Abstract
Aims:
To compare insulin dose adjustments made by physicians to those made by an artificial intelligence-based decision support system, the Advisor Pro, in people with type 1 diabetes (T1D) using an insulin pump and self-monitoring blood glucose (SMBG).
Methods:
This was a multinational, non-interventional study surveying 17 physicians from 11 countries. Each physician was asked to provide insulin dose adjustments for the settings of the pump including basal rate, carbohydrate-to-insulin ratios (CRs), and correction factors (CFs) for 15 data sets of pumps and SMBG of people with T1D (mean age 18.4 ± 4.8 years; eight females; mean glycated hemoglobin 8.2% ± 1.4% [66 ± 11mmol/mol]). The recommendations were compared among the physicians and between the physicians and the Advisor Pro. The study endpoint was the percentage of comparison points for which there was an agreement on the direction of insulin dose adjustments.
Results:
The percentage (mean ± SD) of agreement among the physicians on the direction of insulin pump dose adjustments was 51.8% ± 9.2%, 54.2% ± 6.4%, and 49.8% ± 11.6% for the basal, CR, and CF, respectively. The automated recommendations of the Advisor Pro on the direction of insulin dose adjustments were comparable )49.5% ± 6.4%, 55.3% ± 8.7%, and 47.6% ± 14.4% for the basal rate, CR, and CF, respectively( and noninferior to those provided by physicians. The mean absolute difference in magnitude of change between physicians was 17.1% ± 13.1%, 14.6% ± 8.4%, and 23.9% ± 18.6% for the basal, CR, and CF, respectively, and comparable to the Advisor Pro 11.7% ± 9.7%, 10.1% ± 4.5%, and 25.5% ± 19.5%, respectively, significant for basal and CR.
Conclusions:
Considerable differences in the recommendations for changes in insulin dosing were observed among physicians. Since automated recommendations by the Advisor Pro were similar to those given by physicians, it could be considered a useful tool to manage T1D.
Keywords: insulin pump settings adjustments, Advisor Pro, automated decision support, self-monitoring of blood glucose, type 1 diabetes
Introduction
Intensive insulin treatment based on the monitoring of blood glucose levels is essential for the management of people with type 1 diabetes (T1D) to prevent diabetes-related complications. 1 Nevertheless, the majority of people with T1D do not attain the desired glycemic targets and are, therefore, prone to develop these complications.2,3 In an attempt to help the patients achieve glycemic targets and to overcome the complexity of managing diabetes, new strategies are being developed. The use of digital tools, decision support systems (DSSs), and telemedicine is emerging with the aim of supporting people with diabetes and their healthcare providers in diabetes-related decisions and daily management.4,5
One of the major challenges in accomplishing good glycemic control is the constant need to adjust insulin doses owing to the frequent changes in insulin demands and sensitivity.6,7 For patients using an insulin pump, adjusting the insulin pump settings is particularly complex due to the need to modify a variety of parameters for different times of the day. Optimal dose adjustment of these parameters, which include the basal rate, carbohydrate-to-insulin ratios (CRs), and correction factors (CFs), needs to take into consideration the insulin delivery rate (all data of insulin delivery available from the pump data: the basal hourly timed delivery rate including temporary basal rate and suspend, insulin boluses including meals and correction, amount and timing of each bolus), carbohydrates consumed, and glucose levels obtained from self-monitoring blood glucose (SMBG) or a continuous glucose monitoring (CGM) device. Thus, the task of properly and frequently adjusting insulin doses for patients using pumps requires expertise and experience from the healthcare provider, which is time-consuming and may become not feasible as 50%-60% of individuals with T1D use an insulin pump.2,8,9
DSSs provide new tools in the treatment of T1D as they aid in adjusting insulin doses during and between clinic visits. The DreaMed Advisor Pro (DreaMed Diabetes Ltd., Petah Tikva, Israel) is a Food and Drug Administration (FDA)-cleared and CE-marked DSS that assist healthcare professionals in the management of people with T1D using an insulin pump and CGM or SMBG. The Advisor Pro utilizes data on glucose, insulin doses, and carbohydrate intake from various patient devices, to automatically provide exact insulin dosing recommendations and other treatment tips. In a previous study, we compared insulin pump setting adjustments made by 26 different physicians and by the Advisor Pro to the same set of 15 patient data using an insulin pump and continuous glucose sensor. Full agreement among physicians in the direction of insulin adjustments was in the range of 41%-46% and 10%-12% for full disagreement for each of the pump parameters, which was comparable with the adjustments suggested by the Advisor Pro. 10 These results imply that for treating people with T1D, an artificial intelligence (AI)-based algorithm can provide recommendations for insulin dose adjustments, which are similar to those given by experienced physicians. In the current study, we compared different expert physicians’ dose adjustments, but this time based on SMBG data, as SMBG is still more in use worldwide than continuous glucose sensors for various reasons.11,12 Therefore, there is a need for a DSS for insulin pump dose adjustments that are also based on SMBG. We tested whether the level of agreement on the direction of insulin adjustments (increase, decrease, or no change) among expert physicians and between the physicians and the Advisor Pro is comparable when based on SMBG data.
Methods
Study Design
This was a non-interventional survey study. Endocrinologists experienced in the treatment of T1D who participated in the Advanced Technologies and Treatments for Diabetes Medtronic master school (Rome, June 2018) were invited to participate in the survey. Those who agreed were asked to provide insulin dose adjustment recommendations based on data downloaded from the CareLink Pro Ver 4.0A (Medtronic Diabetes, Northridge, CA, USA) of 15 randomly selected people with T1D. Three-week CareLink upload data (standard patient profiles) on SMBG and insulin pump, including details about the current pump settings (basal rate, CR, CF, bolus calculator glucose targets, and active insulin time), were sent to the participants via email. The CareLink pump download file also contained insulin delivery data, including insulin boluses and data from the bolus calculator, with amount of carbohydrates for each meal when entered. In addition to the 21-day CareLink download, each case was supplemented with anonymized information about the patient, including gender, age, glycated hemoglobin (HbA1c), weight, height, and body mass index. An example of the provided form for the physicians is added in the supplementary material. The physicians were asked to review each case and to provide insulin dose adjustment recommendations in a unified form. In addition, they were asked to add recommendations for behavioral changes based on the data, if needed. The study protocol was approved by the Rabin Medical Center Institutional Review Board (No. 000917).
The DreaMed Advisor Pro
The Advisor Pro (DreaMed Diabetes Ltd., Petah Tikva, Israel) is an AI-based DSS that was cleared by the FDA. It assists healthcare professionals in the management of people with T1D who use an insulin pump and monitor their glucose levels by CGM or SMBG. The Advisor Pro collects diabetes-related information from various brands of devices downloaded to a Diabetes Management System (ie, glucose data from a glucose meter or/and a CGM device, insulin delivery data from the insulin pump, and carbohydrate count from the pump bolus calculator). Then, the Advisor Pro analyzes the existing information, identifies glucose patterns as well as insulin dosing events and their probable causes. Based on these analyses, the Advisor Pro produces recommendations for exact insulin pump dosing adjustments, including basal, CR, and CF plans. In addition, the Advisor generates suggestions for personalized diabetes management tips such as the timing of insulin meal boluses or absence of bolus delivery before meals. These personalized insights and suggestions can be the basis for a conversation between the healthcare provider and the patient during clinic visits or between them. The Advisor Pro recommendations were evaluated in a previous small feasibility study that showed that it was capable of providing insulin dose adjustments similar to those given by experienced physicians. 10 The principles of the Advisor Pro technology are illustrated in Figure 1. More details on Advisor Pro are provided in the supplementary material, including an example of the Advisor Pro recommendation report in Supplemental Figure S1.
Figure 1.
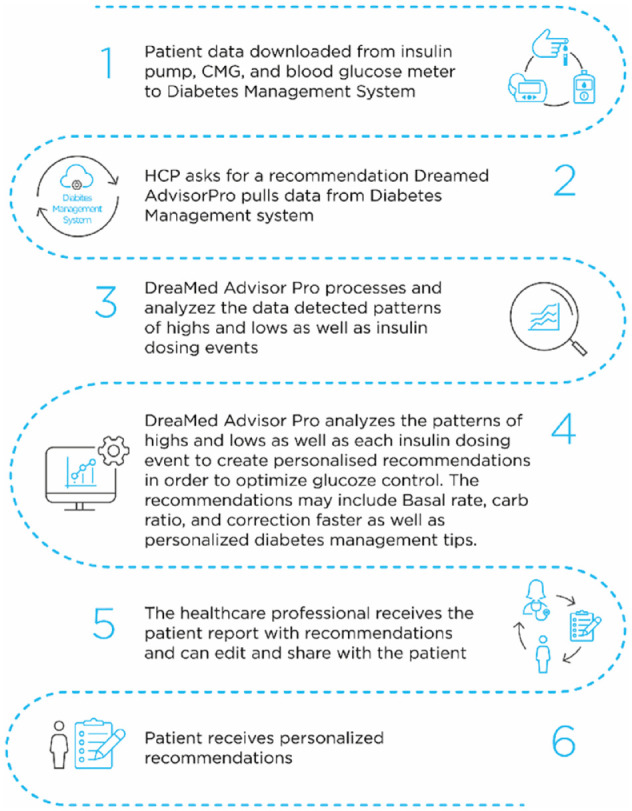
The DreaMed Advisor Pro workflow.
Outcomes and Data Analysis
The level of agreement on the direction of the insulin dose adjustments (increase, decrease, or no change) among the physicians and compared to the Advisor Pro was evaluated as we previously described. 10 In brief, for each hour of the day, the insulin dose recommendations were compared for the relative changes to the patient’s current pump settings in the basal, CR, and CF plans among the physicians and between the physicians and the automated algorithm (further explanation on the method in the supplementary appendix). Hence, a total of 1080 comparison points were available for analysis (24 hours, 15 patients, and 3 pump setting parameters).
The primary endpoint of the study was the percentage of comparison points for which there was a full agreement on the direction of the suggested adjustment in the treatment plan (increase, decrease, or no change in the insulin dose) or a full disagreement (opposite directions of insulin dose adjustments, means one wanted to increase and other to decrease). The secondary endpoint was the percentage of comparison points that were in partial positive disagreement (ie, one physician advised increasing the insulin dose and another advised no change) and partial negative disagreement (ie, one physician advised decreasing the insulin dose and another advised no change). In addition, we compared the magnitude of dosing adjustments relative to the current pump settings.
Statistical Analysis
A one-tailed, noninferiority t-test was used to assess whether the agreement (right tail) and the disagreement (left tail) between physicians and the Advisor Pro (17 pairs and total of 18,360 comparison points) was not inferior to the agreement and disagreement between pairs of physicians (136 pairs and total of 146,880 comparison points). The interquartile range of the level of agreement between the physicians was defined for the noninferiority t-test margin. For the level of agreement between the physicians and Advisor Pro the limit was set for the 25th percentile (one quartile) and for the level of disagreement greater than the 75th percentile (one quartile) that is found between the physicians. The null hypothesis was that there is a difference in agreement/disagreement between physicians and the Advisor Pro. Therefore, any significant P-value (<.05) indicated that there was no significant difference between the physicians and Advisor Pro recommendations, indicating the noninferiority of the Advisor Pro. The number of similar or dissimilar time periods for each parameter basal, CR, and CF change from the original program was compared among physicians and the Advisor Pro using a general linear model repeated-measures analysis with pairwise comparisons. The number of parameters that were changed for the same period of time among physicians and the Advisor Pro was compared using Pearson’s χ2 tests. The magnitude of the insulin dose adjustment between physicians and the Advisor Pro for the three variables (basal, CR, and CF) was compared using a one-tailed, noninferiority t-test. The null hypothesis was that the magnitude of the difference in dose adjustments between physicians and the Advisor Pro was greater than the magnitude of the difference in dose adjustments between each pair of physicians. Therefore, any significant P-value (<.05) indicated that the Advisor Pro is not inferior compared to the physicians (ie, comparable) regarding recommendations on the magnitude of insulin dose adjustments. The noninferiority margin was the 75th percentile of the mean absolute difference.
In addition, we conducted a separate comparison between Advisor Pro recommendations only for pump dosing adjustments made once based on CGM data and another based on SMBG data, which was artificially simulated based on CGM data. A total of 923 different data sets (each data set included 21 days from the insulin pump and CGM devices) was used for the comparison. A special method was used to simulate SMBG points from the CGM data. The simulation creates, on average, 3-9 SMBG data points (see the detailed method in the supplementary appendix). Recommendations of the Advisor Pro, which were made based on the CGM data, were compared to its recommendations that were made based on the artificial simulated SMBG data, and the level of agreement and disagreement was evaluated.
Results
Seventeen physicians from 11 countries (Croatia, Spain, Finland, Serbia, Italy, Greece, Belgium, South Africa, Czech Republic, United Arab Emirates, and Israel) participated in the survey. Of the responders, 16 physicians practice in academic centers and 1 in a private care clinic. The average number of years since medical school graduation in this group was 19.1±7.8 years. Each physician provides diabetes care to an average of 284.7 ± 116.3 people with T1D, of whom 38.2% ± 18.1% use pump therapy and 49.5% ± 26.8% use continuous or flash glucose monitoring. All 17 physicians reviewed all 15 cases as described.
The characteristics of the 15 patients whose data were used for the study survey are presented in Table 1. Additional data regarding insulin pump parameters for each individual case including average daily blood glucose measurements, average glucose level, and total daily insulin dose are presented in Supplemental Table S1. The cases were heterogeneous in means of initial pump parameters, for example, basal rates for each case range from 2 to 7 different rates and percentage of basal from total daily dose ranges from 20% to 60%.
Table 1.
Patient Data Set Characteristics.
| N | 15 |
|---|---|
| Age (years) | 18.4 ± 4.8 |
| Gender (M/F) | 7/8 |
| Weight (kg) | 66.0 ± 14.0 |
| Height (cm) | 166.1 ± 9.7 |
| BMI | 23.9 ± 4.8 |
| BMI-SDS* | 0.4 ± 1 |
| HbA1c (%) | 8.2 ± 1.4 |
| HbA1c range (%) | 5.4-11.4 |
| HbA1c (mmol/mol) | 65.9 ± 15.1 |
| HbA1c range (mmol/mol) | 35.5-101.1 |
| TDD (U) | 52.1 ± 22.1 |
| TDD (U/kg) | 0.76 ± 0.19 |
| Bolus ratio (%) | 55.1 ± 11.3 |
| Average daily SMBG | 6.2 ± 1.5 |
The data are presented as means and standard deviations.
The BMI-SDS was calculated for 12 pediatric participants (under 20 years of age).
BMI, body mass index; HbA1c, glycated hemoglobin; SMBG, self-blood glucose monitoring; TDD, total daily dose.
Primary Endpoint Outcomes
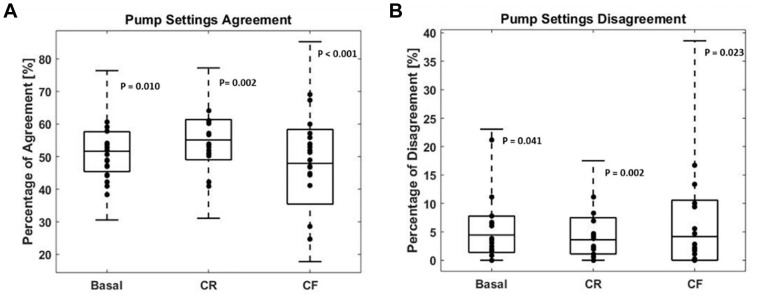
The levels of full agreement and disagreement for each of the pump parameter adjustments among the participating physicians, and between the physicians and Advisor Pro are presented in Figure 2.
Figure 2.
Level of agreement and disagreement in the direction of insulin dose adjustments for basal, CR, and CF plans.CF, correction factor; CR, carbohydrate-to-insulin ratio.
Level of agreement (A) and disagreement (B) per pump setting. The box plot represents the among-physician comparisons showing the median, interquartile range, minimum, and maximum (N = 136) per pump setting feature. Dots represent the Advisor Pro–physician comparisons (N = 17) per pump setting feature.
The level of full agreement on the direction of insulin dose adjustment among the physicians was 51.8% ± 9.2%, 54.2% ± 6.4%, and 49.8% ± 11.6% for the basal rate, CR, and CF, respectively, while the level of agreement between the Advisor Pro and the physicians was 49.5% ± 6.4%, 55.3% ± 8.7%, and 47.6% ± 14.4% for the basal rate, CR, and CF, respectively.
The level of full disagreement on the direction of insulin dose adjustment among the physicians was 5.4% ± 5.3%, 3.5% ± 3%, and 4.2% ± 5.1% for the basal rate, CR, and CF, respectively, while the level of disagreement between the Advisor Pro and the physicians was 5.5% ± 5.1%, 4.6% ± 4.1%, and 6.4% ± 7.2% for the basal rate, CR, and CF, respectively.
Secondary Endpoint Outcomes
Table 2 depicts the percentages of partial disagreement on the direction of insulin dose changes among the physicians and between the physicians and the Advisor Pro. For the basal rate, similar percentages of partial positive (to increase insulin vs no change) disagreement were observed among the physicians and between the physicians and the Advisor Pro (average 32.9% and 28.5%, respectively). In addition, also similar percentage of partial negative (to decrease insulin vs no change) disagreement was observed among the physicians and between the physicians and the Advisor Pro (average 12.2% and 14.1%, respectively) for the basal rate. For the bolus parameters (CR and CF), the mean percentage of partial negative disagreement was similar in both groups (around 30%), whereas the mean percentage of partial positive disagreement was significantly different between the two groups (within the range of 11%-14% and 16%-21% for the CR and CF, respectively; Table 2).
Table 2.
Results of Comparison for Partial Disagreement on Insulin Dosing Adjustments.
| Parameter, mean (SD) | Among physicians | Between Advisor Pro and physicians | P-value |
|---|---|---|---|
| Recommendation for no change vs recommendation to deliver more insulin via basal (%) | 32.9 ± 5.8 | 28.5 ± 8.6 | <.001 |
| Recommendation for no change vs recommendation to deliver less insulin via basal (%) | 12.2 ± 4.0 | 14.1± 7.7 | <.001 |
| Recommendation for no change vs recommendation to deliver more insulin via CR (%) | 10.8 ± 5.9 | 14.2 ± 6.3 | .12 |
| Recommendation for no change vs recommendation to deliver less insulin via CR (%) | 31.5 ± 5.0 | 26.0 ± 6.8 | <.001 |
| Recommendation for no change vs recommendation to deliver more insulin via CF (%) | 16.2 ± 12.8 | 20.6 ± 13.4 | .05 |
| Recommendation for no change vs recommendation to deliver less insulin via CF (%) | 29.9 ± 8.2 | 25.4 ± 12.5 | <.001 |
CF, correction factor; CR, carbohydrate-to-insulin ratio; SD, standard deviation.
Values are mean ± SD.
The Magnitude of Insulin Dosing Adjustments
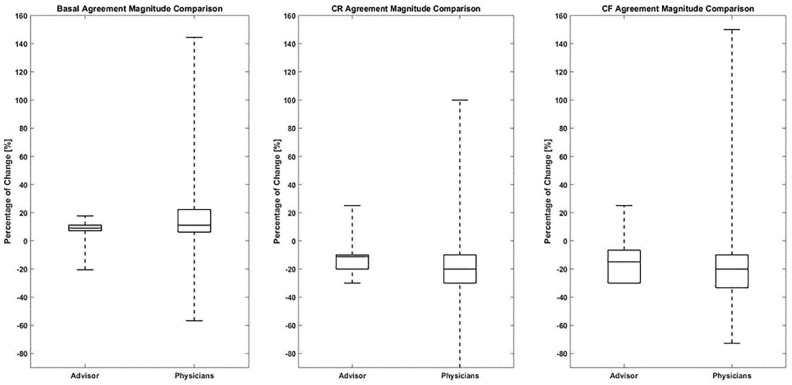
The magnitude of insulin dose adjustments was calculated as the percentage of change from the current patient pump settings. Figure 3 represents the magnitude of change for the points of full agreement among the physicians and between the physicians and the Advisor Pro for basal, CR, and CF pump parameters. The magnitude of insulin dose adjustments was comparable between the physicians and the Advisor Pro for basal rate and CR dosing parameters. The mean absolute difference in magnitude of change between physicians for the basal rate was 17.1% ± 13.1% and between physicians and Advisor Pro was 11.7% ± 9.7% (P < .001) (Figure 3). For the CR, the mean absolute difference in magnitude of change between physicians was 14.6% ± 8.4% and between physicians and Advisor Pro was 10.1% ± 4.5% (P < .001) (Figure 3). A nonsignificant difference was found in the magnitude of change for the CF. The mean absolute difference in the magnitude of change between physicians was 23.9% ± 18.6% and between physicians and Advisor Pro was 25.5% ± 19.5% (not significant) (Figure 3). The reason for the insignificant result was outlier data from one physician. The absolute magnitude of change between this physician and the other physicians was 61% ± 25% and between this physician and Advisor Pro was 97% while the majority ranged around 25%.
Figure 3.
Magnitude of insulin pump dosing adjustments in cases of full agreement for basal, CR, and CF plans.
CF, correction factor; CR, carbohydrate-to-insulin ratio. Percentage of change in insulin dosing pump parameters: basal, CR, and CF plans in cases of full agreement (increase or decrease) on the direction of insulin dosing adjustment (1157 cases for the basal rate, 996 cases for the CR, and 550 cases for the CF).
The box plot displays the median and interquartile range and the dashed line the minimum and maximum of the percentage of dose change. P values for the three parameters: basal P < .001, CR P < .01 and CF not significant.
Advisor Pro Recommendations Based on CGM Compared to SMBG Data Points
A high level of agreement was found on the direction of insulin dose adjustments between Advisor Pro recommendations based on CGM and those based on simulated SMBG (58.7% ± 22.3%, 64.7% ± 22.9%, and 62.4% ± 26.2% for average 3 simulated SMBG data points per day, for basal, CR, and CF, respectively, Supplemental Table S2). A significant positive correlation was found between the number of SMBG-simulated points and the percent of agreement on insulin dosing adjustments. The level of disagreement was low, less than 2% for all numbers of average simulated SMBG data points (Supplemental Table S1 and Supplemental Figure S2).
Discussion
Insulin dose adjustments for people with T1D treated with an insulin pump and SMBG recommended by the automated algorithm Advisor Pro were found to be comparable and noninferior to those given by experienced physicians with regard to the direction and magnitude of insulin adjustments. Considerable variability was found in the recommendations of the physicians, with the percentage of complete agreement on the direction of insulin dose adjustments being only around 50%. However, the level of complete disagreement between the physicians was 5% for all insulin pump parameters.
The current survey showed that the level of complete agreement among the physicians as well as between the physicians and the Advisor Pro was higher, while the level of complete disagreement was significantly lower compared to recommendations based on CGM data, which were reported in our previous study. 10 First and foremost, this finding demonstrates the prospects of the Advisor Pro in everyday practice. The results of this survey showed that for people with T1D using an insulin pump and SMBG, the Advisor Pro recommendations are noninferior to those given by experienced physicians from academic centers, suggesting that it can be used to adjust insulin pump settings based on either CGM or SMBG data. Second, to our understanding, the higher level of agreement and lower level of disagreement in the direction of insulin dose adjustments observed in this survey (pump and SMBG data) compared to the previous survey (pump and CGM data) are due to the amount and complexity of the data that are generated by CGM as compared to SMBG. The higher level of agreement found in the set of recommendations based on SMBG compared to CGM data relates to the higher cases where no change in insulin dosing was recommended. Thus, more information and data complexity may lead to less agreement and diversity in decision making on insulin dose adjustments. This may also explain the difference between the level of partial positive disagreement between physicians and between physicians and Advisor Pro. The complexity of data analysis may be part of the answer to the technological paradox. Although more people are using insulin pumps and CGM, the glycemic control did not improve and even deteriorated during the last years. 3
The Diabetes Control and Complications Trial showed that intensive insulin therapy achieved a 2% reduction in HbA1c by using weekly in-person clinic visits until target glucose ranges were achieved, followed by monthly visits and weekly telephone contacts. 13 These findings underscore the need for frequent professional monitoring to improve glycemic control. Frequent monitoring allows adjustments of insulin dosing that are essential to overcoming changes in insulin sensitivity, alterations in exercise and/or dietary patterns, and, importantly, changes in growth and development that are fundamental to pediatric diabetes care.
The current recommendation is to perform clinical visits every three to four months14,15; however, in its current status, the global healthcare system cannot provide such support. In practice, the frequency of patient visits is lower due to distance, loss of working days, and so on. In the course of a year, only 0.1% of the patient’s time is accompanied by a healthcare professional. 16 Thus, access to quality care and self-management tools is needed. Digital tools and DSS can help with insulin titration efforts and hold promise for improvement of glycemic control.
The most important parameter for a DSS is the assurance that adequate treatment safety is not compromised by the attempts to improve glycemic control. The low disagreement percentage of the Advisor Pro and the magnitude of the insulin pump parameter changes, both comparable to those of the physicians, imply that the safety of the patients is noninferior to current practice. Moreover, as suggested by others, the frequent titrations of insulin are likely to improve glycemic control while maintaining the safety. 17 One of the main factors in ensuring the safety of the Advisor Pro recommendations is achieved by limiting the changes in insulin factors allowed by the algorithm. Currently, the allowed changes in the basal rate are set at ±20% from the current setting, while the CR and CF allowed changes are set at ±30%. It is interesting to note that the greatest variance in the magnitude of change made by physicans was for the CF parameter as much as a mean absolute change of 100%-150%. This variance occurred mainly during the night time and may reflect different attitudes of physicians regarding conservative treatment during this period.
In addition to evaluating the automated Advisor Pro recommendations compared to those given by experienced diabetologists, the methodology of our study enabled us to analyze the consistency of insulin dosing recommendations among experienced physicians. The percentage of disagreement in the direction of insulin dose adjustments observed among the physicians was low. However, considerable variability was found in their recommendations with an agreement percentage of only about 50%. Previous studies demonstrated substantial differences in insulin dosing recommendations among different healthcare providers. 18 These variations may be related to differences in attitude among different insulin prescribers such as primary physicians, endocrinologists, and nurse practitioners. In our study, all participating physicians were endocrinologists, and yet considerable differences in insulin dosing were observed, signifying the complexity of insulin pump setting adjustments. We may postulate that even lower agreement percentages would have been observed if the survey included other healthcare providers.
The current survey was designed to demonstrate the variability in insulin dosing recommendations between experienced physicians from academic centers and at the same level with the Advisor Pro. Noninferiority was demonstrated in the level of complete agreement on the direction of needed insulin adjustments (increase, decrease, or no change) and their magnitude.
This was not a clinical study and has a few limitations. The study survey included only experienced physicians in endocrinology, mainly from Europe; hence, we are limited in the ability to generalize the findings to other prescribers such as primary care physicians and nurse practitioners or prescribers from different geographical locations. In addition, the reviewed cases included adolescents and young adults (ages ranged from 12 to 29 years). Therefore, including different age groups may yield different results. Importantly, the study was not designed to evaluate the efficacy of dosing adjustments. Clinical studies are needed to evaluate the efficacy of the Advisor Pro as an everyday clinical tool. Indeed, a multicenter multinational parallel-design study including 108 participants (ages 10-21) was recently completed. The study compared the glycemic control using insulin pump adjustments made by the Advisor Pro and by physicians from specialized academic diabetes centers (ClinicalTrials.gov identifier: NCT03003806).
The advantage of the study is its methodology that enables a direct comparison between dosing decisions made by AI automatization and humans. The previous study, which was similar, enabled us to compare the level of agreement based on data derived from CGM and SMBG.
Conclusion
In conclusion, considerable differences in the recommendations for changes in insulin pump dosing parameters based on SMBG data were observed among experienced physicians. Automated recommendations by an artificial DSS, the DreaMed Advisor Pro, were similar to those given by experienced physicians. Automated systems can facilitate diabetes management, empower patients, and provide remote between visits consultation in the era of shortage in healthcare providers and limited access to medical care in rural areas. The need for remote consultation has become even more critical during the Covid-19 outbreak when in-person visits have become limited. We anticipate that shortly, the Advisor Pro and other automated algorithms will be able to provide proper and consistent insulin dosing adjustments during clinical visits and between them. Further clinical studies are needed to evaluate the efficacy of the Advisor Pro as an everyday clinical tool.
Supplemental Material
Supplemental material, Supplementary_Data_-_Rev-Clean for Adjustment of Insulin Pump Settings in Type 1 Diabetes Management: Advisor Pro Device Compared to Physicians’ Recommendations by Revital Nimri, Tal Oron, Ido Muller, Ivana Kraljevic, Montserrat Martín Alonso, Paivi Keskinen, Tanja Milicic, Asaf Oren, Athanasios Christoforidis, Marieke den Brinker, Lutgarda Bozzetto, Andrea Mario Bolla, Michal Krcma, Rosa Anna Rabini, Shadi Tabba, Lizl Smith, Andriani Vazeou, Giulio Maltoni, Elisa Giani, Eran Atlas and Moshe Phillip in Journal of Diabetes Science and Technology
Acknowledgments
The authors wish to thank Shiri Diskin, PhD, for her assistance in editing this manuscript. Part of this work was presented at the 12th International Conference on Advanced Technologies & Treatments for Diabetes, Berlin, Germany, February 20-23, 2019.
Footnotes
Abbreviations: AI, artificial intelligence; BMI, body mass index; CE, conformity european; CF, correction factor; CGM, continuous glucose monitoring; CR, carbohydrate to insulin ratio; DCCT, the diabetes control and complications trial; DSS, decision support systems; FDA, food and drug administration; NS, not significant; SD, standard deviation; SMBG, self-monitoring blood glucose; T1D, type 1 diabetes mellitus.
Authors’ Contributions: RN, IM, EA, and MP contributed to the study concept and design, collected data, participated in data analysis and interpretation, drafted, reviewed, and edited the manuscript. IM and EA performed the statistical analyses. TO participated in the study survey, drafted, reviewed, and edited the manuscript. IK, MMA, PK, TM, AO, AC, MdB, LB, AMB, MK, RAR, ST, LS, AV, GM, and EG participated in the study survey, reviewed, and edited the manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RN reports receiving grants from Helmsley Charitable Trust, Dexcom and Insulet; personal fees and other from DreaMed Diabetes Ltd; personal fees from Novo Nordisk and Eli Lilly; grants from Medtronic; In addition, RN owns DreaMed Diabetes Ltd stock and has a patent DreaMed Diabetes Ltd licensed. ID and EA are DreaMed Diabetes Ltd employees and own DreaMed Diabetes Ltd stock. MP reports receiving grants from Helmsley Charitable Trust, Dexcom and Insulet; personal fees and other from DreaMed Diabetes Ltd; grants and personal fees from Medtronic, Novo Nordisk, grants from Roche, Eli Lilly, and Sanof; grants from Lexicon, OPKO; personal fees from RSP Systems and Qulab Medical; In addition, MP owns DreaMed Diabetes Ltd stock and has a patent DreaMed Diabetes Ltd licensed. All other authors declared no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Revital Nimri  https://orcid.org/0000-0003-3571-4938
https://orcid.org/0000-0003-3571-4938
Michal Krcma  https://orcid.org/0000-0002-1564-2503
https://orcid.org/0000-0002-1564-2503
Supplemental Material: Supplemental material for this article is available online.
References
- 1. The Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971-978. [DOI] [PubMed] [Google Scholar]
- 3. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21:66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prahalad P, Tanenbaum M, Hood K, Maahs DM. Diabetes technology: improving care, improving patient-reported outcomes and preventing complications in young people with Type 1 diabetes. Diabet Med. 2018;35:419-429. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2020;43(suppl 1):s77-s88. [DOI] [PubMed] [Google Scholar]
- 6. Petitti DB, Klingensmith GJ, Bell RA, et al. Glycemic control in youth with diabetes: the SEARCH for diabetes in Youth Study. J Pediatr. 2009;155:668-672.e661-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Diabetes Association. Glycemic targets. Diabetes Care. 2020;43(suppl 1):s66-s76. [DOI] [PubMed] [Google Scholar]
- 8. McKnight JA, Wild SH, Lamb MJ, et al. Glycaemic control of type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabet Med. 2015;32:1036-1050. [DOI] [PubMed] [Google Scholar]
- 9. van den Boom L, Karges B, Auzanneau M, et al. Temporal trends and contemporary use of insulin pump therapy and glucose monitoring among children, adolescents, and adults with type 1 diabetes between 1995 and 2017. Diabetes Care. 2019;42:2050-2056. [DOI] [PubMed] [Google Scholar]
- 10. Nimri R, Dassau E, Segall T, et al. Adjusting insulin doses in patients with type 1 diabetes who use insulin pump and continuous glucose monitoring: Variations among countries and physicians. Diabetes Obes Metab. 2018;20:2458-2466. [DOI] [PubMed] [Google Scholar]
- 11. Anderson JE, Gavin JR, Kruger DF. Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther. 2020;22:169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ontario HQ. Continuous monitoring of glucose for type 1 diabetes: a health technology assessment. Ont Health Technol Assess Ser. 2018;18:1-160. [PMC free article] [PubMed] [Google Scholar]
- 13. Diabetes Control and Complications Trial (DCCT): results of feasibility study. The DCCT Research Group. Diabetes Care. 1987;10:1-19. [DOI] [PubMed] [Google Scholar]
- 14. Pihoker C, Forsander G, Fantahun B, et al. ISPAD Clinical Practice Consensus Guidelines 2018: The delivery of ambulatory diabetes care to children and adolescents with diabetes. Pediatr Diabetes. 2018;19(suppl 27):84-104. [DOI] [PubMed] [Google Scholar]
- 15. Chiang JL, Maahs DM, Garvey KC, et al. Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care. 2018;41:2026-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marrero DG. Diabetes care and research: what should be the next Frontier? Diabetes Spectr. 2016;29:54-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergenstal RM, Johnson M, Passi R, et al. Automated insulin dosing guidance to optimise insulin management in patients with type 2 diabetes: a multicentre, randomised controlled trial. Lancet. 2019;393:1138-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simon AC, Schopman JE, Hoekstra JB, et al. Factors that drive insulin-dosing decisions of diabetes care providers: a vignette-based study in the Netherlands. Diabet Med. 2015;32:69-77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Data_-_Rev-Clean for Adjustment of Insulin Pump Settings in Type 1 Diabetes Management: Advisor Pro Device Compared to Physicians’ Recommendations by Revital Nimri, Tal Oron, Ido Muller, Ivana Kraljevic, Montserrat Martín Alonso, Paivi Keskinen, Tanja Milicic, Asaf Oren, Athanasios Christoforidis, Marieke den Brinker, Lutgarda Bozzetto, Andrea Mario Bolla, Michal Krcma, Rosa Anna Rabini, Shadi Tabba, Lizl Smith, Andriani Vazeou, Giulio Maltoni, Elisa Giani, Eran Atlas and Moshe Phillip in Journal of Diabetes Science and Technology