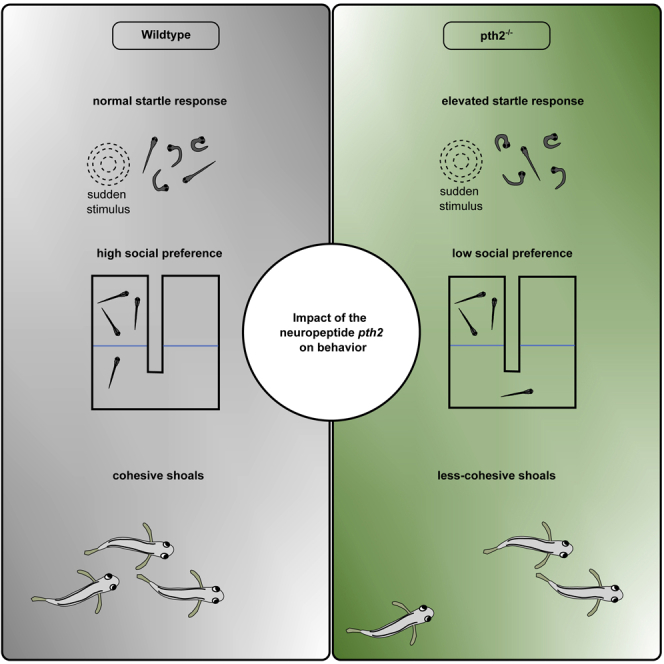
Summary
Behavior is context-dependent and often modulated by an animal’s internal state. In particular, different social contexts can alter anxiety levels and modulate social behavior. The vertebrate-specific neuropeptide parathyroid hormone 2 (pth2) is regulated by the presence of conspecifics in zebrafish. As its cognate receptor, the parathyroid hormone 2 receptor (pth2r), is widely expressed across the brain, we tested fish lacking the functional Pth2 peptide in several anxiety-related and social behavior paradigms. Here, we show that the propensity to react to sudden stimuli with an escape response was increased in pth2−/− zebrafish, consistent with an elevated anxiety level. While overall social preference for conspecifics was maintained in pth2−/− fish until the early juvenile stage, we found that both social preference and shoaling were altered later in development. The data presented suggest that the neuropeptide Pth2 modulates several conserved behaviors and may thus enable the animal to react appropriately in different social contexts.
Subject areas: Biological sciences, Behavioral neuroscience, Molecular neuroscience
Graphical abstract
Highlights
-
•
Lack of the neuropeptide pth2 increases startle responsiveness in zebrafish
-
•
Social preference is decreased at the late juvenile stage in pth2 mutants
-
•
Pth2 mutant fish form less cohesive shoals
Biological sciences; Behavioral neuroscience; Molecular neuroscience
Introduction
Different aspects of animal behavior, such as aggression (Tulogdi et al., 2014; Zelikowsky et al., 2018; Agrawal et al., 2020), anxiety (Meyer et al., 2017; Shams et al., 2017), or interaction with conspecifics (Shams et al., 2018; Groneberg et al., 2020), are strongly modulated by an animal’s social context. In several cases, neuropeptides have been shown to regulate this behavioral plasticity (Zelikowsky et al., 2018; Agrawal et al., 2020; Gemmer et al., 2021). Recently, we found that the expression of the neuropeptide pth2 (initially described as tuberoinfundibular peptide of 39 residues or tip39 (Usdin, 1997)) is quantitatively regulated by the density of conspecifics in zebrafish (Anneser et al., 2020). In rodents, pth2 is involved in the regulation of maternal behavior (Coutellier et al., 2011), pain processing (Dimitrov et al., 2013), oxytocinergic signaling (Cservenák et al., 2017a), and fear learning (Coutellier and Usdin, 2011). In addition, social housing with conspecifics was recently found to increase pth2 levels in a thalamic nucleus (Keller et al., 2022). In zebrafish, the cognate receptor of this neuropeptide, pth2r, is expressed in approximately 10% of all neurons, suggesting a potential broad-scale influence (Anneser et al., 2020). It has not been described, however, whether the presence or absence of pth2 alters behavior or other biological features in teleosts.
To identify potential roles of pth2, we obtained a line (pth2sa23129) carrying a premature stop codon which terminates translation at amino acid 55 (out of 157) (Kettleborough et al., 2013). In the experiments described below, we tested how the absence of Pth2 influences anxiety-related and social behaviors in larval and late juvenile zebrafish.
Results
Pth2sa23129 fish lack a functional Pth2 peptide
To investigate the potential roles of Pth2, we in-crossed heterozygous mutants and analyzed the viability of homozygous pth2−/− fish by observing them for several weeks. We validated the complete absence of Pth2 both by sequencing and by immunostaining (Figure 1). The loss of Pth2 did not lead to differences in either the survival rate or body length of mutant fish (Figure S1). This observation is consistent with data from rodents, in which the deletion of PTH2 did not induce obvious external phenotypic effects except for decreased fertility (Usdin et al., 2008). However, in contrast to rodents, matings between pth2−/− zebrafish led to viable offspring.
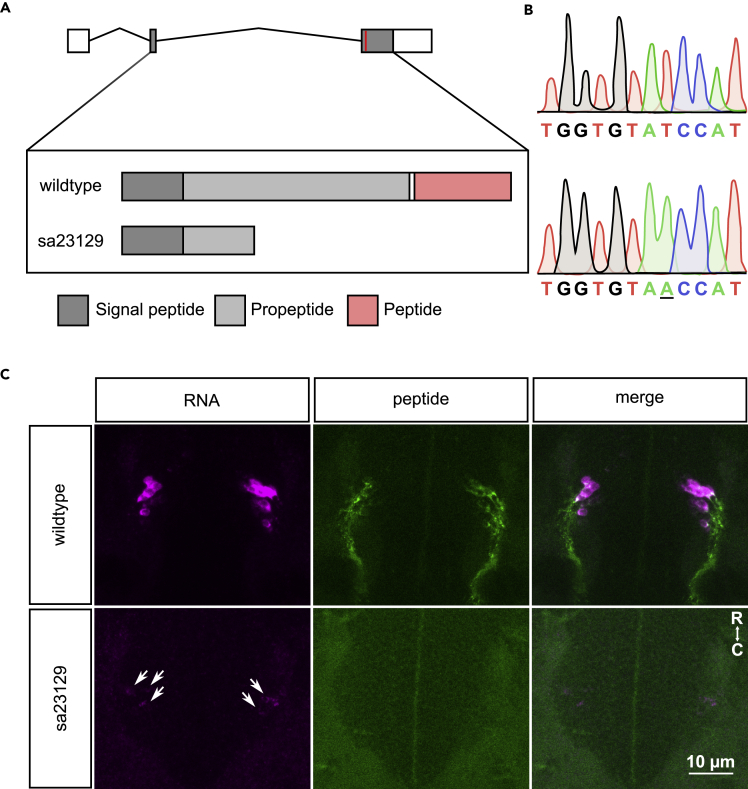
Figure 1.
Validation of pth2sa23129 fish lacking a functional peptide
(A) The pth2 gene consists of three exons; the coding sequence (CDS) is contained in exon 2 and 3. In pth2sa23129 fish, a T/A point mutation induces a premature stop codon within the propeptide sequence, thus preventing the successful translation and cleavage of the Pth2 peptide.
(B) Homozygous mutants were validated by sequencing and the premature ochre stop codon was identified at base pair 165 out of 474 of the CDS.
(C) Pth2+ cells are only found at the lateral edge of the dorsal thalamus, imaged here in a dorsoventral orientation. Transcript levels of pth2 were strongly decreased in pth2−/− animals, as demonstrated by fluorescent in-situ hybridization, although some remaining signal was detected (arrowheads). Abolished Pth2 translation was validated using a custom antibody recognizing a 27-mer epitope immediately preceding the peptide sequence (Anneser et al., 2020). For both wildtype and pth2−/−, 6 larvae at 6 dpf were imaged. Scale bar: 10 μm. R: rostral, (C) caudal. See also Figure S1
Lack of Pth2 increases startle responsiveness
In rodents, administration of Pth2 has anxiolytic effects (LaBuda et al., 2004) and animals lacking Pth2 show increased anxiety-related behavior (Fegley et al., 2008). A common assay to investigate anxiety-related behavior in zebrafish is the startle response (Burgess and Granato, 2008; Reider and Connaughton, 2015; Tomasi et al., 2020), in which fish are presented with a sudden stimulus and react with stereotyped escape responses, so-called C-starts (Burgess and Granato, 2007). Previous work showed that higher rearing densities in zebrafish decreased the likelihood of a startle response (Burgess and Granato, 2008). As pth2 levels are strongly influenced by the number of conspecifics (Anneser et al., 2020), we tested pth2−/− and pth2+/+ fish at 5 days post fertilization (dpf) in a startle paradigm. We measured when and how often mutant and wild-type fish responded to a vibrational cue with an escape (Figure S2A and Figure 2A). We observed a typical bimodal distribution characterizing the startle response with fish either exhibiting a short- or long-latency startle response (SLC or LLC, Figure 2B; Burgess and Granato, 2007) mediated by Mauthner cells (Burgess and Granato, 2007) or a prepontine cell population, respectively (Marquart et al., 2019). When we compared the time-to-response after stimulus onset between pth2−/− and pth2+/+ animals, we found SLCs occurred significantly earlier in mutants (Figure 2C), suggesting that pth2−/− fish respond more quickly to potential threats (Troconis et al., 2017). In general, pth2−/− fish exhibited a higher overall startle response rate (Figure 2D). We analyzed how the proportion of SLCs, LLCs, and non-responding animals per trial were related to the genotype of the fish and found significant interactions between these ratios, indicating that for both SLCs and LLCs, pth2−/− fish showed higher response rates (Figure 2E). To test whether these interactions result in joint response properties reliably indicating the genotype of the tested fish, we fit a logistic regression model relating response ratios and genotype and found the increased overall response rate of mutant fish to be a significant predictor of the pth2−/− genotype (Figures S2B–S2D).
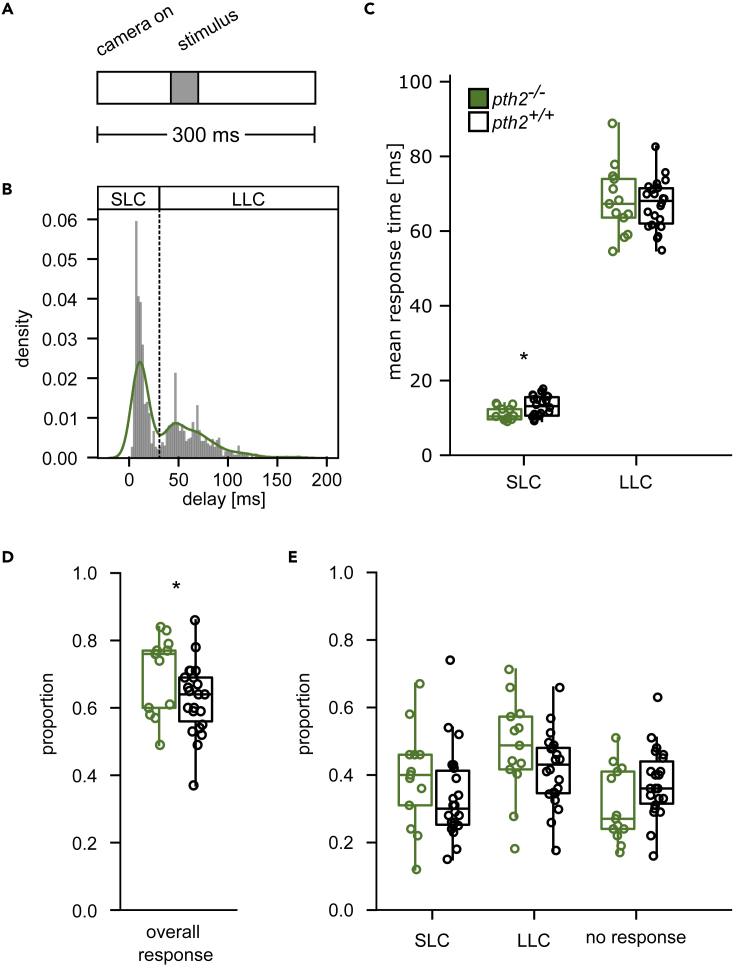
Figure 2.
pth2−/− fish display increased startle propensity
(A) Experimental scheme. Ten larval fish (5 dpf) were recorded with 500 Hz for 300 ms. After a baseline of 100 ms, a 40-ms pulse was delivered using a bass shaker.
(B) The histogram and overlaid kernel density estimate show the delay with which animals respond after the onset of the stimulus with a C-start. We recorded 1613 escape responses from 220 wild-type fish and observed the bimodal distribution characteristic of Mauthner-cell-mediated short-latency C-starts (SLC) (Burgess and Granato, 2007) and long-latency C-starts (LLC) mediated by a prepontine cell group (Marquart et al., 2019).
(C) Delay after stimulus until escape onset is shown as a boxplot for wild-type and pth2−/− animals. Single dots represent the mean of individual experiments. We tested the mean response rate differences between genotypes using a Mann-Whitney-U test and found SLCs in pth2−/− fish occured slightly earlier (pU=79 = 0.015, Cohen’s d = 0.81, μpth2−/− = 10.9 ms, μpth2+/+ = 12.8 ms), while LLCs were indistinguishable between genotypes (pU=138 = 0.439, Cohen’s d = 0.13, μpth2−/− = 66.9 ms, μpth2+/+ = 66.0 ms) (D) Boxplots show the fraction of animals that react to a stimulus with an escape response. Fractions were calculated for groups of 10 animals, with n = 13 for pth2−/− and n = 22 for wildtype. The proportion of pth2−/− animals performing an escape response as reaction to a startle stimulus was significantly increased as compared to wildtype (Mann-Whitney-U test, pt=92.0 = 0.042, Cohen’s d = 0.68, μpth2−/− = 0.7, μpth2+/+ = 0.62).
(E) The boxplots show the proportion of pth2−/− and wild-type animals responding to a startle stimulus with an SLC, LLC, or the absence of a response. While no main effects were observed, we found the interaction between all response types was significantly related to the genotype in a logistic regression model (pF=6.95 = 0.013), arguing for a cross-over interaction. See also Figure S2.
Maintenance of social preference is disrupted in pth2−/− fish
As pth2 levels are strongly influenced by the social environment of an animal (Anneser et al., 2020), we queried whether the absence of pth2 might alter social preference for conspecifics or other features of their social interactions. We tested the propensity of animals to stay close to conspecifics in two different paradigms. First, we placed animals in a U-shaped chamber which allowed the fish to freely explore both arms (Dreosti et al., 2015); at the end of only one arm, the fish could see conspecifics, separated by a transparent wall. After a habituation period, three conspecifics were placed in one of the arms and the time the experimental fish spent in the corresponding area was measured (Dreosti et al., 2015) (see Figure 3A). At the early juvenile stage (21 dpf), both pth2+/+ and pth2−/− animals showed a clear preference for the arm containing the conspecifics and no difference between genotypes was apparent (Figure 3B). As the development and maintenance of social preference is modulated by social context well after 21 dpf (Gemmer et al., 2021), we raised wild-type and mutant animals until the late juvenile stage (56 dpf) and tested them again. At this stage, while pth2+/+ animals still displayed a strong preference for the conspecific-containing arm, pth2−/− fish did not (Figure 3C). Consistent with these results, we found that rearing of wild-type fish in isolation (which reduces pth2 levels) abolished the social preference at 56 dpf as well (Figure 3D). The second social paradigm we tested used a rectangular tank, in which the animals could see (again, separated by a transparent barrier) three age-matched fish, placed in one of two adjacent areas, from any location. We used the fraction of time the animals spent in the half of the arena closer to the social area to measure social preference (Figure 3E). Just as we observed in the U-shaped chamber, no difference was found at 21 dpf, but lack of pth2 led to a trend for a social preference decrease at 56 dpf, although it did not reach statistical significance (Figures 3F and 3G). Again, the social isolation of wild-type fish recapitulated these effects (Figure 3H).
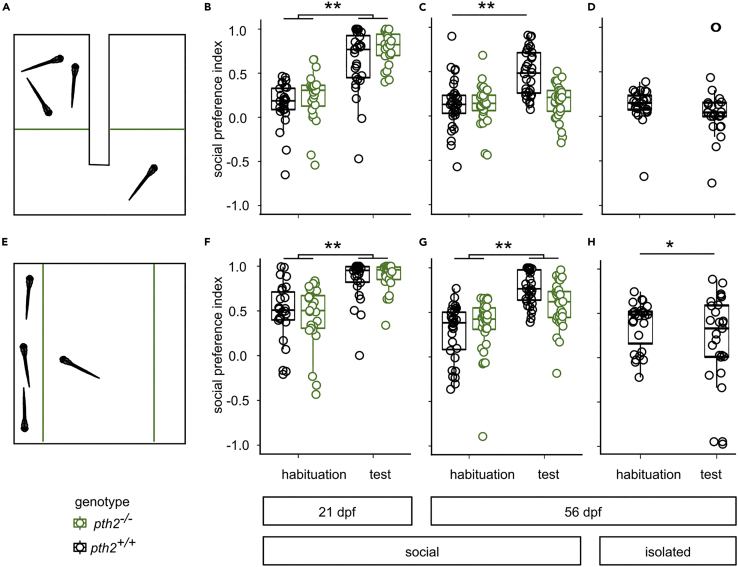
Figure 3.
Maintenance of social preference is disrupted in pth2−/− mutants
(A) Scheme of the U-shaped chamber, in which the experimental fish could spend time in either of two adjacent compartments, separated by acrylic glass (green line) (Dreosti et al., 2015). Visual access to three age-matched wild-type conspecifics was only possible in one of the two areas.
(B) Boxplots indicate the preference for the social area displayed by pth2+/+ (n = 28) and pth2−/− fish (n = 24) at 21 dpf during habituation and in response to the presence of stimulus fish. While animals showed no preference during the habituation period, they spent a significantly increased amount of time close to conspecifics during the test period (two-way ANOVA, pF=93.02 = 5.94e-16).
(C) At 56 dpf, both the presence of conspecifics (two-way ANOVA, pF=20.4 = 0.000015) and the genotype of the animals (two-way ANOVA, pF=13.94 = 0.0003; n = 32 for wildtype and 31 for mutants) contributed significantly to social preference. Additionally, we found a significant interaction between these factors (pF=13.89 = 0.0003). A post-hoc Tukey-Kramer test indicated that pth2+/+ fish displayed a significant increase in SPI under test conditions (pq=8.25 = 0.001), whereas pth2−/− fish did not (pq=0.72 = 0.9).
(D) Boxplots show the effect of rearing animals in isolation on social preference. In 56 dpf animals, the presence of conspecifics led to no detectable change in behavior (n = 25; paired, two-sided t-test: pt=0.78, df =23 = 0.44, effect size = 0.13, mean social preference during habituation: 0.12, during test conditions: 0.08).
(E) Scheme for open field social preference test, in which fish could move freely in a rectangular dish, while having visual access to three conspecifics.
(F) Boxplots indicate social preference of wild-type (n = 26) and pth2−/− fish (n = 25) at 21 dpf during the habituation period and in response to stimulus fish. The presence of conspecifics during the test period altered behavior of all animals significantly (two-way ANOVA, pF=53.41 = 7.35e-11), while genotype did not influence behavior (two-way ANOVA, pF=0.46 = 0.49).
(G) At 56 dpf, the presence of conspecifics (pF=56.23 = 1.14e-11) and its interaction with the test animal’s genotype (pF=7.25 = 0.008) significantly altered social preference (for pth2−/−, n = 32, for pth2+/+, n = 31). A post-hoc Tukey-Kramer test indicated a significant preference for the arm with conspecifics (when compared to the habituation period) in pth2+/+ fish (pq=10.15 = 0.001, effect size: 1.93). In pth2−/− fish, the same effect was present, although less pronounced (pq=4.88 = 0.004, effect size: 0.82).
(H) After 8 weeks of social isolation, pth2+/+ fish displayed no social preference toward conspecifics and rather exhibited an avoidance reaction (two-sided, paired t-test: pt=2.06 = 0.049, n = 27)
Shoal cohesion is decreased in pth2−/− fish
Zebrafish groups display collective behavior, characterized by the coordinated swimming of individuals (Miller and Gerlai, 2012). Several individual genes have been shown to contribute to this behavior (Tang et al., 2020). As our previous results indicated that pth2 is regulated by the presence of conspecifics, we tested whether its absence might influence collective behavior. At 56 dpf, groups of 20 pth2+/+ or pth2−/− fish were placed in a circular tank and their behavior was recorded for 30 min. Animals of either genotype immediately adapted their movement to the group and explored the entire tank together (Figures 4A and S3A). Across the duration of the trials, pth2+/+ fish reached higher average swim velocities (Figure 4B). As zebrafish move faster in more polarized groups (Couzin et al., 2002; Miller and Gerlai, 2012; Tang et al., 2020), we analyzed cohesiveness and alignment of the animals. We measured inter-individual distances including the nearest neighbor distance, the median distance, and the maximum distance, and found that the maximum distance between individuals was consistently increased in pth2−/− animals (Figures 4C and S3B), suggesting a lower degree of cohesion. To measure the alignment between the animals, we computed the degree to which individual trajectories could be explained by the movement of the group centroid (Figure 4D). To this end, we computed a polarization parameter p that was determined by calculating how much variance within the matrix consisting of all 20 individual trajectories was explained by the two first principal components obtained in a principal component analysis, which closely reflected centroid movement. This polarization parameter was significantly lower in pth2−/− animals across all trials (Figures 4E and 4F), further supporting the notion that collective motion of pth2−/− fish is less coordinated. When we counted the number of times that individual animals clearly broke away from the main group, we also found that both the total number of excursions and the time spent away from the main group were higher in the groups of pth2−/− fish (see Figures 4G and 4H).
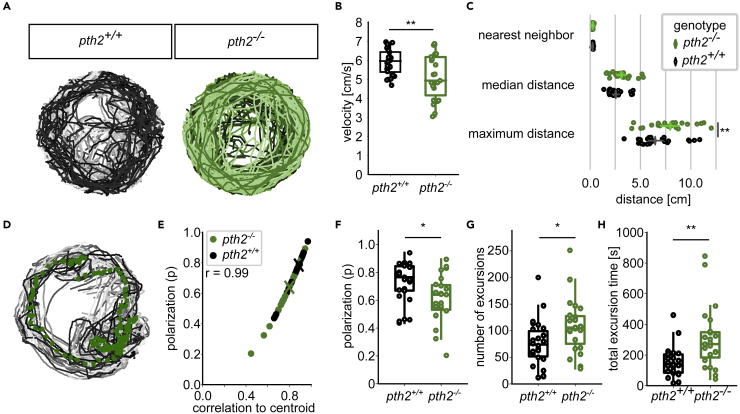
Figure 4.
Fish move in a less cohesive manner in groups of pth2−/− animals (A) Example traces of 20 fish over 1 min in the behavioral tank
Fish movement was strongly coordinated across all replicates. For pth2−/−, n = 26, for wildtype, n = 29.
(B) Boxplots depict the median movement speed of fish over 30 min. Wild-type animals moved on average faster than fish without pth2 (unpaired, two-sided t-test, pt=2.938, df=50 = 0.01, median velocity of wildtype: 5.9 cm/s, for mutant: 4.9 cm/s).
(C) Dot plots highlight the increased distance between pth2−/− fish when compared to wild-type animals. In an ANOVA, genotype was found to be a significant factor for distance (pF=10.15 = 0.002), Tukey’s post-hoc range test then showed the maximum distance was significantly different between genotypes (pq=5.21 = 0.004, median maximum distance in wildtype: 4.60 cm, for mutants: 6.49 cm). All datapoints shown are the median across all fish within one experiment.
(D) Trajectories of wild-type animals over 1 min with the trajectory of the shoal centroid overlaid as green dotted line.
(E) Polarization of animals was strongly correlated with the mean of the individual animals' correlation to the shoal centroid. Median values for pth2−/− and pth2+/+ are indicated by the colored x (Pearson’s r = 0.99, pt=94.92 = 1.2e-55).
(F) The animals' polarization was significantly increased in wild-type animals (unpaired, two-sided t-test, pt=2.62,df=50 = 0.022, effect size: 0.75, mean of mutants: 0.61, mean of wildtype = 0.72).
(G) Wild-type animals were more likely to consistently move in coherent groups, as evidenced by the increased propensity of pth2−/− animals to split away from the main group (unpaired, two-sided t-test, pt=2.51, df=50 = 0.032, effect size = 0.72, mean number of excursions in mutants: 108.9, in wildtypes: 75.5).
(H) The smaller number of excursions in wild-type animals was not compensated for by a longer total excursion time (unpaired, two-sided t-test, pt=3.40, df=50 = 0.002, effect size: 0.97, mean total excursion time in mutants: 303.9 s, in wildtype: 151.9 s)
Discussion
In many animal species, the social context modulates the abundance of neuropeptides and thus influences physiology and behavior (Pan et al., 2009; Slavich and Cole, 2013; French et al., 2016; Zelikoswky et al., 2018). Here, we demonstrate that the socially regulated neuropeptide pth2 influences anxiety-related and social behaviors in zebrafish. pth2 has been shown to regulate similar behaviors in other species, suggesting an evolutionarily conserved role for this peptide across vertebrates. For example, several lines of evidence suggest the involvement of pth2 in the regulation of anxiety: intracerebroventricular administration of the peptide in rodents led to an increase of time spent in the open arms of an elevated plus maze (LaBuda et al., 2004). In other experiments, mice lacking a functional gene copy of either the neuropeptide or its receptor showed elevated levels of freezing when placed in a context where they had previously received foot shocks (Coutellier and Usdin, 2011). Here, we showed that zebrafish lacking Pth2 have a higher propensity to react to sudden stimuli with an escape response. Startle responses are stereotyped but can be modulated; states of elevated anxiety have been shown to increase startle responsiveness in rats (Walker and Davis, 1997) and humans (Grillon et al., 1999). Interestingly, social deprivation led to the same phenomenon in rats and monkeys (Wilkinson et al., 1994; Parr et al., 2002), suggesting pth2 is involved in the regulation of anxiety states.
Increased anxiety has often been associated with social isolation. A common finding is that rodents tend to visit the open arms in elevated plus mazes less after experiencing social isolation (Pan et al., 2009; Kumari et al., 2016; Nakagawa et al., 2019). The acquisition and retention of fear memory has also been shown to be enhanced in socially isolated animals (Lukkes et al., 2009; Liu et al., 2015; Zelikowsky et al., 2018). One of the key benefits of sociality is the protection from predators by diluting the risk of being targeted (Hamilton, 1971; Vine, 1971). As such, a logical consequence of social isolation is to increase vigilance and respond to potentially threatening stimuli at a lower threshold (Hawkley and Cacioppo, 2010). For example, fruit flies display a graded decrease in freezing in response to threatening stimuli that is proportional to group size (Ferreira and Moita, 2020). Our results suggest that Pth2 might contribute to the regulation of appropriate vigilance states in vertebrates. In our experiments, we analyzed the effect of complete and chronic ablation of pth2. As pth2 is quickly and bidirectionally regulated by the social environment (Anneser et al., 2020), it remains to be determined how quickly the observed behavioral effects are induced and whether they scale with group size.
In three different paradigms assessing social interaction, we found pth2−/− animals displayed altered behavior. In zebrafish, social preference develops with age and reaches peak levels at around three weeks of development (Dreosti et al., 2015). Here, we showed, that up until 21 dpf, no difference was found between pth2−/− and pth2+/+ animals in either of two social preference paradigms. However, 56 dpf pth2−/− animals showed a clear decrease in social preference, suggesting a role for pth2 in the maintenance of social preference at later developmental stages. Previous studies have shown that social preference can be altered by rearing conditions (Tunbak et al., 2020; Gemmer et al., 2021), which also affect the maintenance of social preference at later developmental stages (Gemmer et al., 2021). Consistent with these findings, pth2−/− animals form less cohesive shoals than pth2+/+ fish and swim in a less polarized manner. Features of collective behavior such as group alignment and shoal cohesion are genetically controlled (Tang et al., 2020; Aspiras et al., 2021), suggesting that pth2 might be one of the effector genes that contributes to the regulation of group behavior.
In previous studies, the impact of pth2 on social behavior had only been analyzed in depth in rodents. Social behavior in mammals is quite distinct from fish, so that a direct comparison between previous results and ours is hard to make. Previous studies have convincingly shown that the neuropeptide contributes to rodent maternal behavior (Cservenák et al., 2010, 2013; Gellén et al., 2017; Dobolyi et al., 2018) and the posterior intralaminar thalamus, where one of the thalamic Pth2+ populations is found in mammals, integrates social cues, eventually activating oxytocinergic neurons (Cservenák et al., 2017a; Dobolyi et al., 2018; Valtcheva et al., 2021).
Several different neuromodulatory systems contribute to the regulation of anxiety (Zelikowsky et al., 2018; Shiozaki et al., 2020) and social behavior (Stednitz et al., 2018; Tang et al., 2020). Indeed, work on the genetic control of shoaling behavior has identified over 40 genes that contribute to kinematic features of collective motion (Tang et al., 2020). Across metazoans, it has been shown repeatedly that different neuromodulators act together to encode brain states or co-tune network function (Marder and Bucher, 2007; Lovett-Barron et al., 2020; Xu et al., 2020). In this context, the analysis of behavioral contributions of individual neuropeptides must be considered a first step toward a more comprehensive analysis of the way pth2 interacts with other modulatory substances and alters circuit dynamics. For example, previous work in rodents showed that Pth2+ cells project to neurons in the preoptic area expressing oxytocin (Dobolyi et al., 2018) and galanin (Cservenák et al., 2017b), suggesting that activity of Pth2+ cells could be altering the state of those modulatory networks.
Neuropeptidergic modulation of neuronal circuits is an ancient motif of neuronal information processing (Jékely, 2013, 2021) and although adaptation of neuropeptidergic circuits for new functions is common, some neuropeptides are remarkably consistent in the kind of modulation they induce (Minakata, 2010). Both zebrafish and human Pth2 peptides efficiently activate Pth2r in the other species, arguing for a well-conserved system (Papasani et al., 2004). Here, we provide evidence that lack of Pth2 induces changes in anxiety levels and social behavior in zebrafish, behaviors that have been shown to be affected by Pth2 in other species. In several other paradigms, social behavior and anxiety have been found to be co-modulated (Felix-Ortiz et al., 2016; Zou et al., 2016; Zelikowsky et al., 2018). Pth2 might thus serve a conserved function in the regulation of these important behavioral features.
Limitations of the study
In this study, we explored the effects of a loss-of-function mutation in the gene pth2 on the startle response and social behavior in zebrafish. Although we observed clear behavioral changes in startle responsiveness, we did not test whether improved sensory perception or a reduced threshold in the effector circuits is responsible for the behavioral difference. Additionally, the startle response is just one of many possible measures of anxiety levels in zebrafish. This study did not test whether the absence of Pth2 leads to similar effects in other behavioral settings. Our study addressed the impact of a single mutation, which might be linked to other alleles closeby. Although animals were outcrossed several times before experiments, and independent crosses were used for all individual experiments, the results presented should be revisited using independent mutants. In our experiments testing social preference, we found that the absence of Pth2 led to a decline in social preference in 56-dpf old animals, which was recapitulated by raising animals in isolation. However, we did not investigate whether at this stage short-term isolation (which also shuts down Pth2 transcription) is sufficient to disrupt social preference. Finally, in our shoaling experiment we did not investigate individual differences owing to uncertainties about tracking individual fish for the entirety of the trial. Further work will be necessary to elucidate the precise forms of interaction deficits in pth2−/− fish.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| anti-pth2 (raised in guinea-pig) | Peptide Specialty Laboratories (custom antibody) (Anneser et al., 2020) |
RRID:AB_2905556 |
| Goat Anti-Guinea pig IgG H&L (Alexa Fluor® 594) | Abcam | ab150188; RRID:AB_2905555 |
| Chemicals, peptides, and recombinant proteins | ||
| NaCl | Sigma | Cat#S5886-1KG |
| KCl | Sigma | Cat#P9541-1KG |
| CaCl2 | Sigma | Cat#C5670 |
| MgSO4 | Sigma | Cat#M7506-500G |
| NaOH | Sigma | Cat#S2770 |
| Tris-HCl, 1 M | Sigma | Cat#10812846001 |
| Deposited data | ||
| Data repository | This study. | https://doi.org/10.17617/3.6v |
| Analysis Code repository | This study | https://github.com/Anneser/zfish_PTH2 |
| Experimental models: Organisms/strains | ||
| zebrafish: pth2sa23129 | European Zebrafish Resource Center | RRID:ZIRC_ZL12243.19 |
| zebrafish: Konstanz wildtype | MPI Brain Research | N/A |
| Oligonucleotides | ||
| CTATGTTTCTCTTCTGCTGGTGAC | This study | pth2 reverse primer |
| GCTGTTAGGCGAGTGTC | This study | pth2 forward primer |
| Software and algorithms | ||
| Zen 3.4 (blue edition) | Zeiss | N/A |
| Pylon Recorder Software | MPI for Brain Research: Scientific Computing; https://software.scic.brain.mpg.de/projects/PylonRecorder/PylonRecorder | N/A |
| Jupyter notebook 6.0.0 | Project Jupyter: https://jupyter.org/index.html | N/A |
| Arduino IDE 1.8.13 | Arduino: https://www.arduino.cc/en/Main/Software_ | N/A |
| Matlab 9.11.0.1751886 (R2021b) | MathWorks: https://ch.mathworks.com/de/products/matlab.html | N/A |
| TRex | Walter and Couzin, 2021 | N/A |
| Anaconda Navigator 1.9.7 (64-bit version) | Anaconda: https://anaconda.org/anaconda/anaconda-navigator | N/A |
Resource availability
Lead contact
Further information and requests for resources should be directed to the lead contact, Erin Schuman (erin.schuman@brain.mpg.de)
Material availability
This study did not generate new unique reagents
Experimental model and subject details
Adult zebrafish of the lines Konstanz wildtype (KN) and Pth2sa23129 (RRID:ZIRC_ZL12243.19, referred to as pth2−/−) were kept at 28°C on a light cycle of 14-h light/10-h dark and housed in 3.5 L ZebTEC tanks at a density of 5–35 fish of mixed sexes. Heterozygous Pth2sa23129 fish were obtained from the European Zebrafish Resource Center (EZRC, Karlsruhe, Germany) and outcrossed to our standard wildtype strain three times to reduce potential background mutations. Fish were then maintained as homozygous mutants. Mutants and wildtypes used in experiments were the offspring of mutant adults and sibling wildtype fish. All experimental groups were obtained from independent crosses of different parents. Fish were fed with brine shrimp (Artemia salina) and/or GEMMA Micro three times per day. In addition, vinegar eelworms (Turbatrix aceti) were fed to larval and juvenile fish. Larvae up to 5 days post fertilization (dpf) were kept in dishes filled with E3 medium (5 mM NaCl, 17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4) in a 28°C incubator also in a 14-h light/10-h dark cycle. All animal procedures conformed to the institutional guidelines of the Max Planck Society and were approved by the Regierungspräsidium Darmstadt, Germany (governmental ID: V 54-19 c 20/15-F126/1016). At the developmental stages used in this study, the animal’s sex coudl not be clearly determined and was not further considered. All animals were screened for obvious phenotypic defects and only healthy animals were included.
Method details
Genotyping
To validate the genotype of the animals used in our experiments, all fish were genotyped after completion of the experiments. Genomic DNA was extracted from parts of the tail by placing tissue in 50 μl 50 mM NaOH. Samples were heated to 95°C for 15 min under vigorous shaking. Tubes were cooled to 4°C and 5 μl of 1 M Tris-HCl (pH 8.0) were added for neutralization. Tubes were centrifuged for 5 min at 8,000 rcf and 1 μl supernatant was used for PCR. The pth2sa23129 fish are characterized by a T→A point mutation in the pth2 gene that leads to a premature stop codon at amino acid 55/157. The flanking region was amplified using the primer pair 5’-GCTGTTAGGCGAGTGTC-3’ and 5’-CTATGTTTCTCTTCTGCTGGTGAC–3’, genotype was then determined by sequencing.
Whole-mount in-situ hybridization and immunohistochemistry
Fixation and staining were performed as described previously (Herget et al., 2014). Pth2 mRNA was visualized using a previously published in-situ probe (Anneser et al., 2020). A custom antibody raised in guinea pig against zebrafish Pth2 (Anneser et al., 2020) was used in a dilution of 1:500. Secondary antibodies were tagged with Alexa594 dyes and used in a dilution of 1:1000. After in-situ hybridization and immunohistochemistry, animals were transferred stepwise into 87% glycerol and mounted dorsally for imaging using an inverted confocal microscope (LSM-780, Zeiss, Jena, Gemany). For all conditions, animals were imaged with a 20x air objective, using a 488 nm (92 μW) or a 594 nm laser (39 μW).
Startle response
For this behavioral paradigm, animals were reared in specified densities of 10 animals per 20 ml of E3 in dishes of 6 cm diameter as of 3 dpf. At 5 dpf, fish were transferred within their dish to the behavioral chamber, which was placed in a fully enclosed steel casing that reduced outside visual and auditory stimulation. After 5 min of habituation, the experiment commenced. Vibrational cues were delivered with a Monacor EX-1W bass shaker (Conrad Electronics, 1594623), which was activated by a function generator (Hewlett Packard, 3312A Function Generator), amplified by a Kemo M034N power amplifier (Conrad Electronics, 191010). Stimulus frequency was set to 70 Hz and generated as a sine wave with an intensity pre-determined to induce startle responses in approximately 70% of wildtype fish. Delivery was controlled by an Arduino Mega 2560 Rev3 (Arduino), which jointly triggered the bass shaker and a Redlake MotionXtra HG-SE high-speed camera, which recorded at 500 Hz at 512 × 512 pixel resolution. Homogeneous illumination was achieved with an LED-72T ring light around the objective, placed 10 cm above the setup. 100 ms after camera onset, a 40 ms startle cue was delivered. In total, animal behavior was recorded for 300 ms. This was repeated 10 times with a 15 s inter-trial interval, which was previously reported to prevent habituation (Burgess and Granato, 2007). Using a custom-written MATLAB script, we manually assessed the onset of startle-responses, which were defined by the characteristic C-shape in which the animal bends (Burgess and Granato, 2007). Startle responses in zebrafish occur in two waves, the short-latency C-start (SLC) and the long-latency C-start (LLC). Using the latency histogram of the escape onset, which was calculated as the time between the onset of the startle stimulus delivery and the first frame in which the animal began to bend into the characteristic C-shape, we found the typical bimodal distribution reported previously (Burgess and Granato, 2007) and determined the trough between the two kinds of responses to occur at 30 ms after stimulus onset. This value was then used to distinguish between SLCs and LLCs. The average fraction of fish responding with an SLC, an LLC, or not at all was computed across all ten trials for each experiment.
Social preference testing
Fish were raised in densities of 30 fish per 3.5 liter until 21 dpf. At this day, animals were placed in one of two behavioral chambers. For the open field social preference task, the setup consisted of a rectangular chamber (height 10 mm) with a test area of 25 × 75 mm and adjacent stimulus areas of 8 × 75 mm (21 dpf) or 50 × 75 mm and 16 × 75 mm (56 dpf). For the other social preference task, animals were placed in a U-shaped chamber of previously published design (Dreosti et al., 2015), with a total area of 40 × 32 mm. Areas for the stimulus fish were 15 × 15 mm in dimension and the width of the connection between the two arms was 6 mm (21 dpf). At 56 dpf, total area was 72 × 60 mm with stimulation area of 28 × 28 mm and the width of the connection 15 mm. Water level was adjusted to 5 mm. White backlight illumination was provided from below with a computer screen and the animals were recorded at a temporal resolution of 20 Hz. To reduce outside stimulation, behavioral chambers were placed in a sound-absorbing steel box. Before each experiment, the chambers were cleaned with hot water (∼60°C) and refilled with fresh ZebTEC stand-alone system water (28.5°C). Experimental fish were placed in the middle of the behavioral chamber and recorded for 10 min using the Pylon recorder software (https://software.scic.brain.mpg.de/projects/PylonRecorder/PylonRecorder) without any stimulus fish present (habituation period). Afterwards, three stimulus fish were placed in one of the adjacent stimulus areas and behavior was recorded for another 15 min. To compute the social preference index, the location of the animal was tracked using custom-written software and the fraction of time the animals spent either in the “social” half of the test area (open field social preference) or in the arm of the U-shaped chamber which allowed visual access to conspecifics was calculated in MATLAB, following a previously published formula (Dreosti et al., 2015). Data was excluded from further analysis if the test fish’s average swim speed was below an age-and tank-specific threshold. This threshold was determined as 60% of the average swimming speed of each group (socially reared pth2+/+, socially reared pth2−/−, at 8 wpf also: isolation reared pth2+/+). In total, 20 fish were excluded from further analysis.
Collective behavior
Fish were raised until 2 months of age at densities of 50 fish per 3.5 L. To observe collective behavior, 20 fish were placed in a setup consisting of a white round exploration tank with a diameter of 70 cm. The tank was placed in a basin filled with 29.5°C water (heated by a pump) to maintain the temperature of the water inside the behavioral chamber for the 30 min of experiment. A high-resolution camera (Basler acA4112-30um), positioned 73 cm above the chamber, recorded the fish (30 frames per second) using the Pylon recorder software and 1200 white LEDs in the ceiling provided homogeneous illumination (approximately 650 Lux). A white box surrounding the setup reduced visual and acoustic disturbances of the fish. For each replicate, the chamber was cleaned and refilled with 3.0 Lliter 28.5°C warm system water. Before the experiments, home tanks were moved from the ZebTEC system to a 28.5°C incubator in the experimental room. Approximately 10 min before the experiment started, 20 fish of similar size were transferred from their home tank to a 1L breeding cage (TECNIPLAST) with a nursery insert (TECNIPLAST, Part Number: ZB300BTI). This way, all 20 fish were moved to the center of the behavioral chamber at once. Recording started immediately and continued for 30 min. Individual trajectories were obtained using the TRex software (Walter and Couzin, 2021) with a tracking threshold of 80.
Quantification and statistical analysis
Outcome measures
For the collective behavior experiment, several measurements were computed from the obtained individual trajectories. For each frame, all pairwise distances between the 20 fish were computed. From this distance matrix, we computed the median of the nearest-neighbor (NN), the median of the maximum-distance and the median distance across all fish. Velocity was calculated as the distance travelled between consecutive frames, and then recorded as cm per second. Ultimately, the median velocity across all fish was computed. Additionally, the inter-quartile range for velocity was calculated to obtain a measurement for consistency of movement. Cumulative shoal distance was calculated by computing the centroid of the group of fish and cumulatively adding the distance this centroid covered between consecutive frames. To analyze group cohesion, we calculated the area occupied by the smallest convex hull we could fit around the group using standard algorithms (Barber et al., 1996) implemented in scipy (Virtanen et al., 2020). Additionally, we calculated dispersion of the animals as follows: we first calculated the randomized nearest-neighbor distance by temporally shuffling locations of animals within a video (NNshuffled). Dispersion was then computed according to the following formula: dispersion = log(NN / NNshuffled) (Harpaz et al., 2021). To further examine the coordination of swimming, we calculated a polarization parameter p as follows: X- and y-components of the individual trajectories were assembled in a 40 × 54,000 matrix and decomposed to obtain the first two principal components (PCs), which largely resembled the trajectory described by the centroid of the group. The sum of the variance explained by these two PCs was calculated to obtain the parameter p, which allowed us to exactly quantify the degree to which the individual trajectories were correlated. Across the videos, we often observed individual animals or small groups that split away from the main group. To automatically identify these animals, we modified an approach described before by Miller and Gerlai in 2011 (Miller and Gerlai, 2011). In short, we calculated for each video the overall distribution of nearest-neighbor distances (dNND) and used the mode of the distribution as a first threshold to extract movement segments for each individual, during which their nearest-neighbor distance exceeded this threshold. For each segment, the maximum nearest-neighbor distance was stored and used to compute a second, maximum nearest-neighbor distribution. We used the p = 0.015 quantile of this distribution as a threshold to automatically identify proper excursions. To account for different sizes of splinter groups, we replaced dNND with NNDn – NNDn-1, with n being the splinter group size. For example, if two fish moved away from the group, their respective NND would be small, but the distance to the second closest neighbor large. Since we recorded 20 fish, we obtained 10 different excursion types, which we then binarized for further analysis. We used this algorithm to compute the number of excursions performed during one experiment, the total time the animals spent away from the main group, the average excursion time and the average number of fish taking part in an excursion. To evaluate the extent to which the animals display thigmotaxis, we computed a boldness score, for which we assessed what fraction of the total time the animals spent in the middle of the arena. We computed two concentric circles, the outer one being the boundary of the arena, the inner one being defined by a radius which was the outer circle’s radius divided by the square root of 2. This implied that the area covered by the inner circle was equal to the area of the outer circle minus the inner circle’s area and if the animals had no preference for any part of the arena, they should spend 50% of their time in each of the circles.
Data analysis
Significance values are reported as follows: ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001. All replicates are biological replicates obtained from different breeding pairs. For the social preference tasks, we performed ANOVA to analyze whether the variables genotype of the animals and presence of conspecifics significantly altered the social preference index. In case of significant interactions, Tukey-Kramer post-hoc tests were performed. For the startle response, we compared the average fraction of animals per experiment that responded to stimulation with an escape response with the fraction of non-responders using an unpaired, one-sided t-test. We then segmented the responses into SLCs and LLCs and fit a logistic regression model relating genotype with the individual response variables using a subset of the data. We bootstrapped the accuracy of the model by repeated sampling and evaluated with a hold-out test-set containing 30% of the entire dataset. To visualize the model, we projected the logistic decision boundary we obtained with the entire dataset onto the first two principal components of the Eigen-reduced matrix containing SLC, LLC, and no-response rates for all experimental replicates.
The collective behavior data was analyzed as follows: Cumulative shoal distance, velocity, boldness, dispersion, the polarity parameter p and hull area were compared between genotypes using two-sided, unpaired t-tests. To test nearest-neighbor distance, median distance, and maximum distance between animals, we first conducted an ANOVA to see whether the genotype affected these features. We then used the post-hoc Tukey-Kramer test to identify the features affected by genotype. Unpaired, two-sided t-tests were used to compare all other values.
The survival of animals was analyzed by comparing the Kaplan-Meier Estimates of pth2−/− and pth2+/+ fish animals using a log rank test (Kaplan and Meier, 1958), their size at 14 dpf was measured from snout to the onset of the tail fin and compared with a one-sided, unpaired t-test.
All data analysis was performed using custom-written python (python 3.7) scripts in the jupyter notebook 6.0.0 environment, embedded in the anaconda navigator 1.9.7 (64-bit version). Graphs and figures were compiled in Inkscape 0.92. Results are reported as boxplots with individual data points overlaid, as violin plots either with quantiles indicated or with individual data points on top, or as scatterplots with the mean and its confidence interval (0.95) overlaid.
Acknowledgments
We thank the following individuals: Florian Vollrath who wrote the MATLAB script to track startle responses and compute social preference, Norman Heller and Andreas Umminger who built the chamber for analysis of startle behavior, and Fabian Bayer, Erik Papuschin, and Dr. Claudio Polisseni who built the tracking setup for collective behavior. We also thank Friedrich Kretschmer who discussed important aspects of data analysis with us. Erin Schuman is funded by the Max Planck Society.
Author contributions
L.A. and E.M.S. conceived the project. L.A., A.G., T.E., and I.C.A. performed the experiments. L.A., A.G., and T.E. analyzed the data. A.-Y. L. and S.R. provided valuable input throughout the study. L.A. and E.M.S. wrote the manuscript and prepared the figures. All authors read and reviewed the manuscript.
Declaration of interests
The authors declare no competing interests.
Published: March 18, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.103868
Supplemental information
Data and code availability
-
•
All data is openly available from EDMOND: https://doi.org/10.17617/3.6v.
-
•
All relevant analysis code is available from GitHub: https://github.com/Anneser/zfish_PTH2.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Agrawal P., Kao D., Chung P., Looger L. The neuropeptide Drosulfakinin regulates social isolation-induced aggression in Drosophila. J. Exp. Biol. 2020;223 doi: 10.1242/jeb.207407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneser L., Alcantara I.C., Gemmer A., Mirkes K., Ryu S., Schuman E.M. The neuropeptide Pth2 dynamically senses others via mechanosensation. Nature. 2020;588:653–657. doi: 10.1038/s41586-020-2988-z. [DOI] [PubMed] [Google Scholar]
- Aspiras A.C., Harpaz R., Chambule S., Tseng S., Engert F., Bahl A. Collective behavior emerges from genetically controlled simple behavioral motifs in zebrafish. Preprint at bioRxiv. 2021 doi: 10.1126/sciadv.abi7460. 2021.03.03.433803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber C.B., Barber C., Dobkin D., Huhdanpaa H. The Quickhull algorithm for convex hulls. ACM Trans. Math. Softw. 1996;22:469–483. [Google Scholar]
- Burgess H.A., Granato M. Sensorimotor gating in larval zebrafish. J. Neurosci. 2007;27:4984–4994. doi: 10.1523/JNEUROSCI.0615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess H.A., Granato M. The neurogenetic frontier-lessons from misbehaving zebrafish. Brief. Funct. Genomics Proteomics. 2008;7:474–482. doi: 10.1093/bfgp/eln039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutellier L., Logemann A., Rusnak M., Usdin T. Maternal absence of the parathyroid hormone 2 receptor affects postnatal pup development. J. Neuroendocrinology. 2011:612–619. doi: 10.1111/j.1365-2826.2011.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutellier L., Usdin T.B. Enhanced long-term fear memory and increased anxiety and depression-like behavior after exposure to an aversive event in mice lacking TIP39 signaling. Behav. Brain Res. 2011;222:265–269. doi: 10.1016/j.bbr.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzin I.D., Krause J., James R., Ruxton G., Franks N. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 2002;218:1–11. doi: 10.1006/jtbi.2002.3065. [DOI] [PubMed] [Google Scholar]
- Cservenák M., et al. Thalamic neuropeptide mediating the effects of nursing on lactation and maternal motivation. Psychoneuroendocrinology. 2013;38:3070–3084. doi: 10.1016/j.psyneuen.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenák M., Bodnár I., Usdin T., Palkovits M., Nagy G.M., Dobolyi A. Tuberoinfundibular peptide of 39 residues is activated during lactation and participates in the suckling-induced prolactin release in rat. Endocrinology. 2010;151:5830–5840. doi: 10.1210/en.2010-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenák M., Keller D., Kis V., et al. A thalamo-hypothalamic pathway that activates oxytocin neurons in social contexts in female rats. Endocrinology. 2017;158:335–348. doi: 10.1210/en.2016-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenák M., Kis V., Keller D., et al. Maternally involved galanin neurons in the preoptic area of the rat. Brain Struct. Funct. 2017:222. doi: 10.1007/s00429-016-1246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov E.L., Kuo J., Kohno K., Usdin T. Neuropathic and inflammatory pain are modulated by tuberoinfundibular peptide of 39 residues. Proc. Natl. Acad. Sci. United States America. 2013;110:13156–13161. doi: 10.1073/pnas.1306342110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A., Cservenák M., Young L.J. Thalamic integration of social stimuli regulating parental behavior and the oxytocin system. Front. Neuroendocrinol. 2018:102–115. doi: 10.1016/j.yfrne.2018.05.002. Academic Press Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreosti E., Lopes G., Kampff A., Wilson S. Development of social behavior in young zebrafish. Front. Neural Circuits. 2015;9 doi: 10.3389/fncir.2015.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D.B., Holmes A., Riordan T., Faber C., Weiss J., Ma S., Batkai S., Pacher P., Dobolyi A., Murphy A., et al. Increased fear- and stress-related anxiety-like behavior in mice lacking tuberoinfundibular peptide of 39 residues. Genes, Brain Behav. 2008;7:933–942. doi: 10.1111/j.1601-183X.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz A.C., Burgos-Robles A., Bhagat N., Leppla C., Tye K. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/J.NEUROSCIENCE.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira C.H., Moita M.A. Behavioral and neuronal underpinnings of safety in numbers in fruit flies. Nat. Commun. 2020;11:4182. doi: 10.1038/s41467-020-17856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French J.A., Taylor J., Mustoe A., Cavanaugh J. Neuropeptide diversity and the regulation of social behavior in new world primates. Front. Neuroendocrinol. 2016:18–39. doi: 10.1016/j.yfrne.2016.03.004. Academic Press Inc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellén B., Zelena D., Usdin T., Dobolyi A. The parathyroid hormone 2 receptor participates in physiological and behavioral alterations of mother mice. Physiol. Behav. 2017;181:51–58. doi: 10.1016/j.physbeh.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Gemmer A., Mirkes K., Anneser L., Eilers T., Kibat C., Ryu S., Schuman E.M. Oxytocin receptors influence the development and maintenance of social behavior in zebrafish (Danio rerio) Preprint at bioRxiv. 2021 doi: 10.1101/2021.06.26.449566. 2021.06.26.449566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Merikangas K., Dierker L., Snidman N., Arriaga R., Kagan J., Dikel T., Nelson C. Startle potentiation by threat of aversive stimuli and darkness in adolescents: a multi-site study. Int. J. Psychophysiology. 1999;32:63–73. doi: 10.1016/S0167-8760(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Groneberg A.H., Marques J., Martins A., Polavieja G., Orger M. Early-life social experience shapes social avoidance reactions in larval zebrafish. Current Biology. 2020;30:4009–4021. doi: 10.1016/j.cub.2020.07.088. [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Geometry for the Selfish Herd. J. Theor. Biol. 1971 doi: 10.1016/0022-5193(71)90189-5. [DOI] [PubMed] [Google Scholar]
- Harpaz R., Nguyen M., Bahl A., Engert F. Precise visuomotor transformations underlying collective behavior in larval zebrafish. Preprint at bioRxiv. 2021 doi: 10.1101/2021.05.24.445521. 2021.05.24.445521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley L.C., Cacioppo J.T. Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 2010;40:218–227. doi: 10.1007/s12160-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herget U., Wolf A., Wullimann M., Ryu S. Molecular neuroanatomy and chemoarchitecture of the neurosecretory preoptic-hypothalamic area in zebrafish larvae. J. Comp. Neurol. 2014;522:1542–1564. doi: 10.1002/cne.23480. [DOI] [PubMed] [Google Scholar]
- Jékely G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. U S A. 2013;110:8702–8707. doi: 10.1073/pnas.1221833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jékely G. The chemical brain hypothesis for the origin of nervous systems. Philos. Trans. R. Soc. B Biol. Sci. 2021;376:1821. doi: 10.1098/rstb.2019.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- Keller D., Láng T., Cservenák M., Puska G., Barna J., Csillag V., Farkas I., Zelena D., Dóra F., Barteczko L., et al. A thalamo-preoptic pathway promoting social touch. biorxiv. 2022 doi: 10.1101/2022.01.11.475648. [DOI] [PubMed] [Google Scholar]
- Kettleborough R.N.W., et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496:494–497. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A., et al. Social isolation mediated anxiety like behavior is associated with enhanced expression and regulation of BDNF in the female mouse brain. Physiol. Behav. 2016;158:34–42. doi: 10.1016/j.physbeh.2016.02.032. [DOI] [PubMed] [Google Scholar]
- LaBuda C.J., Dobolyi A., Usdin T.B. Tuberoinfundibular peptide of 39 residues produces anxiolytic and antidepressant actions. NeuroReport. 2004;15:881–885. doi: 10.1097/00001756-200404090-00030. [DOI] [PubMed] [Google Scholar]
- Liu J.-H., You Q.-L., Wei M.-D., Wang Q., Luo Z.-Y., Lin S., Huang L., Li S.-J., Li X.-W., Gao T.-M. Social isolation during adolescence strengthens retention of fear memories and facilitates induction of late-phase long-term potentiation. Mol. Neurobiol. 2015;52:1421–1429. doi: 10.1007/S12035-014-8917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barron M., Chen R., Bradbury S., Andalman A., Wagle M., Guo S., Deisseroth K. Multiple convergent hypothalamus–brainstem circuits drive defensive behavior. Nat. Neurosci. 2020;23:959–967. doi: 10.1038/s41593-020-0655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukkes J.L., Mokin M., Scholl J., Forster G. Adult rats exposed to early-life social isolation exhibit increased anxiety and conditioned fear behavior, and altered hormonal stress responses. Horm. Behav. 2009;55:248–256. doi: 10.1016/J.YHBEH.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Marder E., Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu. Rev. Physiol. 2007:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marquart G.D., Tabor K., Bergeron S., Briggman K., Burgess H. Prepontine non-giant neurons drive flexible escape behavior in zebrafish. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer N., Jenikejew J., Richter S., Kaiser S., Sachser N. Social experiences during adolescence affect anxiety-like behavior but not aggressiveness in male mice. Behav. Brain Res. 2017;326:147–153. doi: 10.1016/j.bbr.2017.03.017. [DOI] [PubMed] [Google Scholar]
- Miller N., Gerlai R. Redefining membership in animal groups. Behav. Res. Methods. 2011;43:964–970. doi: 10.3758/s13428-011-0090-z. [DOI] [PubMed] [Google Scholar]
- Miller N., Gerlai R. From schooling to shoaling: patterns of collective motion in zebrafish (Danio rerio) PLoS One. 2012;7:e48865. doi: 10.1371/JOURNAL.PONE.0048865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minakata H. Oxytocin/vasopressin and gonadotropin-releasing hormone from cephalopods to vertebrates. Ann. N Y Acad. Sci. 2010:33–42. doi: 10.1111/j.1749-6632.2010.05569.x. Blackwell Publishing Inc. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., To M., Saruta J., Yamamoto Y., Shimizu T., Kamata Y., Matsuo M., Tsukinoki K. Effect of social isolation stress on saliva BDNF in rat. J. Oral Sci. 2019;61:516–520. doi: 10.2334/JOSNUSD.18-0409. [DOI] [PubMed] [Google Scholar]
- Pan Y., Liu Y., Young K., Zhang Z., Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci. Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasani M.R., Gensure R., Yan Y., Gunes Y., Postlethwait J., Ponugoti B., John M., Jüppner H., Rubin D. Identification and characterization of the zebrafish and fugu genes encoding tuberoinfundibular peptide 39. Endocrinology. 2004;145:5294–5304. doi: 10.1210/en.2004-0159. [DOI] [PubMed] [Google Scholar]
- Parr L.A., Winslow J.T., Davis M. Rearing experience differentially affects somatic and cardiac startle responses in rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2002;116:378–386. doi: 10.1037//0735-7044.116.3.378. [DOI] [PubMed] [Google Scholar]
- Reider M., Connaughton V.P. Developmental exposure to methimazole increases anxiety behavior in zebrafish. Behav. Neurosci. 2015;129:634–642. doi: 10.1037/bne0000087. [DOI] [PubMed] [Google Scholar]
- Shams S., Amlani S., Buske C., Chatterjee D., Gerlai R. Developmental social isolation affects adult behavior, social interaction, and dopamine metabolite levels in zebrafish. Dev. Psychobiol. 2018 doi: 10.1002/dev.21581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams S., Seguin D., Facciol A., Chatterjee D., Gerlai R. Effect of social isolation on anxiety-related behaviors, cortisol, and monoamines in adult zebrafish. Behav. Neurosci. 2017;131:492–504. doi: 10.1037/bne0000220. [DOI] [PubMed] [Google Scholar]
- Shiozaki K., Kawabe M., Karasuyama K., Kurachi T., Hayashi A., Ataka K., Iwai H., Takeno H., Hyasaka O., Kotani T., et al. Neuropeptide Y deficiency induces anxiety-like behaviours in zebrafish (Danio rerio) Scientific Rep. 2020;10:1–13. doi: 10.1038/s41598-020-62699-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich G.M., Cole S.W. The emerging field of human social genomics. Clin. Psychol. Sci. 2013;1:331–348. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stednitz S.J., McDermott E., Ncube D., Tallafuss A., Eisen J., Washbourne P. Forebrain control of behaviorally driven social orienting in zebrafish. Curr. Biol. 2018;28 doi: 10.1016/j.cub.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Davidson J., Zhang G., Conen K., Fang J., Serluca F., Li J., Xiong X., Coble M., Tsai T., et al. Genetic control of collective behavior in zebrafish. iScience. 2020;23:100942. doi: 10.1016/j.isci.2020.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi J., Zai C., Zai G., Herbert D., King N., Freeman N., Kennedy J., Tiwari A. The effect of polymorphisms in startle-related genes on anxiety symptom severity. J. Psychiatr. Res. 2020;125:144–151. doi: 10.1016/j.jpsychires.2020.03.019. [DOI] [PubMed] [Google Scholar]
- Troconis E.L., Ordoobadi A., Sommers T., Aziz-Bose R., Carter A., Trapani J. Intensity-dependent timing and precision of startle response latency in larval zebrafish. J. Physiol. 2017;595:265–282. doi: 10.1113/JP272466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulogdi Á., Toth M., Barsvari B., Biro L., Mikics E., Haller J. Effects of resocialization on post-weaning social isolation-induced abnormal aggression and social deficits in rats. Developmental Psychobiology. 2014;56:49–57. doi: 10.1002/dev.21090. [DOI] [PubMed] [Google Scholar]
- Tunbak H., Vazquez-Prada M., Ryan T., Kampff A., Dreosti E. Whole-brain mapping of socially isolated zebrafish reveals that lonely fish are not loners. eLife. 2020;9 doi: 10.7554/eLife.55863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin T.B. Evidence for a Parathyroid Hormone-2 receptor selective ligand in the hypothalamus. Endocrinology. 1997;138:831–834. doi: 10.1210/endo.138.2.5031. [DOI] [PubMed] [Google Scholar]
- Usdin T.B., Paciga M., Riordan T., Kuo J., Parmelee A., Petukova G., Daniel Camerini-Otero R., Mezey E. Tuberoinfundibular peptide of 39 residues is required for germ cell development. Endocrinology. 2008;149:4292–4300. doi: 10.1210/en.2008-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtcheva S., Issa H., Martin K., Jung K., Kwon H., Froemke R. Neural Circuitry for Maternal Oxytocin Release Induced by Infant Cries. Preprint at bioRxiv. 2021 doi: 10.1038/s41586-023-06540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vine I. Risk of visual detection and pursuit by a predator and the selective advantage of flocking behaviour. J. Theor. Biol. 1971 doi: 10.1016/0022-5193(71)90061-0. [DOI] [PubMed] [Google Scholar]
- Virtanen P., Gommers R., Oliphant T., Haberland M., Reddy T., Cournapeau D., Burovski E., Peterson P., Weckesser W., Bright J., et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods. 2020;17:261–272. doi: 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.L., Davis M. Anxiogenic effects of high illumination levels assessed with the acoustic startle response in rats. Biol. Psychiatry. 1997;42:461–471. doi: 10.1016/S0006-3223(96)00441-6. [DOI] [PubMed] [Google Scholar]
- Walter T., Couzin I.D. Trex, a fast multi-animal tracking system with markerless identi cation, and 2d estimation of posture and visual elds. eLife. 2021;10:1–73. doi: 10.7554/eLife.64000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson L.S., Killcross S., Humby T., Hall F., Geyer M., Robbins T. Social isolation in the rat produces developmentally specific deficits in prepulse inhibition of the acoustic startle response without disrupting latent inhibition. Neuropsychopharmacology. 1994;10:61–72. doi: 10.1038/npp.1994.8. [DOI] [PubMed] [Google Scholar]
- Xu S., Yang H., Menon V., Lemire A., Wang L., Henry F., Turaga S., Sternson S. Behavioral state coding by molecularly defined paraventricular hypothalamic cell type ensembles. Science. 2020;370 doi: 10.1126/science.abb2494. [DOI] [PubMed] [Google Scholar]
- Zelikoswky M., Ding K., Anderson D.J. Neuropeptidergic control of an internal brain state produced by prolonged social isolation stress. Cold Spring Harb. Symp. Quant. Biol. 2018;89:97–103. doi: 10.1101/sqb.2018.83.038109. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M., Hui M., Karigo T., Choe A., Yang B., Blanco M., Beadle K., Gradinaru V., Deverman B.E., Anderson D.J. The neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress article the neuropeptide Tac2 controls a distributed brain state induced by chronic social isolation stress. Cell. 2018;173:1265–1268.e19. doi: 10.1016/j.cell.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D., Chen L., Deng D., Jiang D., Dong F., McSweeney C., Zhou Y., Liu L., Chen G., Wu Y., et al. DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction. Curr. Mol. Med. 2016;16:91. doi: 10.2174/1566524016666151222150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data is openly available from EDMOND: https://doi.org/10.17617/3.6v.
-
•
All relevant analysis code is available from GitHub: https://github.com/Anneser/zfish_PTH2.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.