Abstract
Background:
Recent guidelines have been developed for continuous glucose monitoring (CGM) metrics in persons with diabetes. To understand what glucose profiles should be judged as normal in clinical practice and glucose-lowering trials, we examined the glucose profile of healthy individuals using CGM.
Methods:
Persons without diabetes or prediabetes were included after passing a normal oral glucose tolerance test, two-hour value <8.9 mmol/L (160 mg/dL), fasting glucose <6.1 mmol/L (110 mg/dL), and HbA1c <6.0% (<42 mmol/mol). CGM metrics were evaluated using the Dexcom G4 Platinum.
Results:
In total, 60 persons were included, mean age was 43.0 years, 70.0% were women, mean HbA1c was 5.3% (34 mmol/mol), and mean body mass index was 25.7 kg/m2. Median and mean percent times in hypoglycemia <3.9 mmol/L (70 mg/dL) were 1.6% (IQR 0.6-3.2), and 3.2% (95% CI 2.0; 4.3), respectively. For glucose levels <3.0 mmol/L (54 mg/dL), the corresponding estimates were 0.0% (IQR 0.0-0.4) and 0.5% (95% CI 0.2; 0.8). Median and mean time-in-range (3.9-10.0 mmol/L [70-180 mg/dL]) was 97.3% (IQR 95.4-98.7) and 95.4% (95% CI 94.0; 96.8), respectively. Median and mean standard deviations were 1.04 mmol/L (IQR 0.92-1.29) and 1.15 mmol/L (95% CI 1.05; 1.24), respectively. Measures of glycemic variability (standard deviation, coefficient of variation, mean amplitude of glycemic excursions) were significantly greater during daytime compared with nighttime, whereas others did not differ.
Conclusions:
People without prediabetes or diabetes show a non-negligible % time in hypoglycemia, median 1.6% and mean 3.2%, which needs to be accounted for in clinical practice and glucose-lowering trials. Glycemic variability measures differ day and night in this population.
Keywords: continuous glucose monitoring metrics, healthy population, hypoglycemia, time in range
Introduction
A cornerstone in the treatment of type 1 and 2 diabetes is maintaining glucose levels within targets,1,2 which have traditionally been estimated by HbA1c based on relationships with long-term diabetes complications. 3 With more widespread use of continuous glucose monitoring (CGM), other glycemic metrics have become the focus of attention.4-7
CGM uses a subcutaneous sensor to estimate blood glucose levels up to every five minutes, which are then displayed on an insulin pump, a handheld receiver, or mobile phone. 8 However, CGM does not directly measure levels in blood glucose; thus, questions remain about its accuracy and the time lag between actual and subcutaneously estimated levels.9,10 CGM exists both as a real-time system continuously showing glucose levels for the patient and as intermittent scanning CGM (isCGM) when glucose levels are displayed when the CGM sender is actively scanned.
Today, CGM is commonly used among persons with type 1 diabetes, where it has been shown to lower HbA1c and reduce time in hypoglycemia compared with capillary self-monitoring.6,11,12 In evaluations of novel glucose-lowering treatments in both type 1 and 2 diabetes, masked CGM is used in evaluating relevant glucose patterns.7,11-13
In clinical practice, it is important to understand the normal average time of hypoglycemia according to CGM in persons without diabetes. Initial guidelines have recently been proposed for targets of glycemic metrics based on CGM. 5 According to these guidelines, individuals with type 1 and 2 diabetes should spend less than 4.0% (about 60 minutes) per day at <3.9 mmol/L (<70 mg/dL), less than 1.0% at <3.0 mmol/L (<54 mg/dL), more than 70% at 3.9-10 mmol/L (70-180 mg/dL), and less than 25% at >10mmol/L (>180 mg/dL).
Few studies have evaluated CGM metrics, and simultaneously confirmed reliability, in persons without diabetes or prediabetes using modern CGM sensors. The aim of this study was to develop relevant CGM reference values by evaluating time-in-hypoglycemia and other glycemic metrics in persons without prediabetes or diabetes.
Methods
This study was performed at the diabetes research unit at the NU-Hospital Group, Uddevalla, Sweden, and approved by the regional ethics committee at the University of Gothenburg, Sweden. Persons from the general population >18 years with fasting plasma glucose (FPG) levels <6.1 mmol/L (110 mg/dL), two-hour plasma glucose after 75-g oral glucose tolerance test (OGTT) <8.9 mmol/L (160 mg/dL), and HbA1c <6.0% (<42 mmol/mol) were included. The World Health Organization (WHO) criteria for diagnosis of glucose status in adults were used. 14 Exclusion criteria were current pregnancy, cognitive dysfunction and disease making use of CGM difficult, continuous use of paracetamol or medications known to influence glucose levels (eg, corticosteroids), and current use of a CGM sensor. At least half of the individuals included had to have a body mass index of 18-25 kg/m2. All participants gave written and verbal informed consent.
Collection of CGM Data
Study personnel inserted CGM sensors (Dexcom G4 Platinum, DG4P, original algorithm for estimating glucose levels; Dexcom, San Diego, CA) subcutaneously in the abdominal area. Dexcom G4 Platinum is a real-time CGM system and measures interstitial glucose levels. Participants were instructed on sensor use, and sensors were calibrated according to the manufacturer’s instructions. CGM values were masked but stored on the system during days 1-7 and unmasked during days 8-14. The masked period was performed so that information from the CGM system does not influence the daily habits for the participants and hence constituted the main evaluation period for reference CGM metrics. The unmasked period was performed to make it possible for the patient to control with capillary testing if CGM values were correct at low and high glucose levels. Each sensor was set for a minimum of six days and maximum of seven. After the sensor was removed, insertion sites were observed for bleeding, inflammation, or infection and photographed in the event of abnormal findings.
Self-Measurement of Blood Glucose
All participants were taught to perform self-measurement of blood glucose (SMBG) with a HemoCue® meter DM RT 201 (Ängelholm, Sweden), which was used to calibrate CGM, and advised to measure capillary glucose values at least three times per day (fasting blood glucose in the morning, during lunch and evening). During days 8-14, participants measured capillary glucose levels when the CGM warned for low glucose levels. When blood glucose was <4.0 mmol/L (72 mg/dL), capillary values were measured at 0 minutes, 15-30 minutes, and then hourly if CGM continued to show levels <4.0 mmol/L. Warnings were also set at 11 mmol/L (198 mg/dL) for high levels, and participants performed capillary testing at the same time intervals until the system showed levels <11 mmol/L. All HemoCue meters were calibrated before use by an absolute isotope dilution GC-MC measurement system. 15 The total error of analytical reproducibility imprecision of HemoCue is <6.5%. 16 Capillary tests with HemoCue as references for CGM have shown high validity in comparison to venous tests. 17
Recording of Glucose Data
CGM data were downloaded at all clinical visits. Participants recorded capillary and CGM values at the same time point in a written diary, and research staff reviewed levels by comparing the values in the CGM and HemoCue system.
Participants were encouraged to behave normally when CGM or capillary values were low unless symptoms consistent with hypoglycemia, such as severe fatigue or heart palpitations, occurred and recorded whether they consumed food or drink when glucose levels were below 4.0 mmol/L. Each participant experience with CGM was evaluated using three predefined questions. The first question “How was your experience seeing your daily blood glucose profile with the help of CGM?” was rated by participants on a Visual Analog Scale (VAS) ranging from 0 to 100 (higher number equal to better). The two other questions were categorical with two alternatives (Yes/No) “Do you consider CGM to be an educational tool for patients who are at risk of getting diabetes?” and “Do you consider CGM to be an eye-opener about diet and other lifestyle changes?”
Primary and Secondary Endpoints
Endpoints were predefined before enrolment and registered on ClinicalTrials.gov with other trial information. The primary endpoint was percentage of time-below-target glucose <4.0 mmol/L (72 mg/dL) estimated on days 1-7 with masked CGM. Secondary endpoints estimated by masked CGM in days 1-7 included percentage of time below <3.0 mmol/L (54 mg/dL), time above >10 mmol/L (180 mg/dL), mean glucose levels described by a 24-hour profile each day, mean absolute relative difference (MARD) estimated by HemoCue and CGM values, mean absolute difference (MAD), and Pearson correlation coefficient estimated from HemoCue and CGM values. An additional secondary endpoint was the difference in mean glucose levels during days 1-7 (masked CGM) and 8-14 (unmasked CGM).
Exploratory Endpoints
Exploratory endpoints included percentages of time below <4.0 mmol/L (72 mg/dL) and <3.0 mmol/L (54 mg/dL) during night and day on days 1-7, time-in-target 4-8 mmol/L (72-144 mg/dL), time-in-range, glucose levels >14 mmol/L (252 mg/dL), and glycemic variability measured by standard deviation (SD), coefficient of variation, and mean amplitude of glycemic excursions (MAGE). MAGE is the mean of blood glucose values exceeding 1 SD from the 24-hour mean glucose. 18
Since 3.9 mmol/L (70 mg/dL) is used more commonly as a cut point for hypoglycemia internationally than 4.0 mmol/L, 4 data are also presented using the lower cut point. These include time in hypoglycemia, time-in-target, and time-in-range.
Among exploratory endpoints was the percentage of glucose values checked with HemoCue that were in the same glucose range when the CGM system showed glucose values <4.0 mmol/L and <3.0 mmol/L on days 8-14. The different CGM metrics were also analyzed for daytime and nighttime using two different time periods (06:00-23:59 and 06:00-21:59 vs 24:00-05:59 and 22:00-05:59). MARD, MAD, and mean difference of CGM and capillary values were estimated as overall measures for CGM accuracy.
Statistical Analysis
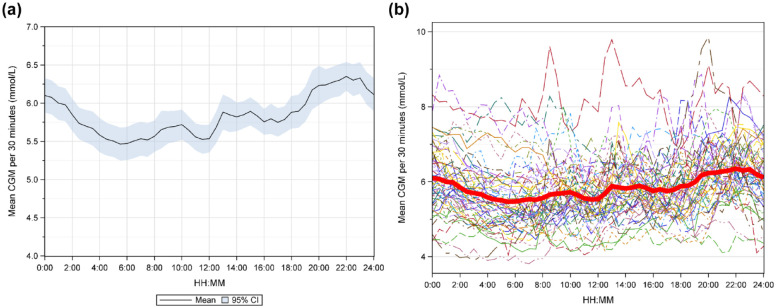
Patient characteristics were described by mean ± standard deviation. Endpoint variables were described by means with 95% confidence intervals using the inversion of Fisher’s nonparametric permutation test, and median (interquartile range [IQR]). Categorical variables were described by number and percentage. For comparison within groups, Fisher’s nonparametric permutation test for matched pairs was used. All individuals with >3 time points having evaluable CGM values and >3 time points with reference capillary values during the study were included in the intent-to-treat (ITT) population. When calculating mean glucose levels per 30 minutes (Figure 1) the mean glucose level per 30-minute period was first calculated for each participant and then the mean value of these estimates was calculated.
Figure 1.
(a) Mean glucose level with 95% CI in persons without prediabetes or diabetes estimated by masked CGM from 00:00-23:59. (b) Mean glucose level is shown for each individual and the red curve shows the mean glucose level for all individuals.
Results
Between October 2016 and March 2017, 74 subjects were enrolled in the study. Of those, 14 were excluded, 13 due to prediabetes found at OGTT, and one with type 2 diabetes. Mean age was 43.2 ±15.3 years, 70% were female. Mean BMI was 25.7 ± 3.8 kg/m2 and 15% were smokers. Mean HbA1c was 5.3 ± 0.29% (34.3 ± 3.1 mmol/mol). Mean fasting blood glucose (at 0 minutes OGTT) was 5.35 ± 0.42 mmol/L (96 ± 7.56 mg/dL). After about 60 minutes OGTT, mean glucose level was 8.41 ± 1.76 mmol/L (151.5 ± 31.7 mg/dL), and 6.82 ± 1.11 mmol/L (123 ± 20 mg/dL) after 120 minutes.
CGM Metrics During Masked CGM
Results of common CGM metrics are shown in Table 1. The primary endpoint, percentage of time below <4.0 mmol/L during masked CGM in days 1-7, showed a skewed distribution with a median time of 1.92% (IQR 0.67-3.35) or 27.6 minutes, and a mean time of 3.54% (95% CI 2.26; 4.84) or 51.0 minutes. Using the 3.9 mmol/L cut point resulted in 1.6% (IQR 0.57-3.18) or 23.1 minutes, and 3.2% (95% CI 2.00; 4.33) or 45.4 minutes, respectively.
Table 1.
Estimates of Common CGM Metrics in Persons Without Prediabetes or Diabetes During Masked and Open CGM.
| Difference | ||||
|---|---|---|---|---|
| Variable | Blinded period (n = 60) | Unblinded period (n = 60) | Unblinded period - Blinded period | P value |
| % time <4.0 mmol/L (72 mg/dL) | 3.54 (2.26; 4.84) 1.92 (0.67; 3.35) | 2.58 (1.66; 3.56) 1.33 (0.53; 3.53) | −0.96 (–1.96; 0.04) – 0.54 (–1.88; 0.46) | .059 |
| % time <3.9 mmol/L (70 mg/dL) | 3.16 (2.00; 4.33) 1.6 (0.57; 3.18) | 2.28 (1.42; 3.18) 1.07 (0.33; 3.06) | −0.87 (–1.85; 0.09) –0.47 (–1.77; 0.54) | .076 |
| % time <3.0 mmol/L (54 mg/dL) | 0.49 (0.17; 0.83) 0 (0; 0.36) | 0.46 (0.14; 0.78) 0 (0; 0.20) | −0.04 (–0.52; 0.44) 0 (–0.28; 0.08) | .88 |
| Mean glucose levels (mmol/L) | 5.83 (5.68; 5.99) 5.81 (5.41; 6.21) | 5.82 (5.69; 5.95) 5.76 (5.63; 6.03) | −0.02 (–0.11; 0.08) 0.04 (–0.17; 0.23) | .76 |
| Mean glucose levels (mg/dL) | 105.0 (102.2; 107.8) 104.6 (97.4; 111.7) | 104.8 (102.4; 107.1) 103.8 (101.3; 108.6) | −0.27 (–1.97; 1.45) 0.71 (–2.99; 4.19) | .76 |
| % time >10.0 mmol/L (180 mg/dL) | 1.44 (0.61; 2.28) 0.25 (0; 1.29) | 0.98 (0.38; 1.60) 0.18 (0; 0.71) | −0.46 (–1.11; 0.18) –0.02 (–0.59; 0.25) | .17 |
| % time >14.0 mmol/L (252 mg/dL) | 0.05 (0.00; 0.11) 0 (0; 0) | 0.06 (0.00; 0.124) 0 (0; 0) | 0.01 (–0.07; 0.09) 0 (0; 0) | .85 |
| % time 3.9 to 10.0 mmol/L (70-180 mg/dL) | 95.4 (94.0; 96.8) 97.3 (95.4; 98.7) | 96.7 (95.7; 97.8) 98.4 (95.7; 99.2) | 1.34 (0.22; 2.46) 0.8 (–0.47; 2.6) | .019 |
| % time 4.0 to 10.0 mmol/L (72-180 mg/dL) | 95.0 (93.5; 96.5) 97.2 (95.1; 98.4) | 96.4 (95.3; 97.5) 98.1 (95.2; 99.1) | 1.42 (0.28; 2.57) 0.86 (–0.61; 2.78) | .015 |
| % time 3.9 to 8.0 mmol/L (70-144 mg/dL) | 90.7 (88.5; 92.8) 92.9 (88.9; 96) | 92.4 (90.7; 94.1) 93.9 (91.2; 96.8) | 1.72 (0.11; 3.33) 0.99 (–1.55; 4.42) | .037 |
| % time 4.0 to 8.0 mmol/L (72-144 mg/dL) | 90.3 (88.1; 92.5) 92.8 (88.5; 95.6) | 92.1 (90.4; 93.8) 93.7 (90.8; 96.4) | 1.80 (0.18; 3.43) 0.93 (–1.58; 4.52) | .030 |
| SD glucose levels (mmol/L) | 1.15 (1.05; 1.24) 1.04 (0.92; 1.29) | 1.11 (1.03; 1.19) 1.05 (0.9; 1.22) | −0.04 (–0.12; 0.05) –0.02 (–0.17; 0.09) | .39 |
| CV (SD/Mean) glucose levels (mmol/L) | 0.20 (0.18; 0.21) 0.18 (0.16; 0.22) | 0.19 (0.18; 0.20) 0.18 (0.16; 0.20) | −0.01 (–0.02; 0.01) –0.00 (–0.03; 0.02) | .41 |
| MAGE (mmol/L) | 2.63 (2.43; 2.82) 2.44 (2.13; 2.96) | 2.58 (2.39; 2.76) 2.42 (2.11; 2.82) | −0.05 (–0.22; 0.13) –0.01 (–0.29; 0.31) | .59 |
Note. For continuous variables, mean (95% CI for mean using the inversion of Fisher’s nonparametric permutation test)/median (Q1; Q3) is presented. For comparison within groups, the Fisher’s nonparametric permutation test for matched pairs was used. CV, Coefficient of Variation.
The median percentage of time below <3.0 mmol/L was 0.00% (IQR 0.00-0.36), mean 0.49% (95% CI 0.17; 0.83). Mean time-in-range was 95.4% (95% CI 94.0; 96.8), median 97.3% (IQR 95.4-98.7). The mean SD was 1.15 mmol/L (95% CI 1.05; 1.24) [20.7 mg/dL (95% CI 18.9; 22)] and median 1.04 mmol/L (IQR 0.92-1.29) [18.7 mg/dL (95% CI 16.9; 23)].
The mean 24-hour glucose level with 95% CI is shown in Figure 1(a) and together with the mean level for each individual in Figure 1(b). Levels were at their lowest around 06:00 and before lunch time, while the highest levels were found around 22:00. There was also an increase shortly after lunch time. Consecutively decreasing glucose levels were found overnight from 22:00 until 06:00. Time-in-hypoglycemia at different glucose values as a continuous function is shown in Figure 2. Median and mean percentage of glucose levels <3.5 mmol/L (63 mg/dL) was 0.39% and 1.26%, respectively, and <2.5 mmol/L (45 mg/dL) was 0% and 0.24%, respectively.
Figure 2.
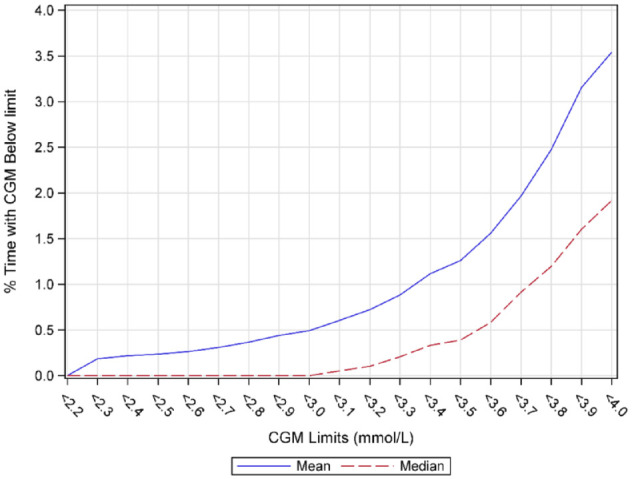
Median and mean % time below different low glucose levels in persons without diabetes or prediabetes.
Glucose Levels During Day- and Nighttime With Masked CGM
The different CGM metrics divided into day- and nighttime (06:00-21:59 vs 22:00-05:59, respectively) are shown in Table 2. Time-in-hypoglycemia <3.9 mmol/L was 3.31% (95% CI 2.15; 4.48) during daytime and 2.89% (95% CI 1.28; 4.56) during nighttime, P = .58. Corresponding mean and median times with glucose values <3.0 mmol/L were 0.45% (95% CI 0.13; 0.77) and 0.59% (95% CI 0.10; 1.13) and 0% (IQR 0-0.17) and 0% (IQR 0-0), respectively. Mean and median percentage of time with glucose values >10.0 mmol/L during day and night were 1.38% (95% CI 0.64; 2.10) versus 1.53% (95% CI 0.45; 2.65), and 0.35% (IQR 0-1.15) and 0% (IQR 0-0.78), respectively. Glycemic variability was greater during the daytime for all evaluated glycemic variability metrics (SD, Coefficient of Variation (CV), and MAGE). The mean SD was 1.16 mmol/L during daytime and 1.01 mmol/L at night (P < .001). Mean CV was 0.20 (95% CI 0,18; 0.21) and 0.17 (95% CI 0.16; 0.18), P < .001, respectively. Results were similar regardless of time period (data not shown).
Table 2.
Common CGM Metrics in Persons Without Prediabetes or Diabetes During Day- and Nighttime.
| Difference | ||||
|---|---|---|---|---|
| Variable | Day 06:00-21:59 (n = 60) | Night 22:00-05:59 (n = 60) | Night 22:00-05:59 - Day 06:00-21:59 | P value |
| % time <4.0 mmol/L (72 mg/dL) | 3.71 (2.44; 4.99) 1.91 (0.85; 4.12) | 3.25 (1.51; 5.06) 0.88 (0; 3.13) | −0.46 (–1.99; 1.07) –0.75 (–2.16; 0.49) | .56 |
| % time <3.9 mmol/L (70 mg/dL) | 3.31 (2.15; 4.48) 1.67 (0.69; 3.74) | 2.89 (1.28; 4.56) 0.63 (0; 2.4) | −0.42 (–1.89; 1.04) –0.68 (–1.92; 0.41) | .58 |
| % time <3.0 mmol/L (54 mg/dL) | 0.45 (0.13; 0.77) 0 (0; 0.17) | 0.59 (0.10; 1.13) 0 (0; 0) | 0.14 (–0.39; 0.68) 0 (0; 0) | .67 |
| Mean glucose levels (mmol/L) | 5.81 (5.66; 5.97) 5.77 (5.46; 6.23) | 5.87 (5.67; 6.07) 5.80 (5.35; 6.31) | 0.06 (–0.09; 0.21) 0.07 (–0.37; 0.50) | .45 |
| Mean glucose levels (mg/dL) | 104.7 (101.8; 107.5) 103.8 (98.4; 112.1) | 105.7 (102.1; 109.2) 104.3 (96.3; 113.6) | 1.03 (–1.67; 3.73) 1.29 (–6.56; 9.06) | .45 |
| % time >10.0 mmol/L (180 mg/dL) | 1.38 (0.64; 2.10) 0.35 (0; 1.15) | 1.53 (0.45; 2.65) 0 (0; 0.78) | 0.15 (–0.57; 0.87) 0 (–0.39; 0) | .70 |
| % time >14.0 mmol/L (252 mg/dL) | 0.06 (0.00; 0.13) 0 (0; 0) | 0.04 (0.00; 0.10) 0 (0; 0) | −0.02 (–0.12; 0.08) 0 (0; 0) | .69 |
| % time 3.9 to 10.0 mmol/L (70-180 mg/dL) | 95.3 (94.0; 96.6) 97.4 (94.7; 98.7) | 95.6 (93.6; 97.5) 98.1 (95.3; 99.8) | 0.28 (–1.20; 1.76) 0.53 (–0.89; 2.54) | .71 |
| % time 4.0 to 10.0 mmol/L (72-180 mg/dL) | 94.9 (93.5; 96.3) 97 (94.3; 98.6) | 95.2 (93.2; 97.3) 97.9 (95; 99.7) | 0.31 (–1.23; 1.86) 0.55 (–0.99; 2.57) | .69 |
| % time 3.9-8.0 mmol/L (70 to 144 mg/dL) | 90.4 (88.2; 92.5) 92.6 (87.3; 96.2) | 91.3 (88.7; 93.9) 94.3 (88.1; 98.1) | 0.87 (–0.93; 2.71) 1.05 (–2.83; 5.22) | .34 |
| % time 4.0-8.0 mmol/L (72 to 144 mg/dL) | 90.0 (87.8; 92.2) 92.5 (86.6; 95.9) | 90.9 (88.3; 93.6) 94.3 (87.8; 97.7) | 0.91 (–0.94; 2.78) 0.87 (–2.77; 5.42) | .33 |
| SD glucose levels (mmol/L) | 1.16 (1.06; 1.26) 1.07 (0.91; 1.33) | 1.01 (0.91; 1.11) 0.94 (0.74; 1.17) | −0.15 (–0.23; –0.07) –0.16 (–0.32; 0.05) | <.001 |
| CV (SD/Mean) glucose levels (mmol/L) | 0.20 (0.18; 0.21) 0.19 (0.17; 0.21) | 0.17 (0.16; 0.18) 0.16 (0.14; 0.19) | −0.03 (–0.04; –0.02) –0.02 (–0.05; –0) | <.001 |
| MAGE (mmol/L) | 2.58 (2.37; 2.79) 2.35 (2.02; 2.9) | 2.20 (1.99; 2.41) 2.13 (1.55; 2.56) | −0.38 (–0.56; –0.20) –0.35 (–0.66; 0.01) | <.001 |
Note. For continuous variables, mean (95% CI for mean using the inversion of Fisher’s nonparametric permutation test)/median (Q1; Q3) is presented.
For comparison within groups, the Fisher’s nonparametric permutation test for matched pairs was used.
Unmasked CGM, Days 8-14
Unmasked CGM was mainly performed to evaluate capillary glucose values during low and high glucose levels. However, different glycemic metrics were also evaluated during unmasked CGM, Table 1. The secondary endpoint, difference in mean glucose level between days 1-7 and 8-14 showed similar mean glucose levels of 5.83 mmol/L (105 mg/dL) (95% CI 5.68; 5.99) and 5.82 mmol/L (104.9 mg/dL) (95% CI 5.69; 5.96), P = .76. Among other comparisons, there was a tendency for more optimal glucose levels during days 8-14, although patients were instructed to behave normally. Time-in-target and time-in-range increased in days 8-14 compared with days 1-7, from 90.3% to 92.1% (P = .03) and 95.0% to 96.4% (P = .015).
Overall, 44 of 60 (73%) participants had CGM measurements <4.0 mmol/L, and a corresponding capillary measurement within maximum five minutes from the CGM value. Of 274 CGM values <4.0 mmol/L, 26 (9.5%) were confirmed with a capillary measurement. Thirteen (50%) of the 26 confirmed CGM measurements were from the same participant. The remaining 13 measurements were from eight participants. Eleven (18%) had CGM values <3.0 mmol/L and a corresponding capillary measurement within five minutes. Only one (4%) of 27 CGM measurements were confirmed to be <3.0 mmol/L by capillary measurements.
Accuracy Evaluations of CGM Variables
MARD was 12.0% (95% CI 10.7;13.2) during masked CGM and 13.3% (95% CI 12.3;14.3) during unmasked CGM. Corresponding values for MAD were 0.71 mmol/L (95% CI 0.63; 0.79) [12.8 mg/dL (95% CI 11; 14) ] during masked CGM and 0.80 mmol/L (95% CI 0.72; 0.88) [14.5 mg/dL (95% CI 13; 15.9)] during unmasked CGM. MARD and MAD during unmasked CGM when CGM measurements <4.0 mmol/L were excluded were 12.0% (95% CI 11.1; 13.0) and 0.74 mmol/L (95% CI 0.67; 0.82). Mean difference between capillary glucose and CGM values was −0.08 mmol/L (95% CI −0.14; −0.02) in days 1-7 and -0.21 mmol/L (95% CI −0.29; −0.13) in days 8-14. When CGM values <4.0 mmol/L during week two were excluded, the mean difference was −0.09 mmol/L (95% CI −0.17; −0-02).
The Pearson correlation coefficient between all measured capillary and CGM values was 0.66 in days 1-7 and 0.74 in days 8-14.
CGM Experience
No participants had blood, other fluids, or visible skin reactions at the sensor site when the sensor was removed. On a VAS scale (0-100) where a higher number was better, participants rated as a mean their experience of receiving continuous information on their blood glucose level to 85. All participants found CGM to be a great educational tool for patients at risk of diabetes and 96.7% thought CGM was an eye-opener regarding diet and lifestyle changes.
Discussion
Principal Findings
In this study of individuals without prediabetes or diabetes, median and mean time in hypoglycemia <3.9 mmol/L (70 mg/dL) was 1.6% and 3.2%, respectively. When <4.0 mmol/L (72 mg/dL) was used as a cut point for hypoglycemia, corresponding estimates were 1.9% and 3.5%, respectively. Median and mean times in hypoglycemia <3.0 mmol/L (<54 mg/dL) were 0.0% and 0.5%, respectively. Time-in-range was 97.0%, SD 1.0 mmol/L (18 mg/dL), and the coefficient of variation was 20.0%. Glycemic variability was greater during the daytime than nighttime, whereas other glycemic metrics were similar regardless of time of day.
When unmasked CGM was used by participants during days 8-14, they rated it as being an essential educational tool in preventing diabetes and changing lifestyle habits in their ability to see how glucose levels react to diet and physical activity. When capillary blood tests were performed to confirm low glucose levels, only a minority of measurements were confirmed to be low.
Earlier Studies
In a multicenter, prospective study, persons with HbA1c <5.7% (39 mmol/mol) without performing OGTT wore masked Dexcom G6 CGM for up to 10 days. 19 Time-in-hypoglycemia <3.9 mmol/L (70 mg/dL) was 1.1%, slightly lower compared with the current study (1.6%). The difference may be explained by approximately 20% of individuals excluded due to prediabetes or diabetes detected with OGTT in the current study. Others have also evaluated glycemic metrics primarily in persons without glucose disturbances,19-26 although current CGM metrics used in guidelines were generally not a focus of attention since these studies were performed before current guidelines were introduced. 5 Estimates for different metrics have generally differed, with SD 0.8-1.5 mmol/L (13.5-27 mg/dL)20-24 and time-in-hypoglycemia <3.9 mmol/L between 1.5% and 1.7%.20,23 Earlier studies also did not use masked CGM,21-26 which may influence glucose levels to some extent.11,13 Others used earlier generation CGM sensors, which were generally less accurate than the Dexcom G4.10,24 Finally, OGTT was not performed in some studies; thus, it is likely a significant number of persons with prediabetes or diabetes were included.19,21,24-26
To date, no studies have evaluated the accuracy of CGM sensors in persons without diabetes or prediabetes.19-23,25,26 It is of interest that we did not find a systematic deviation in accuracy, despite controlling for one, as this could influence estimated time in hypoglycemic range. People without diabetes or prediabetes have glucose levels close to the hypoglycemic range over extended periods of time where a systematic deviation could shift values registered in that range. In persons with diabetes, we found the Freestyle Libre to regularly report glucose levels 0.5 mmol/L lower than capillary reference samples, which implies a need to control for accuracy in healthy individuals as well. 9
Explanations and Interpretations
Why persons without prediabetes or diabetes have non-negligible times in hypoglycemia when estimated by CGM may be due to several factors. First, the 3.9 mmol/L cut point is likely not physiological, as some persons have intermittently lower glucose levels. Second, CGM sensors are not completely accurate in estimating blood glucose levels,9,10 implying that glucose levels close to the hypoglycemic threshold may sometimes be recorded as in the hypoglycemic range. Although the current study was not designed to evaluate CGM accuracy in the general population, at least three capillary glucose levels were measured each day. MARD, a common metric of accuracy, was 12% in this study, which is similar to earlier studies of Dexcom G4 accuracy evaluated in persons with type 1 diabetes. 10 The reason why glycemic variability is greater during daytime is likely due to glycemic excursions in connection to meals, as mean glucose levels were higher after lunch time and around 22:00.
There are several reasons why few CGM values <4.0 mmol/L (72 mg/dL) were confirmed by capillary testing. One is the lag time between capillary glucose levels and as estimated by CGM. 8 In other words, when CGM glucose values are low, the actual value may have been low a few minutes earlier but has since risen when the CGM reading is taken. Another is due to the relative imprecision of CGM as described above. Thus, small differences in blood glucose levels may sometimes fall below or rise above a certain cut point.
It is noteworthy that pressure-induced low interstitial glucose can lead to inaccurate recordings of estimated blood glucose levels. 27 This is likely a greater problem nighttime, than daytime, when persons may lie on the sensor. However, time in hypoglycemia was of the same magnitude daytime and nighttime in the current study.
Implications
First, it is important to have robust estimates of glycemic metrics from a population without diabetes or prediabetes as a basis for CGM metric guidelines. Health professionals and patients need to be informed when levels have reached values similar to that of the general population. A recent study of a semi-closed loop system in persons with type 1 diabetes found median and mean times in hypoglycemia <3.9 mmol/L of 1.4% and 1.6%, respectively, compared to our findings of 1.6% and 3.2% in persons without glucose disturbances. 28 For the <3.0 mmol/L cut point, estimates were 0.2% and 0.3% in the semi-closed loop system compared with 0.0% and 0.5% in the current study. Hence, persons with type 1 diabetes and semi-closed loop systems had a similar degree of time-in-hypoglycemia as persons without diabetes. Moreover, it is possible that persons with prediabetes or type 2 diabetes using glucose-lowering treatments that do not increase hypoglycemia risk and may approach other glycemic metrics in persons without prediabetes or diabetes.
Furthermore, when novel glucose-lowering treatments are evaluated both in persons with type 1 and type 2 diabetes,6,7,11,13 a certain amount of time-in-hypoglycemia registered via glucose sensor should be viewed as normal, at least for the 3.9 mmol/L cut point. However, none or very little time in hypoglycemia<3.0 mmol/L should exist where the median and mean time was 0.0% and 0.5%, respectively, in the current study.
Moreover, our results show that crucial CGM metrics such as time-in-hypoglycemia and time-in-range do not differ significantly between day and night. This is of particular interest when therapies such as prandial versus basal insulins are evaluated. For basal insulins, the risk of nocturnal hypoglycemia is a common key endpoint.29-31 It is also of concern in clinical practice since patients and healthcare professionals need to understand normal day- and nighttime CGM levels. Our results also show that it is crucial to clearly express whether median or mean time is used since mean time-in-hypoglycemia was considerably greater. It is also important to evaluate different sensors in the general population. Key CGM metrics such as time-in-hypoglycemia may be greatly influenced by systematic deviations or lower accuracy.
Finally, participants were very positive about using CGM despite not having diabetes, and the majority rated it useful to improving their lifestyle. Although participants were not advised to change lifestyle during unmasked CGM, glucose profiles improved to some extent.
Strengths and Limitations
Strengths of the current study include that persons with prediabetes and diabetes were carefully excluded. Including persons with impaired glucose tolerance would likely have influenced estimations. Moreover, overall CGM accuracy was estimated during both masked and unmasked CGM use. A sensor with high accuracy was used and capillary glucose levels were checked during unmasked CGM to confirm low and high levels. Common glucose metrics were also evaluated overall and separately by day- and nighttime.
Limitations include that novel generations of sensors such as the Dexcom G6 may be more accurate. 32 However, direct comparisons with Dexcom G4 are lacking; thus, caution should be exercised in comparing the degree of accuracy since MARD is strongly dependent on mean glucose level.9,10
On the other hand, patients calibrated the Dexcom G4, which may ensure validity to some extent, and it has been more accurate than the Enlite sensor used in several earlier studies of CGM metrics in persons with diabetes.10,33,34 It is also noteworthy that we used the WHO criteria for excluding prediabetes of fasting glucose <6.1 mmol/L, whereas ADA criteria are somewhat lower of 5.6 mmol/L. 14
Conclusion
In the current study, data generally showed a skewed distribution in different glycemic metrics. The current study may help caregivers, patients, and researchers understand weather treatment effects are similar to those in persons without prediabetes or diabetes. It is important to present both median and mean time of several glycemic metrics due to their skewed distribution. Since the distribution was skewed, the median time-in-hypoglycemia <3.9 mmol/L (1.6% in this study) and <3.0 mmol/L (0% in this study) should be viewed as the most correct estimates. However, since the corresponding mean times were 3.2% and 0.5%, this needs to be considered in future CGM evaluations.
Acknowledgments
We thank all participants who made the study possible. We also thank Joseph Murphy for language editing.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin; IQR, interquartile range; ITT, intent-to-treat; MAD, mean absolute difference; MAGE, mean amplitude of glycemic excursions; MARD, mean absolute relative difference; OGTT, oral glucose tolerance test; SD, standard deviation; SMBG, self-measurement of blood glucose.
Authors’ Note: Parts of this study were presented as a poster at the ATTD (Advanced Technologies & Treatments for Diabetes) in Madrid, Spain, February 18-22, 2020.
Authors’ Contributions: The steering committee consisted of M.L. (PI), S.S., and A.P.. S.S. wrote the first draft of the manuscript. S.S. and M.L. designed the study. A.P. performed statistical calculations; all authors took part in interpreting the data. All authors revised the manuscript and approved the final version. S.S. and M.L. take responsibility for the contents of the article.
Trial Registration: clinicaltrials.gov Identifier: NCT03471949.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Sheyda Sofizadeh has been a consultant or Novo Nordisk, Bayer, Pfizer, Sanofi, and Boehringer Ingelheim. Marcus Lind has received grants from Dexcom and Novo Nordisk and been a consultant for Astra Zeneca, Boehringer Ingelheim, Dexcom, Eli Lilly, Merck Sharp & Dohme, and Novo Nordisk. Arndís F Ólafsdóttir and Anders Pehrsson have no conflicts of interest to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Swedish state (ALF-grant), The Fyrbodal Research Council, and the Novo Nordisk Foundation.
ORCID iD: Sheyda Sofizadeh  https://orcid.org/0000-0003-3701-5065
https://orcid.org/0000-0003-3701-5065
References
- 1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 2. Nasr CE, Hoogwerf BJ, Faiman C, Reddy SS. United Kingdom Prospective Diabetes Study (UKPDS). Effects of glucose and blood pressure control on complications of type 2 diabetes mellitus. Cleve Clin J Med. 1999;66:247-253. [DOI] [PubMed] [Google Scholar]
- 3. Lind M, Pivodic A, Svensson AM, Ólafsdóttir AF, Wedel H, Ludvigsson J. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ. 2019; 366: l4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42:400-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lind M, Hirsch IB, Tuomilehto J, et al. Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: randomised clinical trial (MDI Liraglutide trial). BMJ. 2015;351:h5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hirsch Irl B. Clinical review: realistic expectations and practical use of continuous glucose monitoring for the endocrinologist. J Clin Endocrinol Metab. 2009;94:2232-2238. [DOI] [PubMed] [Google Scholar]
- 9. Ólafsdóttir AF, Attvall S, Sandgren U, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor freestyle libre in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19:164-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matuleviciene V, Joseph JI, Andelin M, et al. A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian REAL-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther. 2014;16:759-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;24;317:379-387. [DOI] [PubMed] [Google Scholar]
- 12. Ólafsdóttir AF, Polonsky W, Bolinder J, et al. A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD-3). Diabetes Technol Ther. 2018;20:274-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sofizadeh S, Imberg H, Ólafsdóttir AF, et al. Effect of liraglutide on times in glycaemic ranges as assessed by CGM for type 2 diabetes patients treated with multiple daily insulin injections. Diabetes Ther. 2019;10:2115-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Metcalf PA, Scragg RK. Comparison of WHO and ADA criteria for diagnosis of glucose status in adults. Diabetes Res Clin Pract. 2000;49:169-180. [DOI] [PubMed] [Google Scholar]
- 15. Hannestad U, Lundblad A. Accurate and precise isotope dilution mass spectrometry method for determining glucose in whole blood. Clin Chem. 1997;43:794-800. [PubMed] [Google Scholar]
- 16. Kos S, van Meerkerk A, van der Linden J, Stiphout T, Wulkan R. Validation of a new generation POCT glucose device with emphasis on aspects important for glycemic control in the hospital care. Clin Chem Lab Med. 2012;50:1573-1580. [DOI] [PubMed] [Google Scholar]
- 17. Andelin M, Kropff J, Matuleviciene V, et al. Assessing the accuracy of continuous glucose monitoring (CGM) calibrated with capillary values using capillary or venous glucose levels as a reference. J Diabetes Sci Technol. 2016;10:876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baghurst PA. Calculating the mean amplitude of glycemic excursions from continuous glucose monitoring data: an automated algorithm. Diabetes Technol Ther. 2011;13:296-302. [DOI] [PubMed] [Google Scholar]
- 19. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104:4356-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group; Fox LA, Beck RW, Xing D. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33:1297-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13:921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou J, Li H, Ran X, et al. Establishment of normal reference ranges for glycemic variability in Chinese subjects using continuous glucose monitoring. Med Sci Monit. 2011;17:CR9-CR13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deiss D, Abtahi T, Rastogi R, Kelley EL. Glucose variability of individuals without diabetes using a long-term continuous glucose monitoring system (Abstract). Diabetes. 2018;67(suppl 1):1542. [Google Scholar]
- 24. Akintola AA, Noordam R, Jansen SW, et al. Accuracy of continuous glucose monitoring measurements in normo-glycemic individuals. PLoS One. 2015;10: e0139973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noordam R, Huurman NC, Wijsman CA, et al. High adiposity is associated with higher nocturnal and diurnal glycaemia, but not with glycemic variability in older individuals without diabetes. Front Endocrinol. 2018; 9:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borg R, Kuenen JC, Carstensen B, et al. Real-life glycaemic profiles in non-diabetic individuals with low fasting glucose and normal HbA1c: the A1C-Derived Average Glucose (ADAG) study. Diabetologia. 2010;53:1608-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mensh BD, Wisniewski NA, Neil BM, Burnett DR. Susceptibility of interstitial continuous glucose monitor performance to sleeping position. J Diabetes Sci Technol. 2013;7:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381:1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lane W, Bailey TS, Gerety G, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: the SWITCH 1 randomized clinical trial. JAMA. 2017;318:33-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heller SR, DeVries JH, Wysham C, Hansen CT, Hansen MV, Frier BM. Lower rates of hypoglycaemia in older individuals with type 2 diabetes using insulin degludec versus insulin glargine U100: results from SWITCH 2. Diabetes Obes Metab. 2019;21:1634-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schiavon M, Visentin R, Giegerich C, et al. In silico head-to-head comparison of insulin glargine 300 U/mL and insulin degludec 100 U/mL in type 1 diabetes. Diabetes Technol Ther. 2020;22:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol Ther. 2018;20:395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Damiano ER, McKeon K, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors: the Navigator, G4 Platinum, and Enlite. J Diabetes Sci Technol. 2014;8:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Calhoun P, Lum J, Beck RW, Kollman C. Performance comparison of the medtronic sof-sensor and enlite glucose sensors in inpatient studies of individuals with type 1 diabetes. Diabetes Technol Ther. 2013;15:758-761. [DOI] [PMC free article] [PubMed] [Google Scholar]