Abstract
Alterations in consciousness state are a defining characteristic of focal epileptic seizures. Consequently, understanding the complex changes in neurocognitive networks which underpin seizure-induced alterations in consciousness state is important for advancement in seizure classification. Comprehension of these changes are complicated by a lack of data standardization; however, the use of a common terminological system or ontology in a patient registry minimizes this issue. In this paper, we introduce an integrated knowledgebase called Epilepsy-Connect to improve the understanding of changes in consciousness states during focal seizures of pharmacoresistant epilepsy patients. This registry catalogues over 809 seizures from 70 patients at University Hospital’s Epilepsy Center who were undergoing stereotactic electroencephalography (SEEG) monitoring as part of an evaluation for surgical intervention. Although Epilepsy-Connect focuses on consciousness states, it aims to enable users to leverage data from an informatics platform to analyze epilepsy data in a streamlined manner.
Epilepsy-Connect is available at https://bmhinformatics.case.edu/Epilepsyconnect/login/.
1. Introduction
Epilepsy is a serious neurological disease that is defined as having at least two unprovoked seizures in a 24-hour time period and is the fourth leading non-communicable disease impacting global years lived with disability (YLD)[1], [2]. More than 52 million people worldwide are affected by epilepsy, resulting in 18.3 million global YLD[2]. Approximately one third of these people suffer from refractory or drug-resistant epilepsy, leaving them with uncontrolled seizures[3].
Epilepsy is a spectrum condition that broadly encompasses all seizure disorders, with alterations of consciousness during seizure events being a core component of epileptic seizures[4]. While successful treatment of epilepsy requires refined classification of the disorder, informative classification guidelines that focus on changes in consciousness state have been difficult to achieve. In 2017, the International League Against Epilepsy (ILAE) updated the definition of epilepsy to include several new focal and generalized seizure types[5]. Despite offering more granular classifiers such as awareness, these ILAE seizure classifications have been criticized for a lack of clarity and brevity[6]–[8]. The “Four-dimensional epilepsy classification” is an approach that aims to classify seizures based exclusively on ictal symptomatology, and it includes precise and detailed descriptions of the nature of changes in consciousness state[7], [9]. This proposed approach to use clinical features to better delineate focal seizures reduces the dependency on an abnormal, correctly interpreted, available electroencephalograph (EEG)[8].
A better understanding of the changes in consciousness state during seizures is essential for both improvement in treatment of epilepsy as well as potentially enhancing the safety of epilepsy patients during seizure events [4], [8], [10], [11]. In addition to limitations of existing classification systems with respect to consciousness state during epileptic seizures, the lack of standardization in case ascertainment, data collection methods, and data reporting methods also impedes advancement in epilepsy research related to consciousness. In this paper, we propose that the design and implementation of a patient registry focused on alterations of consciousness state in epilepsy using a common terminological system or ontology can address many of the data management challenges faced by clinical researchers [12]–[16]. Ontologies are knowledge reference models that often use description logic to represent the relationships between concepts or terms in a way that allows computational logical reasoning, which allows for mass integration and analysis of large amounts of heterogeneous data; this logic is widely used to enhance patient classification and other data analysis tasks [14], [16]. Therefore, ontologies can play a key role in managing multi-dimensional data from different sources in a consciousness focused patient registry.
Patient registries have become a common way to facilitate sharing comprehensive, diagnostic, condition-specific data in order to enhance clinical and scientific research [12]. There are several existing registries that cover various aspects of epilepsy, each providing unique insights into different aspects of therapies, rare variants, and general health related to epilepsy (Table 1). To the best of our knowledge, there is no existing registry that focuses on changes in consciousness states in epilepsy patients and the use of the registry data for computational analysis of the patient data to study changes in brain network topology during seizures. Furthermore, most existing epilepsy registries do not include publicly accessible data, making it difficult for researchers to perform exploratory data analysis or relate these highly specific findings to more general topics. To address this significant gap in the study of consciousness states in epilepsy patients, we introduce an integrated knowledgebase called Epilepsy-Connect that uses ontology for standardization of data in a new patient registry to study changes in consciousness states during epileptic seizures (https://bmhinformatics.case.edu/Epilepsyconnect). The broader objective of the Epilepsy-Connect informatics platform is to enable a greater understanding of the consciousness state system, ultimately improving diagnostic accuracy, patient care, and the prediction of surgical necessity and/or outcomes.
Table 1: An example of epilepsy registries already in existence and their respective purposes.
| Registry Name | Purpose |
|---|---|
| Rare Epilepsy Network Registry (REN)[17] | To catalogue and understand 32 different rare epilepsies with the goal improving treatments and quality of life[17] |
| North American SUDEP Registry (NASR)[18] | To obtain medical records, family interviews, and other reports of cases of sudden unexpected death in epilepsy[18] |
| Stockholm Incidence Registry of Epilepsy (SIRE)[19] | To identify prospective epilepsy cases in patients with newly diagnosed single unprovoked seizures[19] |
| International Ion Channel Epilepsy Patient Registry (IICEPR)[20] | To collect data on patients with epilepsy caused by an ion channel mutation[20] |
| Medtronic Registry for Epilepsy (MORE)[21] | To evaluate the long-term effects of deep brain stimulation for the treatment of refractory epilepsy[21] |
| International Registry of Antiepileptic Drugs and Pregnancy (EURAP)[22] | To determine risks associated with antiepileptic drug use during pregnancy[22] |
| Epilepsy Birth Control Registry (EBCR) [23] | To evaluate the contraceptive practices of women with epilepsy[23] |
1.1. Background
Significance of Consciousness State in Epilepsy Neurological Disorder. Up to 70% of focal epilepsy patients do not have freedom from seizures despite optimal drug therapy[3]. However, surgery to resect the epileptogenic zone often does not result in seizure-free patients, and they continue to experience significant deterioration in their quality of life due to repeated seizures, including loss or change in consciousness state the endangers their safety [11], [24]. The analysis of changes in consciousness states during recurrent seizures has been suggested as a potential method to improve evaluation of patients for surgery[3], [24]. Consciousness is a comprehensive yet complicated term lacking one concrete definition[4], [25]. Consciousness, while not concretely defined, is believed to depend upon continuous integration and processing of information[4]. Interruptions in this continuous integration and processing of information–often presenting as changes in awareness, attention, arousal, responsiveness, ability to interact, and/or memory–are noted as alterations in consciousness state[4], [11]. Focal seizures span various degrees of impairments or alterations of consciousness, including: (1) no impairment, (2) observable motor or autonomic components, (3) sensory or psychic auras, (4) dyscognition, (5) dialepsis, (6) ictal delirium, (8) evolution to a bilateral, convulsive seizure, and (9) epileptic coma[4], [26].
Losses of, or alterations in, consciousness state in patients with epilepsy are complex phenomena that involve changes in neurocognitive networks such as the default mode network (DMN), salience network (SN), and central executive network (CEN)[27]–[30]. These impairments may be identified by various neurological characteristics and can be measured objectively by assessing a patient’s amnesia of events occurring during epileptic seizures and their responsiveness to external stimuli during epileptic seizure [4], [11]. Several studies have been conducted to link changes in consciousness to specific brain regions[11], [31], [32] and to changes in brain activity in several cortical regions and subcortical arousal systems[11], [27]–[30], [33], [34]. These studies, however, fail to distinguish between the various states of consciousness. Each state of consciousness has its own unique clinical presentation, and it has been noted that each state of consciousness has unique characteristics during a seizure. We propose to analyze signal recordings from refractory patients to characterize changes in brain network topology and correlate the changes to alterations in consciousness during seizure events using de-identified patient data from the Epilepsy Center at the University Hospitals Cleveland Medical Center (UH-CMC).
Epilepsy-Connect Patient Registry Data. The UH-CMC is a Tier 4 epilepsy center that conducts research on the causes and therapies of epilepsy with refractory epilepsy patients receiving care in the epilepsy monitoring unit (EMU). In the EMU, patients undergo continuous monitoring of brain activity for five days, including continuous video monitoring. During this period, anti-seizure medications are temporarily reduced or stopped in order to safely induce medically supervised seizures so that epileptologists may determine the epileptogenic zone and, by extension, determine the best course of therapy for the patient. Patients are monitored using intracranial electrodes that record stereotactic electroencephalography (SEEG) to localize seizure foci. We use de-identified patient data from the UH-CMC with Institutional Review Board (IRB) approval to populate the Epilepsy-Connect patient registry.
Network Analysis of SEEG Data for Characterizing Alterations of Consciousness State in Epilepsy Patients. It is well understood that focal seizures originate within networks where initial activation is limited to one cerebral hemisphere[26], [35]. Current focal epilepsy models acknowledge that the epileptogenic zone is a distributed network involving inter-regional anatomo-functional relationships among brain areas[24], [36]. These networks have not been fully characterized yet, and research for more accurate prediction of surgical intervention outcomes using these networks is ongoing[24], [36].
One increasingly popular body of research has emphasized the utility of network analysis methods that model epileptic seizure networks as graph models consisting of brain locations as nodes and the interaction between them as edges in a graph to understand each of these potential network mechanisms[37]. Network topology has been used to study general changes in ictal network properties spanning the duration of the seizure[38], [39] or at seizure onset[40]. Research has even considered changes in interictal network topology[37], [38], [41], [42]. While there is an abundance of literature focused on developing general understandings of epilepsy networks, little is known about the relationship between these networks and the differences in the various impaired states of consciousness.
The Epilepsy-Connect knowledgebase aims to serve as an integrated neuroinformatics resource with a patient registry and network analysis tools, which can be directly applied to the patient data. The Epilepsy-Connect knowledgebase features a query interface that allows users to create patient cohorts based on specific inclusion/exclusion criteria. In the next section, we describe the implementation details of the Epilepsy-Connect knowledgebase, including the workflow used to populate the registry, the development of the informatics component, and the functionalities currently supported by this integrated neuroinformatics tool.
2. Methods
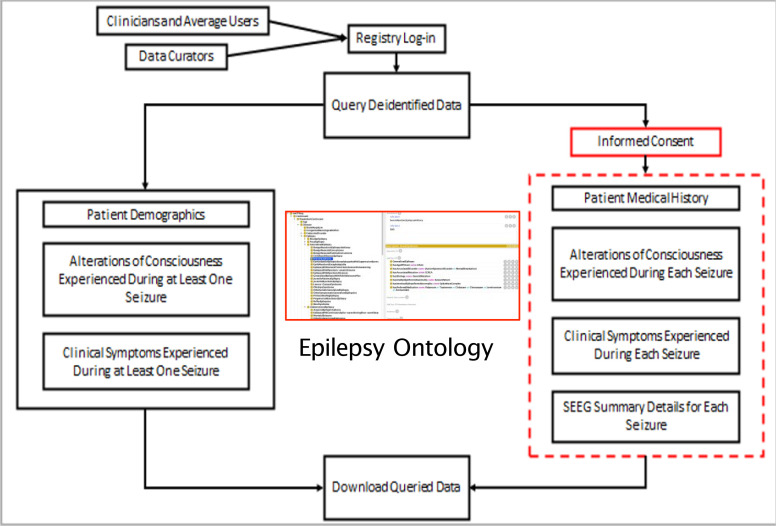
The Epilepsy-Connect knowledgebase was developed in close collaboration with clinicians at the UH-CMC to support clinical research together with patient care. Figure 1shows the workflow used in the development of the Epilepsy-Connect knowledgebase. The three primary objectives of the Epilepsy-Connect knowledgebase are:
Figure 1:
Epilepsy-Connect knowledgebase workflow development
Support the creation of a patient registry using standardized terminology modeled in a widely used epilepsy-focused ontology [16];
Enable researchers to perform patient-cohort queries over the patient registry data for hypothesis development and validation related to alteration of consciousness state in pharmacoresistant epilepsy patients who undergo evaluation for surgical evaluation; and
Support users to manage their cohort queries and query results, which can be used for data sharing as well as dissemination of their study results.
2.1. The architecture and development of the Epilepsy-Connect platform.
The Epilepsy-Connect platform consists of a set of modules that support: (1) creation of a cohort query using a visual query interface, and (2) management of user information, including query history. The current version of Epilepsy-Connect has been developed using the Django web application framework, which uses the Python programming language and features a large number of libraries and modules that support variety of data processing and analysis tasks including access to ontology files. The architecture of Epilepsy-Connect allows it to integrate and manage data from the UH-CMC using software modules for data pre-processing and conversion to a common data model following the well-known Extract Transform Load (ETL) approach. We note that as part of the data processing step, multiple terms in the study data conform to the ontology classes in the Epilepsy Ontology, which facilitates the standardization of the data terms in the patient registry module of the platform.
The Epilepsy-Connect platform uses the Model View Template (MVT) approach with data in the registry component managed using an object relational data Model, the user interface is managed by the View component, and the user interaction with various features of the software is mediated by the Template, which conforms to the Django framework. The Epilepsy-Connect platform is accessed via a Web browser with role-based access control (RBAC) with users assigned to a user group. The access to data in the registry component is governed by the UH-CMC IRB with a data user agreement (DUA) required for download of the data. Users follow an intuitive query process to create a study cohort using a visual query composition process (results are discussed in Section 3).
2.2. Study Data.
At present, the Epilepsy-Connect registry hosts de-identified retrospective patient data from UH-CMC who were evaluated for surgical intervention. The preoperative evaluation data were gathered from each patient’s discharge summary following invasive EEG/video evaluation. This data was split into patient demographics, medical history, seizure etiology, medical imaging records and findings, neuropsychological testing information, and clinically observed representations of patient alterations of consciousness. Further, de-identified SEEG recordings for each patient are processed using an in-house signal processing workflow called the NeuroIntegrative Connectivity (NIC) tool to segment and transform signal data stored in the European Data Format (EDF) to a Javascript Object Notation (JSON) format called Cloudwave Signal Format (CSF) [43], [44]. The SEEG recordings are analyzed by the UH-CMC clinicians during EEG reading sessions to determine seizure details and define ictal and interictal periods. Further, clinicians also note the specific electrode contact involved in seizure events, which are used as input parameters for the computational neuroscience workflow used in this project to characterize topological changes associated with change in consciousness state of these patients. The seizure details, ictal time periods, and the list of electrode contacts together with other relevant patient details are stored in the Epilepsy-Connect knowledge as part of the ETL process.
Summary statistics of the data were calculated in R (version 4.0.4). A total of 70 patients, ranging from the age of 15 to 69 (median age of 35), were included in the registry. Table 2 shows a summary of the patient demographics. The number of males and females included was approximately equal, and age did not differ significantly between the sexes (p = 0.97, χ2 = 0.001). A total of 809 seizures were analyzed for the registry. The seizures for one patient were excluded from this calculation since the patient was reported to have had “one seizure every two minutes” over multiple days within the study. Of these seventy participants, sixty-one reported having at least one seizure with an alteration of consciousness. Forty-two patients were reported to have had an aura during at least one seizure; twenty-seven patients experienced dialepsis during at least one seizure; six patients presented with dyscognition in at least one seizure; and thirty-one patients had at least one seizure without any alterations of consciousness. Access to the patient registry data through the Epilepsy-Connect query interface is available as a “guest user” and a “registered user”. A registered user account will be created after the user has completed the data user agreement with IRB approval. A registered user has full access to the Epilepsy-Connect knowledgebase; however, a “guest user” can explore a restricted set of data elements through the user interface.
Table 2. Summary statistics for participants.
| Percentof People (n = 70) | |
| Mean Age (Standard Deviation) | 35.49 (12.89) |
| % Male | 33 (47%) |
| % Right-Handed | 65 (94%) |
| Epileptogenic Zone (%) | |
| Bitemporal | 8 (11%) |
| Left Hemisphere | 34 (49%) |
| Right Hemisphere | 28 (40%) |
| Alteration of Consciousness (%) | |
| Aura | 42 (60%) |
| Dialepsis | 27 (39%) |
| Dyscognition | 6 (9%) |
| None | 31 (44%) |
2.3. Epilepsy Ontology and Standardization of Terms.
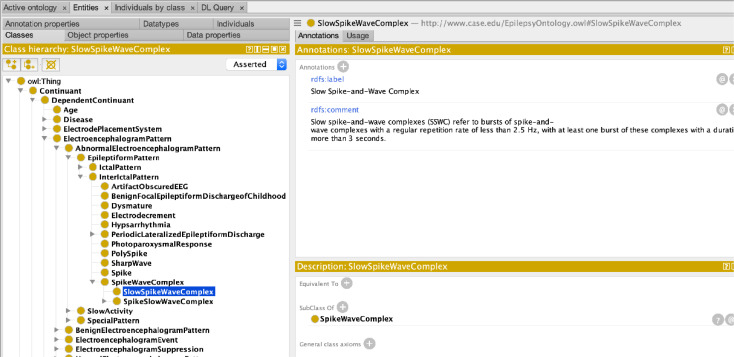
The Epilepsy-Connect uses the Epilepsy Ontology (listed in the National Center for Biomedical Ontologies as the Epilepsy and Seizure Ontology, EpSO) as a reference ontology to standardize the storage and querying of data [16]. The Epilepsy Ontology has been iteratively developed using input from multiple epilepsy domain experts as part of the ILAE Big Data task force to comprehensively model various aspects of epilepsy and seizures, including genetics, neuropathology, medication, and brain anatomy. Epilepsy Ontology uses the description-logic-based Web Ontology Language (OWL) to construct a class hierarchy, link together the ontology classes using properties, and apply sophisticated class-level restrictions to model epilepsy information at a fine-level of granularity. The Epilepsy Ontology uses ontology-engineering best practices to re-use existing ontology terms from the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT), the Gene Ontology, RxNorm for drug information, and the Foundational Model of Anatomy (FMA) for modeling brain regions. A phased approach was used to extend the Epilepsy Ontology for use in the Epilepsy-Connect project.
In the first phase, we enumerated all the data elements in the Epilepsy-Connect registry, which can be broadly classified into six categories of patient evaluation results (e.g., etiology, neurological review, and family history); results of neurological exam (e.g., memory, attention, motor, and reflexes); details of imaging procedures (e.g., imaging modality and status); seizure events (e.g., start and end of seizure events, onset and end frequency); alterations of consciousness state (e.g., aura, dialeptic, or apraxia); and details of alterations of consciousness events (e.g., post-seizure memory retention, fine distal movement). In the next phase, we modeled these data elements in the Epilepsy Ontology, for example Figure 2shows a part of the class hierarchy describing EEG patterns. These ontology terms are being used in the Epilepsy-Connect project for both annotation of database as well as to support the functionalities of the user interface. In particular, we are integrating the Epilepsy Ontology in the user interface to support patient cohort queries using the ontology-based database access (OBDA) framework. The use of OBDA will allow users to leverage semantic matching of query terms to information in the patient registry without being constrained by string matching only. At present, the Epilepsy-Connect query interface uses the ontology terms to create a customized database for supporting the query composition task using drop-down menu values. The use of the Epilepsy Ontology in the patient registry will also enable easier sharing of data using common terminology for data annotation.
Figure 2:
A section of the class hierarchy in the Epilepsy Ontology modeling EEG patterns.
Network Analysis of SEEG Data using Patient Cohort Query. In addition to supporting patient cohort queries, the Epilepsy-Connect knowledgebase is being integrated with a network analysis tool called the Neuro-Integrative Connectivity (NIC) platform, which was developed in our prior work[43]. The NIC platform converts SEEG European Data Format (EDF) files into Cloudwave Signal Format (CSF) files, a JavaScript Object Notation (JSON) based human-readable format previously developed by our lab[44]. Once files have been converted, the NIC tool may be used to investigate statistical correlations of brain functional connectivity. Users are able to choose between Pearson Correlation[45], Mean Phase Coherence[46], and Non-linear correlation[47] for their measurement(s) of correlation. Furthermore, the NIC workflow allows users to analyze the change in network graph topology throughout seizures via methods in algebraic topology. Outputs include the clustering coefficient, Betti numbers, characteristic path length, and global efficiency. The NIC tool is available for download at https://bmhinformatics.case.edu/nicworkflow/accounts/login/.
After the user creates a study cohort using the Epilepsy-Connect user interface, the NIC tool will be able to be invoked to process and compute network measures over the SEEG data. At present, we created a cohort with patients with two seizures for further analysis. The corresponding SEEG files were processed using the NIC platform to characterize the network characteristics of the epileptic seizures, including global and local connectivity with corresponding disruption of the small-world network topology, which is a characteristic of brain network in healthy subjects. In the next section, we describe the results generated by the Epilepsy-Connect knowledgebase via the user interface.
3. Results
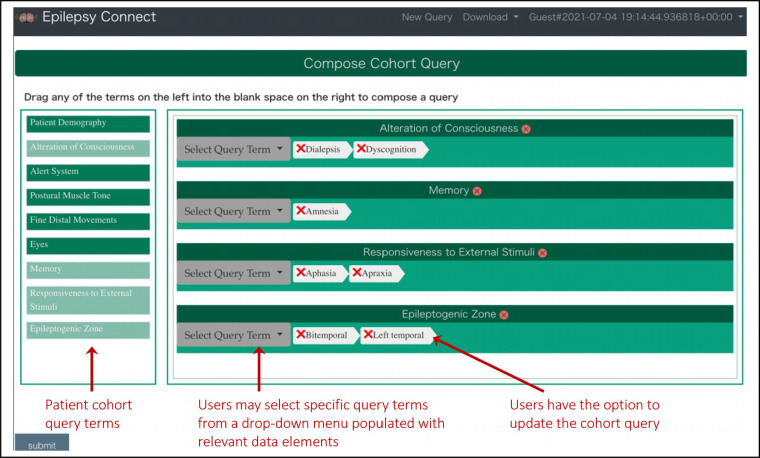
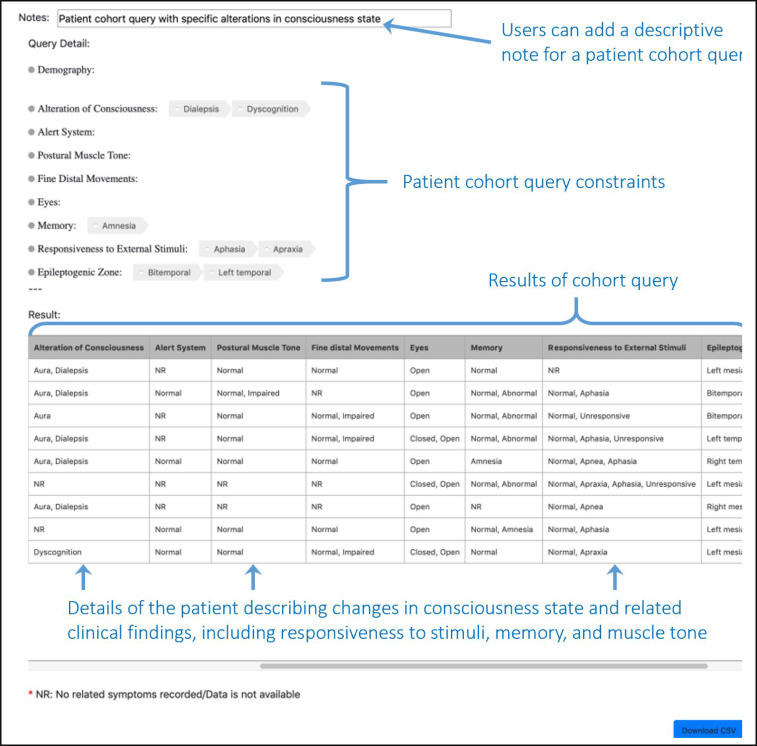
Epilepsy-Connect User Interface. The Epilepsy-Connect user interface consists of an intuitive query composition module (Figure 3), which supports both drop-down menu values as well as a “Drag-and-drop” feature. Using a stepwise process, users can select at least one variable from a list of curated patient cohort variables. Variables provide a drop-down list of factors which can be used to impose search criteria limits; numeric variables will provide sliders whereas qualitative responses will allow for radio button selection. At this time, queries are limited to “OR” logic. Once a user has completed a query, they will be directed to the results page to view their results. Restricted access users will have the option at this point to download the resulting dataset in a comma separated values (CSV) format. For an example of functionality, we have chosen to illustrate the query criteria that a patient had at least one dialeptic seizure (Figure 4).
Figure 3:
A view of the query composition interface of the Epilepsy-Connect knowledgebase with support for patient cohort queries focused on studying alterations of consciousness states in patients.
Figure 4:
Results of a patient cohort query using the criteria of Dialepsis for the consciousness state of patients.
Additional features have been implemented for restricted access users. Users with a registered account will be able to review the data in the registry as a whole by visiting the “data” tab in the upper right-hand corner of the page. Furthermore, they will be able to revisit their prior queries, by utilizing the “history” tab in the upper right-hand corner of the page.
4. Discussion & Limitations
We created an integrated knowledgebase called Epilepsy-Connect to improve the understanding of changes in consciousness states during focal seizures of pharmacoresistant epilepsy patients. This registry catalogues over 809 seizures from 70 patients at the Epilepsy Monitoring Unit within University Hospital’s Epilepsy Center who were undergoing SEEG monitoring as part of an evaluation for surgical intervention. Public use data for the registry includes information on general patient demographics, clinical symptoms that patients experienced during at least one of their seizures, and the alterations of consciousness that patients experienced during at least one of their seizures. We propose to deploy Epilepsy-Connect in the EMU of UH-CMC to support clinical research and improve patient care.
The broader objective of the Epilepsy-Connect informatics platform is to enable greater understanding of the consciousness state system that could be useful in improving diagnostic accuracy and patient care, as well as predicting surgical necessity and/or outcomes. To demonstrate and validate the use of Epilepsy-Connect for this purpose, we composed a simple query of the public access data to examine patients who had experienced at least one dialeptic seizure during the recorded evaluation. The resulting cohort included six patients. While the results of this case study are enlightening, further analysis is needed to fully comprehend the complexities of the various networks associated with alterations of consciousness states. Comparisons across the selected cohort could reveal network changes that occur during seizures in which a patient presents with dialepsis. Moreover, comparisons across multiple cohorts could foster understanding of network changes that occur during any seizure-induced alteration of consciousness state.
Although Epilepsy-Connect was designed with a focus on consciousness states, it includes a large range of data that can be utilized to explore epilepsy from several perspectives. Epilepsy-Connect’s standardized and comparable data collection makes it ideal for sharing comprehensive diagnostic data in order to improve research into epilepsy treatment methods and new intervention techniques, while still providing unique insights into seizure-induced alterations of consciousness states[12]–[16]. In an effort to make Epilepsy-Connect more universal, future releases are expected to add information such as weighted diffusion tensor imaging results for further connectome analysis, patient surgery decisions and outcomes, and additional patient neuropsychological testing. Furthermore, the modular architecture of the registry allows for queries to be easily modified, thus streamlining data collection procedures for any interested investigators.
5. Conclusions
Alterations of consciousness state are important in defining epileptic seizures[6]–[8], [10], and developing comprehension of seizure-induced changes to consciousness state is essential for improving seizure classification[8], [10]. One aspect of this is to understand the complex changes in neurocognitive networks which underpin alterations of consciousness states during epileptic seizures[27]–[30]. However, this is not easy, as the lack of standardization in case ascertainment, data collection methods, and data reporting methods tend to make data difficult to compare or validate across studies[12]–[15]. Patient registries work to minimize these issues[12]. In this paper, we introduced an integrated knowledgebase called Epilepsy-Connect that supports standardized and comparable data collection of patients with refractory focal epilepsy, and showed one potential use case for improving the understanding of changes in consciousness states during epileptic seizures. Although Epilepsy-Connect was designed with a focus on consciousness states, it aims to enable users to leverage data from an informatics platform to analyze epilepsy data in a streamlined manner in order to bolster scientific advancement.
Acknowledgements
This project was supported in part by the Clinical and Translational Science Collaborative (CTSC) of Cleveland which is funded by the National Institutes of Health (NIH), National Center for Advancing Translational Science (NCATS), Clinical and Translational Science Award (CTSA) grant, UL1TR002548. The content is solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This project was also supported in part by the NSF grant #1636850.
Figures & Table
References
- [1].Fisher R. S., et al. “ILAE Official Report: A practical clinical definition of epilepsy,”. Epilepsia. 2014;55(4):475–482. doi: 10.1111/epi.12550. doi: [DOI] [PubMed] [Google Scholar]
- [2].Institute for Health Metrics and Evaluation (IMHE), University of Washington. “GBD 2019 Cause and Risk Summaries: Epilepsy — Level 1 impairment profile,”. IMHE. Oct. 15, 2020.
- [3].West S., et al. “Surgery for epilepsy,”. Cochrane Database Sys. Rev. 2019. doi: 10.1002/14651858.CD010541.pub3. [DOI] [PMC free article] [PubMed]
- [4].Lüders H., et al. “Proposal: Different types of alteration and loss of consciousness in epilepsy,”. Epilepsia. 2014;55(8):1140–1144. doi: 10.1111/epi.12595. doi: [DOI] [PubMed] [Google Scholar]
- [5].Fisher R. S., et al. “Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology,”. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.13670. doi: [DOI] [PubMed] [Google Scholar]
- [6].Lüders H., et al. “Critique of the 2017 epileptic seizure and epilepsy classifications,”. Epilepsia. 2019;60(6):1032–1039. doi: 10.1111/epi.14699. doi: [DOI] [PubMed] [Google Scholar]
- [7].Rosenow F., et al. “Could the 2017 ILAE and the four-dimensional epilepsy classifications be merged to a new ‘Integrated Epilepsy Classification’?,”. Seizure. 78:31–37. doi: 10.1016/j.seizure.2020.02.018. May 2020, doi: 10.1016/j.seizure.2020.02.018. [DOI] [PubMed] [Google Scholar]
- [8].Palmini A., et al. “From theory to practice: Critical points in the 2017 ILAE classification of epileptic seizures and epilepsies,”. Epilepsia. 2020;61(2):350–353. doi: 10.1111/epi.16426. doi: [DOI] [PubMed] [Google Scholar]
- [9].Lüders H., et al. “Classification of paroxysmal events and the four-dimensional epilepsy classification system,”. Epileptic Disord. 2019;21(1):1–29. doi: 10.1684/epd.2019.1033. doi: [DOI] [PubMed] [Google Scholar]
- [10].Fisher R. S. “The New Classification of Seizures by the International League Against Epilepsy 2017,”. Curr. Neurol. Neurosci. Rep. 17(6):48. doi: 10.1007/s11910-017-0758-6. Jun. 2017, doi: 10.1007/s11910-017-0758-6. [DOI] [PubMed] [Google Scholar]
- [11].Blumenfeld H. “Impaired consciousness in epilepsy,”. Lancet Neurol. 11(9):814–826. doi: 10.1016/S1474-4422(12)70188-6. Sep. 2012, doi: 10.1016/S1474-44221270188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Washington, D.C: National Academies Press (US); 2012. Institute of Medicine (US) Committee on the Public Health Dimensions of the Epilepsies et al., Epilepsy Across the Spectrum. [Google Scholar]
- [13].McIntosh A. M., Wilson S. J., Berkovic S. F. “Seizure outcome after temporal lobectomy: current research practice and findings,”. Epilepsia. 42(10):1288–1307. doi: 10.1046/j.1528-1157.2001.02001.x. Oct. 2001, doi: 10.1046/j.1528-1157.2001.02001.x. [DOI] [PubMed] [Google Scholar]
- [14].Haendel M. A., Chute C. G., Robinson P. N. “Classification, Ontology, and Precision Medicine,”. N. Engl. J. Med. 379(15):1452–1462. doi: 10.1056/NEJMra1615014. Oct. 2018, doi: 10.1056/NEJMra1615014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Téllez-Zenteno J. F., Dhar R., Wiebe S. “Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis,”. Brain. 128(5):1188–1198. doi: 10.1093/brain/awh449. May 2005, doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- [16].Sahoo S. S., et al. “Epilepsy and seizure ontology: towards an epilepsy informatics infrastructure for clinical research and patient care,”. J. Am. Med. Inform. Assoc JAMIA. 21(1):82–89. doi: 10.1136/amiajnl-2013-001696. Jan. 2014, doi: 10.1136/amiajnl-2013-001696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Epilepsy Foundation, “Access the Rare Epilepsy Network Registry,”. Epilepsy Foundation. https://www.epilepsy.com/
- [18].Verducci C., et al. “SUDEP in the North American SUDEP Registry: The full spectrum of epilepsies,”. Neurology. 93(3):e227–e236. doi: 10.1212/WNL.0000000000007778. Jul. 2019, doi: 10.1212/WNL.0000000000007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Adelöw C., et al. “Newly diagnosed single unprovoked seizures and epilepsy in Stockholm, Sweden: First report from the Stockholm Incidence Registry of Epilepsy (SIRE),”. Epilepsia. 50(5):1094–1101. doi: 10.1111/j.1528-1167.2008.01726.x. May 2009, doi: 10.1111/j.1528-1167.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- [20].University of Michigan Hospital and Dravet Syndrome Foundation & Ion Channel Epilepsy Alliance. “International Ion Channel Epilepsy Patient Registry.”. https://sites.google.com/umich.edu/iicepr .
- [21].Lehtimäki K., et al. “The Surgical Approach to the Anterior Nucleus of Thalamus in Patients With Refractory Epilepsy: Experience from the International Multicenter Registry (MORE),”. Neurosurgery. 84(1):141–150. doi: 10.1093/neuros/nyy023. Jan. 2019, doi: 10.1093/neuros/nyy023. [DOI] [PubMed] [Google Scholar]
- [22].Tomson T., et al. “EURAP: An International Registry of Antiepileptic Drugs and Pregnancy,”. Epilepsia. 45(11):1463–1464. doi: 10.1111/j.0013-9580.2004.451101.x. 2004, doi: [DOI] [PubMed] [Google Scholar]
- [23].Herzog A. G., Mandle H. B., Cahill K. E., Fowler K. M., Hauser W. A., Davis A. R. “Contraceptive practices of women with epilepsy: Findings of the epilepsy birth control registry,”. Epilepsia. 2016;57(4):630–637. doi: 10.1111/epi.13320. doi: [DOI] [PubMed] [Google Scholar]
- [24].Palma L. de, Benedictis A., De Specchio N., Marras C. E. “Epileptogenic Network Formation,”. Neurosurg. Clin. N. Am. 31(3):335–344. doi: 10.1016/j.nec.2020.03.012. Jul. 2020, doi: 10.1016/j.nec.2020.03.012. [DOI] [PubMed] [Google Scholar]
- [25].Gloor P. “Consciousness as a neurological concept in epileptology: a critical review,”. Epilepsia. 1986;27(Suppl 2):S14–26. doi: 10.1111/j.1528-1157.1986.tb05737.x. doi: 10.1111/j.1528-1157.1986.tb05737.x. [DOI] [PubMed] [Google Scholar]
- [26].Berg A. T., et al. “Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005-2009,”. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. doi: [DOI] [PubMed] [Google Scholar]
- [27].Danielson N. B., Guo J. N., Blumenfeld H. “The default mode network and altered consciousness in epilepsy,”. Behav. Neurol. 24(1):55–65. doi: 10.3233/BEN-2011-0310. Jan. 2011, doi: 10.3233/BEN-2011-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Picard F., Kurth F. “Ictal alterations of consciousness during ecstatic seizures,”. Epilepsy Behav. 30:58–61. doi: 10.1016/j.yebeh.2013.09.036. Jan. 2014, doi: 10.1016/j.yebeh.2013.09.036. [DOI] [PubMed] [Google Scholar]
- [29].Burianová H., et al. “Altered functional connectivity in mesial temporal lobe epilepsy,”. Epilepsy Res. 137:45–52. doi: 10.1016/j.eplepsyres.2017.09.001. Nov. 2017, doi: 10.1016/j.eplepsyres.2017.09.001. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Z., et al. “Epileptic discharges specifically affect intrinsic connectivity networks during absence seizures,”. J. Neurol Sci. 336(1):138–145. doi: 10.1016/j.jns.2013.10.024. Jan. 2014, doi: 10.1016/j.jns.2013.10.024. [DOI] [PubMed] [Google Scholar]
- [31].Lee K. H., et al. “Pathophysiology of altered consciousness during seizures: Subtraction SPECT study,”. Neurology. 59(6):841–846. doi: 10.1212/wnl.59.6.841. Sep. 2002, doi: 10.1212/WNL.59.6.841. [DOI] [PubMed] [Google Scholar]
- [32].Xie F., Xing W., Wang X., Liao W., Shi W. “Altered states of consciousness in epilepsy: a DTI study of the brain,”. Int. J. Neurosci. 127(8):667–672. doi: 10.1080/00207454.2016.1229668. Aug. 2017, doi: 10.1080/00207454.2016.1229668. [DOI] [PubMed] [Google Scholar]
- [33].Arthuis M., et al. “Impaired consciousness during temporal lobe seizures is related to increased long-distance cortical-subcortical synchronization,”. Brain. 132(8):2091–2101. doi: 10.1093/brain/awp086. Aug. 2009, doi: 10.1093/brain/awp086. [DOI] [PubMed] [Google Scholar]
- [34].Li R., et al. “Disruption of functional connectivity among subcortical arousal system and cortical networks in temporal lobe epilepsy,”. Brain Imaging Behav. 14(3):762–771. doi: 10.1007/s11682-018-0014-y. Jun. 2020, doi: 10.1007/s11682-018-0014-y. [DOI] [PubMed] [Google Scholar]
- [35].Blume W. T., Lüders H. O., Mizrahi E., Tassinari C., Boas W. V. E., Engel J. “Glossary of Descriptive Terminology for Ictal Semiology: Report of the ILAE Task Force on Classification and Terminology,”. Epilepsia. 2001;42(9):1212–1218. doi: 10.1046/j.1528-1157.2001.22001.x. doi: [DOI] [PubMed] [Google Scholar]
- [36].Amorim-Leite R., Remick M., Welch W., Abel T. J. “History of the Network Approach in Epilepsy Surgery,”. Neurosurg. Clin. N. Am. 31(3):301–308. doi: 10.1016/j.nec.2020.03.011. Jul. 2020, doi: 10.1016/j.nec.2020.03.011. [DOI] [PubMed] [Google Scholar]
- [37].Bartolomei F., Bettus G., Stam C. J., Guye M. “Interictal network properties in mesial temporal lobe epilepsy: A graph theoretical study from intracerebral recordings,”. Clin. Neurophysiol. Dec. 2013;124(12):2345–2353. doi: 10.1016/j.clinph.2013.06.003. doi: 10.1016/j.clinph.2013.06.003. [DOI] [PubMed] [Google Scholar]
- [38].Wilke C., Worrell G., He B. “Graph analysis of epileptogenic networks in human partial epilepsy,”. Epilepsia. 2011;52(1):84–93. doi: 10.1111/j.1528-1167.2010.02785.x. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ponten S. C., Douw L., Bartolomei F., Reijneveld J. C., Stam C. J. “Indications for network regularization during absence seizures: Weighted and unweighted graph theoretical analyses,”. Exp. Neurol. 217(1):197–204. doi: 10.1016/j.expneurol.2009.02.001. May 2009, doi: 10.1016/j.expneurol.2009.02.001. [DOI] [PubMed] [Google Scholar]
- [40].Kramer M. A., Kolaczyk E. D., Kirsch H. E. “Emergent network topology at seizure onset in humans,”. Epilepsy Res. May 2008;79(2):173–186. doi: 10.1016/j.eplepsyres.2008.02.002. doi: 10.1016/j.eplepsyres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- [41].Bernhardt B. C., Chen Z., He Y., Evans A. C., Bernasconi N. “Graph-Theoretical Analysis Reveals Disrupted Small-World Organization of Cortical Thickness Correlation Networks in Temporal Lobe Epilepsy,”. Cereb. Cortex. Sep. 2011;21(9):2147–2157. doi: 10.1093/cercor/bhq291. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- [42].Vaessen M. J., et al. “White Matter Network Abnormalities Are Associated with Cognitive Decline in Chronic Epilepsy,”. Cereb. Cortex. Sep. 2012;22(9):2139–2147. doi: 10.1093/cercor/bhr298. doi: 10.1093/cercor/bhr298. [DOI] [PubMed] [Google Scholar]
- [43].Sahoo S. S., et al. “NeuroIntegrative Connectivity (NIC) informatics tool for brain functional connectivity network analysis in cohort studies,” in AMIA Annual Symposium Proceedings. 2020. pp. 1090–1099. [PMC free article] [PubMed]
- [44].Jayapandian C., et al. “A scalable neuroinformatics data flow for electrophysiological signals using MapReduce,”. Front. Neuroinformatics. 2015;9:4. doi: 10.3389/fninf.2015.00004. doi: 10.3389/fninf.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pearson K. “Note on regression and inheritance in the case of two parents,”. Proc. R. Soc. Lond. Jan. 1895;58(347-352):240–242. doi: 10.1098/rspl.1895.0041. [Google Scholar]
- [46].Mormann F., Lehnertz K., David P., Elger C. E. “Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients,”. Phys. Nonlinear Phenom. 144(3):358–369. Oct. 2000, doi: 10.1016/S0167-27890000087-7. [Google Scholar]
- [47].Pijn J. P., da Silva F. Lopes. Boston, MA: Birkhäuser; 1993. “Propagation of Electrical Activity: Nonlinear Associations and Time Delays between EEG Signals,” in Basic Mechanisms of the EEG, S. Zschocke and E.-J. Speckmann, Eds. pp. 41–61. doi: 10.1007/978-1-4612-0341-4_4. [Google Scholar]