Abstract
The development of painful diabetic neuropathy (PDN) is a common complication of chronic diabetes that can be associated with significant disability and healthcare costs. Prompt symptom identification and aggressive glycemic control is essential in controlling the development of neuropathic complications; however, adequate pain relief remains challenging and there are considerable unmet needs in this patient population. Although guidelines have been established regarding the pharmacological management of PDN, pain control is inadequate or refractory in a high proportion of patients. Pharmacotherapy with anticonvulsants (pregabalin, gabapentin) and antidepressants (duloxetine) are common first-line agents. The use of oral opioids is associated with considerable morbidity and mortality and can also lead to opioid-induced hyperalgesia. Their use is therefore discouraged. There is an emerging role for neuromodulation treatment modalities including intrathecal drug delivery, spinal cord stimulation, and dorsal root ganglion stimulation. Furthermore, consideration of holistic alternative therapies such as yoga and acupuncture may augment a multidisciplinary treatment approach. This aim of this review is to focus on the current management strategies for the treatment of PDN, with a discussion of treatment rationale and practical considerations for their implementation.
Keywords: diabetes, neuromodulation, neuropathy, pain, painful diabetic neuropathy, pharmacotherapy
Introduction
Approximately half of the estimated 425 million patients with diabetes worldwide will be affected by diabetic neuropathy.1-4 Neuropathic complications reportedly comprise up to 27% of the cost of diabetes. 5 Less easily quantified are the burdens of depression, lost productivity, and impaired quality-of-life suffered by patients.6,7 Up to a third of patients with diabetes will also develop painful diabetic neuropathy (PDN), further exacerbating disability.8,9 Thus, the treatment of PDN is focused on strict glycemic control to deter disease progression and pain management. In one study, patients with PDN spent US$7066 annually more than patients without pain, highlighting significant economic costs. 10
Both the American Academy of Neurology 11 and European Federation of Neurological Societies 12 have published guidelines regarding the clinical management of PDN, although the randomized clinical trials (RCTs) forming the basis of these guidelines were generally based on short-term data. 13 In the clinical setting, PDN often involves polypharmacy, which highlights the difficulty in obtaining pain relief with single medications.14,15 This review focuses on the current management strategies for the treatment of PDN including pharmacological, neuromodulation, and alternative therapies.
Clinical Presentation and General Management
PDN is a clinical diagnosis, and there is considerable variability in its presentation.1,4 Burning pain is often the initial presenting complaint, and concurrent paresthesias are common.16,17 A distal, symmetric “stocking” distribution is the most common manifestation. 17 Rarer atypical forms include diabetic radiculoplexopathies (often unilateral/asymmetric), chronic inflammatory demyelinating polyradiculoneuropathy, and autonomic neuropathies. 17 These subtypes are less studied and can be particularly difficult to manage. Onset may be insidious, and therefore a careful history probing for less overt symptoms may be essential for diagnosis. 1
PDN is a diagnosis of exclusion; as such, other metabolic derangements and causes of peripheral neuropathy must be ruled out. This may be particularly difficult given the common associated comorbidities, including metabolic syndrome. 16 To further complicate diagnosis, up to half of patients may be asymptomatic. 1 Therefore, the American Diabetes Association recommends temperature and monofilament testing to screen for neuropathy in patients initially diagnosed with type 2 diabetes, and within five years of a type 1 diabetes diagnosis.1,18 Both subtypes should be tested annually after their initial evaluation, and some research has shown certain prediabetic patients should also be screened.1,18
There are no proven treatments able to reverse the nerve damage; therefore, preventative measures including proper foot care and good glycemic control are the cornerstones of treatment.1,2,4 Measures to slow or halt symptom progression include lifestyle modifications, such as an improved diet, exercise, and weight loss, as well as medications to optimize glycemic control.17,19 Maintaining glycemic control has been associated with significantly lower incidence and slower progression of neuropathy, with particular benefit (up to 81% relative risk reduction) demonstrated in patients with type 1 diabetes.19,20
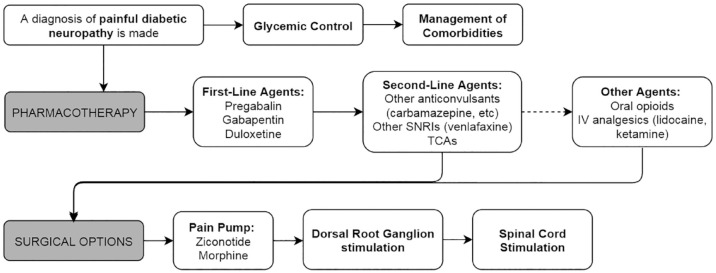
Pain management is a particular challenge posed by PDN and an important treatment consideration.14,21 For the remainder of this review, we will focus on PDN management and discuss accepted pharmacotherapies, neuromodulatory techniques, and alternative therapies used for pain relief. Figure 1 illustrates a flowchart of recommended management strategies.
Figure 1.
Flowchart of painful diabetic neuropathy management options.
Pharmacotherapy
It is a common misconception that all pain medications are to be taken “as needed.” While this may hold true for musculoskeletal pain, the management of neuropathic pain requires routine and regimented administration regardless of pain severity in order to provide sustained relief. Therefore, it is crucial that specialists in diabetes management educate patients on the need to adhere to the appropriate dosage regimen.
Current pharmacotherapeutic strategies include antidepressants, anticonvulsants, and opioids, which have been demonstrated to be superior to placebo for pain control in PDN. 13 Other therapies such as topical analgesics and intravenous (IV) medications warrant further study. Published guidelines from the American Academy of Neurology recommend pregabalin as a first-line treatment. 11 In addition to pregabalin, first-line treatment recommendations from the European Federation of Neurological Societies include serotonin-norepinephrine reuptake inhibitors (SNRI), tricyclic antidepressants (TCA), and gabapentin. 12 Table 1 outlines pharmacotherapeutic agents, including dose considerations, mechanisms, and side effects.22,23
Table 1.
Pharmacotherapy for Painful Diabetic Neuropathy.
| Dose range | Starting dose | Dose escalation | Mechanism | Side effects | Notes | |
|---|---|---|---|---|---|---|
| Pregabalin | 150-600 mg | 75 mg bid | Escalate to 150 mg bid within 1 week of initiation based on
tolerability; Max Dose: 300 mg bid |
Inhibition of voltage gated calcium channel and ATP gated potassium channel | Dizziness, blurred vision, drowsiness. | NNT = 5-8; NNH = 9-16 |
| Gabapentin | 300-3600 mg | 300 mg daily (Day 1) | Day 2: 300 mg bid; Day 3: 300 mg tid; Max Dose: 1800-3600 mg/day in 3 divided doses. |
Inhibition of voltage gated calcium channel | Confusion, dizziness, gastrointestinal issues, abnormal thinking | NNT = 3; NNH = 4 |
| Duloxetine | 60-120 mg | 30 mg bid | Lower dose based on tolerability. Max Dose: 60 mg/day. |
SNRI | Gastrointestinal issues, somnolence, hyperhidrosis | NNT = 5; NNH = 17 |
| Venlafaxine | 37.5-225 mg | 37.5 mg bid | Escalate to 150 mg daily: Max Dose: 225 mg daily |
SNRI or SSRI at low doses | Gastrointestinal issues, somnolence, hyperhidrosis, prolonged QT | NNT = 3; NNH = 16 |
| Amitriptyline | 10-150 mg | 10 mg daily at bedtime |
Max Dose: 150 mg daily at bedtime |
Inhibition of voltage-gated sodium channels, NDMA receptors, and reuptake of serotonin and norepinephrine | Gastrointestinal issues, orthostatic hypotension, dry mouth, urinary retention, and QTc prolongation. | NNT = 1-3; NNH = 28 (for major adverse effects), 6 (for minor adverse effects) |
| Tapentadol | 100-250 mg | 50 mg bid | Escalate to 100-250 mg bid as tolerated; Max Dose: 250 mg bid |
µ-opioid receptor agonist and norepinephrine reuptake inhibitor | Addiction, paradoxical hyperalgesia, respiratory depression, gastrointestinal issues. | NNT = 9-10; NNH = 5 |
| Topical Capsaicin | 0.075-8% | Apply 4 times daily | Not applicable | Vanilloid receptor agonist | Burning sensation and dermal irritation | NNT = 7 |
| Topical Lidocaine | 5% | 1 patch every 12 hours | 12-hour patch free interval. Max Dose: 3 patches daily |
Inhibition of voltage gated sodium channels. | Dermal irritation | NNT = 4 |
| α-Lipoic acid | 100-1800 mg | 600 mg | Not applicable | Antioxidant | Nausea, gastrointestinal issues | Not studied. |
| Actovegin | 600- 2000 mg | 600 mg tid | Not applicable | Anti-hypoxic agent | Nausea | Not studied. |
Abbreviations: bid, twice daily; NNH, number needed to harm; NNT, number needed to treat; SNRI, selective norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitors; tid, thrice daily.
Anticonvulsants
Pregabalin is a Food and Drug Administration (FDA)-approved anticonvulsant used for the management of PDN, administered daily at 150-600 mg. Evidence shows significant pain reduction with comparable efficacy to duloxetine and gabapentin,24-27 as well as improvements in sleep and global impression scales without affecting nerve conduction.26,28 Gabapentin is characterized as second-line therapy, typically administered at a total daily dose of 300-3600 mg. 29 It is well-tolerated, demonstrating up to 50% pain relief compared to placebo, and noninferior pain relief compared to amitriptyline and pregabalin.30,31 At higher doses, both pregabalin and gabapentin are administered in divided doses.
Secondary to their pharmacokinetics, pain relief is observed within one to four weeks from initiation of either pregabalin or gabapentin. 23 During this time, however, side effects may be experienced including confusion, dizziness, drowsiness, and gastrointestinal issues.26,32 The use of anticonvulsants is also associated with the development of tachyphylaxis. It is important to emphasize to patients to continue taking their medication in order for pain control to be realized, and dose adjustments can be made to balance pain relief and side effects. Furthermore, pregabalin is often cost-prohibitive or not commonly covered by insurance.
Other anticonvulsants for PDN management include carbamazepine, valproic acid, and lamotrigine. While these medications have demonstrated improvement in PDN symptoms, they are not first-line and have shown inconsistent results.33-35
Antidepressants
Antidepressants, such as duloxetine, are considered first-line pharmacotherapy for PDN. While initially prescribed for depression, duloxetine is an SNRI that is widely studied in PDN management, and is prescribed as a daily dose of 60-120 mg. Duloxetine improves long-term pain outcomes, depressive symptoms, and overall quality-of-life in PDN patients.36,37 Effects are typically observed as early as three to five days from drug initiation. It is associated with mild elevations of fasting plasma glucose and weight gain, but insignificant changes in hemoglobin A1c. 38 Adverse effects include gastrointestinal issues, somnolence, and hyperhidrosis. 36
Venlafaxine is an SNRI that is administered at a daily dose of 37.5-225 mg, 29 and functions as a selective serotonin reuptake inhibitor at low doses. While not FDA-approved for PDN, studies show significant pain improvement compared to placebo; however, there is comparatively better literature on the use of duloxetine in PDN, and thus venlafaxine should be considered only if duloxetine fails or the side effects are intolerable. Venlafaxine is noninferior to TCAs and gabapentin, 39 but inferior to pregabalin. 40 Adverse effects are similar to those of duloxetine; however, patients should also be routinely monitored for QTc prolongation. 39
TCAs, specifically amitriptyline, are also used in PDN management; however their use requires careful monitoring and tends to be avoided in elderly patients and those with significant comorbidities. Amitriptyline is administered daily at 25-150 mg. 29 Compared to duloxetine,41,42 gabapentin, 43 and pregabalin, 44 amitriptyline confers noninferior pain relief. TCAs concurrently target histaminergic, adrenergic, and cholinergic receptors leading to a robust side effect profile, including gastrointestinal issues, orthostatic hypotension, dry mouth, urinary retention, and QTc prolongation. 42 The use of this drug is recommended only as a last resort given its high risk of side effects.
Opioids
Chronic opioid therapy for PDN can be helpful for a subset of people; however, their use is associated with certain unique concerns. For example, now well-recognized is the concern of misuse and abuse with long-term opioid treatment: misuse is the use of a drug in a nonindicated manner, whereas abuse is when use becomes detrimental or unlawful. 45 Furthermore, it is important to understand the distinction between tolerance (physiological adaptation resulting in reduced drug efficacy) and physical dependence (physiological adaptation in which withdrawal can be induced with drug cessation or rapid dose reduction) with chronic opioid use. 45 Evaluating a person with PDN for the potential use of chronic opioid therapy should include obtaining a history of past/current substance abuse and addiction so that the prescriber can address all relevant issues before prescribing opioids.
Since the development of tolerance may lead to dose escalation, the prescriber needs to be aware of dose-dependent risks of opioid therapy. Opioids should always be used at the lowest effective dose. Side effects and aberrant behaviors must be monitored consistently by the prescriber and opioid-sparing approaches including interventional treatments need to be considered and routinely incorporated. Additional concerns of chronic therapy include the development of opioid-induced hyperalgesia—although not well understood, some patients will actually experience increased pain. 46 If identified, dose reduction can be very effective.
Tapentadol is an opioid that is FDA-approved specifically in PDN management as third-line therapy, 47 administered at an optimal dose of 100-250 mg twice daily. Several studies demonstrate that extended-release tapentadol significantly reduces pain intensity compared to placebo.48-50 Levorphanol, a synthetic opioid, has also been reported to significantly reduce neuropathic pain when used in high-strength formulations (around 9 mg/day), with efficacy comparable to gabapentin and TCAs. 51 Methadone, another synthetic opioid, has demonstrated efficacy in treating neuropathic pain, including patients who have previously failed trials of conventional opioids. 52 Opioid-naïve patients are typically started at doses of 2.5-5 mg every 8-12 hours, and those patients currently taking other opioids require careful dose conversion. 53 Although effective for general neuropathic pain, both levorphanol and methadone require further studies to evaluate their use in PDN.
Topical Analgesics
Capsaicin is a topical analgesic utilized in low (0.075%) and high (8%) dose formulations for PDN management. Significant reductions in neuropathic pain intensity have been reported,54,55 although results can be inconsistent.56,57 Additional studies have reported 8% capsaicin as noninferior to pregabalin, duloxetine, and gabapentin with a comparatively more tolerable side effect profile. 55 Adverse effects include dermal irritation. 58 Qutenza is an 8% capsaicin patch approved for the treatment of PDN in Europe, and is currently pending FDA-approval.
Topical lidocaine (5%) can be utilized for the treatment of localized neuropathic pain, 59 with several studies demonstrating significant pain reduction, improvement in quality-of-life, and patient satisfaction compared to placebo.60,61 Noninferiority testing with pregabalin, gabapentin, capsaicin and amitriptyline has demonstrated sustained efficacy with comparatively improved tolerability.62,63 Side effects similarly include dermal irritation. 64
Other topical analgesics include ketamine cream and clonidine gel. 65 One study demonstrated that topical 5% ketamine did not improve PDN symptoms compared to placebo. 66 There is currently limited and inconsistent evidence on the clinical utility of clonidine for PDN.67,68
Novel Agents
α-Lipoic acid is a potent antioxidant used in multivitamin formulas. 69 Its properties, including reduced oxidative stress, sustained microvascular blood flow, and improved nerve conduction velocity,70,71 prompted investigation into its effects on PDN. Various placebo-controlled RCTs administered α-Lipoic acid with an oral or IV infusion of 600 mg once to thrice daily. Overall, improved neuropathic sensory symptoms were reported, without significant improvement in neuropathy impairment scores, levels of inflammatory or oxidative stress markers, or nerve conduction.72-76 In the United States, it is available as a nutritional supplement and does not require a prescription. This has raised concerns regarding drug purity and safety.
Actovegin is a deproteinized ultrafiltrate of calf serum with potent anti-hypoxic effects. It has been investigated in several trials assessing a potential neuroprotective role in PDN, with regimens consisting of daily infusions (2000 mg) for 20 days followed by three times a day oral formulations (200 mg) for 140 days. These studies demonstrated improvement in neuropathic symptoms and vibration thresholds compared to placebo with a tolerable safety profile.77,78 These novel agents require further investigation to further define their role in PDN management.
Intravenous Therapies
IV Lidocaine
Lidocaine is a local anesthetic with antiarrhythmic properties that also exerts analgesic effects. A recent systematic review evaluating IV lidocaine as a treatment for neuropathic pain reported superiority to placebo in the early post-infusion period. 79 However, infusions over four weeks had no further significant effect. While IV lidocaine was associated with an increased risk of minor adverse effects, no serious adverse effects were reported. Studies specific to the use of IV lidocaine for PDN were included in this review;80-82 common infusion dosages were 5 and 7 mg/kg, providing varying degrees of pain relief from 10 to 28 days following infusion.
The effects of IV lidocaine are mainly limited to the immediate post-infusion period.53,83,84 As such, its use may be better suited for acute adjunctive care while allowing for up-titration of oral analgesics to therapeutic dosages, as well as incorporation of nonpharmacologic modalities. Results are mixed, with other research showing no significant short- or long-term analgesic benefit up to four weeks post-infusion. 85 Overall, IV lidocaine is a safe alternative to opioid medications for acute management of PDN. Additional well-designed studies are needed to provide clear guidelines on clinical use.
IV Ketamine
Ketamine is a dissociative anesthetic that, when given in sub-anesthetic doses, has analgesic and anti-hyperalgesic effects. The duration of analgesic effect with a single dose of IV ketamine is approximately 60 minutes, and up to six hours when administered orally. 53 Although most studies have evaluated IV ketamine, oral administration has been reported to significantly improve neuropathic pain compared to methadone in one study. 86 A study evaluating patients with peripheral neuropathy treated with ketamine bolus and infusion reported a significant reduction in allodynia and spontaneous pain. 87 A recent systematic review showed that patients treated with IV ketamine demonstrated some type of pain relief for a variety of neuropathic pain syndromes, with comparatively poorer results for oral and topical administration. 88
In one study, the most common side effect was dizziness (44% for infusion, 22% for oral administration). Other side effects included sedation, loss of appetite, nausea, and vomiting. 89 Importantly, the use of IV ketamine has addictive potential similar to that of opioids. As it can be considered a drug of abuse, its use should be considered only in carefully selected patients, and under the strict supervision of a pain physician. Patient evaluation and screening should be similar to patients taking opioid therapy, including delineating a history of substance abuse and addiction. Overall, the use of ketamine in all forms for the treatment of PDN requires further study.
Neuromodulation
Neuromodulation therapies are nonablative pain treatments that are both titratable and reversible; these include intrathecal (IT) pain therapy and spinal cord stimulation (SCS). Both modalities have been demonstrated to be effective treatments option for neuropathic pain, including failed back surgery syndrome and complex regional pain syndrome (CRPS). However, few studies have assessed the use of IT therapy or SCS specifically in the treatment of PDN. Currently, the use of neuromodulation in the treatment of PDN is underutilized, which can be attributed to the lack of high-quality studies supporting its use, as well as limited referral to neuromodulation specialists. Accordingly, these are rare interventions that require careful consideration of a patient’s comorbidities and goals of therapy. Potential patients should be referred to a pain management specialist to determine their candidacy, and ideally their care should be discussed at a multidisciplinary pain conference. The use of neuromodulation therapies should be considered only following the failure of conservative management with evidence-based medications such as anticonvulsants and antidepressants.
Intrathecal Pain Therapy
The use of IT pain therapy is indicated for the treatment of chronic neuropathic pain, including peripheral neuropathy related to diabetes. Morphine (a µ-receptor agonist) and ziconotide (a nonopioid calcium channel blocker) are the only FDA-approved agents for the IT treatment of pain, and are both recommended by the Polyanalgesic Consensus Conference as first-line monotherapy in the treatment of localized or diffuse nonmalignant neuropathic pain. 90 IT therapies are recommended for the treatment of refractory pain, defined as a failure of conservative management with multiple evidence-based treatments. 91 Importantly, IT therapy should not be used as salvage therapy following the failure of high-dose systemic opioids. 90 IT therapy can be used concurrently with oral therapies, and doses should be carefully managed by a pain specialist.
Ziconotide has certain advantages due to its nonopioid properties that make it a preferable first-line monotherapy. In particular, its use is not associated with an elevated risk of respiratory depression, and there is no withdrawal with abrupt discontinuation. However, a history of psychosis or concurrent use of anti-epileptics or sedatives is a contraindication. 92 Initial IT dosing starts at 1.2 mcg/day, with titration of 1.1-2.8 mcg/day at two to four week intervals. 93
The use of IT morphine is also considered first-line; however, the risk of respiratory depression can be deadly, especially with the concurrent use of hypnotics or sedatives. Additional concerns include the development of tolerance, withdrawal with drug discontinuation or pump malfunction, and the rare development of a catheter tip granuloma which can cause neurological injury. 94 The recommended starting dosage for IT morphine is 0.1-0.5 mg/day, which should be titrated conservatively with consideration for the aforementioned side effects. 90
Tonic SCS
Conventional, tonic SCS utilizes low-frequency stimulation of 40-100 Hz to create paresthesias that overlaps painful anatomic regions. 95 In practice, lower extremity stimulation can typically be achieved with lead placement between T9-T11, although sometimes retrograde lead placement is required to achieve foot coverage. In the first clinical study of SCS in medically-refractory PDN, permanent SCS leads were implanted between T9-T11, with patients achieving significant pain relief at a median of 14 months, and with reduced or eliminated oral analgesic intake. 96 Significant long-term pain relief was demonstrated with follow-up at 3.3 and 7.5 years. 97 Similar small, uncontrolled studies demonstrated promising results with long-term SCS treatment.98-100
The success of these pilot studies lead to the 2014 publication of two prospective, multi-center RCTs comparing the efficacy of conventional medical management with and without SCS.101,102 In the study reported by de Vos et al, 36 PDN patients had SCS electrodes implanted between T9-T12. 101 At six months follow-up, mean pain intensity was significantly decreased in the SCS group, with improved quality-of-life metrics and decreased analgesic intake. In the concurrent study by Slangen et al, 17 PDN patients underwent SCS implantation. Treatment success (≥50% improvement in pain severity) at six months follow-up was reported in 59% of SCS patients versus 7% of those receiving medical management alone. 102 The patients in this latter study were reassessed at 24 months and continued to have sustained pain relief. 103
Sub-Paresthesia SCS
Although high-quality evidence has been reported for the use of tonic SCS in neuropathic pain, a significant proportion of patients in these studies did not achieve adequate pain relief. Subsequently, sub-paresthesia waveforms have been developed, including high-frequency and burst stimulation, in which minimal to no paresthesia is perceived. 95 Compared to tonic SCS, both high-frequency and burst SCS have been demonstrated to provide superior relief of back and leg pain in prospective RCTs.104,105
There has been recent enthusiasm in applying these novel waveforms to improve the clinical outcomes of patients with PDN. The SENZA-PDN study is an ongoing prospective, multicenter RCT, with patients assigned to high-frequency SCS at 10 000 Hz plus medical management versus medical management alone (NCT03228420). 106 The primary endpoint is a composite measure of both safety and effectiveness at three months, with follow-up to continue for 24 months. Enrollment was recently completed in 2019, with preliminary results presented at the 2020 North American Neuromodulation Society Annual Meeting; 107 patients with PDN demonstrated improved pain severity, sleep, and walking tolerance at three months, and without higher rates of infection.
Dorsal Root Ganglion Stimulation
Conventional SCS targets the dorsal columns and thus has certain coverage limitations, and dorsal root ganglion (DRG) stimulation has emerged as a therapeutic modality to improve coverage of specific dermatomal distributions. Compared to tonic SCS, DRG stimulation in patients with complex regional pain syndrome has been reported to provide higher rates of treatment success. 108
As patients with PDN predominantly have lower extremity and foot pain, the specificity of coverage provided by DRG stimulation is attractive, although the current evidence is limited. In one retrospective study, seven PDN patients were implanted with a permanent system after a successful trial, and four patients with 12-month follow-up data reported a 64.16% mean relative reduction in perceived pain; 109 the majority of patients were implanted at L5. Based on dermatomal coverage, it is recommended to target L5, possibly with the addition of S1. A staged trial may consist of unilateral electrode placement, with the addition of bilateral electrode(s) for permanent implantation should the trial be successful. In clinical practice, the use of DRG stimulation may be preferable to dorsal column SCS due to its dermatomal specificity.
Other DRG studies have been preclinical. Using conventional and burst DRG stimulation in rat models of PDN, Franken et al observed attenuation of mechanical hypersensitivity using both waveforms compared to sham stimulation.110,111 Although preliminary, these data suggest a potential role for DRG stimulation in the management of refractory PDN.
Other Stimulation Modalities
Despite being considered low-risk procedures, IT and SCS therapies are invasive and require the chronic implantation of hardware. Transcutaneous electrical nerve stimulation (TENS) is a noninvasive neuromodulation modality that delivers electricity via patches placed on painful areas, with efficacy demonstrated for some patients with musculoskeletal pain. The mechanism of action in PDN is unclear, and has been attributed to improved microcirculation and/or the stimulation of cutaneous afferents, leading to nociceptive inhibition. 112
Some of the earliest studies evaluating TENS in the treatment of PDN reported decreased pain scores and improvement in neuropathic symptoms, although with a high placebo effect with sham stimulation.113,114 Additional studied therapies include external muscle stimulation,115,116 pulsed electromagnetic fields, 117 and frequency rhythmic electrical modulation. 118 Although multiple studies have been performed, they are limited by their short duration, small patient numbers, lack of study rigor, and significant placebo effects. 112
Alternative Therapies
The use of nonpharmacological interventions for PDN has a great deal of support, and the most studied modalities include lifestyle modification with diet and exercise, dietary supplements, and holistic therapies such as yoga and acupuncture. 119
Yoga has been reported to improve multiple health parameters in patients with diabetes including reduced BMI, systolic blood pressure, and fasting glucose, as well as a reduction in oxidative stress parameters.120,121 In a study of older female patients with PDN, meditation and progressive relaxation resulted in significant daily pain reduction for at least 24 hours compared to baseline. 122 Acupuncture has also been shown to be an effective treatment for PDN; one study reported significant pain relief when participants were treated once per week for 10 consecutive weeks with individualized acupuncture regimens. 123 Subsequent systematic reviews have reported favorable outcomes with acupuncture, although the practice is quite variable, employing different methodologies and outcome measures.124,125
Low-level laser therapy (aka photobiomodulation) employs the use of red and near infra-red light over painful areas to purportedly improve tissue healing and promote pain relief. Some studies have demonstrated short-term pain relief in patients with PDN;126,127 however, the lasting efficacy is unclear, with some studies reporting no benefit with longer-term follow-up.128,129 As such, the use of alternative therapies should be integrated into a comprehensive management strategy and should not be used as sole interventions.
Conclusion
PDN is a relatively common complication of diabetes associated with significant disability and cost. Although primary prevention of diabetes is required at a societal level, the treatment of diabetic complications, including aggressive glycemic control, is essential in controlling the development of neuropathic complications. Guidelines exist regarding the pharmacological management of PDN, and first-line agents include anticonvulsants (pregabalin, gabapentin) and antidepressants (duloxetine). Oral opioids may be helpful in a subset of patients; however, their use should be carefully monitored, and should always be used at the lowest effective dose. Despite multiple conservative treatment options, there are considerable unmet needs in this patient population, and neuromodulation therapies, including IT drug delivery and SCS, have the potential to improve pain management outcomes. Improving pain outcomes will require an individualized patient approach, with consideration for pharmacotherapy, surgical treatments including neuromodulation, and other holistic alternative therapies.
Footnotes
Abbreviations: DRG, dorsal root ganglion; FDA, Food and Drug Administration; IT, intrathecal; PDN, painful diabetic neuropathy; RCT, randomized control trial; SCS, spinal cord stimulation; SNRI, serotoninnorepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; TEN, transcutaneous electrical nerve stimulation.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Argoff is a Speakers Bureau member for Allergan, Inc., AstraZeneca Pharmaceuticals LP, Depomed, Inc., Iroko Pharmaceuticals, LLC, Millennium Laboratories, LLC, Teva Pharmaceuticals Industries Ltd., and XenoPort, Inc.; Advisory Board member for AstraZeneca Pharmaceuticals LP, Collegium Pharmaceutical, Inc., Depomed, Inc., Endo Pharmaceuticals Inc., Pfizer Inc., Purdue Pharma L.P., Shionogi & Co. Ltd., Teva Pharmaceuticals Industries Ltd., Vertex, and XenoPort, Inc.; Research support from Eli Lilly and Company, Endo Pharmaceuticals Inc., and Forest Laboratories, Inc. Dr Pilitsis is a consultant for Boston Scientific, Nevro, TerSera, Medtronic, Saluda and Abbott and receives grant support from Medtronic, Boston Scientific, Abbott, Nevro, TerSera, NIH 2R01CA166379-06 and NIH U44NS115111. She is medical advisor for Aim Medical Robotics and Karuna and has stock equity. All other authors declare no conflicts of interest.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Michael D. Staudt  https://orcid.org/0000-0002-9123-136X
https://orcid.org/0000-0002-9123-136X
References
- 1. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care. 2017;40(1):136-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hicks CW, Selvin E. Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep. 2019;19(10):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iqbal Z, Azmi S, Yadav R, et al. Diabetic peripheral neuropathy: epidemiology, diagnosis, and pharmacotherapy. Clin Ther. 2018;40(6):828-849. [DOI] [PubMed] [Google Scholar]
- 4. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- 5. Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26(6):1790-1795. [DOI] [PubMed] [Google Scholar]
- 6. Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American diabetes association. Diabetes Care. 2005;28(4):956-962. [DOI] [PubMed] [Google Scholar]
- 7. Zafeiri M, Tsioutis C, Kleinaki Z, Manolopoulos P, Ioannidis I, Dimitriadis G. Clinical characteristics of patients with co-existent diabetic peripheral neuropathy and depression: a systematic review [published online ahead of print September 26, 2018]. Exp Clin Endocrinol Diabetes. doi: 10.1055/a-0741-6937 [DOI] [PubMed] [Google Scholar]
- 8. Abbott CA, Malik RA, van Ross ERE, Kulkarni J, Boulton AJM. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alleman CJM, Westerhout KY, Hensen M, et al. Humanistic and economic burden of painful diabetic peripheral neuropathy in Europe: a review of the literature. Diabetes Res Clin Pract. 2015;109(2):215-225. [DOI] [PubMed] [Google Scholar]
- 10. Kiyani M, Yang Z, Charalambous LT, et al. Painful diabetic peripheral neuropathy: health care costs and complications from 2010 to 2015. Neurol Clin Pract. 2020;10(1):47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American academy of neurology, the american association of neuromuscular and electrodiagnostic medicine, and the American academy of physical medicine and rehabilitation. Neurology. 2011;76(20):1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113-e88. [DOI] [PubMed] [Google Scholar]
- 13. Griebeler ML, Morey-Vargas OL, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014;161(9):639-649. [DOI] [PubMed] [Google Scholar]
- 14. Mai LM, Clark AJ, Gordon AS, et al. Long-term outcomes in the management of painful diabetic neuropathy. Can J Neurol Sci. 2017;44(4):337-342. [DOI] [PubMed] [Google Scholar]
- 15. Staudt MD, Clark AJ, Gordon AS, et al. Long-term outcomes in the management of central neuropathic pain syndromes: a prospective observational cohort study. Can J Neurol Sci. 2018;45(5):545-552. [DOI] [PubMed] [Google Scholar]
- 16. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A, KORA Study Group. Neuropathic pain in diabetes, prediabetes and normal glucose tolerance: the MONICA/KORA Augsburg surveys S2 and S3. Pain Med. 2009;10(2):393-400. [DOI] [PubMed] [Google Scholar]
- 17. Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14(8):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S124-S138. [DOI] [PubMed] [Google Scholar]
- 19. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 21. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Snyder MJ, Gibbs LM, Lindsay TJ. Treating painful diabetic peripheral neuropathy: an update. Am Fam Physician. 2016;94(3):227-234. [PubMed] [Google Scholar]
- 23. Azmi S, ElHadd KT, Nelson A, et al. Pregabalin in the management of painful diabetic neuropathy: a narrative review. Diabetes Ther. 2019;10(1):35-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quilici S, Chancellor J, Löthgren M, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009;9:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253-260. [DOI] [PubMed] [Google Scholar]
- 26. Arezzo JC, Rosenstock J, Lamoreaux L, Pauer L. Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanenberg RJ, Irving GA, Risser RC, et al. Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clin Proc. 2011;86(7):615-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104-2110. [DOI] [PubMed] [Google Scholar]
- 29. Rudroju N, Bansal D, Talakokkula ST, et al. Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. 2013;16(6):E705-E714. [PubMed] [Google Scholar]
- 30. Moore RA, Wiffen PJ, Derry S, Toelle T, Rice ASC. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014;2014(4):CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hemstreet B, Lapointe M. Evidence for the use of gabapentin in the treatment of diabetic peripheral neuropathy. Clin Ther. 2001;23(4):520-531. [DOI] [PubMed] [Google Scholar]
- 32. Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280(21):1831-1836. [DOI] [PubMed] [Google Scholar]
- 33. Eisenberg E, Lurie Y, Braker C, Daoud D, Ishay A. Lamotrigine reduces painful diabetic neuropathy: a randomized, controlled study. Neurology. 2001;57(3):505-509. [DOI] [PubMed] [Google Scholar]
- 34. Papanas N, Ziegler D. Emerging drugs for diabetic peripheral neuropathy and neuropathic pain. Expert Opin Emerg Drugs. 2016;21(4):393-407. [DOI] [PubMed] [Google Scholar]
- 35. Gill D, Derry S, Wiffen PJ, Moore RA. Valproic acid and sodium valproate for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2011;2011(10):CD009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raskin J, Pritchett YL, Wang F, et al. A double-blind, randomized multicenter trial comparing duloxetine with placebo in the management of diabetic peripheral neuropathic pain. Pain Med. 2005;6(5):346-356. [DOI] [PubMed] [Google Scholar]
- 37. Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006;67(8):1411-1420. [DOI] [PubMed] [Google Scholar]
- 38. Hardy T, Sachson R, Shen S, Armbruster M, Boulton AJM. Does treatment with duloxetine for neuropathic pain impact glycemic control? Diabetes Care. 2007;30(1):21-26. [DOI] [PubMed] [Google Scholar]
- 39. Rowbotham MC, Goli V, Kunz NR, Lei D. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706. [DOI] [PubMed] [Google Scholar]
- 40. Razazian N, Baziyar M, Moradian N, Afshari D, Bostani A, Mahmoodi M. Evaluation of the efficacy and safety of pregabalin, venlafaxine, and carbamazepine in patients with painful diabetic peripheral neuropathy. A randomized, double-blind trial. Neurosciences (Riyadh). 2014;19(3):192-198. [PMC free article] [PubMed] [Google Scholar]
- 41. Kaur H, Hota D, Bhansali A, Dutta P, Bansal D, Chakrabarti A. A comparative evaluation of amitriptyline and duloxetine in painful diabetic neuropathy: a randomized, double-blind, cross-over clinical trial. Diabetes Care. 2011;34(4):818-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256. [DOI] [PubMed] [Google Scholar]
- 43. Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Intern Med. 1999;159(16):1931-1937. [DOI] [PubMed] [Google Scholar]
- 44. Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P., Amitriptyline vs. pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabet Med. 2009;26(10):1019-1026. [DOI] [PubMed] [Google Scholar]
- 45. Jamison RN, Mao J. Opioid analgesics. Mayo Clin Proc. 2015;90(7):957-968. [DOI] [PubMed] [Google Scholar]
- 46. Rivat C, Ballantyne J. The dark side of opioids in pain management: basic science explains clinical observation. Pain Rep. 2016;1(2):e570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patil PR, Wolfe J, Said Q, Thomas J, Martin BC. Opioid use in the management of diabetic peripheral neuropathy (DPN) in a large commercially insured population. Clin J Pain. 2015;31(5):414-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin. 2011;27(1):151-162. [DOI] [PubMed] [Google Scholar]
- 49. Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth. 2014;113(1):148-156. [DOI] [PubMed] [Google Scholar]
- 50. Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37(8):2302-2309. [DOI] [PubMed] [Google Scholar]
- 51. Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003;348(13):1223-1232. [DOI] [PubMed] [Google Scholar]
- 52. Moulin DE, Palma D, Watling C, Schulz V. Methadone in the management of intractable neuropathic noncancer pain. Can J Neurol Sci. 2005;32(3):340-343. [DOI] [PubMed] [Google Scholar]
- 53. Mendlik MT, Uritsky TJ. Treatment of neuropathic pain. Curr Treat Options Neurol. 2015;17(12):50. [DOI] [PubMed] [Google Scholar]
- 54. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18(1):42-53. [DOI] [PubMed] [Google Scholar]
- 55. van Nooten F, Treur M, Pantiri K, Stoker M, Charokopou M. Capsaicin 8% patch versus oral neuropathic pain medications for the treatment of painful diabetic peripheral neuropathy: a systematic literature review and network meta-analysis. Clin Ther. 2017;39(4):787-803.e18. [DOI] [PubMed] [Google Scholar]
- 56. Low PA, Opfer-Gehrking TL, Dyck PJ, Litchy WJ, O’Brien PC. Double-blind, placebo-controlled study of the application of capsaicin cream in chronic distal painful polyneuropathy. Pain. 1995;62(2):163-168. [DOI] [PubMed] [Google Scholar]
- 57. Kulkantrakorn K, Chomjit A, Sithinamsuwan P, Tharavanij T, Suwankanoknark J, Napunnaphat P. 0.075% capsaicin lotion for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. J Clin Neurosci. 2019;62:174-179. [DOI] [PubMed] [Google Scholar]
- 58. Peppin JF, Pappagallo M. Capsaicinoids in the treatment of neuropathic pain: a review. Ther Adv Neurol Disord. 2014;7(1):22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21(3):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wolff RF, Bala MM, Westwood M, Kessels AG, Kleijnen J. 5% lidocaine-medicated plaster vs other relevant interventions and placebo for post-herpetic neuralgia (PHN): a systematic review. Acta Neurol Scand. 2011;123(5):295-309. [DOI] [PubMed] [Google Scholar]
- 61. Mick G, Correa-Illanes G. Topical pain management with the 5% lidocaine medicated plaster–a review. Curr Med Res Opin. 2012;28(6):937-951. [DOI] [PubMed] [Google Scholar]
- 62. Baron R, Mayoral V, Leijon G, Binder A, Steigerwald I, Serpell M. 5% lidocaine medicated plaster versus pregabalin in post-herpetic neuralgia and diabetic polyneuropathy: an open-label, non-inferiority two-stage RCT study. Curr Med Res Opin. 2009;25(7):1663-1676. [DOI] [PubMed] [Google Scholar]
- 63. Wolff RF, Bala MM, Westwood M, Kessels AG, Kleijnen J. 5% lidocaine medicated plaster in painful diabetic peripheral neuropathy (DPN): a systematic review. Swiss Med Wkly. 2010;140(21-22):297-306. [DOI] [PubMed] [Google Scholar]
- 64. Navez ML, Monella C, Bösl I, Sommer D, Delorme C. 5% lidocaine medicated plaster for the treatment of postherpetic neuralgia: a review of the clinical safety and tolerability. Pain Ther. 2015;4(1):1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Javed S, Petropoulos IN, Alam U, Malik RA. Treatment of painful diabetic neuropathy. Ther Adv Chronic Dis. 2015;6(1):15-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mahoney JM, Vardaxis V, Moore JL, Hall AM, Haffner KE, Peterson MC. Topical ketamine cream in the treatment of painful diabetic neuropathy: a randomized, placebo-controlled, double-blind initial study. J Am Podiatr Med Assoc. 2012;102(3):178-183. [DOI] [PubMed] [Google Scholar]
- 67. Wrzosek A, Woron J, Dobrogowski J, Jakowicka-Wordliczek J, Wordliczek J. Topical clonidine for neuropathic pain. Cochrane Database Syst Rev. 2015;8(9):CD010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Campbell CM, Kipnes MS, Stouch BC, et al. Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy. Pain. 2012;153(9):1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790(10):1149-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Papanas N, Ziegler D. Efficacy of α-lipoic acid in diabetic neuropathy. Expert Opin Pharmacother. 2014;15(18):2721-2731. [DOI] [PubMed] [Google Scholar]
- 71. Vallianou N, Evangelopoulos A, Koutalas P. Alpha-lipoic acid and diabetic neuropathy. Rev Diabet Stud. 2009;6(4):230-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ziegler D, Low PA, Litchy WJ, et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011;34(9):2054-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabet Med. 2004;21(2):114-121. [DOI] [PubMed] [Google Scholar]
- 74. Mendoza-Núñez VM, García-Martínez BI, Rosado-Pérez J, Santiago-Osorio E, Pedraza-Chaverri J, Hernández-Abad VJ. The effect of 600 mg alpha-lipoic acid supplementation on oxidative stress, inflammation, and RAGE in older adults with type 2 diabetes mellitus. Oxid Med Cell Longev. 2019;2019:3276958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Reljanovic M, Reichel G, Rett K, et al. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha lipoic acid in diabetic neuropathy. Free Radic Res. 1999;31(3):171-179. [DOI] [PubMed] [Google Scholar]
- 76. Ametov AS, Barinov A, Dyck PJ, et al. The sensory symptoms of diabetic polyneuropathy are improved with alpha-lipoic acid: the SYDNEY trial. Diabetes Care. 2003;26(3):770-776. [DOI] [PubMed] [Google Scholar]
- 77. Ziegler D, Edmundson S, Gurieva I, Mankovsky B, Papanas N, Strokov I. Predictors of response to treatment with actovegin for 6 months in patients with type 2 diabetes and symptomatic polyneuropathy. J Diabetes Complications. 2017;31(7):1181-1187. [DOI] [PubMed] [Google Scholar]
- 78. Ziegler D, Movsesyan L, Mankovsky B, Gurieva I, Abylaiuly Z, Strokov I. Treatment of symptomatic polyneuropathy with actovegin in type 2 diabetic patients. Diabetes Care. 2009;32(8):1479-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhu B, Zhou X, Zhou Q, Wang H, Wang S, Luo K. Intra-venous lidocaine to relieve neuropathic pain: a systematic review and meta-analysis. Front Neurol. 2019;10:954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kastrup J, Petersen P, Dejgård A, Angelo HR, Hilsted J. Intravenous lidocaine infusion–a new treatment of chronic painful diabetic neuropathy? Pain. 1987;28(1):69-75. [DOI] [PubMed] [Google Scholar]
- 81. Bach FW, Jensen TS, Kastrup J, Stigsby B, Dejgård A. The effect of intravenous lidocaine on nociceptive processing in diabetic neuropathy. Pain. 1990;40(1):29-34. [DOI] [PubMed] [Google Scholar]
- 82. Viola V, Newnham HH, Simpson RW. Treatment of intractable painful diabetic neuropathy with intravenous lignocaine. J Diabetes Complications. 2006;20(1):34-39. [DOI] [PubMed] [Google Scholar]
- 83. Wren K, Lancaster RJ, Walesh M, et al. Intravenous lidocaine for relief of chronic neuropathic pain. AANA J. 2019;87(5):351-355. [PubMed] [Google Scholar]
- 84. Tremont-Lukats IW, Hutson PR, Backonja M-M. A randomized, double-masked, placebo-controlled pilot trial of extended IV lidocaine infusion for relief of ongoing neuropathic pain. Clin J Pain. 2006;22(3):266-271. [DOI] [PubMed] [Google Scholar]
- 85. Moulin DE, Morley-Forster PK, Pirani Z, Rohfritsch C, Stitt L. Intravenous lidocaine in the management of chronic peripheral neuropathic pain: a randomized-controlled trial. Can J Anaesth. 2019;66(7):820-827. [DOI] [PubMed] [Google Scholar]
- 86. Rigo FK, Trevisan G, Godoy MC, et al. Management of neuropathic chronic pain with methadone combined with ketamine: a randomized, double blind, active-controlled clinical trial. Pain Physician. 2017;20(3):207-215. [PubMed] [Google Scholar]
- 87. Felsby S, Nielsen J, Arendt-Nielsen L, Jensen TS. NMDA receptor blockade in chronic neuropathic pain: a comparison of ketamine and magnesium chloride. Pain. 1996;64(2):283-291. [DOI] [PubMed] [Google Scholar]
- 88. Aiyer R, Mehta N, Gungor S, Gulati A. A systematic review of NMDA receptor antagonists for treatment of neuropathic pain in clinical practice. Clin J Pain. 2018;34(5):450-467. [DOI] [PubMed] [Google Scholar]
- 89. Cvrcek P. Side effects of ketamine in the long-term treatment of neuropathic pain. Pain Med. 2008;9(2):253-257. [DOI] [PubMed] [Google Scholar]
- 90. Deer TR, Pope JE, Hayek SM, et al. The polyanalgesic consensus conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines [published online ahead of print January 2, 2017]. Neuromodulation. doi: 10.1111/ner.12538 [DOI] [PubMed] [Google Scholar]
- 91. Deer TR, Caraway DL, Wallace MS. A definition of refractory pain to help determine suitability for device implantation. Neuromodulation. 2014;17(8):711-715. [DOI] [PubMed] [Google Scholar]
- 92. Deer TR, Pope JE, Hanes MC, McDowell GC. Intrathecal therapy for chronic pain: a review of morphine and ziconotide as firstline options. Pain Med. 2019;20(4):784-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prusik J, Argoff C, Peng S, Pilitsis JG. Use of low dose ziconotide as first-line intrathecal monotherapy. Neuromodulation. 2017;20(4):386-391. [DOI] [PubMed] [Google Scholar]
- 94. Deer TR, Pope JE, Hayek SM, et al. The polyanalgesic consensus conference (PACC): recommendations for intrathecal drug delivery: guidance for improving safety and mitigating risks. Neuromodulation. 2017;20(2):155-176. [DOI] [PubMed] [Google Scholar]
- 95. Sheldon B, Staudt MD, Williams L, Harland TA, Pilitsis JG. Spinal cord stimulation programming: a crash course [published online ahead of print April 15, 2020]. Neurosurg Rev. doi: 10.1007/s10143-020-01299-y [DOI] [PubMed] [Google Scholar]
- 96. Tesfaye S, Watt J, Benbow SJ, Pang KA, Miles J, MacFarlane IA. Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348(9043):1698-1701. [DOI] [PubMed] [Google Scholar]
- 97. Daousi C, Benbow SJ, MacFarlane IA. Electrical spinal cord stimulation in the long-term treatment of chronic painful diabetic neuropathy. Diabet Med. 2005;22(4):393-398. [DOI] [PubMed] [Google Scholar]
- 98. Kumar K, Toth C, Nath RK. Spinal cord stimulation for chronic pain in peripheral neuropathy. Surg Neurol. 1996;46(4):363-369. [DOI] [PubMed] [Google Scholar]
- 99. de Vos CC, Rajan V, Steenbergen W, van der Aa HE, Buschman HPJ. Effect and safety of spinal cord stimulation for treatment of chronic pain caused by diabetic neuropathy. J Diabetes Complications. 2009;23(1):40-45. [DOI] [PubMed] [Google Scholar]
- 100. Pluijms WA, Slangen R, Bakkers M, et al. Pain relief and quality-of-life improvement after spinal cord stimulation in painful diabetic polyneuropathy: a pilot study. Br J Anaesth. 2012;109(4):623-629. [DOI] [PubMed] [Google Scholar]
- 101. de Vos CC, Meier K, Zaalberg PB, et al. Spinal cord stimulation in patients with painful diabetic neuropathy: a multicentre randomized clinical trial. Pain. 2014;155(11):2426-2431. [DOI] [PubMed] [Google Scholar]
- 102. Slangen R, Schaper NC, Faber CG, et al. Spinal cord stimulation and pain relief in painful diabetic peripheral neuropathy: a prospective two-center randomized controlled trial. Diabetes Care. 2014;37(11):3016-3024. [DOI] [PubMed] [Google Scholar]
- 103. van Beek M, Slangen R, Schaper NC, et al. Sustained treatment effect of spinal cord stimulation in painful diabetic peripheral neuropathy: 24-month follow-up of a prospective two-center randomized controlled trial. Diabetes Care. 2015;38(9):e132-e134. [DOI] [PubMed] [Google Scholar]
- 104. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851-860. [DOI] [PubMed] [Google Scholar]
- 105. Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation. 2018;21(1):56-66. [DOI] [PubMed] [Google Scholar]
- 106. Mekhail NA, Argoff CE, Taylor RS, et al. High-frequency spinal cord stimulation at 10 kHz for the treatment of painful diabetic neuropathy: design of a multicenter, randomized controlled trial (SENZA-PDN). Trials. 2020;21(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Petersen EA, Stauss TG, Scowcroft J, et al. 10 kHz spinal cord stimulation for treatment of painful diabetic neuropathy-a multicenter randomized controlled trial. North American Neuromodulation Society 23rd Annual Meeting; Las Vegas, NV, 2020. [Google Scholar]
- 108. Deer TR, Levy RM, Kramer J, et al. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017;158(4):669-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Eldabe S, Espinet A, Wahlstedt A, et al. Retrospective case series on the treatment of painful diabetic peripheral neuropathy with dorsal root ganglion stimulation. Neuromodulation. 2018;21(8):787-792. [DOI] [PubMed] [Google Scholar]
- 110. Franken G, Debets J, Joosten EAJ. Dorsal root ganglion stimulation in experimental painful diabetic peripheral neuropathy: burst vs. conventional stimulation paradigm. Neuromodulation. 2019;22(8):943-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Franken G, Debets J, Joosten EAJ. Nonlinear relation between burst dorsal root ganglion stimulation amplitude and behavioral outcome in an experimental model of painful diabetic peripheral neuropathy. Neuromodulation. 2020;23(2):158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Thakral G, Kim PJ, LaFontaine J, Menzies R, Najafi B, Lavery LA. Electrical stimulation as an adjunctive treatment of painful and sensory diabetic neuropathy. J Diabetes Sci Technol. 2013;7(5):1202-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kumar D, Marshall HJ. Diabetic peripheral neuropathy: amelioration of pain with transcutaneous electrostimulation. Diabetes Care. 1997;20(11):1702-1705. [DOI] [PubMed] [Google Scholar]
- 114. Kumar D, Alvaro MS, Julka IS, Marshall HJ. Diabetic peripheral neuropathy. Effectiveness of electrotherapy and amitriptyline for symptomatic relief. Diabetes Care. 1998;21(8):1322-1325. [DOI] [PubMed] [Google Scholar]
- 115. Reichstein L, Labrenz S, Ziegler D, Martin S. Effective treatment of symptomatic diabetic polyneuropathy by high-frequency external muscle stimulation. Diabetologia. 2005;48(5):824-828. [DOI] [PubMed] [Google Scholar]
- 116. Humpert PM, Morcos M, Oikonomou D, et al. External electric muscle stimulation improves burning sensations and sleeping disturbances in patients with type 2 diabetes and symptomatic neuropathy. Pain Med. 2009;10(2):413-419. [DOI] [PubMed] [Google Scholar]
- 117. Weintraub MI, Herrmann DN, Smith AG, Backonja MM, Cole SP. Pulsed electromagnetic fields to reduce diabetic neuropathic pain and stimulate neuronal repair: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(7):1102-1109. [DOI] [PubMed] [Google Scholar]
- 118. Bosi E, Bax G, Scionti L, et al. Frequency-modulated electromagnetic neural stimulation (FREMS) as a treatment for symptomatic diabetic neuropathy: results from a double-blind, randomised, multicentre, long-term, placebo-controlled clinical trial. Diabetologia. 2013;56(3):467-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Baute V, Zelnik D, Curtis J, Sadeghifar F. Complementary and alternative medicine for painful peripheral neuropathy. Curr Treat Options Neurol. 2019;21(9):44. [DOI] [PubMed] [Google Scholar]
- 120. Hegde SV, Adhikari P, Shetty S, Manjrekar P, D’Souza V. Effect of community-based yoga intervention on oxidative stress and glycemic parameters in prediabetes: a randomized controlled trial. Complement Ther Med. 2013;21(6):571-576. [DOI] [PubMed] [Google Scholar]
- 121. Hegde SV, Adhikari P, Kotian S, Pinto VJ, D’Souza S, D’Souza V. Effect of 3-month yoga on oxidative stress in type 2 diabetes with or without complications: a controlled clinical trial. Diabetes Care. 2011;34(10):2208-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hussain N, Said ASA. Mindfulness-based meditation versus progressive relaxation meditation: impact on chronic pain in older female patients with diabetic neuropathy. J Evid Based Integr Med. 2019;24:2515690X19876599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Bailey A, Wingard D, Allison M, Summers P, Calac D. Acupuncture treatment of diabetic peripheral neuropathy in an American Indian community. J Acupunct Meridian Stud. 2017;10(2):90-95. [DOI] [PubMed] [Google Scholar]
- 124. Dimitrova A, Murchison C, Oken B. Acupuncture for the treatment of peripheral neuropathy: a systematic review and meta-analysis. J Altern Complement Med. 2017;23(3):164-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nash J, Armour M, Penkala S. Acupuncture for the treatment of lower limb diabetic peripheral neuropathy: a systematic review. Acupunct Med. 2019;37(1):3-15. [DOI] [PubMed] [Google Scholar]
- 126. Cg SK, Maiya AG, Hande HM, Vidyasagar S, Rao K, Rajagopal KV. Efficacy of low level laser therapy on painful diabetic peripheral neuropathy. Laser Ther. 2015;24(3):195-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Swislocki A, Orth M, Bales M, et al. A randomized clinical trial of the effectiveness of photon stimulation on pain, sensation, and quality of life in patients with diabetic peripheral neuropathy. J Pain Symptom Manage. 2010;39(1):88-99. [DOI] [PubMed] [Google Scholar]
- 128. Zinman LH, Ngo M, Ng ET, Nwe KT, Gogov S, Bril V. Low-intensity laser therapy for painful symptoms of diabetic sensorimotor polyneuropathy: a controlled trial. Diabetes Care. 2004;27(4):921-924. [DOI] [PubMed] [Google Scholar]
- 129. M A, Ummer VS, Maiya AG, Hande M. Low level laser therapy for the patients with painful diabetic peripheral neuropathy - a systematic review. Diabetes Metab Syndr. 2019;13(4):2667-2670. [DOI] [PubMed] [Google Scholar]