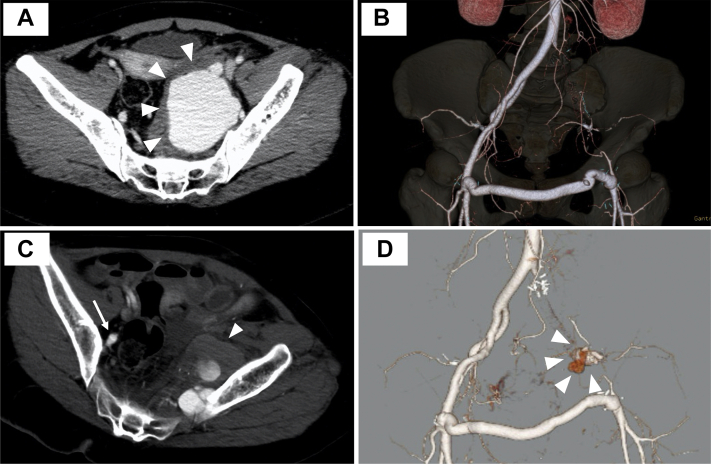
A 40-year-old woman with NF-1 was brought to our hospital because of sudden left buttock pain. She had experienced spontaneous left IIA aneurysm rupture with an arteriovenous fistula 7 years previously (Fig 1, A). She had been treated using open surgical repair, with ligation of her common iliac artery, external iliac artery, and IIA and performance of femorofemoral bypass (Fig 1, B). She had experienced massive bleeding during this surgery, requiring two-stage abdominal gauze packing. She had been taking aspirin since that surgery. Contrast-enhanced computed tomography (CT) showed a newly developed left IIA aneurysm with a diameter of 50 mm (Fig 1, C and D). The aneurysm appeared to have multiple feeding arteries. Considering the difficulty of reaching the aneurysm for EVT through the antegrade approach and the presence of feeding arteries associated with the previous surgery, conservative treatment with aspirin discontinuation was selected, in accordance with her stable vital signs and normal hemoglobin level (13.9 g/dL).
Fig 1.
Computed tomography findings at the previous operation and the present admission. A, Computed tomography scan showing an internal iliac artery (IIA) aneurysm with a diameter of 70 mm in the left pelvis (arrowheads) due to spontaneous IIA rupture and arterial venous formation. B, Ligation of the left external iliac artery and IIA and femorofemoral bypass had been performed 7 years previously. C, The aneurysm had developed in the left IIA and its branches with a diameter of 50 mm in the left pelvic cavity (arrowhead) at admission. The aneurysm was located at the same level as the right IIA (arrow). D, Three-dimensional volume rendering of computed tomography data.
However, the diameter of the aneurysm had increased to 55 mm within 3 days, and reintervention was required. To overcome the approach limitations, surgical access through the left inferior gluteal artery (IGA) was considered for EVT, as previously described by Magishi et al.5 The patient was placed in the right lateral position under local anesthesia and sedation. After making an ∼8-cm incision in the left buttock, the gluteus maximus muscle was split, and the distal branch of the IGA was identified (Fig 2, A). A 4F sheath was inserted into the IGA, and the aneurysm was visualized using angiography (Fig 2, B). Although we could not catheterize the aneurysm, embolization coils were placed near the aneurysm (Fig 2, C). The procedure was concluded after embolization of a feeding artery and the aneurysm could no longer be visualized. However, postoperative CT showed continued enlargement of the aneurysm regardless of embolization of the feeding artery, indicating that the procedure had been insufficient to thrombose the aneurysm (Fig 2, D). This finding suggested that the previous procedure had insufficiently controlled the aneurysmal perfusion owing to the presence of multiple feeding vessels. Thus, we decided to approach the aneurysm itself to access all the feeding arteries. She was placed in the right lateral position under local anesthesia and sedation. The aneurysm was located between the left ilium and sacral vertebra and was confirmed by applying an ultrasound echo probe to her buttocks focused at a depth of ∼3 cm. Once reverse flow from a puncture needle was confirmed, a 4F sheath was inserted into the aneurysm directly under ultrasound guidance (Fig 3, A). We initially identified the first feeding vessel via angiography of the aneurysm (Fig 3, B). Next, we engaged the microcatheter and placed microcoils (Fig 3, C). We also accessed the second feeding artery and performed embolization using the coils (Fig 3, D). The aneurysm itself was embolized, and no flow was detected on ultrasound. CT showed decreased enhancement of the aneurysm 5 days after the procedure (Fig 3, E). Complete thrombosis of the aneurysm was achieved, with a decrease in size seen 2 months after the procedure (Fig 3, F).
Fig 2.
Findings from the first operation. We performed endovascular treatment by the retrograde approach through the inferior gluteal artery (IGA). A, The distal branch of the left IGA (arrowheads) was identified. B, Angiography of the IGA showed the bleeding point (dotted circle). C, Embolization coils (dotted circle) were placed near the bleeding point. D, Postoperative computed tomography scan showing continued enlargement of the bleeding cavity (arrowheads).
Fig 3.
Findings from the second operation and postoperative computed tomography. We attempted to perform embolization of the feeding vessels in the bleeding cavity using a direct percutaneous puncture approach in the second operation. A, The operation was performed with the patient in the right lateral position, and a 4F sheath was advanced to the bleeding cavity under ultrasound guidance. B, Angiography of the bleeding cavity showed the first feeding vessel (dotted circle). C, Embolization was performed for the first feeding vessel using microcoils (arrowheads). D, The second feeding vessel was found and embolized using coils (arrowheads). E, Computed tomography scan showing decreased enhancement in the cavity at 5 days postoperatively. F, Computed tomography scan showing complete thrombosis of the aneurysm at 2 months postoperatively.
Discussion
We used a novel approach for the embolization of an IIA aneurysm by direct percutaneous puncture. NF-1 is an autosomal dominant disorder linked to chromosome 17.6 Its incidence is ∼1 in 3000 persons, and it can affect any tissue of the body, including connective tissue, nerve tissue, and vascular tissue.7 Vascular lesions are less common causes of comorbidity in those with NF-1, with a reported prevalence of 2% to 3.6%.6,8 However, spontaneous arterial rupture and aneurysm formation have been previously reported as fatal vascular complications, with early endovascular or surgical hemostasis recommended. With open surgical repair, in some cases, it can be difficult to stop bleeding from the artery and aneurysm owing to vascular fragility.1 In the present case, the patient had also experienced massive bleeding during the first surgery performed 7 years previously (Fig 1, A and B). For EVT in a patient with NF-1, attention to guidewire manipulation is required, because a previous case report stated that injury to the left colic artery during stent placement can cause subsequent aneurysm rupture owing to vascular fragility.9
In the present case, the feeding vessels were thought to be the distal branches of the IIA. However, EVT using an antegrade approach to the IIA was difficult because of the previous surgery. Open surgery should be avoided because of postoperative adhesion formation and difficulty controlling bleeding owing to the vascular fragility. For EVT, several approaches are possible to reach the feeding vessels and aneurysm, including a surgical approach involving the IGA for EVT in the IIA system and a direct puncture technique to access the aneurysm.5 Although we initially attempted the former approach, the retrograde approach through the IGA was not possible because a catheter could not be advanced to the aneurysm. Thus, endovascular intervention via the retrograde approach through the IGA was not attempted. However, approaching through the profunda femoral artery might have been a possible option, according to a retrospective review of Fig 1, D. Nevertheless, the direct percutaneous puncture approach was effective for successfully reaching the feeding arteries of the aneurysm. The advantages of this approach are as follows: (1) the feeding vessels of the aneurysm can be almost certainly identified; (2) hemostasis materials can be easily placed in the aneurysm and its feeding vessels; and (3) open surgical repair can be avoided. However, its disadvantages include the possibility of bleeding and aneurysm formation at the puncture site if embolization is incomplete and the difficulty of compression at the puncture site, although the application of cyanoacrylate glue on the aneurysm might help resolve this issue. Hemostatic approaches, such as those involving gelatin sponges and n-butyl-2-cyanoacrylate, were available; however, coil embolization of the feeding arteries was successfully performed in our patient. Gelatin sponges and n-butyl-2-cyanoacrylate could be useful in the treatment of patients with selective cannulation difficulty to the target vessels and large diameter aneurysms. This direct puncture technique was previously applied in the treatment of various vascular diseases, such as embolization for a type II endoleak after endovascular abdominal aortic aneurysm repair.10,11 To the best of our knowledge, the direct puncture technique is a novel approach for treating IIA aneurysms. Therefore, this procedure is not the first choice for hemostasis but can be applied in the treatment of other aneurysms for which a transarterial approach for EVT and/or surgical hemostasis might be difficult.
Conclusions
We have reported a novel approach of direct percutaneous puncture for IIA aneurysm embolization associated with vascular fragility in a patient with NF-1. This approach can be useful for cases in which open surgical repair or the usual EVT approaches are difficult to perform.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Aizawa K., Iwashita C., Saito T., Misawa Y. Spontaneous rupture of an intercostal artery in a patient with neurofibromatosis type 1. Interact Cardiovasc Thorac Surg. 2010;10:128–130. doi: 10.1510/icvts.2009.222125. [DOI] [PubMed] [Google Scholar]
- 2.Han K.S., Lee K.M., Kim B.J., Kwun B.D., Choi S.K., Lee S.H. Life-threatening hemothorax caused by spontaneous extracranial vertebral aneurysm rupture in neurofibromatosis type 1. World Neurosurg. 2019;130:157–159. doi: 10.1016/j.wneu.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Ishizu A., Ooka T., Murakami T., Yoshiki T. Rupture of the thyrocervical trunk branch from the subclavian artery in a patient with neurofibromatosis: a case report. Cardiovasc Pathol. 2006;15:153–156. doi: 10.1016/j.carpath.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda W., Taniguchi S., Fukuda I. Endovascular treatment of ruptured intercostal arteriovenous fistulas associated with neurofibromatosis type 1. Ann Vasc Dis. 2012;5:109–112. doi: 10.3400/avd.cr.11.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magishi K., Izumi Y., Tanaka K., Shimizu N., Uchida D. Surgical access of the gluteal artery to embolize a previously excluded, expanding internal iliac artery aneurysm. J Vasc Surg. 2007;45:387–390. doi: 10.1016/j.jvs.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 6.Lin A.E., Birch P.H., Korf B.R., Tenconi R., Niimura M., Poyhonen M., et al. Cardiovascular malformations and other cardiovascular abnormalities in neurofibromatosis 1. Am J Med Genet. 2000;95:108–117. doi: 10.1002/1096-8628(20001113)95:2<108::aid-ajmg4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Oderich G.S., Sullivan T.M., Bower T.C., Gloviczki P., Miller D.V., Babovic-Vuksanovic D., et al. Vascular abnormalities in patients with neurofibromatosis syndrome type 1: clinical spectrum, management, and results. J Vasc Surg. 2007;46:475–484. doi: 10.1016/j.jvs.2007.03.055. [DOI] [PubMed] [Google Scholar]
- 8.Brasfield R.D., Das Gupta T.K. Von Recklinghausen’s disease: a clinicopathological study. Ann Surg. 1972;175:86–104. doi: 10.1097/00000658-197201000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moro K., Kameyama H., Abe K., Tsuchida J., Tajima Y., Ichikawa H., et al. Left colic artery aneurysm rupture after stent placement for abdominal aortic aneurysm associated with neurofibromatosis type 1. Surg Case Rep. 2019;5:12. doi: 10.1186/s40792-019-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zener R., Oreopoulos G., Beecroft R., Rajan D.K., Jaskolka J., Tan K.T. Transabdominal direct sac puncture embolization of type II endoleaks after endovascular abdominal aortic aneurysm repair. J Vasc Interv Radiol. 2018;29:1167–1173. doi: 10.1016/j.jvir.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Fenelli F., Cannavale A., Chisci E., Citone M., Falcone G.M., Michelagnoli S., et al. Direct percutaneous embolization of aneurysm sac: a safe and effective procedure to treat post-EVAR type II endoleaks. Radiol Med. 2021;126:258–263. doi: 10.1007/s11547-020-01247-2. [DOI] [PubMed] [Google Scholar]