Abstract
Flubendiamide (FBD) is the first commercially available phthalic acid diamide that targets ryanodine receptors (RyRs) in insects, which play a major role in lepidoptera control. However, excessive use of FBD can influence the quality of treated products leading to toxic effects on human health. The availability of rapid and convenient methods for evaluating FBD amount in the environment is necessary. Therefore, analytical methods were developed for the determination of residues of FBD and its metabolite desiodo in different food matrices like tomato, cabbage, pigeon pea, apple, chilli and rice. The current review carries forward methods for FBD residues analysis in foods by using several chromatographic techniques including sample preparation steps. The comparison between the different methods employed for quantitative and qualitative analysis of food quality and safety is also discussed. Liquid chromatography (LC) is the predominant analytical method for assessing the quality of foods treated with FBD. Studies related to LC coupled multichannel detector (Ultraviolet (UV), Mass spectrometry (MS)) are also applied to detect pesticide residues. Extraction and clean up steps are essential to obtain reliable results. Moreover, this review reports the allowed limits of residues for the safety of consuming products treated with FBD.
Keywords: Flubendiamide, RyRs, Lepidoptera pest, Chromatographic analysis, Food samples
Graphical abstract
Highlights
-
•
State of the art of flubendiamide as diamide insecticide is presented.
-
•
Significance of extraction and cleanup process used for sample preparation is exhibited.
-
•
Chromatographic methods used in FBD analysis in food samples are discussed.
1. Introduction
Pesticides are substances or mixtures of compounds that are used to manage pests including insects, weeds, mammals, and microbes (Percival and Schroeder, 2017). These chemical substances can come from variety of natural sources such as animals, plants, and bacteria. Approximately, 5.2 billion pounds of pesticides are used worldwide each year to control pests and diseases in many fruit and vegetable crops (Mahmood et al., 2016). Their use is not only limited to agricultural crops, but they are also used in households to control mosquitoes, rats, fleas, ticks and other insect pests (Olszewski et al., 2010). For this reason, pesticides are often found in our food products in addition to their presence in the environment. However, pesticides are among the most dangerous chemicals, with their stability and mobility in the environment (Damalas and Eleftherohorinos, 2011). Thus, the successive use of pesticides in crops can cause intense risks on biodiversity (Tossou et al., 2019).
There are different classes of pesticides organized according to their application, including: i) herbicides, used to control weeds and other plants; ii) fungicides, used to control fungi; and iii) insecticides, which play an important role in the control of insect pests (Özkara et al., 2016).
The majority of pesticides are used as insecticides to control a wide variety of insects. These are classified into different categories, such as cholinesterase inhibitors (organophosphates and carbamates), pyrethroids, neonicotinoids, and ryanoids (Ishaaya, 2012). The latter category of insecticides includes several chemical compounds such as FBD, known as diamide insecticides (Caballero et al., 2013; Sharma et al., 2019; Gill and Garg, 2014). FBD is one of the most widely used insecticides on crops, vegetables, and fruits because of its exceptional pest control effectiveness, extremely high intrinsic potency, remarkable selectivity, minimal ecotoxicity, and low residual levels (Secretariat and Center, 2003; Lehotay et al., 2005). However, due to its widespread use, environmental risks and food safety have become an important concern. Therefore, it is important to develop rapid and practical methods for the determination of FBD residues in food products. However, the detection of FBD residues in foods is quite difficult due to the presence of a large amount of interferences in the matrix and also for the low concentrations of pesticide residues in the food samples. Therefore, extraction and cleaning techniques are needed first to prepare the food matrix. This review presents a general approach on insecticidal diamides considering FBD as an example. Extraction and clean-up methods used in the literature for the preparation of the food matrix treated with FBD were reviewed. Moreover, emphasis was put on the most recent research reports regarding the determination of FBD's residues and it's dissipation in different food matrices.
2. Ryanodine receptor-targeting insecticides
Ryanodine receptor-targeting insecticides (Ryanoids) are a novel class of insecticides (Coronado et al., 1994; Fill and Copello, 2002; Lahm et al., 2005, 2007, 2009; Cordova et al., 2006; Li et al., 2012, 2020; Pawar and Bhilave, 2020). This type of insecticide is naturally extracted from tropical South American plant (Ryania speciosa Vahl) (Rogers et al., 1948; Jefferies et al., 1991; Ruest et al., 2002; Sattelle et al., 2008). Ryanodine receptors (RyRs) are intracellular calcium channels in insects placed in the sarcoplasmic and endoplasmic reticulum in neuromuscular tissues and they are important for the control of calcium ions release. They are activated by calcium influx mediated by voltage gated calcium channels upon depolarization of the cell membrane (Brillantes et al., 1994; Marx et al., 1998; Messutat et al., 2001; Masaki et al., 2006; Shiomi et al., 2010; Wang et al., 2013; Liu et al., 2014; Vemu and Dumka, 2014; Yang et al., 2014). There are three types of ryanodine receptors: RyR1 is primarily expressed in skeletal muscle, RyR2 in myocardium, and RyR3 in the brain (Meissner and El-Hashem, 1992; Ogawa, 1994; Laitinen et al., 2001). The ryanodine shows high binding affinity for a class of ligand-gated calcium channels. This affinity is measured by the equilibrium dissociation constant (KD). This means that as the KD value is lower, the binding affinity of the ligand for its target is higher for the ryanodine receptor and the calcium trigger ligand, showing the high strength of the binding interaction between a ryanodine and a calcium ligand (Ito et al., 1986; Bull et al., 1989; McGrew et al., 1989; Chu et al., 1990; Ellisman et al., 1990; Stein et al., 1992; Chameau et al., 2001; Ebbinghaus-Kintscher et al., 2006; Rizzuto and Pozzan, 2006; Thomas and Williams, 2012; Santulli and R Marks, 2015). Ryanodine (ryanodyl 3-(pyrrole-2-carboxylate)) is a complex bridged diterpene heptol that is divided into two classes: alkaloid type and nonalkaloid type. The nonalkaloids type were isolated from Persea indica (Lauraceae) while the alkaloidal ryanoids (ryanodines and spiganthines) were isolated from Spigelia anthelmia (Logani-aceae) (Jansen et al., 2009). There are many studies pursued on the synthetic derivatives and structure-activity of the Ryania alkaloids (Soloway, 1976; Waterhouse et al., 1984; Ruest et al., 1985; Jefferies et al., 1992; Isman, 1997; Ujváry, 1999; Cabras et al., 2001; Gossauer, 2003; Nauen, 2006; Mao and Henderson, 2007; Akhtar et al., 2008; Sattelle et al., 2008; Dimetry, 2012; Feng et al., 2012; Wagner et al., 2012; Zhao et al., 2012; Grdiša and Gršić, 2013). Recently, two classes of chemicals that target the RyRs of insects have emerged as new insecticides and have attracted considerable attention. The first chemical class is anthranilic diamides and the second is phthalic acid diamides (Pence and Williams, 2010). These diamides are rapidly replacing the major uses of previous classes of insecticides because of their high efficacy against major pests resistant to these earlier chemicals and appear to have low toxicity to human health and the environment (He et al., 2019; Jactel et al., 2019; Li et al., 2020).
2.1. Anthranilic diamides
Anthranilic diamides are very demanding commercial class of insecticides. This class was discovered by the Dupont company during the last decade (Zhou et al., 2019; Boaventura et al., 2020). The most important characteristics of these diamides are their broad spectrum insecticidal efficacy and their environmental and ecological safety (Boaventura et al., 2020). A class of anthranilic diamides was being developed with two representative compounds including chlorantraniliprole and cyantraniliprole (Fig. 1). Chlorantraniliprole was the first commercialized diamide and exhibits an exceptional activity against lepidopteran pests (European Food Safety Authority et al., 2019; Kadala et al., 2019; Zhou et al., 2019; Jouraku et al., 2020; Satpathy et al., 2020). It activates ryanodine receptors by stimulation of calcium release from the sarcoplasmic reticulum of muscle cells causing impaired regulation, paralysis, and ultimately death of target species (Ahlawat et al., 2019; Chen et al., 2019; He et al., 2019; Héma et al., 2019; Jallow et al., 2019; Plata-Rueda et al., 2019; Silva et al., 2019; Passos et al., 2020; Shah and Shad, 2020; Williams et al., 2020). However, the researcher continued to pursue a wide range of polar groups on the anthranilic core with an emphasis on nitrile substitution (Barrania, 2019; Bolzan et al., 2019; Carscallen et al., 2019; Mao et al., 2019; Gao et al., 2019; Meng et al., 2019; O'Neal et al., 2019; Sharma et al., 2019; Sreedhar, 2019; Truong and Pessah, 2019; Jiang et al., 2020). This effort resulted in the discovery of cyantraniliprole, a second product candidate in this chemical class possessing excellent interspectral activity against a range of insect orders, including lepidopteran and hemipteran pests (Hopkinson and Pumpa, 2019; Plata-Rueda et al., 2019; Ran et al., 2019; Zhang et al., 2019). This insecticide product exhibits a very low toxicity to vertebrates and non-target organisms.
Fig. 1.
Chemical structure of clorantraniliprole and cyantraniliprole.
2.2. Phthalic diamides
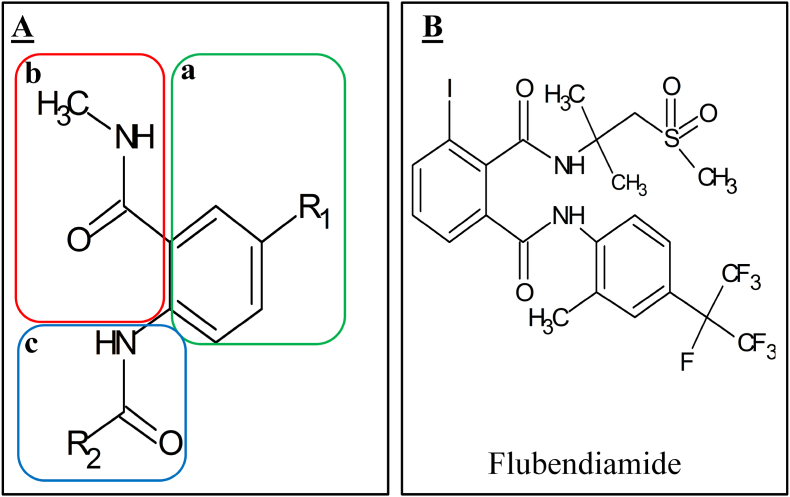
The phthalic diamide shares the same mode of action to anthranilic diamides. The chemical structure of phthalic acid diamides is marked by three parts as shown in Fig. 2A: (a) the phthaloyl moiety that have yielded the important commercial product namely FBD, (b) the aliphatic amide moiety, and (c) the aromatic amide moiety (Boaventura et al., 2020; Gonring et al., 2019; Lin et al., 2020; Zhang et al., 2020; Zuo et al., 2020). The initial leads of this compound had discovered by Nihon nohyaku in 1993 in a pyridine dicarboxamide herbicide and is being co-developed by N. nohyaku and bayer in july 2005 (Bolzan et al., 2019).
Fig. 2.
Chemical structure of (A) phthalic diamide and (B) flubendiamide.
3. Flubendiamide
Flubendiamide or 1, 2-benzenedicarboxamides N0-[1, 1-dimethyl-2-(methyl-sulfonyl) ethyl]-3-iodo-N-{4-[2, 2, 2tetrafluoro-1-(trifluoromethyl) ethyl]-0-tolyl} is a new insecticide belongs to phthalic acid diamides, where its chemical structure reveals several interesting features (Tohnishi et al., 2005). This diamide compound is characterized by three substituents (Fig. 2B): a heptafluoro isopropyl group in the anilide moiety, a sulfonylalkyl group in the aliphatic amide moiety, and an iodine atom at the 3-position of the phthalic acid moiety (Singh Battu et al., 2008; Kato et al., 2009; Paramasivam and Banerjee, 2011). The heptafluoro-isopropyl side chain confers to the compound lipophilic character and is required for the very high insecticidal activity. FBD has also an iodine atom substituent, which are accounts for superior activity by comparison with the chloro analogue. The introduction of this halogen done by Sandmeyer reaction (Fig. 3A) on the corresponding amino phthalic (Li et al., 2006). The two amines are subsequently introduced in a regioselective manner from the phtalanhydride and the isoimide (Fig. 3B). The introduction of heptafluoroisopropyl is fixed via a radical reaction of 2- bromo-heptafluoropropane on the aniline side (Fig. 3C) (Jeanguenat, 2013). FBD is widely used as a strong potent for controlling lepidopterous pests including Helicoverpaspp, Heliothisspp, Spodopteraspp, Plutellaspp, Trichoplusiaspp and Hyrotisspp (Das et al., 2017). It is also very safe for mammalians (Isaacs et al., 2012). In addition, it has recently been approved for use on major crops such as diamondback moth, cabbage whitefly, grapevine caterpillar, corn, cotton, tobacco, seeds and stone nuts, grapes and vegetables (cucurbits, fruiting vegetables and okra vegetables) (LU and LI, 2012).
Fig. 3.
Schematic illustration of the synthesis of flubendiamide.
3.1. Mechanism of action
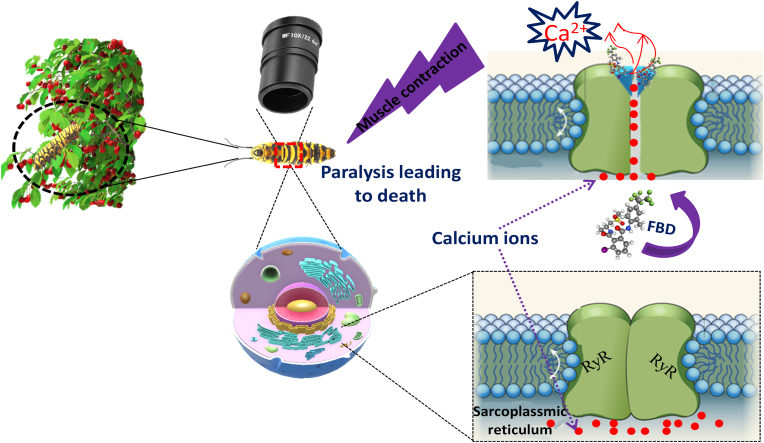
The mode of action of FBD has been detailed by Ebbinghaus-Kintscher and colleagues (Ebbinghaus-Kintscher et al., 2006). They affirm that FBD is a selective activator of the insect ryanodine receptor, inducing ryanodine-sensitive cytosolic Ca2+ transients that were independent of the extracellular Ca2+ concentration (Fig. 4). A study reported by Lummen et al. (Lümmen et al., 2007) on isolated neuronal cells showed that FBD activates the intracellular release of ryanodine-sensitive calcium. Another separate study effected by Masaki et al. (2006) showed that FBD stimulates Ca2+ pump activity by reducing the coupling between RyRs and the pump, resulting a decrease in internal calcium concentration. This specific mode of action of FBD produced several disruptions of muscle function in the target insect with symptoms of poisoning including rapid cessation of feeding, contractile paralysis, and regurgitation resulting in insect death.
Fig. 4.
Mode of action of flubendiamide on lepidoptera pest.
3.2. Acute toxicity
FBD shows low oral, dermal and inhalation toxicity (EFSA, 2013). However, their successive use can lead to the accumulation of its residues on crops at harvest time (Regueiro et al., 2015). The presence of trace amounts of FBD residues and degradation of organic compounds in agricultural crops may result in potential health risks. Although in small quantities, their accumulation in the body can have adverse effects on human health, which should be controlled to ensure “food safety” (Boobis et al., 2008). For this reason, the European Union directives have set maximum residue limits (MRLs) of 0.2 ppm for FBD in food, agricultural products or feed (Tohnishi et al., 2005; Wilkowska and Biziuk, 2011). FBD presents a low acute risk toward birds and mammals, beneficial insects including honey bees and natural lepidoptera predators, and terrestrial plants. Although, the continued use of FBD will result in negative effects due to one degradation product of FBD in water is highly toxic to fish (Tohnishi et al., 2005; Lahm et al., 2009; EFSA, 2015). FBD is less absorbed in rats when administered orally and has a wide distribution with the highest concentrations in target organs such as the liver, thyroid gland, kidney, bloodstream. Numerous studies confirm that FBD does not have genotoxic effects and does not possess mutagenic and carcinogenic activity, selective neurotoxic and immunotoxic action (Ludlow, 2010). Various studies on the degradation of FBD in soil, which generally degrades in two phases, the first one being faster, leading to photolysis, and the second one being quite slow, establishing that the substance is sufficiently stable in soil under aerobic and anaerobic conditions (Ludlow, 2010). FBD records a low risk assessment towards earthworms, soil macro-organisms and soil microorganisms.
4. Sample preparation methods for determination of FBD residues
A variety of pre-treatment and extraction techniques were used for determing FBD residues in food samples. In laboratories, there are no standard methods for extracting pesticides. Furthermore, the extraction procedure follows a common path that includes the release of the desired analyte from the matrices, followed by a cleanup process that refers to a step or series of steps in the analytical procedure in which the majority of the potential interference co-extracts are removed by physical or chemical methods (Fig. 5).
Fig. 5.
Analytical procedure for determining pesticide in food matrices.
4.1. Pre-treatment and extraction methods
The process of extracting pesticides from the sample is a fundamental aspect of the analytical process (Jing and Amirav, 1997). Solid-liquid extraction (SLE) is the initial step in the sample preparation process for most agro-environmental samples such as crops and soil, as well as for animal samples such as eggs and tissues. As described earlier, FBD is highly soluble in water, which paves the way for a wide range of extractants capable of efficiently extracting FBD from solid samples (Ebbinghaus-Kintscher et al., 2006). Acetone, acetonitrile and methanol are mainly used for the extraction of FBD (Table 1). Especially, acetonitrile has an exceptional permeability to the water-soluble component of the sample due to its good miscibility with water. In addition, the solvent minimizes the co-extraction of hydrophobic components such as lipids, pigments and wax; the addition of salts also allows them to be quickly separated from the aqueous phase. It is preferable to shake the samples to extract them, as long as this gives acceptable results for the samples taken and/or the standard reference materials (if available). This practice is acceptable for the extraction of many pesticide residues from many crops, but may be problematic for systemic pesticides present in some pasty foods where agitation does not allow easy penetration into the matrix. Soxhlet extraction has been used since the early 1900s (De Castro and Priego-Capote, 2010). Despite the fact that this technique is time consuming and requires the use of relatively large volumes of solvent, it is cost-effective and robust. Thus, the efficiency of the process is high, which makes it still useful today (Brits et al., 2016). Increasingly, new extraction techniques are being sought, with shorter extraction time and minimal use of solvents including ultra-turax macerators and sonication. These are faster and more efficient techniques for extracting organic analytes from solids or semi-solids.
Table 1.
Clean up preparation in the analysis of flubendiamide residues in foods.
| Aghris et al. | |||||
|---|---|---|---|---|---|
| Matrix | Clean-up method | Sample preparation | Recovery (%) | RSD (%) | Ref |
|
Pigeon/Pea Cabbage/Tomato |
D-SPE |
Extraction: 10 mL acetonitrile, 50 mL polypropylene, 1.0 g NaCl, and 4.0 g MgSO4 Clean-up: 10 mg of PSA + 150 mg of MgSO4 |
85–99 | – | (Paramasivam and Banerjee, 2011) |
| Cabbage | LLP |
Extraction:1.5 g sodium chloride, 6 g anhydrous magnesium sulfate, 30 mL of acetonitrile. Clean-up: MgSO4 (150 mg), PSA (25 mg), C18 (25 mg). |
72.4–119 | 15 | Chen et al. (2014) |
| Cabbage | D-SPE |
Extraction: 30 mL acetonitrile, 1.5 g sodium chloride, 6 g anhydrous magnesium sulfate Clean-up: 150 mg MgSO4, 25 mg PSA |
80.7–99.4 | 4.90 | Sharma et al. (2014) |
| Cucumber | D-SPE |
Extraction: 30 mL acetonitrile – 50 mL Teflon -3 g sodium chloride, 9 g anhydrous sodium sulfate (remove moisture). Clean-up: 12 mL acetonitrile +400 mg PSA +1.15 g anhydrous magnesium sulfate. |
91–101 | 2.77 | Sahoo et al. (2009) |
| Cabbage, Cauliflower, Brinjal/Chilli | D-SPE |
Extraction: 15 mL acetonitrile, 3 g sodium chloride, 9 g anhydrous sodium sulfate Clean-up: 40 mg of PSA + 140 mg of MgSO4 |
90.2–97.9 | 1.05–5.26 | Mukherjee et al. (2012) |
| Chili | SPE |
Extraction: 100 mL acetonitrile, 50 mL chloroform. Clean-up: 500 mg activated charcoal |
89–95 | 6 | (Gopal and Mishra, 2008) |
| Tomato | SPE |
Extraction: 100 mL acetonitrile, 50 mL chloroform. Clean-up: 500 mg activated charcoal |
84.4–96.4 | 2.30 | Sharma and Parihar (2013) |
| Brinjal | SPE |
Extraction: 100 mL acetonitrile, 50 mL chloroform. Clean-up: 500 mg activated charcoal |
89–95.8 | 6 | (Chen et al., 2014) |
| Chilli | SPE |
Extraction: 100 mL acetonitrile, 50 mL chloroform. Clean-up: 500 mg activated charcoal |
89–101 | – | Takkar et al. (2012) |
| Jatropha Plant Leaves | AC |
Extraction:100 mL acetonitrile + 0.002 M hydrochloric acid Clean-up: 10 g of alumina +50 mL of 9:1 hexane: ethyl acetate |
– | – | Sharma et al. (2011) |
| Melon | LLP |
Extraction: acetone (100 mL) dichloromethane (100 mL) Clean-up: n-hexane (6 mL) + acetonitrile (8 mL) |
92–103.06 | 1.7–3.4 | Kabir et al. (2018) |
| Rice | LLP |
Extraction:1.5 g of sodium chloride, 6 g of anhydrous magnesium sulfate Clean-up: MgSO4 (150 mg), PSA (25 mg), and C18 (25 mg) |
92–101 | – | (Wu et al., 2014) |
| Cabbage | SPE |
Extraction:1.5 g of sodium chloride, 6 g of anhydrous magnesium sulfate Clean-up: MgSO4 (150 mg), PSA (25 mg), and C18 (25 mg) |
92–99.8 | – | Chen et al. (2015) |
D-SPE: Dispersive solid phase extraction, PSA: Primary secondaryamine, GPC: Gel permeation chromatography, SPE: Solid phase extraction, LLP: Liquid Liquid partitioning, AC: Adsorption chromatography.
Singh Battu et al. (Singh Battu et al., 2008) analyzed the residues of FBD in a number of crops included cabbage, tomato, pigeonpea and chilli based on extraction with high-speed homogenization using LC equipped with diode array detector (DAD). In this study, they evaluated different extractants like dichloromethane, acetone, acetate, ethyl acetate hexane and acetonitrile. The results showed that acetone and dichloromethane had low solvating capacity and very low recoveries, less than 20%. Similarly, with ethyl acetate and hexane, the recovery percentages were very low. However, acetonitrile was selected as the optimal extractant because the matrix interference peaks were smaller and good recoveries were achieved (85%–94%). Because acetonitrile is convenient for application to samples with high sugar content and because it evaporates easily, it has also been selected as an extractant for FBD residues. Tian et al. developed a new method for simultaneous determination of residues of FBD in edible mushrooms (Tian et al., 2020). The samples were prepared using the QuEChERS methodology. This methodology was chosen because it allows the simultaneous extraction of polar and non-polar compounds. The extraction was performed with the use of 10 mL of acetonitrile; the mixture was then shaken for 10 min. Next, 1 g of NaCl and 4 g of anhydrous MgSO4 were added and the final extract were shaken again for 5 min. This method was successful in separating and extracting the sample, and also had satisfactory recoveries which ranged from 73.5 to 110.2%. In addition to the extractants described above, MeOH has been used for the extraction of FBD (Diandian and Wenzhu, 2012; Chen et al., 2014; Ma et al., 2015; Wang et al., 2018). Chen et al. (2014) used MeOH to extract FBD in cabbage. Wang et al. (2021) used MeOH–water (90:10) containing 0.1% formic acid to extract six amide pesticides including cyantraniliprole, mandipropamid, boscalid, fluopicolide, thifluzamide and flubendiamide, in vegetables and fruits. As shown in Table 1, in a few cases, MeOH is used as an extractant for FBD. However, instead of concentrating the sample extract under reduced pressure, adjusting the concentration of MeOH with water allows a smooth transition to the next clean-up process of the sample extract. Therefore, MeOH is also regarded as an effective extractant for FBD.
4.2. Clean-up approaches
Clean up is an essential step to clear away co-extractives interfering substances from the matrice (Ballesteros and Ramos-Martos, 2010). Therefore, following the extraction procedure, clean-up of the sample extract is positioned as the next important step (Mekonen et al., 2014). For most samples, it is critical to clean up the sample extracts using an appropriate procedure and to reduce matrix interference in chromatographic analysis to the greatest extent possible (Schenck and Hobbs, 2004). To eliminate matrix interference before the use of highly selective and sensitive analytical equipment such as liquid chromatography tandem mass spectrometry (LC–MS/MS), a time-consuming clean-up process was required. Apparently, column chromatography and liquid-liquid extraction (LLE) were applied to clean up the sample extracts. The most extensively utilized clean-up method as a replacement to the above-mentioned is solide phase extraction (SPE). Among the many adsorbents used in SPE are normal phase adsorbents such as silica gel and Florisil, reverse phase adsorbents such as C18 and C8, ion exchange adsorbents and GCB. In SPE, a sample extract dissolved in a tiny amount of organic solvent is placed into a cartridge that has been pre-equilibrated with an appropriate amount of organic solvent or water. Extracted pesticides are eluted selectively after the matrix components are washed away while target pesticides are kept on the adsorbent. SPE is a clean-up procedure that outperforms standard column chromatography in terms of the amount of organic solvent used, speed of operation, and simplicity. As expected, SPE has been shown to clean up sample extracts in residue analysis procedures for FBD. Several adsorbents have been applied to clean-up FBD in sample extracts by SPE such as primary secondary amine (PSA) (Paramasivam and Banerjee, 2011; Mukherjee et al., 2012; Chen et al., 2015; Słowik-Borowiec and Szpyrka, 2018; Sharma et al., 2019; Park et al., 2021; Reddy et al., 2021; Wang et al., 2021), activated charcoal (Sahoo et al., 2009; Sharma et al., 2018; Takkar et al., 2012; Sharma and Parihar, 2013), C18 (Abbas et al., 2017; Ares et al., 2017; Kabir et al., 2018; Kralj et al., 2018; Sherma and Rabel, 2018; Huh et al., 2019; Li et al., 2020; Sharma et al., 2019; Wang et al., 2021), silica gel (Kabir et al., 2018; Sherma and Rabel, 2018; Ma et al., 2021; Souza et al., 2017), Florsil (Hwang et al., 2018; Malhat et al., 2018; Słowik-Borowiec and Szpyrka, 2018;; Lee et al., 2019; Ruiz et al., 2020; Kim et al., 2021), graphitized carbon black (GCB) (Lee et al., 2018; Słowik-Borowiec and Szpyrka, 2018; Wang et al., 2021). And hydrophilic-lipophilic balanced (HLB) (Ballesteros and Ramos-Martos, 2010; Abbas et al., 2017; Casado et al., 2018; Hou et al., 2019; Lu et al., 2019; Li et al., 2020; Jiafeng et al., 2021; Park et al., 2021). According to reports, HLB is effective for cleaning sample extracts containing pesticides with a wide range of physicochemical properties, ranging from hydrophilic pesticides like FBD to hydrophobic pesticides. HLB has been applied as a clean-up method of samples of animal origin (Li et al., 2020; Jiafeng et al., 2021) and in food samples (Hou et al., 2019; Ballesteros and Ramos-Martos, 2010). In d-SPE, various adsorbents were utilized in the QuEChERS procedure based on the properties of the samples. In the original QuEChERS (Sharma et al., 2019), the mixture of MeCN extract (12 mL), PSA (400 mg), and anhydrous MgSO4 (115 mg) was stirred by hand or vortex. After centrifugation of the mixture, the supernatant was collected to complete the cleanup. The method has favorable accuracy with RSD values of 1.05–5.26%. Furthermore, combining different adsorbents can lead to larger clean-up effect. In fact, d-SPE has been extensively used to clean up extracts of vegetables, fruits, cereals, and others (see Table 1). As a conclusion, d-SPE is a tailor clean-up method that may be used by arbitrarily combining adsorbents based on matrix component characteristics in samples. Lipid-rich foods like olives and avocados are regarded as a difficult sample matrix for developing a pesticide residue analytical method. Furthermore, contamination of the ion source with lipid could result in a reduction of analytical sensitivity due to ion suppression. Because of these reasons, lipid removal is essential. To remove lipids and proteins in a sample extract, freezing this latter can be done to precipitate these components (Hildmann et al., 2015; Bernal et al., 2019). Nguyen Huu Vinh et al. (Vinh et al., 2010) applied a frozen low temperature clean-up to MeCN extracts of 262 pesticides including FBD in milk, butter and peanut samples. This method is the simplest approach to remove lipids from a sample extract. However, the procedure is time consuming and does not completely eliminate lipids. Afterward, clean-up processing is frequently required. Gel permeation chromatography (GPC) also aids in the separation of low-molecular-mass chemicals like insecticides from higher-molecular-mass matrix components like lipids. Hildmann et al. (2015) applied a small-scale GPC that halves the consumption of extractant (mobile phase) to the removal of lipids in a multi-class residue analytical method for 78 pesticides including FBD in egg samples. Although GPC has been effective at removing high molecular mass lipids, it has been difficult to remove low molecular mass lipids. For that reason, the sample extract was finally cleaned up with C18 SPE.
5. Analytical techniques for FBD detection in food matrices
Consumption of fruits and vegetables is considered part of a regular, balanced diet and a healthy, active lifestyle. In order to achieve good quality and better yields, insecticides are used in the cultivation of fruits and vegetables. Apart from their benefits, insecticide residues left on fruits and vegetables can be very harmful when consumed by humans. To this end, several analytical techniques such as gas chromatography (GC), LC-MS/MS, and high performance liquid chromatography (HPLC) coupled with detectors such as nitrogen and phosphorus detector, photometric flame detector, diode array detector, fluorescence detector and MS detector to determine pesticide residues in food matrices. Table 2 summarizes various methods for determining FBD in food matrices.
Table 2.
Analytical techniques for determining flubendiamide in food matrices.
| Aghris et al. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Matrix | Sample treatment (time) | Reagents (g, mL) | Recoveries | Baseline separation | LOQs (μg/Kg) | System (SP, time) | Ref. | ||
| Vegetables | SE + clean-up + EV (>12 h) | 0.5 g, > 900 mL | 85–99% | Yes | 10 | LC-PDA (C18, 15 min) | (Singh Battu et al., 2008) | ||
| Capsicum fruit | QuEChERS (<30 min) | 8.1 g, 15 mL of ACN | 96–100% | Yes | 50 | LC-PDA; LC-MS/MS (C18, 20 min) | Buddidathi et al. (2016) | ||
| Fruits and vegetables | QuEChERS + EV (<30 min) | 3.0 g, 20 mL of ACN | 87–112% | Yes | 0.8 | LC-MS/MS (C8, 8 min) | Caboni et al. (2008) | ||
| Cabbage | QuEChERS + EV (<30 min) | 7.7 g, 30 mL of ACN | 81–92% | Yes | 0.3 (LLOD) | LC-MS/MS (C18, 12 min) | (Chen et al., 2014) | ||
| Cabbage | QuEChERS (<30 min) | 7.7 g, 30 mL of ACN | 80–91% | Yes | 15 (LLOD) | LC-MS/MS (C18, 12 min) | Chen et al. (2014) | ||
| Cardamon | QuEChERS (NP) | NS (ACN) | >83% | NP | 50 | LC | (De Castro and Priego-Capote, 2010) | ||
| Rice | SE + CP + EV (>5 h) | 9 g, > 400 mL | 80–92 | Yes | 25 | LC-UV (NS, 14 min) | (Gopal & March et al., 2009) |
||
| Pea | SE + clean-up + EV (>12 h) | 0.5 g, > 900 mL | 87–96% | Yes | 50 | LC-UV-Vis (C18, 5 NS) | Kale et al. (2012) | ||
| Fodder berseem clover | QuEChERS (<20 min) | 21.1 g, 15 mL of ACN | 87–99% | Yes | 10 | LC-MS/MS (C18, 5 min) | Kaur et al. (2016) | ||
| Tomato | SE + clean-up + EV (>24 h) | 25.5 g, 400 mL | 98–102% | Yes | 10 | LC-PDA (C18, 12.5 min) | Mohapatra et al. (2011) | ||
| Cabbage, tomato and pea | QuEChERS + EV (<30 min) | 5.2 g, 10 mL of ACN | 85–99% | Yes | 10 | LC-UV-Vis (C18, 13 min) | Paramasivam & Banerjee (2011) | ||
| Tomato | QuEChERS + EV (<30 min) | 5.2 g, 10 mL of ACN | 97–99% | Yes | 10 | LC-UV-Vis (C18, 13 min) | Paramasivam & Banerjee (2012) | ||
| Cabbage | QuEChERS + EV (<30 min) | 5.2 g, 10 mL of ACN | 96–98% | Yes | 10 | LC-UV-Vis (C18, 13 min) | Paramasivam & Banerjee (2013) | ||
| Gherkin | QuEChERS + EV (<30 min) | 5.7 g, 20 mL of ACN | 87–93% | Yes | 10 | LC-DAD (C18, 12 min) | (Paramasivam et al., 2014) | ||
| Bee pollen | QuEChERS (<30 min) | 1.75 g, 7 mL (5 mL of ACN) | 80–84 | No | 5 | LC-MS/MS (C8, 19 min) | (Percival and Schroeder, 2017 | ||
| Tomato | SE + clean-up + EV (>12 h) | 0.5 g, > 900 mL | 86–96% | Yes | 10 | LC-PDA (C18, NS) | (Sharma & Pawar and Bhilave, 2020) |
||
| Cabbage | SE + clean-up + EV (>12 h) | 25.5 g, 400 mL | 81–100% | Yes | 10 | LC-PDA (C18, 15) | (Sharma et al., 2014a) | ||
| Tomato | SE + clean-up + EV (>12 h) | 0.5 g, > 900 mL | 85–101% | Yes | 10 | LC-PDA (C18, 15 min) | (Sharma et al., 2014b) | ||
| Chili | SE + clean-up + EV (>12 h) | 0.5 g, > 900 mL | 79–98% | Yes | 10 | LC-PDA (C18, 15 min) | Sharma et al. (2015) | ||
| Gherkin | QuEChERS + EV (<30 min) | 8.4 g, 15 mL of ACN | 98–101% | Yes | 10 | LC-PDA (C18, 20 min) | (Souza et al., 2017) | ||
| Brinjal | SE + clean-up + EV (>12 h) | 0.5 g, > 900 mL | 89–96% | Yes | 50 | LC-PDA (C18, 15 min) | Takkar et al. (2012) | ||
| Cabbage | QuEChERS (<20 min) | 7.75 g, 10 mL (5 mL of ACN) | – | No | 1.1 × 10–6 (LOD) | PD-LVI-LC-MS/MS (C18, 15 min) | (Zhang et al., 2016) | ||
| Tomato | SE + clean-up + EV (2–3 h) | 50 g, 100 mL | 82–90% | Yes | 10 | LC-DAD (C18, 10 min) | (Kooner et al., 2010) | ||
| Korean melon | SE + clean-up + EV (<30 min) | 20 g, 900 mL | 92–103.6% | Yes | 20 | HPLC-UV-Vis (C18, 13 min) | Kabir et al. (2017) | ||
| Okra | SE + clean-up + EV (40 min) | 10 g, 50 mL | 85%–88% | Yes | 0.01 | HPLC-UV-Vis (RP18, 10min) | (Sharma et al., 2011) | ||
| Rice | SE + clean-up + EV (40 min) | 5 g, 30 mL | 86–94% | – | 10 | LC-MS/MS (C18, 30 min) | Chen et al. (2015) | ||
ACN, acetonitrile; CP, column partitioning; DAD, diode array detector; dSPE, dispersive SPE; EV, evaporation; LOD, limit of detection; MS/MS, tandem mass spectrometry; PDA, photodiode array detector; PD-LVI, pre-column dilution large volume injection; QuEChERS, quick, easy, cheap, effective, rugged and safe; SE, solvent extraction.
5.1. Gas chromatography
The most common chromatographic technique is GC, used for separating compounds based on their volatility. A number of studies have been reported for FBD analysis by coupling GC with various detectors such as electron capture detector (ECD), flame photometric detector (FPD), nitrogen phosphorus detector (NPD) and flame ionization detector (FID). In comparison to traditional detectors, the mass detector (MS) has higher sensitivity, accuracy, reproducibility, and effectiveness in removing interferences (Kende et al., 2006). Nowadays, it is possible to combine MS analyzers with triple quadrupole mass spectrometer (QqQ)-MS (Tobin et al., 2014). Further, to eliminate the matrix interference, selective ion monitoring (SIM) is used. Similarly, Sharma et al. (Sharma et al., 2018) reported a method for the identification and quantification of FBD and deltamethrin in cucumber using GC-MS in (SIM) mode based on the use of target and two qualifier ions. Recently, the use of GC methods in detection of FBD are found inappropriate due to its volatility and poor thermal stability.
5.2. Liquid chromatography
The majority of the reviewed studies claim that the detection of FBD have been carried out by LC coupled with various detectors as summarized in Table 2. Detectors such as ultraviolet (UV), photodiode array (PDA), diode array detector (DAD), and mass (MS) detectors are used because of their sensitivity. Gopal and Mishra (2008) developed a method for the determination of FBD in rice. The insecticide was separated and quantified by reversed-phase LC with UV-diode array detection at 220 and 260 nm. Recoveries were ranged from 80% to 92.5% and limit of quantification (LOQ) was 25 μg/kg. Sharma et al. (2015) used PDA detector for the analysis of FBD and its metabolite (des-iodo FBD) in Chili; the recovery was in the range from 79 to 98%.
Recently, MS detection has become the most accepted and successful methodology in FBD analysis due to its increased sensitivity and selectivity. In LC-MS, ionization is usually performed by atmospheric pressure ionization (API) sources, in which the API can ionize both polar and non-polar analytes. In addition, mass analyzer such as triple quadrupole (QqQ) and quadrupole-trap (Q-Trap) are used for qualitative and quantitative analysis of FBD. Ares et al. (2017) developed a method to extract trace FBD from honey of different botanical origins using a single quadrupole. Another study conducted by Chen et al. (2018) reported a method for FBD estimation in cabbage using a QqQ-MS-equipped LC-MS/MS rapid resolution method. Further, Buddidathi et al. (2016) reported a method for simultaneous determination of FBD and its metabolite Des-Iodo FBD in Capsicum and Grape using a modified QuEChERS method equipped with LC-MS/MS. Caboni et al. (2008) developed a method for measuring FBD residues obtained from a variety of vegetables and grains. They then extracted the residue with acetonitrile and homogenized with magnesium sulfate and sodium chloride. After centrifugation, the top layer was analyzed by LC-MS/MS. In reference to the use of LC-MS and MS/MS, a new method has been reported with the use of ultra performance liquid chromatography (UPLC) because of its sensitivity and high chromatographic efficiency in analyzing FBD in fruits and vegetables. Ma et al. (2015) reported a method for the determination of six amide pesticide residues in vegetables and fruits with Electrospray Ionization –Time of Flight (ESI-TOF) detection. Regueiro et al. (2017) have reported a method for the quantification and confirmation capabilities of UPLC coupled with QqQ and hybrid Q-TOF-MS in FBD residue analysis.
5.3. Enzyme linked immunosorbent assay ELISA
In recent years, immunochemical techniques have received much attention for the rapid identification and detection of FBDs in various food matrices due to their attractive features of a rapid, simple, portable and inexpensive detection method. ELISA stands for “enzyme-linked immunosorbent assay,” which is a simple and quick method for detecting soluble substances like antibodies, hormones, peptides, and others. It is considered one of the analytical approaches offering specificity and sensitivity to a specific type of insecticide due to the antigen-antibody interaction (Li et al., 2021). Over the past decades, traditional antibodies, such as polyclonal and monoclonal antibodies (pAbs and mAbs), have been widely used to develop immunoassays for small molecules. Nanobody (Nb)-based immunoassays have recently proven to be a powerful tool for detecting environmental compounds such as insecticides in complex matrices like food matrices. The widespread availability of molecular biotechnology allows for the gene engineering of Nbs to facilitate speed of detection, improve analytical sensitivity, and either increase the specificity or even broaden the application range of Nbs. A study conducted by Zhang et al. showed that the application of an indirect competitive ELISA (icELISA) based on monoclonal antibody could lead to detection of anthranilic diamide insecticides namely cyantraniliprole in pakchoi vegetable (Zhang et al., 2015). The inhibition concentration (IC50) values were ranging from 0.43 to 6.15 μg L−1. The method achieved a detection limit of 1.57 μg L−1. The interesting feature of this method is that no obvious cross-reactivity was observed for the analytes studied. The accuracy of the method was verified using the HPLC method and recoveries were 94–101%. These results proved that the ic-ELISA could be used as a sensitive device for monitoring of target analytes.
5.4. Other extraction and detection approaches
High or low water content can have very different effects on the behavior of food products, leading to a range of undesirable consequences such as microbial growth, mycotoxin formation, alteration of the sensory quality of the final product, unstable production conditions and unclear commercial problems. Therefore, it has issued routine and rapid methods for determining the water content of food samples. The water content of food samples is most often determined using rapid instruments such as capacitance-based or microwave analyzers, which are inexpensive and widely available. Whereas near-infrared spectroscopy is most accurate indirect method for water content determination (Zhanget al., 2019).
Various detection methods have been used to estimate FBD from fruits and vegetables. Traditional analytical methods for detecting pesticides, such as gas and liquid chromatography, have great selectivity and sensitivity. However, these approaches have drawbacks such as being time consuming, needing highly skilled personnel, and requiring the use of expensive instruments. Therefore, advanced approaches for the determination of pesticides were reported using sensor-based techniques such as electrochemical impedance spectroscopy (EIS) (Lahrich et al., 2016), differential pulse voltammetry (DPV) (Farahi et al., 2014, 2015, 2016), square wave anodic stripping voltammetry (SWASV) (El Harmoudi et al., 2013), differential pulse anodic stripping voltammetry (DPASV) (Elkasmi et al., 2016), and square wave voltammetry (SQW) (Ajermoun et al., 2019, 2020; El Harmoudi et al., 2017; El Mhammedi et al., 2010; El Mhammedi et al., 2007a, 2007b; El Mhammedi et al., 2008; El Mhammedi et al., 2009). These electrochemical techniques are very sensitive and offer several advantages including low-cost, simplicity, rapid operation and low detection limits compared to the conventional chromatographic methods (Laghrib et al., 2020).
Recently, numerous sensor (Rawtani et al., 2018; Gao et al., 2019; Aghris et al., 2021a, 2021b, 2022) and biosensors such as optical (Biswas et al., 2016; Zhang et al., 2016; Singh et al., 2017; Wang et al., 2017), electrochemical, piezoelectric (Kaur et al., 2004; March et al., 2009; Cervera-Chiner et al., 2020), and molecular imprinted polymer (MIP) (Wang et al., 2016; Saylan et al., 2017; Tan et al., 2019; Singh et al., 2020; Xu et al., 2020) were majorly used for the detection of pesticides. Apart from the benefits offered by these detection methods, the main disadvantage discovered was the small number of pesticides detected, making the method vulnerable.
6. Conclusions and future perspectives
The determination of FBD residues in food as complex matrices is a major concern to several areas such as health and quality control. Therefore, analytical methods have to be approving for bring out both research and monitoring programs. In this field, reproducible analytical methods are appropriate to get the effective separation, selective identification, and accurate quantification of FBD analyses in food products. The extraction of flubendiamide is often followed by clean-up with SPE, D-SPE or GPC. Then, analytical separation with LC and GC coupled with tandem mass spectrometry for identification and quantification was used. Although the advantages in separation and detection of the chromatographic techniques, clean-up remains important to obtain reliable data. However, the presented techniques used for pesticide residues detection present limitations that are laborious, times consuming and destructive methods. In this context, electrochemical sensors and biosensors have attractive analytical characteristics and may become useful tools in detection of pesticides due to the fact that their determinations are simple, fast, sensitive, selective and had lower detection limits compared to the conventional chromatographic methods. Generally, the monitoring of insecticide levels in the different food matrices appears to be necessary to ensure human safety.
Given the danger of FBD to human health, surveillance activities should be increased, and strict measures should be taken when high levels of FBD are detected in food products. Farmers and extension agents should be trained in good agricultural practices by ministries of agriculture and related agencies to help reduce this threat. Simpler extraction and cleanup procedures need to be developed for FBD analysis. Electrochemical sensors and biosensors are being applied for detection of vast number of pesticides in various food systems. Using Biosensors for pesticide detection, is a highly promising and cost-effective technique. Apart from detection, removal of pesticides from various food categories is much more important. With the help of nanotechnology, it could be possible to eradicate these pesticides from the food matrices. In future, research must be focused on biosensors for FBD insecticide, so that the use of sophisticated instruments can be omitted.
CRediT authorship contribution statement
S. Aghris: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. O. Tahiri Alaoui: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. F. Laghrib: Methodology, Writing – original draft. A. Farahi: Writing – original draft, Supervision. M. Bakasse: Conceptualization, Writing – review & editing. S. Saqrane: Conceptualization, Writing – review & editing. S. Lahrich: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. M.A. El Mhammedi: Conceptualization, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbas M.S., Soliman A.S., El-Gammal H.A., Amer M.E., Attallah E.R. Development and validation of a multiresidue method for the determination of 323 pesticide residues in dry herbs using QuEChERS method and LC-ESI-MS/MS. Int. J. Environ. Anal. Chem. 2017;97:1003–1023. [Google Scholar]
- Aghris S., Ajermoun N., Hrioua A., Laghrib F., El Bouabi S., Saqrane S., Farahi A., Bakasse M., Lahrich S., El Mhammedi M.A. Electrochemical determination of flubendiamide insecticide at graphite/ionic liquid/natural phosphate: application in water and white rice. Cas. Stud. Chem. Envi. Eng. 2022;10179:1–7. [Google Scholar]
- Aghris S., Ajermoun N., Loudiki A., Ettadili F.E., Laghrib F., Farahi A., El Mhammedi M.A. Disposal graphite pencil sensor for trace detection of phthalic acid diamide insecticide flubendiamide in wastewater and white rice. Int. J. Environ. Anal. Chem. 2021:1–13. [Google Scholar]
- Aghris S., Matrouf M., Ettadili F.E., Laghrib F., El Bouabi Y., Saqrane S., El Mhammedi M.A. Electrochemical analysis of flubendiamide in water and white rice using clay microparticles supported on pencil electrode. Microw. J. 2021;168 [Google Scholar]
- Ahlawat S., Gulia S., Malik K., Rani S., Chauhan R. Persistence and decontamination studies of chlorantraniliprole in Capsicum annum using GC–MS/MS. J. Food Sci. Technol. 2019;56:2925–2931. doi: 10.1007/s13197-019-03757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajermoun N., Aghris S., Farahi A., Lahrich S., Saqrane S., Bakasse M., El Mhammedi M.A. Electrochemical reduction of neonicotinoids insecticides catalysed by metallic silver: case of the detection of imidacloprid in tomato and orange juice. Int. J. Environ. Anal. Chem. 2019;101:585–597. [Google Scholar]
- Ajermoun N., Lahrich S., Bouarab L., Bakasse M., Saqrane S., El Mhammedi M.A. Physiological effects of thiamethoxam on Zea mays and its electrochemical detection using a silver electrode. J. Sci. Food Agric. 2020;100:2090–2098. doi: 10.1002/jsfa.10232. [DOI] [PubMed] [Google Scholar]
- Akhtar Y., Yeoung Y.R., Isman M.B. Comparative bioactivity of selected extracts from Meliaceae and some commercial botanical insecticides against two noctuid caterpillars, Trichoplusia ni and Pseudaletia unipuncta. Phytochemistry Rev. 2008;7:77–88. [Google Scholar]
- Ares A.M., Valverde S., Bernal J.L., Toribio L., Nozal M.J., Bernal J. Determination of flubendiamide in honey at trace levels by using solid phase extraction and liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Chem. 2017;232:169–176. doi: 10.1016/j.foodchem.2017.03.162. [DOI] [PubMed] [Google Scholar]
- Ballesteros E., Ramos-Martos N. Olives and Olive Oil in Health and Disease Prevention. Academic Press; 2010. Residues of pesticides and polycyclic aromatic hydrocarbons in olive and olive-pomace oils by gas chromatography/tandem mass spectrometry; pp. 425–436. [DOI] [PubMed] [Google Scholar]
- Barrania A.A. Effects of some insecticides on some biological parameters of cotton leafworm, spodoptera littoralis (Lepidoptera: noctuidae) Alexandria Sci. Exch. J. 2019;40:305–311. [Google Scholar]
- Bernal J., Nozal M.J., Martin M.T., Bernal J.L., Ares A.M. Trace analysis of flubendiamide in bee pollen using enhanced matrix removal-lipid sorbent clean-up and liquid chromatography-electrospray ionization mass spectrometry. Microchem. J. 2019;148:541–547. [Google Scholar]
- Biswas S., Tripathi P., Kumar N., Nara S. Gold nanorods as peroxidase mimetics and its application for colorimetric biosensing of malathion. Sens. Actuators, B. 2016;231:584–592. [Google Scholar]
- Boaventura D., Bolzan A., Padovez F.E., Okuma D.M., Omoto C., Nauen R. Detection of a ryanodine receptor target‐site mutation in diamide insecticide resistant fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2020;76:47–54. doi: 10.1002/ps.5505. [DOI] [PubMed] [Google Scholar]
- Bolzan A., Padovez F.E., Nascimento A.R., Kaiser I.S., Lira E.C., Amaral F.S., Omoto C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: noctuidae) to chlorantraniliprole and cross‐resistance to other diamide insecticides. Pest Manag. Sci. 2019;75:2682–2689. doi: 10.1002/ps.5376. [DOI] [PubMed] [Google Scholar]
- Boobis A.R., Ossendorp B.C., Banasiak U., Hamey P.Y., Sebestyen I., Moretto A. Cumulative risk assessment of pesticide residues in food. Toxicol. Lett. (Amst.) 2008;180:137–150. doi: 10.1016/j.toxlet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Brillantes A.M.B., Ondrias K., Scott A., Kobrinsky E., Ondriašová E., Moschella M.C., Marks A.R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Brits M., De Vos J., Weiss J.M., Rohwer E.R., De Boer J. Critical review of the analysis of brominated flame retardants and their environmental levels in Africa. Chemosphere. 2016;164:174–189. doi: 10.1016/j.chemosphere.2016.08.097. [DOI] [PubMed] [Google Scholar]
- Buddidathi R., Mohapatra S., Siddamallaiah L., Manikrao G., Hebbar S.S. Dissipation pattern of flubendiamide residues on capsicum fruit (Capsicum annuum L.) under field and controlled environmental conditions. J. Envi. Sci. Health. Part B. 2016;51:44–51. doi: 10.1080/03601234.2015.1080496. [DOI] [PubMed] [Google Scholar]
- Bull R., Marengo J.J., Suarez-Isla B.A., Donoso P., Sutko J.L., Hidalgo C. Activation of calcium channels in sarcoplasmic reticulum from frog muscle by nanomolar concentrations of ryanodine. Biophys. J. 1989;56:749–756. doi: 10.1016/S0006-3495(89)82722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero R., Cyman S., Schuster D.J., Portillo H.E., Slater R. Baseline susceptibility of Bemisia tabaci (Genn.) biotype B in southern Florida to cyantraniliprole. Crop Protect. 2013;44:104–108. [Google Scholar]
- Caboni P., Sarais G., Angioni A., Vargiu S., Pagnozzi D., Cabras P., Casida J.E. Liquid chromatography− tandem mass spectrometric ion-switching determination of chlorantraniliprole and flubendiamide in fruits and vegetables. J. Agric. Food Chem. 2008;56:7696–7699. doi: 10.1021/jf8014816. [DOI] [PubMed] [Google Scholar]
- Cabras P., Caboni P., Cabras M. Analysis by HPLC of ryanodine and dehydroryanodine residues on fruits and in ryania powdery wood. J. Agric. Food Chem. 2001;49:3161–3163. doi: 10.1021/jf010224g. [DOI] [PubMed] [Google Scholar]
- Carscallen G.E., Kher S.V., Evenden M.L. Efficacy of chlorantraniliprole seed treatments against armyworm (Mythimna unipuncta [Lepidoptera: noctuidae]) larvae on corn (Zea mays) J. Econ. Entomol. 2019;112:188–195. doi: 10.1093/jee/toy338. [DOI] [PubMed] [Google Scholar]
- Casado J., Santillo D., Johnston P. Multi-residue analysis of pesticides in surface water by liquid chromatography quadrupole-Orbitrap high resolution tandem mass spectrometry. Anal. Chim. Acta. 2018;1024:1–17. doi: 10.1016/j.aca.2018.04.026. [DOI] [PubMed] [Google Scholar]
- Cervera-Chiner L., March C., Arnau A., Jiménez Y., Montoya Á. Detection of DDT and carbaryl pesticides in honey by means of immunosensors based on high fundamental frequency quartz crystal microbalance (HFF-QCM) J. Sci. Food Agric. 2020;100:2468–2472. doi: 10.1002/jsfa.10267. [DOI] [PubMed] [Google Scholar]
- Chameau P., Vrede Y., Fossier P., Baux G. Ryanodine-, IP 3-and NAADP-dependent calcium stores control acetylcholine release. Pflügers Archiv. 2001;443:289–296. doi: 10.1007/s004240100691. [DOI] [PubMed] [Google Scholar]
- Chen J., Xue L., Wei R., Liu S., Yin C.C. The insecticide chlorantraniliprole is a weak activator of mammalian skeletal ryanodine receptor/Ca2+ release channel. Biochem. Biophys. Res. Commun. 2019;50:633–639. doi: 10.1016/j.bbrc.2018.11.180. [DOI] [PubMed] [Google Scholar]
- Chen S., Li M., Zheng G., Wang T., Lin J., Wang S., Yu X. Metabolite profiling of 14 Wuyi Rock tea cultivars using UPLC-QTOF MS and UPLC-QqQ MS combined with chemometrics. Molecules. 2018;23:104. doi: 10.3390/molecules23020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.J., Ren Y.J., Meng Z.Y., Lu C.L., Gu H.T., Zhuang Y.Q. Comparative uptake of chlorantraniliprole and flubendiamide in the rice plant. J. Agric. Sci. 2015;7:238. [Google Scholar]
- Chen X., Wang P., Meng Z., Chen S., Gu H., Sha X. Degradation dynamics and residue analysis of flubendiamide in cabbage and soil by liquid chromatography-tandem mass spectrometry with dispersive solid phase extraction. Agric. Sci. 2014;5:50–857. [Google Scholar]
- Chu A., Diaz-Munoz M., Hawkes M.J., Brush K., Hamilton S.L. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol. Pharmacol. 1990;37:735–741. [PubMed] [Google Scholar]
- Cordova D., Benner E.A., Sacher M.D., Rauh J.J., Sopa J.S., Lahm G.P., Rhoades D.F. Anthranilic diamides: a new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006;84:196–214. [Google Scholar]
- Coronado R., Morrissette J., Sukhareva M., Vaughan D.M. Structure and function of ryanodine receptors. Am. J. Physiol. Cell Physiol. 1994;266:C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- Damalas C.A., Eleftherohorinos I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Publ. Health. 2011;8:1402–1419. doi: 10.3390/ijerph8051402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.K., Mukherjee I., Roy A. Flubendiamide as new generation insecticide in plant toxicology. Adv. Clin. Toxicol. 2017;2:100–122. [Google Scholar]
- De Castro M.L., Priego-Capote F. Soxhlet extraction: past and present panacea. J. Chromatogr. A. 2010;1217:2383–2389. doi: 10.1016/j.chroma.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Diandian W.A.N.G., Wenzhu S.N.S.L.W. Residue analysis of flubendiamide and its metabolite in rice by UPLC. Environ. Chem. 2012;9:11–14. [Google Scholar]
- Dimetry N.Z. Prospects of botanical pesticides for the future in integrated pest management programme (IPM) with special reference to neem uses in Egypt. Arch. Phytopathol. Pflanzenschutz. 2012;45:1138–1161. [Google Scholar]
- Ebbinghaus-Kintscher U., Luemmen P., Lobitz N., Schulte T., Funke C., Fischer R., Tohnishi M. Phthalic acid diamides activate ryanodine-sensitive Ca2+ release channels in insects. Cell Calcium. 2006;39:21–33. doi: 10.1016/j.ceca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- El Harmoudi H., Achak M., Farahi A., Lahrich S., El Gaini L., Abdennouri M., El Mhammedi M.A. Sensitive determination of paraquat by square wave anodic stripping voltammetry with chitin modified carbon paste electrode. Talanta. 2013;115:172–177. doi: 10.1016/j.talanta.2013.04.002. [DOI] [PubMed] [Google Scholar]
- El Harmoudi H., Achak M., Lahrich S., Farahi A., El Gaini L., Bakasse M., El Mhammedi M.A. Square wave voltammetric determination of diquat using natural phosphate modified platinum electrode. Arab. J. Chem. 2017;10:671–676. [Google Scholar]
- El Mhammedi M.A., Achak M., Bakasse M., Bachirat R., Chtaini A. Accumulation and trace measurement of paraquat at kaolin-modified carbon paste electrode. Mater. Sci. Eng. C. 2010;30:833–838. [Google Scholar]
- El Mhammedi M.A., Bakasse M., Bachirat R., Chtaini A. Square wave voltammetry for analytical determination of paraquat at carbon paste electrode modified with fluoroapatite. Food Chem. 2008;110:1001–1006. doi: 10.1016/j.foodchem.2008.03.005. [DOI] [PubMed] [Google Scholar]
- El Mhammedi M.A., Bakasse M., Chtaini A. Electrochemical studies and square wave voltammetry of paraquat at natural phosphate modified carbon paste electrode. J. Hazard Mater. 2007;145:1–7. doi: 10.1016/j.jhazmat.2007.02.054. [DOI] [PubMed] [Google Scholar]
- El Mhammedi M.A., Bakasse M., Chtaini A. Square-Wave voltammetric determination of paraquat at carbon paste electrode modified with hydroxyapatite. Electroanalysis. 2007;19:1727–1733. [Google Scholar]
- El Mhammedi M.A., Bakasse M., Najih R., Chtaini A. Carbon paste electrode modified with kaolin for the detection of diquat. Appl. Clay Sci. 2009;43:130–134. [Google Scholar]
- Elkasmi S., Farahi A., Zriouil M., Ahmamou M., Bakasse M., El Mhammedi M.A. Electrochemical determination of paraquat in potato, lemon, orange and natural water samples using sensitive-rich clay carbon electrode. J. Taiwan Inst. Chem. Eng. 2016;58:165–172. [Google Scholar]
- Ellisman M.H., Deerinck T.J., Ouyang Y., Beck C.F., Tanksley S.J., Walton P.D., Sutko J.L. Identification and localization of ryanodine binding proteins in the avian central nervous system. Neuron. 1990;5:135–146. doi: 10.1016/0896-6273(90)90304-x. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA) Anastassiadou M., Brancato A., Carrasco Cabrera L., Greco L., Jarrah S., Nave S. Setting of import tolerances for chlorantraniliprole in oil palms fruits and oil palms kernels. EFSA J. 2019;17 doi: 10.2903/j.efsa.2019.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority Conclusion on the peer review of the pesticide risk assessment of the active substance flubendiamide. EFSA J. 2013;11:3298. [Google Scholar]
- Farahi A., Achak M., El Gaini L., El Mhammedi M.A., Bakasse M. Electrochemical determination of paraquat in citric fruit based on electrodeposition of silver particles onto carbon paste electrode. J. Food Drug Anal. 2015;23:463–471. doi: 10.1016/j.jfda.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahi A., Achak M., El Gaini L., El Mhammedi M.A., Bakasse M. Silver particles modified carbon paste electrodes for differential pulse voltammetric determination of paraquat in ambient water samples. J. Assoc. Arab Univ. Basic Appl. Sci. 2016;19:37–43. [Google Scholar]
- Farahi A., Lahrich S., Achak M., El Gaini L., Bakasse M., El Mhammedi M.A. Parameters affecting the determination of paraquat at silver rotating electrodes using differential pulse voltammetry. Anal. Chem. Res. 2014;1:16–21. [Google Scholar]
- Feng M., Zhu H., Zhao L. Design, synthesis, insecticidal activity and structure–activity relationship of 3, 3‐dichloro‐2‐propenyloxy‐containing phthalic acid diamide structures. Pest Manag. Sci. 2012;68:986–994. doi: 10.1002/ps.3243. [DOI] [PubMed] [Google Scholar]
- Fill M., Copello J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- Gao W., Li D., You H. Functional characterization and genomic analysis of the chlorantraniliprole-degrading strain Pseudomonas sp. GW13. Bioengineered. 2019;6:106. doi: 10.3390/bioengineering6040106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill H.K., Garg H. Pesticide: environmental impacts and management strategies. Pest. Tox. Asp. 2014;8:187. [Google Scholar]
- Gonring A.H.R., de Andrade Silva F.M., Plata-Rueda R.A., Gorri J.E.R., Fernandes F.L. Comparative bioassay methods to determine diamide susceptibility for two coffee pests. Crop Protect. 2019;121:34–38. [Google Scholar]
- Gopal M., Mishra E. Analytical method for estimation of a new insecticide flubendiamide and its safety evaluation for usage in rice crop. Bull. Environ. Contam. Toxicol. 2008;81:360. doi: 10.1007/s00128-008-9470-1. [DOI] [PubMed] [Google Scholar]
- Gossauer A. Fortschritte der Chemie organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products. Springer; Vienna: 2003. Monopyrrolic natural compounds including tetramic acid derivatives; pp. 1–188. [DOI] [PubMed] [Google Scholar]
- Grdiša M., Gršić K. Botanical insecticides in plant protection. Agric. Conspectus Sci. 2013;78:85–93. [Google Scholar]
- He F., Sun S., Tan H., Sun X., Qin C., Ji S., Jiang X. Chlorantraniliprole against the black cutworm Agrotis ipsilon (Lepidoptera: noctuidae): from biochemical/physiological to demographic responses. Sci. Rep. 2019;9:1–17. doi: 10.1038/s41598-019-46915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héma S.A.O., Ouédraogo I., Bourgou L., Vognan G. Utilisation du chlorantraniliprole dans le contrôle des principaux insectes ravageurs du cotonnier au Burkina Faso. Tropos. 2019;37 [Google Scholar]
- Hildmann F., Gottert C., Frenzel T., Kempe G., Speer K. Pesticide residues in chicken eggs–A sample preparation methodology for analysis by gas and liquid chromatography/tandem mass spectrometry. J. Chromatogr. A. 2015;1403:1–20. doi: 10.1016/j.chroma.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Hopkinson J.E., Pumpa S.M. Baseline susceptibility of Bemisia tabaci MEAM 1 (Hemiptera: aleyrodidae) in Australia to spirotetramat, cyantraniliprole and dinotefuran, with reference to pyriproxyfen cross‐resistance. Aust. Entomol. 2019;58:762–771. [Google Scholar]
- Hou J., Xie W., Hong D., Zhang W., Li F., Qian Y., Han C. Simultaneous determination of ten neonicotinoid insecticides and two metabolites in honey and Royal-jelly by solid− phase extraction and liquid chromatography− tandem mass spectrometry. Food Chem. 2019;270:204–213. doi: 10.1016/j.foodchem.2018.07.068. [DOI] [PubMed] [Google Scholar]
- Huh Y., Kim B.K., Yoo E.J., Lee H., Chung H.M., Choi J.W. Simultaneous and rapid analysis of multi-pesticide residues using on-line SPE-LC-orbitrap mass spectrometry. J. Envi. Anal. Health Toxicol. 2019;22:268–276. [Google Scholar]
- Hwang E.J., Park J.E., Kwon C.H., Kim J.S., Chang H.R. Residue dissipation behavior of bistrifluron and cyenopyrafen in peach for the cultivation periods under field conditions. Korean J. Envi. Agri. 2018;37:41–48. [Google Scholar]
- Isaacs A.K., Qi S., Sarpong R., Casida J.E. Insect ryanodine receptor: distinct but coupled insecticide binding sites for [N-C3H3] chlorantraniliprole, flubendiamide, and [3H] ryanodine. Chem. Res. Toxicol. 2012;25:1571–1573. doi: 10.1021/tx300326m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaaya I., editor. Biochemical Sites of Insecticide Action and Resistance. Springer Science & Business Media; 2012. [Google Scholar]
- Isman M.B. Neem and other botanical insecticides: barriers to commercialization. Phytoparasitica. 1997;25:339. [Google Scholar]
- Ito K., Takakura S., Sato K., Sutko J.L. Ryanodine inhibits the release of calcium from intracellular stores in Guinea pig aortic smooth muscle. Circ. Res. 1986;58:730–734. doi: 10.1161/01.res.58.5.730. [DOI] [PubMed] [Google Scholar]
- Jactel H., Verheggen F., Thiéry D., Escobar-Gutiérrez A.J., Gachet E., Desneux N., Neonicotinoids Working Group. Alternatives to neonicotinoids. Environ. Int. 2019;129:423–429. doi: 10.1016/j.envint.2019.04.045. [DOI] [PubMed] [Google Scholar]
- Jallow M.F., Dahab A.A., Albaho M.S., Devi V.Y., Awadh D.G., Thomas B.M. Baseline susceptibility and assessment of resistance risk to flubendiamide and chlorantraniliprole in Tuta absoluta (Lepidoptera: gelechiidae) populations from Kuwait. Appl. Entomol. Zool. 2019;54:91–99. [Google Scholar]
- Jansen R.S., Rosing H., Schellens J.H., Beijnen J.H. Retention studies of 2′-2′-difluorodeoxycytidine and 2′-2′-difluorodeoxyuridine nucleosides and nucleotides on porous graphitic carbon: development of a liquid chromatography–tandem mass spectrometry method. J. Chromatogr. A. 2009;1216:3168–3174. doi: 10.1016/j.chroma.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Jeanguenat A. The story of a new insecticidal chemistry class: the diamides. Pest Manag. Sci. 2013;69:7–14. doi: 10.1002/ps.3406. [DOI] [PubMed] [Google Scholar]
- Jefferies P.R., Lam W.W., Toia R.F., Casida J.E. Ryania insecticide: structural assignments of four natural 8ax-hydroxy-10-epiryanoids. J. Agric. Food Chem. 1992;40:509–512. [Google Scholar]
- Jefferies P.R., Toia R.F., Casida J.E. Ryanodyl 3-(pyridine-3-carboxylate): a novel ryanoid from Ryania insecticide. J. Nat. Prod. 1991;54:1147–1149. doi: 10.1021/np50076a043. [DOI] [PubMed] [Google Scholar]
- Jiafeng Y., Decheng S., Xiaoyong L., Yang L., Guangyu L., Min B.S. Multiresidue determination of 19 anabolic steroids in animal oil using enhanced matrix removal lipid cleanup and ultrahigh performance liquid chromatography-tandem mass spectrometry. Anal. Methods. 2021;13:2374–2383. doi: 10.1039/d1ay00437a. [DOI] [PubMed] [Google Scholar]
- Jiang J., Wang Y., Mu W., Zhang Z. Sublethal effects of anthranilic diamide insecticides on the demographic fitness and consumption rates of the Coccinella septempunctata (Coleoptera: coccinellidae) fed on Aphis craccivora. Environ. Sci. Pollut. Res. 2020;27:4178–4189. doi: 10.1007/s11356-019-06993-z. [DOI] [PubMed] [Google Scholar]
- Jing H., Amirav A. Pesticide analysis with the pulsed-flame photometer detector and a direct sample introduction device. Anal. Chem. 1997;69:1426–1435. doi: 10.1021/ac961110k. [DOI] [PubMed] [Google Scholar]
- Jouraku A., Kuwazaki S., Miyamoto K., Uchiyama M., Kurokawa T., Mori E., Sonoda S. Ryanodine receptor mutations (G4946E and I4790K) differentially responsible for diamide insecticide resistance in diamondback moth, Plutella xylostella L. Insect Biochem. Mol. Biol. 2020;118 doi: 10.1016/j.ibmb.2019.103308. [DOI] [PubMed] [Google Scholar]
- Kabir M.H., Abd El-Aty A.M., Rahman M.M., Chung H.S., Lee H.S., Jeong J.H., Shim J.H. Dissipation kinetics, pre-harvest residue limits, and dietary risk assessment of the systemic fungicide metalaxyl in Swiss chard grown under greenhouse conditions. Regul. Toxicol. Pharmacol. 2018;92:201–206. doi: 10.1016/j.yrtph.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Kabir M.H., Abd El-Aty A.M., Rahman M.M., Kim S.W., Lee H.S., Chung H.S., Shim J.H. Dissipation kinetics, pre-harvest residue limits, and hazard quotient assessments of pesticides flubendiamide and fluopicolide in Korean melon (Cucumis melo L. var. makuwa) grown under regulated conditions in plastic greenhouses. Environ. Sci. Pollut. Res. 2017;24:22241–22250. doi: 10.1007/s11356-017-9880-x. [DOI] [PubMed] [Google Scholar]
- Kadala A., Charreton M., Charnet P., Collet C. Honey bees long-lasting locomotor deficits after exposure to the diamide chlorantraniliprole are accompanied by brain and muscular calcium channels alterations. Sci. Rep. 2019;9:1–9. doi: 10.1038/s41598-019-39193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale V.D., Walunj A.R., Battu R.S., Sahoo S.K., Singh B., Paramasivam M., Sharma K.K. Assessment of flubendiamide residues in pigeon pea in different agro-climatic zones of India. Environ. Monit. Assess. 2012;184:4267–4270. doi: 10.1007/s10661-011-2261-x. [DOI] [PubMed] [Google Scholar]
- Kato K., Kiyonaka S., Sawaguchi Y., Tohnishi M., Masaki T., Yasokawa N., et al. Molecular characterization of flubendiamide sensitivity in the lepidopterous ryanodine receptor Ca2+ release channel. Biochemistry. 2009;48:10342–10352. doi: 10.1021/bi900866s. [DOI] [PubMed] [Google Scholar]
- Kaur J., Singh K.V., Schmid A.H., Varshney G.C., Suri C.R., Raje M. Atomic force spectroscopy-based study of antibody pesticide interactions for characterization of immunosensor surface. Biosens. Bioelectron. 2004;20:284–293. doi: 10.1016/j.bios.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Kaur R., Mandal K., Sahoo S.K., Kumar R., Arora R., Singh B. Estimation and risk assessment of flubendiamide on fodder berseem clover (Trifolium alexandrinum L.) by QuEChERS methodology and LC-MS/MS. Environ. Sci. Pollut. Res. 2016;23:9791–9798. doi: 10.1007/s11356-016-6109-3. [DOI] [PubMed] [Google Scholar]
- Kende A., Csizmazia Z., Rikker T., Angyal V., Torkos K. Combination of stir bar sorptive extraction–retention time locked gas chromatography–mass spectrometry and automated mass spectral deconvolution for pesticide identification in fruits and vegetables. Microchem. J. 2006;84:63–69. [Google Scholar]
- Kim C.K., Lee J.K., Oh S.A., Kim Y.E., Kwon E.Y., Yang H.R., Hwang L.H. Monitoring of pesticide residues in agricultural products in gangseo, seoul, by introduction of the positive list system. J. Food Hyg. Safe. 2021;36:163–171. [Google Scholar]
- Kooner R., Sahoo S.K., Singh B., Battu R.S. Dissipation kinetics of flubendiamide and thiacloprid on tomato (Lycopersicon esculentum Mill) and soil. Qual. Assur. Saf. Crop. 2010;2:36–40. [Google Scholar]
- Kralj M.B., Divanović H., Košenina S., Kete M., Lebedev A.T., Artaev V.B., Trebše P. Effect of humic acids, nitrate and oxygen on the photodegradation of the flubendiamide insecticide: identification of products. Environ. Chem. Lett. 2018;16:591–597. [Google Scholar]
- Laghrib F., Bakasse M., Lahrich S., El Mhammedi M.A. Electrochemical sensors for improved detection of paraquat in food samples: a review. Mater. Sci. Eng. C. 2020;107 doi: 10.1016/j.msec.2019.110349. [DOI] [PubMed] [Google Scholar]
- Lahm G.P., Cordova D., Barry J.D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009;17:4127–4133. doi: 10.1016/j.bmc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Lahm G.P., Selby T.P., Freudenberger J.H., Stevenson T.M., Myers B.J., Seburyamo G., Cordova D. Insecticidal anthranilic diamides: a new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005;15:4898–4906. doi: 10.1016/j.bmcl.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Lahm G.P., Stevenson T.M., Selby T.P., Freudenberger J.H., Cordova D., Flexner L., Hollingshaus J.G. Rynaxypyr™: a new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett. 2007;17:6274–6279. doi: 10.1016/j.bmcl.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Lahrich S., Hammani H., Boumya W., Loudiki A., Farahi A., Achak M., El Mhammedi M.A. Correlation between electrochemical impedance and spectroscopic measurements in adsorbing paraquat on silver: application in underground water samples. Electroanalysis. 2016;28:1012–1022. [Google Scholar]
- Laitinen P.J., Brown K.M., Piippo K., Swan H., Devaney J.M., Brahmbhatt B., Toivonen L. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- Lee J., Shin Y., Lee J., Lee J., Kim B.J., Kim J.H. Simultaneous analysis of 310 pesticide multiresidues using UHPLC-MS/MS in brown rice, orange, and spinach. Chemosphere. 2018;207:519–526. doi: 10.1016/j.chemosphere.2018.05.116. [DOI] [PubMed] [Google Scholar]
- Lee K.B., Kim N.W., Song N.S., Lee J.H., Jung S.M., Shin M.H., Sung S.Y. A safety survey of pesticide residues in fruit products circulated in chungcheongnam-do province, korea. J. Food Hyg. Safe. 2019;34:421–430. [Google Scholar]
- Lehotay S.J., Maštovská K., Yun S.J. Evaluation of two fast and easy methods for pesticide residue analysis in fatty food matrixes. J. AOAC Int. 2005;88:630–638. [PubMed] [Google Scholar]
- Li H., He S., Liu G., Li C., Ma Z., Zhang X. Residue and dissipation kinetics of toosendanin in cabbage, tobacco and soil using IC-ELISA detection. Food Chem. 2021;335 doi: 10.1016/j.foodchem.2020.127600. [DOI] [PubMed] [Google Scholar]
- Li S., Zhang Q., Chen M., Zhang X., Liu P. Determination of veterinary drug residues in food of animal origin: sample preparation methods and analytical techniques. J. Liq. Chromatogr. Relat. Technol. 2020;43:701–724. [Google Scholar]
- Li X., Degain B.A., Harpold V.S., Marçon P.G., Nichols R.L., Fournier A.J., Ellsworth P.C. Baseline susceptibilities of B‐and Q‐biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Manag. Sci. 2012;68:83–91. doi: 10.1002/ps.2227. [DOI] [PubMed] [Google Scholar]
- Li Y., Li M., Chai B.S., Liu C.L. A new insecticide flubendiamide. PESTICIDES-SHENYANG- 2006;45:697. [Google Scholar]
- Lin L., Hao Z., Cao P., Yuchi Z. Homology modeling and docking study of diamondback moth ryanodine receptor reveals the mechanisms for channel activation, insecticide binding and resistance. Pest Manag. Sci. 2020;76:1291–1303. doi: 10.1002/ps.5640. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li C., Gao J., Wang W., Huang L., Guo X., Wang J. Comparative characterization of two intracellular Ca 2+-release channels from the red flour beetle. Tribolium castaneum. Sci. Rep. 2014;4:1–7. doi: 10.1038/srep06702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Li X.L. The preparation of suspension concentrate of 20% ivermectin· flubendiamide. Guangzhou Chem. Ind. 2012;17:14–20. [Google Scholar]
- Lu Z., Zhang Z., Fang N., Hou Z., Li Y., Lu Z. Simultaneous determination of five diamide insecticides in food matrices using carbon nanotube multiplug filtration cleanup and ultrahigh-performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2019;67:10977–10983. doi: 10.1021/acs.jafc.9b02806. [DOI] [PubMed] [Google Scholar]
- Ludlow K. Australian pesticides and veterinary medicines authority. Encyclopedia of nanoscience and society. 2010;1:38–39. [Google Scholar]
- Lümmen P., Ebbinghaus-Kintscher U., Funke C., Fischer R., Masaki T., Yasokawa N., Tohnishi M. Phthalic acid diamides activate insect ryanodine receptors. ACS Symp. Ser. Am. Chem. Soc. 2007;948:235–248. [Google Scholar]
- Ma L., Chen J., Zhao L., Zhan X. Determination of six amide pesticide residues in vegetables and fruits by solid phase extraction-ultra high performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2015;33:1019–1025. doi: 10.3724/sp.j.1123.2015.05013. [DOI] [PubMed] [Google Scholar]
- Ma W., Li J., Li X., Liu H. Enrichment of diamide insecticides from environmental water samples using metal-organic frameworks as adsorbents for determination by liquid chromatography tandem mass spectrometry. J. Hazard Mater. 2021 doi: 10.1016/j.jhazmat.2021.126839. [DOI] [PubMed] [Google Scholar]
- Mahmood I., Imadi S.R., Shazadi K., Gul A., Hakeem K.R. Plant, Soil and Microbes. Springer; Cham: 2016. Effects of pesticides on environment; pp. 253–269. [Google Scholar]
- Malhat F., Kasiotis K.M., Shalaby S. Magnitude of cyantraniliprole residues in tomato following open field application: pre-harvest interval determination and risk assessment. Environ. Monit. Assess. 2018;190:1–10. doi: 10.1007/s10661-018-6496-7. [DOI] [PubMed] [Google Scholar]
- Mao L., Henderson G. Antifeedant activity and acute and residual toxicity of alkaloids from Sophora flavescens (Leguminosae) against Formosan subterranean termites (Isoptera: rhinotermitidae) J. Econ. Entomol. 2007;100:866–870. doi: 10.1603/0022-0493(2007)100[866:aaaaar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mao T., Li F., Fang Y., Wang H., Chen J., Li M., Cheng X. Effects of chlorantraniliprole exposure on detoxification enzyme activities and detoxification-related gene expression in the fat body of the silkworm, Bombyx mori. Ecotoxicol. Environ. Saf. 2019;176:58–63. doi: 10.1016/j.ecoenv.2019.03.074. [DOI] [PubMed] [Google Scholar]
- March C., Manclús J.J., Jiménez Y., Arnau A., Montoya A. A piezoelectric immunosensor for the determination of pesticide residues and metabolites in fruit juices. Talanta. 2009;78:827–833. doi: 10.1016/j.talanta.2008.12.058. [DOI] [PubMed] [Google Scholar]
- Marx S.O., Ondrias K., Marks A.R. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors) Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- Masaki T., Yasokawa N., Tohnishi M., Nishimatsu T., Tsubata K., Inoue K., Hirooka T. Flubendiamide, a novel Ca2+ channel modulator, reveals evidence for functional cooperation between Ca2+ pumps and Ca2+ release. Mol. Pharmacol. 2006;69:1733–1739. doi: 10.1124/mol.105.020339. [DOI] [PubMed] [Google Scholar]
- McGrew S.G., Wolleben C., Siegl P., Inui M., Fleischer S. Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry. 1989;28:1686–1691. doi: 10.1021/bi00430a039. [DOI] [PubMed] [Google Scholar]
- Meissner G., El-Hashem A. Ryanodine as a functional probe of the skeletal muscle sarcoplasmic reticulum Ca 2+ release channel. Mol. Cell. Biochem. 1992;114:119–123. doi: 10.1007/BF00240306. [DOI] [PubMed] [Google Scholar]
- Mekonen S., Ambelu A., Spanoghe P. Pesticide residue evaluation in major staple food items of Ethiopia using the Quechers method: a case study from the Jimma zone. Environ. Toxicol. Chem. 2014;33:1294–1302. doi: 10.1002/etc.2554. [DOI] [PubMed] [Google Scholar]
- Meng X., Dong F., Qian K., Miao L., Yang X., Ge H., Wang J. Transcriptome analysis reveals global gene expression changes of Chilo suppressalis in response to sublethal dose of chlorantraniliprole. Chemosphere. 2019;234:648–657. doi: 10.1016/j.chemosphere.2019.06.129. [DOI] [PubMed] [Google Scholar]
- Messutat S., Heine M., Wicher D. Calcium-induced calcium release in neurosecretory insect neurons: fast and slow responses. Cell Calcium. 2001;30:199–211. doi: 10.1054/ceca.2001.0227. [DOI] [PubMed] [Google Scholar]
- Mohapatra S., Ahuja A.K., Deepa M., Jagadish G.K., Rashmi N., Sharma D. Development of an analytical method for analysis of flubendiamide, des-iodo flubendiamide and study of their residue persistence in tomato and soil. J. Environ. Sci. Health-B. 2011;46:264–271. doi: 10.1080/03601234.2011.540536. [DOI] [PubMed] [Google Scholar]
- Mukherjee I., Das S.K., Kumar A. A fast method for determination of flubendiamide in vegetables by liquid chromatography. Pestic. Res. J. 2012;24:159–162. [Google Scholar]
- Nauen R. Insecticide mode of action: return of the ryanodine receptor. Pest Manag. Sci. 2006;62:690–692. doi: 10.1002/ps.1254. [DOI] [PubMed] [Google Scholar]
- O'Neal S.T., Reeves A.M., Fell R.D., Brewster C.C., Anderson T.D. Chlorothalonil exposure alters virus susceptibility and markers of immunity, nutrition, and development in honey bees. J. Insect Sci. 2019;19:14. doi: 10.1093/jisesa/iez051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y. Role of ryanodine receptors. Crit. Rev. Biochem. Mol. Biol. 1994;29:229–274. doi: 10.3109/10409239409083482. [DOI] [PubMed] [Google Scholar]
- Olszewski K.L., Mather M.W., Morrisey J.M., Garcia B.A., Vaidya A.B., Rabinowitz J.D., Llinás M. Branched tricarboxylic acid metabolism in Plasmodium falciparum. Nature. 2010;466:774–778. doi: 10.1038/nature09301. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Özkara A., Akyıl D., Konuk M. Environmental Health Risk-Hazardous Factors to Living Species. 2016. Pesticides, environmental pollution, and health. IntechOpen. [Google Scholar]
- Paramasivam M., Banerjee H. Simultaneous determination of flubendiamide its metabolite desiodo flubendiamide residues in cabbage, tomato and pigeon pea by HPLC. Bull. Environ. Contam. Toxicol. 2011;87:452. doi: 10.1007/s00128-011-0389-6. [DOI] [PubMed] [Google Scholar]
- Paramasivam M., Banerjee H. Persistence and dissipation of the insecticide flubendiamide and its metabolite desiodo flubendiamide residues in tomato fruit and soil. Bull. Environ. Contam. Toxicol. 2012;88:344–348. doi: 10.1007/s00128-011-0461-2. [DOI] [PubMed] [Google Scholar]
- Paramasivam M., Banerjee H. Dissipation of flubendiamide residues in/on cabbage (Brassica oleracea L.) Environ. Monit. Assess. 2013;185:1577–1581. doi: 10.1007/s10661-012-2652-7. [DOI] [PubMed] [Google Scholar]
- Paramasivam M., Selvi C., Chandrasekaran S. Persistence and dissipation of flubendiamide and its risk assessment on gherkin (Cucumis anguria L.) Environ. Monit. Assess. 2014;186:4881–4887. doi: 10.1007/s10661-014-3745-2. [DOI] [PubMed] [Google Scholar]
- Park E., Lee J., Lee J., Lee J., Lee H.S., Shin Y., Kim J.H. Method for the simultaneous analysis of 300 pesticide residues in hair by LC-MS/MS and GC-MS/MS, and its application to biomonitoring of agricultural workers. Chemosphere. 2021;277 doi: 10.1016/j.chemosphere.2021.130215. [DOI] [PubMed] [Google Scholar]
- Passos D.A., Silva-Torres C.S.A., Siqueira H.A.A. Behavioral response and adaptive cost in resistant and susceptible Plutella xylostella to Chlorantraniliprole. Bull. Entomol. Res. 2020;110:96–105. doi: 10.1017/S0007485319000300. [DOI] [PubMed] [Google Scholar]
- Pawar P.V., Bhilave M.P. Impact of novel insecticide chlorantraniliprole on alkaline phosphatase activity in freshwater fish cirrhinus mrigala, inter. J. Sci. Technol. 2020;9:2992–2995. [Google Scholar]
- Pence H.E., Williams A. ChemSpider: an online chemical information resource. J Chem. Educ. 2010;87:1123–1124. [Google Scholar]
- Percival R.V., Schroeder C.H. Wolters Kluwer Law & Business; 2017. Environmental Law: Statutory and Case Supplement. [Google Scholar]
- Plata-Rueda A., Martínez L.C., Costa N.C.R., Zanuncio J.C., de Sena Fernandes M.E., Serrão J.E., Fernandes F.L. Chlorantraniliprole–mediated effects on survival, walking abilities, and respiration in the coffee berry borer. Hypothenemus hampei. Ecotoxicol. Environ. Safe. 2019;172:53–58. doi: 10.1016/j.ecoenv.2019.01.063. [DOI] [PubMed] [Google Scholar]
- Ran W.A.N.G., Wang J.D., Che W.N., Yan S.U.N., Li W.X., Chen L.U.O. Characterization of field-evolved resistance to cyantraniliprole in Bemisia tabaci MED from China. J. Integr. Agric. 2019;18:2571–2578. [Google Scholar]
- Rawtani D., Khatri N., Tyagi S., Pandey G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018;206:749–762. doi: 10.1016/j.jenvman.2017.11.037. [DOI] [PubMed] [Google Scholar]
- Reddy S.S., Reddy C.N., Reddy A.A., Rao A.M., Reddy S.N. Dissipation pattern of flubendiamide 480% SC in Dolichos bean. J. Entomol. Zool. Stud. 2021;8:1942–1946. [Google Scholar]
- Regueiro J., Lopez-Fernandez O., Rial-Otero R., Cancho-Grande B., Simal-Gandara J. A review on the fermentation of foods and the residues of pesticides—biotransformation of pesticides and effects on fermentation and food quality. Crit. Rev. Food Sci. Nutr. 2015;55:839–863. doi: 10.1080/10408398.2012.677872. [DOI] [PubMed] [Google Scholar]
- Regueiro J., Negreira N., Hannisdal R., Berntssen M.H. Targeted approach for qualitative screening of pesticides in salmon feed by liquid chromatography coupled to traveling-wave ion mobility/quadrupole time-of-flight mass spectrometry. Food Contol. 2017;78:116–125. [Google Scholar]
- Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Rogers E.F., Koniuszy F.R., Shavel J., Jr., Folkers K. Plant insecticides. I. Ryanodine, A new alkaloid from ryania speciosa Vahl. J. Am. Chem. Soc. 1948;70:3086–3088. doi: 10.1021/ja01189a074. [DOI] [PubMed] [Google Scholar]
- Ruest L., Dodier M., De Sève H., Lessard C., Mongrain P. Ryanoids and related compounds Isolation and characterization of 11 new minor ryanoids from the plant Ryania Speciosa Vahl. Can. J. Chem. 2002;80:483–488. [Google Scholar]
- Ruest L., Taylor D.R., Deslongchamps P. Investigation of the constituents of Ryania speciosa. Can. J. Chem. 1985;63:2840–2843. [Google Scholar]
- Ruiz P., Ares A.M., Valverde S., Martín M.T., Bernal J. Development and validation of a new method for the simultaneous determination of spinetoram J and L in honey from different botanical origins employing solid-phase extraction with a polymeric sorbent and liquid chromatography coupled to quadrupole time-of-flight mass spectrometry. Food Res. Int. 2020;130 doi: 10.1016/j.foodres.2019.108904. [DOI] [PubMed] [Google Scholar]
- Sahoo S.K., Sharma R.K., Battu R.S., Singh B. Dissipation kinetics of flubendiamide on chili and soil. Bull. Environ. Contam. Toxicol. 2009;83:384–387. doi: 10.1007/s00128-009-9782-9. [DOI] [PubMed] [Google Scholar]
- Santulli G., R Marks A. Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr. Mol. Pharmacol. 2015;8:206–222. doi: 10.2174/1874467208666150507105105. [DOI] [PubMed] [Google Scholar]
- Satpathy S., Gotyal B.S., Babu V.R. Role of novel insecticides in crop protection and their selectivity to natural enemies: a review. J. Environ. Biol. 2020;41:149–160. [Google Scholar]
- Sattelle D.B., Cordova D., Cheek T.R. Insect ryanodine receptors: molecular targets for novel pest control chemicals. Invertebr. Neurosci. 2008;8:107. doi: 10.1007/s10158-008-0076-4. [DOI] [PubMed] [Google Scholar]
- Saylan Y., Akgonüllü S., Cimen D., Derazshamshir A., Bereli N., Yılmaz F. Development of surface plasmon resonance sensors based on molecularly imprinted nanofilms for sensitive and selective detection of pesticides. Sensor. Actuator. B Chem. 2017;241:446–454. [Google Scholar]
- Schenck F.J., Hobbs J.E. Evaluation of the quick, easy, cheap, effective, rugged, and safe (QuEChERS) approach to pesticide residue analysis. Bull. Environ. Contam. Toxicol. 2004;73:24–30. doi: 10.1007/s00128-004-0388-y. [DOI] [PubMed] [Google Scholar]
- Secretariat A.S.E.A.N., Center T.B. 2003. Risk Assessment and Risk Management in Implementing the Cartagena Protocol: Proceedings of Asia Regional Workshop. [Google Scholar]
- Shah R.M., Shad S.A. House fly resistance to chlorantraniliprole: cross resistance patterns, stability and associated fitness costs. Pest Manag. Sci. 2020;76:1866–1873. doi: 10.1002/ps.5716. [DOI] [PubMed] [Google Scholar]
- Sharma A., Kumar V., Shahzad B., Tanveer M., Sidhu G.P.S., Handa N., Dar O.I. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019;1:1446. [Google Scholar]
- Sharma B.N., Parihar N.S. Dissipation and persistence of flubendiamide and thiacloprid in/on tomato and soil. Bull. Environ. Contam. Toxicol. 2013;90:252–255. doi: 10.1007/s00128-012-0911-5. [DOI] [PubMed] [Google Scholar]
- Sharma D., Mohapatra S., Ahuja A.K., Divakar J.V., Deepa M. Comparative persistence of flubendiamide residues in chilli following application as individual and combination formulation. Qual. Assur. Saf. Crop Foods. 2011;3:69–73. [Google Scholar]
- Sharma K.K., Bhushan V.S., Rao C.S., Reddy K.N., Banerjee H., Mandal S., Walia S. Persistence, dissipation and consumer risk assessment of a combination formulation of flubendiamide and deltamethrin on cucumber. Food Addit. Contam. 2018;35:498–511. doi: 10.1080/19440049.2017.1416678. [DOI] [PubMed] [Google Scholar]
- Sharma K.K., Mukherjee I., Singh B., Sahoo S.K., Mandal K., Mohapatra S., Walunj A.R. Dissipation pattern and risk assessment of flubendiamide on chili at different agro-climatic conditions in India. Environ. Monit. Assess. 2015;187:1–10. doi: 10.1007/s10661-015-4476-8. [DOI] [PubMed] [Google Scholar]
- Sharma K.K., Mukherjee I., Singh B., Sahoo S.K., Parihar N.S., Sharma B.N., Devi S. Residual behavior and risk assessment of flubendiamide on tomato at different agro-climatic conditions in India. Environ. Monit. Assess. 2014;186:7673–7682. doi: 10.1007/s10661-014-3958-4. [DOI] [PubMed] [Google Scholar]
- Sharma S., Kaur A., Warangtiwar R.K. Dissipation and residual bioefficacy of chlorantraniliprole in tomato and brinjal fruits. Pestic. Res. J. 2019;31:249–258. [Google Scholar]
- Sherma J., Rabel F. Review of thin layer chromatography in pesticide analysis: 2016-2018. J. Liq. Chromatogr. Relat. 2018;41:1052–1065. [Google Scholar]
- Shiomi K., Matsui R., Kakei A., Yamaguchi Y., Masuma R., Hatano H., Turberg A. Verticilide, a new ryanodine-binding inhibitor, produced by Verticillium sp. FKI-1033. J. Antibiot. 2010;63:77–82. doi: 10.1038/ja.2009.126. [DOI] [PubMed] [Google Scholar]
- Silva J.E., Ribeiro L.M.D.S., Vinasco N., Guedes R.N.C., Siqueira H.Á.A. Field-evolved resistance to chlorantraniliprole in the tomato pinworm Tuta absoluta: inheritance, cross-resistance profile, and metabolism. J. Pest. Sci. 2019;92:1421–1431. [Google Scholar]
- Singh Battu R., Singh B., Kooner R., Singh B. Simple and efficient method for the estimation of residues of flubendiamide and its metabolite desiodo flubendiamide. J. Agric. Food Chem. 2008;56:2299–2302. doi: 10.1021/jf073081s. [DOI] [PubMed] [Google Scholar]
- Singh R., Thakur P., Thakur A., Kumar H., Chawla P., Rohit J.V. Colorimetric sensing approaches of surface-modified gold and silver nanoparticles for detection of residual pesticides: a review. Int. J. Environ. Anal. Chem. 2020;19:1–17. [Google Scholar]
- Singh S., Tripathi P., Kumar N., Nara S. Colorimetric sensing of malathion using palladium-gold bimetallic nanozyme. Biosens. Bioelectron. 2017;92:280–286. doi: 10.1016/j.bios.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Słowik-Borowiec M., Szpyrka E. Multiresidue analysis of pesticides in wine and grape using gas chromatography with microelectron capture and nitrogen–phosphorus detection. Food Anal. Methods. 2018;11:3516–3530. [Google Scholar]
- Soloway S.B. Naturally occurring insecticides. Environ. Health Perspect. 1976;14:109–117. doi: 10.1289/ehp.7614109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza C.M., Baldin E.L., Ribeiro L.P., Silva I.F., Morando R., Bicalho K.U., Fernandes J.B. Lethal and growth inhibitory activities of Neotropical Annonaceae-derived extracts, commercial formulation, and an isolated acetogenin against Helicoverpa armigera. J. Pest. Sci. 2017;90:701–709. [Google Scholar]
- Sreedhar U. Field evaluation of new insecticides against budworm, Helicoverpa armigera (Hubner) in flue cured Virginia tobacco. J. Entomol. Zool. Stud. 2019;7:417–420. [Google Scholar]
- Stein M.B., Padua R.A., Nagy J.I., Geiger J.D. High affinity [3H] ryanodine binding sites in postmortem human brain: regional distribution and effects of calcium, magnesium and caffeine. Brain Res. 1992;585:349–354. doi: 10.1016/0006-8993(92)91235-7. [DOI] [PubMed] [Google Scholar]
- Takkar R., Sahoo S.K., Singh G., Battu R.S., Singh B. Dissipation pattern of flubendiamide in/on brinjal (Solanum melongena L.) Environ. Monit. Assess. 2012;184:5077–5083. doi: 10.1007/s10661-011-2323-0. [DOI] [PubMed] [Google Scholar]
- Tan L., Guo M.L., Tan J.A., Geng Y.Y., Huang S.Y., Tang Y.W. Development of high-luminescence perovskite quantum dots coated with molecularly imprinted polymers for pesticide detection by slowly hydrolysing the organosilicon monomers in situ. Sensor. Actuator. B Chem. 2019;291:226–234. [Google Scholar]
- Thomas N.L., Williams A.J. Pharmacology of ryanodine receptors and Ca2+ induced Ca2+ release. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012;1:383–397. [Google Scholar]
- Tian F., Qiao C., Luo J., Guo L., Pang T., Pang R., Xie H. Development and validation of a method for the analysis of five diamide insecticides in edible mushrooms using modified QuEChERS and HPLC-MS/MS. Food Chem. 2020;333 doi: 10.1016/j.foodchem.2020.127468. [DOI] [PubMed] [Google Scholar]
- Tobin R., Walsh T., Garvey J., Larkin T. Detection of pesticide residues in organic and conventional fruits and vegetables available in Ireland using gas chromotography/tandem mass spectrometry (GC-MS/MS) and liquid chromotography/tandem mass spectrometry (LC-MS/MS) detection. J. Nutr. Health Food Sci. 2014;1:7–14. [Google Scholar]
- Tohnishi M., Nakao H., Furuya T., Seo A., Kodama H., Tsubata K., Nishimatsu T. Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J. Pest. Sci. 2005;30:354–360. [Google Scholar]
- Tossou E., Tepa-Yotto G., Douro Kpindou O.K., Sandeu R., Datinon B., Zeukeng F., Akoton R., Tchigossou G.M., Djègbè I., Vontas J., Martin T., Wondji C., Tamò M., Bokonon-Ganta A.H., Djouaka R. Susceptibility profiles of helicoverpa armigera (Hübner)(Lepidoptera: noctuidae) to deltamethrin reveal a contrast between the northern and the southern Benin. Int. J. Environ. Res. Publ. Health. 2019;16:1882. doi: 10.3390/ijerph16111882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong K.M., Pessah I.N. Comparison of chlorantraniliprole and flubendiamide activity toward wild-type and malignant hyperthermia-susceptible ryanodine receptors and heat stress intolerance. Toxicol. Sci. 2019;167:509–523. doi: 10.1093/toxsci/kfy256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujváry I. Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Springer; Tokyo: 1999. Nicotine and other insecticidal alkaloids; pp. 29–69. [Google Scholar]
- Vemu B., Dumka V.K. Hematological alterations on sub-acute exposure to flubendiamide in sprague dawley rats. Toxicol. Int. 2014;21:288. doi: 10.4103/0971-6580.155367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinh N.H., Tuoi D.T.H., Tuan N.C., Huy T.P., Nghia T.V.D., Tin N.H., Thao N.H. Simultaneous determination of pesticide residues in food by gaz and liquid chromatography-tandem mass spectrometry (GC-MS/MS & LC-MSMS) Food Safe. Asia. 2010;11:530. [Google Scholar]
- Wagner H., Hikino H., Farnsworth N.R., editors. Economic and Medicinal Plant Research. Academic press; 2012. pp. 111–153. [Google Scholar]
- Wang J., Liu Y., Gao J., Xie Z., Huang L., Wang W., Wang J. Molecular cloning and mRNA expression of a ryanodine receptor gene in the cotton bollworm, Helicoverpa armigera. Pestic. Biochem. Physiol. 2013;107:327–333. doi: 10.1016/j.pestbp.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Wang K., Zhao L., Zhang C., Zhang H., Lian K. Determination of 12 insect growth regulator residues in foods of different matrixes by modified QuEChERS and UPLC-MS/MS. RSC Adv. 2021;11:12162–12171. doi: 10.1039/d1ra00046b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Liu X., Wu X., Xu J., Dong F., Zheng Y. Evaluation of biochars in reducing the bioavailability of flubendiamide in water/sediment using passive sampling with polyoxymethylene. J. Hazard Mater. 2018;344:1000–1006. doi: 10.1016/j.jhazmat.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Wang Q.W., Jiang J.Q., Sui W., Lin X., Liu B. Sensitive molecularly imprinted fluorescence determination of pyrethroids using green zinc oxide quantum dots. Anal. Lett. 2016;50:1139–1149. [Google Scholar]
- Wang Y.J., Zeinhom M.M.A., Yang M.M., Sun R.R., Wang S.F., Smith J.N., Timchalk C., Li L., Lin Y., Du D. A 3D-printed, portable, optical-sensing platform for smartphones capable of detecting the herbicide 2,4-dichlorophenoxyacetic acid. Anal. Chem. 2017;89:9339–9346. doi: 10.1021/acs.analchem.7b02139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A.L., Holden I., Casida J.E. 9, 21-Didehydroryanodine: a new principal toxic constituent of the botanical insecticide Ryania. J. Chem. Soc., Chem. Commun. 1984;19:1265–1266. [Google Scholar]
- Wilkowska A., Biziuk M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011;125:803–812. [Google Scholar]
- Williams J.R., Swale D.R., Anderson T.D. Comparative effects of technical‐grade and formulated chlorantraniliprole to the survivorship and locomotor activity of the honey bee, Apis mellifera (L.) Pest Manag. Sci. 2020;76:2582–2588. doi: 10.1002/ps.5832. [DOI] [PubMed] [Google Scholar]
- Wu M., Zhang S., Yao R., Wu S., Su J., Gao C. Susceptibility of the rice stem borer, Chilo suppressalis (Lepidoptera: crambidae), to flubendiamide in China. J. Econ. Entomol. 2014;107:1250–1255. doi: 10.1603/ec14053. [DOI] [PubMed] [Google Scholar]
- Xu S.J., Zou Y.W., Zhang H.Q. Well-defined hydrophilic “turn-on”-type ratiometric fluorescent molecularly imprinted polymer microspheres for direct and highly selective herbicide optosensing in the undiluted pure milks. Talanta. 2020;211 doi: 10.1016/j.talanta.2020.120711. [DOI] [PubMed] [Google Scholar]
- Yang Y., Wan P.J., Hu X.X., Li G.Q. RNAi mediated knockdown of the ryanodine receptor gene decreases chlorantraniliprole susceptibility in Sogatella furcifera. Pestic. Biochem. Physiol. 2014;108:58–65. doi: 10.1016/j.pestbp.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Zhang M., Zhao C., Shao Q., Yang Z., Zhang X., Xu X., Hassan M. Determination of water content in corn stover silage using near-infrared spectroscopy. Int. J. Agric. Biol. Eng. 2019;12:143–148. [Google Scholar]
- Zhang R., Liu K., Cui Y., Zhang W., He L., Guo S., Wang B. Development of a monoclonal antibody-based ELISA for the detection of the novel insecticide cyantraniliprole. RSC Adv. 2015;5:35874–35881. [Google Scholar]
- Zhang X., Zhang H., Liu J., Xiong L., Li Z. Synthesis and biological evaluation of novel anthranilic diamides containing N-H/CH 3-1 H-pyrazole. Chem. Res. Chin. Univ. 2020:1–6. [Google Scholar]
- Zhang Y., Guo L., Atlihan R., Chi H., Chu D. Demographic analysis of progeny fitness and timing of resurgence of Laodelphax striatellus after insecticides exposure. Entomol. Gen. 2019;39:221–230. [Google Scholar]
- Zhang S.X., Xue S.F., Deng J.J., Zhang M., Shi G.Y., Zhou T.S. Polyacrylicacidcoated cerium oxide nanoparticles: an oxidase mimic applied for colorimetric assay to organophosphorus pesticides. Biosens. Bioelectron. 2016;85:457–463. doi: 10.1016/j.bios.2016.05.040. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Xu L.P., Tong J., Li Y.Q., Xiong L.X., Li F., Li Z.M. Synthesis, crystal structure and biological activity of novel anthranilic diamide insecticide containing alkyl ether group. Mol. Divers. 2012;16:711–725. doi: 10.1007/s11030-012-9406-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wei W., Zhu L., Li Y. Synthesis and bioactivities evaluation of novel anthranilic diamides containing N‐(tert‐Butyl) benzohydrazide moiety as potent ryanodine receptor activator. Chin. J. Chem. 2019;37:605–610. [Google Scholar]
- Zuo Y.Y., Ma H.H., Lu W.J., Wang X.L., Wu S.W., Nauen R., Yang Y.H. Identification of the ryanodine receptor mutation I4743M and its contribution to diamide insecticide resistance in Spodoptera exigua (Lepidoptera: noctuidae) Insect Sci. 2020;27:791–800. doi: 10.1111/1744-7917.12695. [DOI] [PubMed] [Google Scholar]