Abstract
Background:
Continuous glucose monitors (CGMs) have had a significant impact on the management of diabetes mellitus. We present the results of a multinational evaluation of the Cascade CGM (“C-CGM”) over 14 days of in-clinic and home use.
Method:
Each of the 57 enrolled type 1 diabetes mellitus and type 2 diabetes mellitus subjects wore 2 C-CGMs on the abdomen for 14 days. One part of the evaluation was the performance versus reference glucose values generated for 12 -hour in-clinic sessions on days 1, 4, 7, 10, and 14. Glucose blood samples were drawn every 15 minutes and analyzed with the Yellow Spring Instruments (YSI) 2300 glucose analyzer. The performance assessment on in-clinic days was based on paired YSI/CGM data points and on home-use days was based on paired fingerstick BGM (blood glucose monitoring)/CGM data points.
Results:
A total of 17 823 CGM/YSI data points during in-clinic use was analyzed. The mean absolute relative difference for glucose values between 100 and 400 mg/dL (MARD) and mean absolute difference for values between 40 and 100 mg/dL (MAD) were 11.5% and 15.1 mg/dL, respectively. The system accuracy during home use was 12.7% and 15 mg/dL for MARD and MAD, respectively. There were no serious adverse events or infectious complications reported. A modified algorithm “Hybrid Algorithm” was used in a prospective analysis of the in-clinic data, resulting in a MARD of 9.9% and MAD of 14.5 mg/dL.
Conclusions:
The performance of the C-CGM device over 14 days meets the safety and efficacy standards of CGM systems for managing blood glucose levels in people with diabetes. This was further confirmed when the C-CGM system was given approval for CE Mark in October 2019.
Keywords: continuous glucose monitoring, diabetes, CGMS, diabetes therapy, glucose oxidase
Introduction
Currently, approximately 425 million adults are living with diabetes—by 2045 it is projected that there will be as many as 629 million. Diabetes generated at least $727 billion in healthcare expenditures in 2017, which amounted to 12% of total healthcare spending on adults. 1 There is a high risk of comorbidities developing in patients with diabetes as the result of poorly controlled blood glucose levels, for example, heart disease, stroke, peripheral vascular disease, retinal damage, kidney disease, impotence, and nerve damage. Continuous glucose monitoring (CGM) based on interstitial fluid glucose values has been shown to offer significant benefits for persons with diabetes. Literature reviews have shown the advantages and benefits of CGM use associated with improved glycemic control in adults with type 1 diabetes.2-4 Over the last decade or so, factors such as reduced cost, greater comfort, robustness, wear time, and improved accuracy have significantly increased the frequency of CGM use among people with diabetes.5-8
The new Cascade CGM (C-CGM) system developed by WaveForm Diabetes (Wilsonville, OR, USA) has just become commercially available in Europe under the brand name of GlucoMen Day CGM (distributed by A. Menarini Diagnostics). The C-CGM system is an electrochemical sensor that is inserted into the skin of the abdominal region. The C-CGM is based on glucose oxidase enzyme chemistry that is combined with an electrochemical process. The sensor measures the glucose found in the interstitial space residing in the dermis and subcutaneous regions of the abdominal skin tissue. The C-CGM has several unique features that differentiate it from other CGM systems on the market. A key differentiating feature is that the C-CGM is inserted into the skin without the use of a trocar or needle. The wire filament that is the core of the sensors is designed in a way that when a rapid force is applied to it in the form of a spring the sensors will pass into the skin without the need of a trocar. Compared to other insertion techniques, this results in far less tissue damage and associated bleeding, initial foreign body response. Users have commented that they feel less pain from the insertion process than when doing a fingerstick glucose measurement.
Previously, the performance of the C-CGM was assessed over various sensor wear times in prospective, nonlabeled and nonrandomized clinical trials. One early assessment was done using an earlier version of the algorithm in which the performance over seven days was a mean absolute relative difference for glucose values between 100 and 400 mg/dL (MARD) of 13.9% and the CEG analysis identified 81.8% in zone A and 16.6% in zone B. 9 Another study assessed sensors over 10 days with an MARD of 13.3% and the CEG analysis found 76.8% in zone A and 19.9% in zone B. 10 The wear time was extended to 14 days and the initial analysis of that longer wear devices resulted in an MARD of 12.5% with the CEG analysis showing 87.2% and 12.5% in zones A and B, respectively. 11 Valuable clinical performance and safety data as well as user feedback gained from those trials were used for further refinement of the sensor device, algorithm, and user interface software.
In this paper, we are presenting the methods and results of assessing the performance and safety of the CGM system during in-clinic and at-home use from a multinational clinical study. The initial analysis was done on data generated by an earlier version of the algorithm that was embedded in the device and that provided real-time glucose values. The extensive data generated in this study helped us to identify areas of improvement for the existing algorithm. Those improvements were incorporated in an updated Hybrid Algorithm. The Hybrid Algorithm was then used to create the CGM glucose values based on the original glucose current that was stored in the transmitters. This creates glucose values in a serial manner very similar to that which occurred during the study.
Methods
CGM system description: The C-CGM system consists of the sensor assembly (comprising the sensor, sensor insertion guide, and sensor base plate), the nondisposable transmitter, and an app operating on a smart device. An image of the transmitter and sensor is shown in Figure 1.
Figure 1.
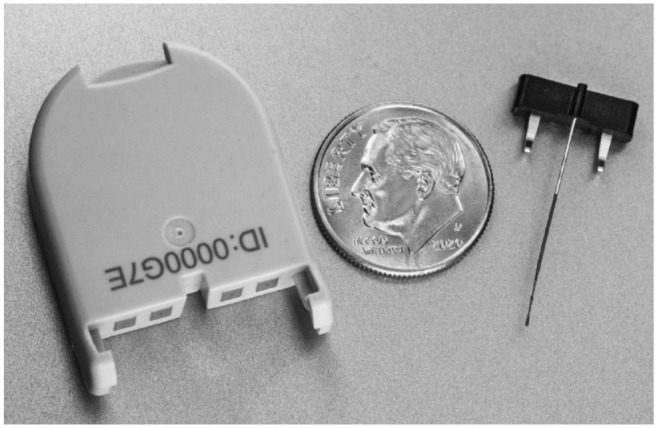
Photo of Cascade continuous glucose monitoring transmitter (left) and sensor (right).
A single-use disposable sensor is inserted into the skin and converts interstitial fluid glucose into an electrical signal. This signal is controlled and processed by a reusable transmitter that is attached to the sensor as part of the body worn unit (BWU). The transmitter contains the potentiostat as well as a microprocessor, memory, battery, BT communication, and several adjunctive sensors. All the signal processing and algorithm functionality of the system resides in the transmitter. This enables the C-CGM to operate completely independently of a receiver or cloud connectivity. The algorithm performs signal processing, sensor calibration, glucose calculation, and auxiliary tasks, such as trend prediction, glucose reading quality assessment, and abnormal glucose detection. The transmitter can be tied via Bluetooth to an app loaded on several formats, including smartphones or watches. The BWU consists of a holding frame for the sensor and the transmitter that is attached to an adhesive patch, which in turn holds the BWU to the skin. The smart device displays glucose levels and glucose trends over time and provides alarms and alerts based on user set glucose levels or rates of change. Calibration of the CGM subsystem is accomplished via capillary blood glucose readings requested by the algorithm at predetermined times or when necessary as prompted by the algorithm. The smart device connects to cloud applications to enable better patient health management.
Clinical study locations: The clinical study was performed on persons with diabetes over the age of 18 and was conducted at 3 different sites in Europe (Study 1: General Hospital Celje Department for the Endocrinology, Angiology and Rheumatology, Oblakova ulica 5 3000 Celje, Slovenia Study 2: Merkur Clinical Hospital University Clinic for Diabetes, Endocrinology and Metabolic Diseases, Zajceva 19 10000 Zagreb, Croatia; Study 3: Clinical Center of Clinical Center of Vojvodina, Novi Sad, Serbia). All three clinical studies were reviewed and approved by the respective national and local ethics committees.
Subject screening, enrollment, and demographics: Sub-jects were enrolled from September 2018 to January 2019 (Clinical study ID: NCT04099043). All potential subjects visited the study site for a screening visit within a period of four weeks prior to the subject’s first study visit. A total of 60 subjects met the screening requirements at the 3 study sites. A total of 57 subjects enrolled in the study, of which 53% were female and 47% male. The study participants were 84% subjects with type 1 diabetes (T1D) and 16% subjects with type 2 diabetes (T2D), thus closely reflecting the anticipated user population for the CGM. The participant ages ranged between 20 and 71 with a mean age of 46 ± 14.5 years (Table 1 for demographics). Forty seven percent of subjects used a combination treatment that included insulin (eg, by multiple daily injections, oral agents or non-insulin injectable hypoglycemic agent treatment) while 53% were using continuous subcutaneous insulin infusion. Subjects had an average body mass index of 25.8 ± 4.6 and hemoglobin A1C (HbA1C) of 7.4% ± 1.4%.
Table 1.
Demographics of Study Participants.
| Mean ± SD | Median | Range | |
|---|---|---|---|
| Age | 46 ± 14.5 | 47 | 20-71 |
| Weight (kg) | 76 ± 15 | 73 | 46-122 |
| Height (cm) | 171.5 ± 9.9 | 171 | 147-190 |
| Body mass index | 25.8 ± 4.6 | 25.2 | 15.9-40.5 |
| HbA1C (%) | 7.4 ± 1.4 | 7.1 | 5.4-11.1 |
HbA1C, hemoglobin A1C; SD, standard deviation.
Study procedure: The study was a prospective, open-labeled and all subjects were provided with a self-monitoring blood glucose meter with test strips (Contour NEXT ONE, Asencia Diabetes Care, Parsippany, NJ, USA). The CGM requires calibration with a blood glucose value once a day. The blood glucose analysis was used for sensor calibration and glucose analysis during the at-home days of the study. Before each in-clinic day, per protocol, subjects were asked not to ingest food for 10 hours before arriving at the clinic. Prior to sensor insertion and glucose modulation session during in-clinic days, subjects underwent intravenous (IV) catheterization of the dorsal hand, lower arm, or antecubital region to obtain blood samples. Venous blood was collected every 15 minutes during in-clinic sessions. The venous blood samples (0.5 mL) were centrifuged, and the plasma part of the sample was used to obtain reference glucose values using a Yellow Spring Instruments (YSI) 2300. On day 1 each subject inserted two sterilized sensors one on the left and one on the right side of the abdomen. The protocol provides for the replacement of a sensor on day 1 if it failed to insert or function properly. If the subject YSI glucose level reached or fell below 3.2 mM, appropriate action was taken by the medical staff to raise blood glucose. The subject could take a meal, but the insulin bolus may have been withheld to achieve a hyperglycemic level of around 15 mM. At the end of the in-clinic day, subjects were permitted to go home if their glucose levels were >5 mM and were stable. At the end of day 14, the sensors were removed from the abdomen by the medical staff and the condition of the skin insertion site was assessed for erythema and/or edema based on the Draize scoring system. 12 The length of sensor wears and reasons for early discontinuation of wear was documented for each sensor. Adhesive assessments were performed at each clinic day visit.
At-home procedure: On the day 1 in-clinic visit, study staff instructed the subjects to record a diary of events, such as shower, bath, exercise (note: swimming was not allowed), start and end time of meals, scheduled BGM measurements, pain or discomfort from the CGM system, and device problems. Subjects were instructed to call site staff if they experienced discomfort, if the device came detached, dislodged, or displayed an error message, or if the device malfunctioned.
CGM calibration: On day 1, calibration was performed twice, first after 45 minutes and then again 360 minutes after insertion. The next calibration was performed 24 hours after the insertion. All other calibrations were scheduled to be performed within 24 hours followed by at least 1 calibration every 24 hours until the end of the wear period. Subjects entered blood glucose values into the CGM receiver loaded with the CGM App. They were instructed not to use the CGM values alone to make insulin dosing decisions.
Insertion pain assessment: Before sensor insertion, subjects performed a finger puncture using a lancet and lancing system at the most frequently used depth setting. Participants were then asked to rate the pain using Gracely Box SL Pain Scale. 13 After participants inserted the two C-CGM sensors, they were asked to rate the pain of the sensor insertion process.
Statistical methods and data analysis: The CGM readings were compared with time-matched glucose values from the reference YSI and BG meter. System agreement within ±15%, ±20%, ±30%, ±40%, and >±40% of the relative difference from the reference YSI values within CGM glucose ranges (40-50, 51-80, 81-180, 181-300, and 300-400 mg/dL) was calculated. Consensus error grid analysis was used to quantify the clinical utility of the CGM system. Precision absolute relative difference between 100 and 400 mg/dL (PARD) and precision absolute difference between 40 and 100 mg/dL (PAD) for sensor pairs worn on the same subject were calculated using a blood glucose cutoff of 100 mg/dL. Data analysis was performed utilizing MATLAB (version R2017b).
Results
Of the 60 subjects who were enrolled in the study, 57 subjects took part in the study and 56 subjects completed all 5 of the in-clinic sessions. Of the 114 successfully inserted sensors, a total of 108 (94.7%) sensors were worn for the full 14 days of the study. There were six sensor systems that required a second sensor insertion. Four were related to poor insertion techniques by the subject and two were due to poor communication between App and the transmitter immediately after insertion. The analysis was conducted on 90 sensors that functioned for the full 14 days of the study, could be calibrated, and blood samples could be obtained for each in-clinic day. The sensors that were excluded were due to incomplete CGM data due to the following causes: loss of communication between the App and the transmitter for prolonged periods, adhesive failures that resulted in large variations in the sensor signals, water ingress caused temporary significant sensors signal shifts, periodic inability to draw blood. The in-clinic data pairs were created by matching each reference YSI glucose value with the nearest neighbor C-CGM value time.
A comparison of the self-assessment of pain of insertion was completed following sensor insertion. Each subject was asked to rate the C-CGM sensor insertion pain compared to their own fingerstick lancing device on a pain scale of 0-20. The Gracely Box SL Pain Scale was used for this assessment. More than 90% of subjects rated the insertion as 4 or less, corresponding to very weak pain, and more than 60% of subjects rated it as a faint pain (Figure 2).
Figure 2.
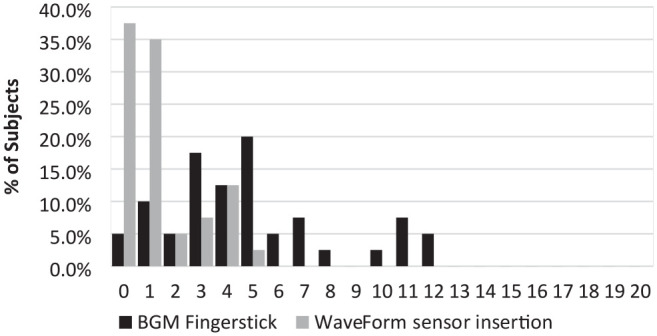
Pain assessment among subjects as experienced after BGM fingerstick and the C-CGM sensor insertion.
C-CGM, Cascade continuous glucose monitoring.
The sensor insertion site and the area under the adhesive were assessed for erythema and edema. Ninety-nine percent of the scores for erythema and edema at the insertion site and under the adhesive were rated as 0 (not present) or 1 (very slight) on the Draize scale (Table 2). No adverse events were recorded.
Table 2.
Assessment of Adverse Effects at Sensor Insertion Site and Under Adhesive After Removal of Sensor Device After 14 Days.
| Draize score |
||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||
| Insertion site | Erythema | 58% | 42% | 0% | 0% | 0% |
| Edema | 99% | 1% | 0% | 0% | 0% | |
| Under adhesive | Erythema | 95% | 4% | 1% | 0% | 0% |
| Edema | 96% | 4% | 0% | 0% | 0% | |
A total of 17 823 CGM-YSI paired data points were recorded within the 40-400 mg/dL functional range of the CGM. We limited the data utilized to those that had calibrated CGM values that were pairable with YSI glucose values. These data pairs were used to assess the performance of the CGM during in-clinic sessions. The home-use performance assessment was done by pairing the Asencia blood glucose readings with the closest CGM value in time. There were a total of 4274 home use data pairs. During in-clinic sessions mean and median ARD between 100 and 400 mg/dL were 11.5% and 9.3%. Mean and median AD was calculated for the reference values between 40 and 100 mg/dL. Those values were 15.1 and 11.6 mg/dL (Table 3). Continuous error grid analysis of the in-clinic data identified that 89.3% of data points were in the most clinically useful zone A, while the combined percentage of data points in zones A and B was 99.3% (Figure 3). The agreement of the C-CGM system to reference blood glucose levels was assessed. The more detailed breakdown of performance was found to be as follows: 68.4% of all CGM readings were within 15/15% of YSI- paired values, 80.5% within 20/20%, 93.2% within 30/30%, and 97.8% within 40/40% of reference value (Table 4).
Table 3.
WaveForm C-CGM System In-Clinic and At-Home Accuracy Performance.
| In-clinic (compared to YSI) |
At-home (compared to BGM) |
|
|---|---|---|
| Number of samples | 17 823 | 4274 |
| Mean ARD (%) 100-400 mg/dL | 11.5 | 12.8 |
| Median ARD (%) 100-400 mg/dL | 9.3 | 9.1 |
| Mean AD (mg/dL) 40-100 mg/dL | 15.1 | 14.9 |
| Median AD (mg/dL) 40-100 mg/dL | 11.6 | 9.3 |
C-CGM, Cascade continuous glucose monitoring; YSI, Yellow Spring Instruments; ARD, absolute relative difference; AD, absolute difference.
Figure 3.
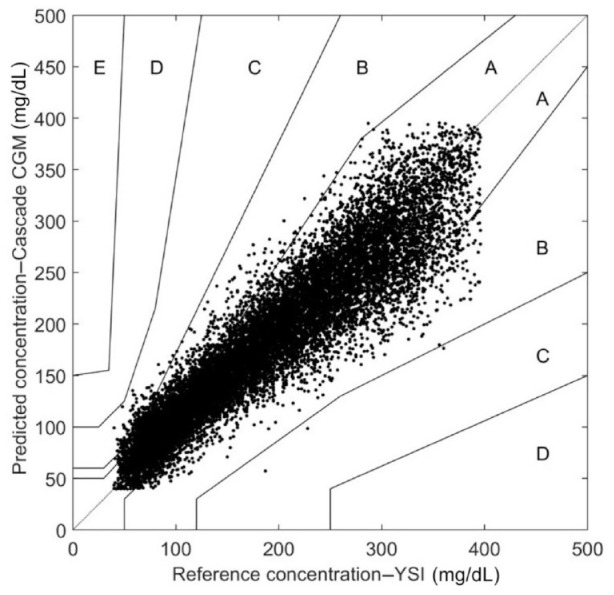
Consensus error grid of in-clinic CGM/YSI pairs. A—89.3%, B—10.0%, C—0.7%, D—0%, E—0%.
CGM, continuous glucose monitoring; YSI, Yellow Spring Instruments.
Table 4.
System Agreement to YSI Reference Within CGM Glucose Ranges During In-Clinic Session.
| CGM glucose ranges (mg/dL) | Number of paired CGM-YSI data points | Percent within 15/15% reference | Percent within 20/20% reference | Percent within 30/30% reference | Percent within 40/40% reference | Percent greater than 40/40% reference |
|---|---|---|---|---|---|---|
| Overall | 17 823 | 68.4 | 80.5 | 93.2 | 97.8 | 2.2 |
| 40-50 | 115 | 51.3 | 61.7 | 81.7 | 94.8 | 5.2 |
| 50-80 | 2116 | 57.2 | 69.1 | 83.9 | 93.4 | 6.6 |
| 80-180 | 7397 | 70.5 | 81.6 | 93.1 | 97.4 | 2.6 |
| 180-300 | 6026 | 72.7 | 85.3 | 96.7 | 99.5 | 0.5 |
| 300-400 | 2167 | 60.9 | 75.2 | 93.4 | 99.1 | 0.9 |
CGM, continuous glucose monitoring; YSI, Yellow Spring Instruments.
The stability of the mean ARD during in-clinic session over the 14 days was evaluated. Mean ARD decreased from day 1 (11.7%) to day 4 (10.3%), followed by a small increase on day 7 (11.2%) and toward day 10 (12.2%) and day 14 (12.2%).
The performance estimations of the home-use data were somewhat lower as indicated by an MARD of 12.8% and a mean absolute difference for values between 40 and 100 mg/dL (MAD) of 14.9 mg/dL (Table 3). Consensus error grid analysis found 86.5% of the data pairs in zone A, and more than 98.6% in zones A and B (Figure 4). These values are to be expected given the less accurate glucose values obtained when using BGM values as a reference. ARD during the home-use days showed a slight decrease in accuracy over time from 11.7% on days 2-3, to 11.4% on days 5-6, 12.2% on days 8-9, and 13.5% on days 12-13. The more detailed performance assessment showed that 65.2% of all CGM readings were within 15/15% of YSI-paired values, 75.6% within 20/20%, 88.1% within 30/30%, and 95.0% within 40/40% of reference value (Table 5).
Figure 4.
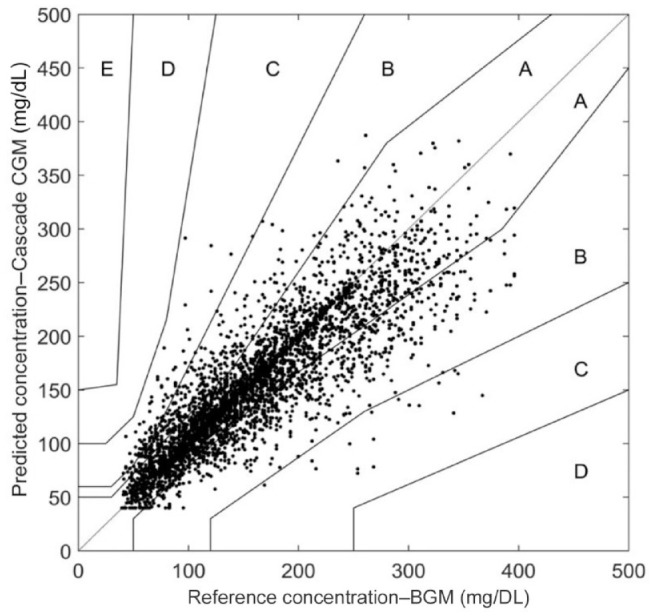
Consensus error grid of home-use CGM/ BGM pairs. Zone A—86.5%, B—12.1%, C—1.5%, D—0%, E—0%.
CGM, continuous glucose monitoring.
Table 5.
System Agreement to BG Reference Within CGM Glucose Ranges During Home Use.
| CGM glucose ranges (mg/dL) | Number of paired CGM-YSI data points | Percent within 15/15% reference | Percent within 20/20% reference | Percent within 30/30% reference | Percent within 40/40% reference | Percent greater than 40/40% reference |
|---|---|---|---|---|---|---|
| Overall | 4274 | 65.2 | 75.6 | 88.1 | 95.0 | 5.0 |
| 40-50 | 50 | 66.0 | 70.0 | 82.0 | 96.0 | 4.0 |
| 50-80 | 505 | 60.1 | 71.7 | 84.4 | 93.1 | 6.9 |
| 80-180 | 2417 | 70.4 | 79.4 | 90.0 | 95.0 | 5.0 |
| 180-300 | 1133 | 60.8 | 73.4 | 88.1 | 96.0 | 4.0 |
| 300-400 | 169 | 33.7 | 51.5 | 74.6 | 94.1 | 5.9 |
CGM, continuous glucose monitoring; YSI, Yellow Spring Instruments.
We identified several areas where the embedded algorithm encountered problems when transitioning from BGM to YSI calibrations that occurred during later in-clinic days. This resulted in some deviations in the conversion of currents into glucose values by the algorithm. We modified the algorithm so that when the algorithm identified a poor transition it would perform calibration for that single day using a single-point calibration. This new algorithm is called the Hybrid Algorithm. It is a hybrid of the blended calibration approach and the single-day calibration approach. The raw data that were generated during the study and had been stored on the transmitters were then used to complete a prospective generation of CGM glucose values using the Hybrid Algorithm. A new performance assessment was then made on the newly generated data pairs. The result was that the overall performance of the CGM was improved with MARD and MAD values of 9.9% and 14.5 mg/dL, respectively (Table 6). The number of pairs in zones A and B in the CEG were 91.4% and 8.0%, respectively. The percent of YSI within 20/20% of CGM readings was 85% (Table 7). PARD and PAD analyzed with Hybrid Algorithm for sensor pairs worn in the same subject were 10.4% and 12.0 mg/dL, respectively (n = 33 sensor pairs).
Table 6.
WaveForm C-CGM System In-Clinic Accuracy Performance After Post Hoc Analysis With Hybrid Algorithm.
| In-clinic (compared to YSI) |
|
|---|---|
| Number of samples | 17 381 |
| Mean ARD (%) 100-400 mg/dL | 9.9 |
| Median ARD (%) 100-400 mg/dL | 7.9 |
| Mean AD (mg/dL) 40-100 mg/dL | 14.5 |
| Median AD (mg/dL) 40-100 mg/dL | 11.3 |
C-CGM, Cascade continuous glucose monitoring; YSI, Yellow Spring Instruments.
Table 7.
System Agreement to YSI Values Within CGM Glucose Ranges During In-Clinic Assessment Based on Hybrid Algorithm Analysis.
| CGM glucose ranges (mg/dL) | Number of paired CGM-YSI data points | Percent within 15/15% reference | Percent within 20/20% reference | Percent within 30/30% reference | Percent within 40/40% reference | Percent greater than 40/40% reference |
|---|---|---|---|---|---|---|
| Overall | 17 381 | 74.0 | 85.0 | 95.2 | 98.5 | 1.5 |
| 40-50 | 91 | 48.4 | 57.1 | 73.6 | 89.0 | 11.0 |
| 50-80 | 1984 | 58.9 | 71.0 | 85.9 | 95.5 | 4.5 |
| 80-180 | 7343 | 73.0 | 83.6 | 94.4 | 97.9 | 2.1 |
| 180-300 | 5913 | 80.7 | 91.4 | 98.7 | 99.8 | 0.2 |
| 300-400 | 2050 | 74.2 | 86.4 | 97.9 | 100 | 0 |
CGM, continuous glucose monitoring; YSI, Yellow Spring Instruments.
Discussion
The primary goal of the in-clinic and at-home study was to validate the safety and performance accuracy of the 14-day WaveForm C-CGM system in a large cohort of people with T1D and T2D over the full range of glucose levels.
A major focus of all CGM devices is safety. Several safety-related parameters were evaluated such as skin irritation from the adhesive or inserted sensor. The sites were assessed by Draize scale scoring from 0 to 4. Ninety-nine percent of the sites were rated 0, which is indicative of no visible or negligible skin reaction. The overall low incidence rate of skin irritation can significantly improve duration of use and tolerability of the C-CGM device in adult users.
Pain experienced during insertion, discomfort while wearing the device, and skin irritation are important contributors to the decision of a CGM user to continue to use their CGM devices. In a survey, the number of children experiencing issues with insertion pain by CGM was more than 78.5%. 14 In the clinical study reported here, 85% of the study participants gave the insertion pain caused by fingerstick a score of 2 or larger (ranging from 2 to 12 on Gracely pain scale), while a large majority of the subjects (72%) rated the insertion pain during insertion of the WaveForm C-CGM system with a score of less than 2. The minimally invasive nature of the insertion process may also be the reason for the low incidence rate of erythema and edema at the insertion site. The advantage of WaveForm’s sensor insertion mechanism to minimize insertion pain in most study subjects may lower the barrier for all users but may do so especially in young children and adolescents.
This protocol was designed to ensure a wide range of hyper- and hypoglycemic blood glucose levels. One hundred fourteen sensors were inserted, and of that a total of 108 sensors were worn for the full 14 days representing a full functionality rate of 94.7%. This shows that the C-CGM device is robust enough to be worn during daily activities. Performance was found to be 11.5% MARD and 15.1 mg/dL MAD of during in-clinic use, and 12.8% and 14.9 mg/dL during home use. After completing the study, complications in the algorithm related to the incorporation of calibrations were corrected in an updated algorithm. The application of the updated Hybrid Algorithm resulted in an improvement in MARD from 11.5% to 9.9% and an increase in CGM values within 15/15% of reference values from 68.4% to 74%. To make a comparison to other published CGM data, MAD (40-80 mg/dL) and MARD (81-400 mg/dL) were 10.5% and 15.6 mg/dL, respectively. There is a plan to implement parts of the Hybrid Algorithm as a modification to the present embedded algorithm.
Overall, the data from the multinational study verified the safety, performance, and user convenience of the 14-day WaveForm C-CGM system in people with T1D and T2D.
Acknowledgments
We are grateful for the support from medical support staff of the following clinical centers: General Hospital Celje in Celje, Slovenia; Merkur Clinical Hospital University Clinic for Diabetes, Endocrinology and Metabolic Diseases in Zagreb, Croatia; and Clinical Center of Clinical Center of Vojvodina, Novi Sad, Serbia. We are especially indebted to Mojca Fir, Vizera d.o.o. for her organizational skill and assistance in making this multinational study possible.
Footnotes
Abbreviations: BT, bluetooth; BWU, body worn unit; CGM, continuous glucose monitoring; C-CGM, cascade continuous glucose monitoring; CEG, consensus error grid; CSII, continuous subcutaneous insulin infusion; MARD, mean absolute relative difference for glucose values between 100-400 mg/dl; MAD, mean absolute difference for values between 40-00 mg/dl; PARD, precision absolute relative difference between 100-400 mg/dl; PAD, precision absolute difference between 40 and 100 mg/dL; SMBG, self monitoring blood glucose; T1DM//T2DM, type I or II diabetes mellitus; YSI, yellow spring instruments.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors are employees of WaveForm Technologies, Inc. (An AgaMatrix Holdings LLC Company).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by WaveForm Technologies.
ORCID iD: Ralph Dutt-Ballerstadt  https://orcid.org/0000-0003-3046-4335
https://orcid.org/0000-0003-3046-4335
References
- 1. IDF Diabetes Atlas Eighth Edition 2017. Available at: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html.
- 2. Rodbard D. Continuous Glucose Monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19:25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hirsch IB, Battelino T, Peters AL, Chamberlain JJ, Aleppo G, Bergenstal RM. Role of Continuous Glucose Monitoring in Diabetes Treatment. Arlington, VA: American Diabetes Association, 2018. [Google Scholar]
- 4. Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and application. Diabetes Metab J. 2019;43:383-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Karinka AA, Bailey TS, Brazg RL, et al. 910-P: improved accuracy of 14-day factory-calibrated FreStyle Libre System with new glucose algorithm. Diabetes. 2019;68(suppl 1). [Google Scholar]
- 6. Welsh JB, Gao P, Derdzinski M, et al. Accuracy, utilization, and effectiveness comparison of different continuous glucose monitoring systems. Diabetes Technol Ther. 2019;21:128-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christiansen MP, Garg SK, Brazg R, et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christiansen MP, Klaff LJ, Bailey TS, Brazg R, Carlson G, Tweden KS. A prospective multicenter evaluation of the accuracy and safety of an implanted continuous glucose sensor: the PRECISION study. DiabetesTechnolTher. 2019; 21:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rebec M, Anderson E, Bruce R, Dutt-Ballerstadt R. Assessment of the performance of the WaveForm CGM system. J Diabetes Sci Technol. 2018; 12:426-532.29493358 [Google Scholar]
- 10. Rebec M, Anderson E, Bruce R, Dutt-Ballerstadt R. Results of first 10-day use study for WaveForm CGM. J Diabetes Sci Technol. 2018;12:426-532.29493358 [Google Scholar]
- 11. Rebec M, Anderson EM, Dutt-Ballerstadt R, Haidar A, Singh A, Janez A. Accuracy assessment of the WaveForm C-CGM system versus FreeStyle Libre over 14 days. Diabetologia. 2018;61(suppl 1):1-620 .30132038 [Google Scholar]
- 12. Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Ther. 1944;82:377-390. [Google Scholar]
- 13. Gracely RH, Dubner R. Pain assessment in humans: a reply to Hall. Pain. 1981;11:109-120. [DOI] [PubMed] [Google Scholar]
- 14. Engler R, Routh TL, Lucsiano Y. Adoption barriers for continuous monitoring and their potential reduction with a fully implantable system: results from patient preference surveys. Clin Diabetes.2018;36:50-58. [DOI] [PMC free article] [PubMed] [Google Scholar]


