Abstract
Diabetes distal symmetrical peripheral neuropathy (DSPN) is the most prevalent form of neuropathy in industrialized countries, substantially increasing risk for morbidity and pre-mature mortality. DSPN may manifest with small-fiber disease, large-fiber disease, or a combination of both. This review summarizes: (1) DSPN subtypes (small- and large-fiber disease) with attention to clinical signs and patient symptoms; and (2) technological diagnosis and screening for large- and small-fiber disease with inclusion of a comprehensive literature review of published studies from 2015-present (N = 66). Review findings, informed by the most up-to-date research, advance critical understanding of DSPN large- and small-fiber screening technologies, including those designed for point-of-care use in primary care and endocrinology practices.
Keywords: diabetes, diagnostic technologies, distal symmetrical peripheral neuropathy, large-fiber neuropathy, small-fiber neuropathy
Introduction
Distal symmetric peripheral neuropathy (DSPN) is the most common form of diabetes neuropathy, affecting 50% of adults with diabetes during their lifetime. DSPN afflicts the feet, and less frequently, the hands.1-4 In addition to severe pain, imbalance, and associated risk for fall, a highly concerning sequela of DSPN is foot ulceration.5,6 Foot ulceration confers heightened risk for gangrene and lower extremity amputation. Lower extremity amputations increase risk for mortality with a 5-year survival rate of 40%–48% post-amputation. 7 The economic cost of foot ulcerations and amputations is exceptional, with the cost of foot ulcerations due to diabetes alone estimated at $9-$13 billion annually in the United States. 8 Hence, development of reliable, accurate technological methods to detect DSPN, particularly in the early course of its development (even in asymptomatic stages), is critically needed to slow DSPN progression with prompt interventions to prevent or decrease adverse outcomes and contain healthcare costs.9-16
The pathogenesis of diabetes DSPN is not fully understood but is believed to involve multiple mechanisms.4,17,18 There is strong evidence suggesting that diabetes DSPN is caused by hyperglycemia-induced nerve fiber injury and microvascular ischemia with ensuing nerve fiber degeneration and loss. Hyperglycemia causes direct nerve toxicity through several pathways, such as increased oxidative stress, advanced glycation end-product accumulation, and impaired axonal transport, thereby precipitating nerve degeneration.4,19 At the same time, long-standing hyperglycemia effects the microvasculature, causing nerve ischemia & degeneration. 20 Hypertension, dyslipidemia, elevated body mass index, and smoking may also contribute to DSPN incidence.21-26
DSPN is a progressive condition with no cure. Hence, timely diagnosis of DSPN is essential to prevent or mitigate DSPN-related morbidity and mortality. Prompt diagnosis of DSPN is indicated in prediabetes, new-onset T2D, and well-established T2D and T1D. Among those with prediabetes, DSPN may afflict 10%-30% or more, indicating neuropathic damage may occur at glucose levels not meeting the threshold for diagnosis of T2D.4,27 DSPN prevalence, among patients with newly diagnosed T2D, is estimated at 21%. 28 After 10 years of T2D duration, DSPN rates climb exponentially to 50%. 4 Among patients with T1D, DSPN rates are or exceed 20% after 20 years of disease duration.15,29 Since DSPN rates increase dramatically with longer diabetes duration, early and ongoing surveillance is warranted. Risk reduction strategies for DSPN and its sequelae are optimization of blood glucose, blood pressure, and lipid levels in addition to healthy lifestyle behaviors (daily foot care, weight loss, increased physical activity, smoking cessation).4,21-26,30-33
On the frontline diagnosing and managing prediabetes and diabetes, primary care providers play a critical role in early detection of DSPN. Yet, in primary care, research indicates DSPN may be underdiagnosed or not diagnosed promptly with underutilization of diagnostic tests and sometimes misperceptions about causes or management.34-36
Hence, this study examines: (1) DSPN subtypes (small- and large-fiber disease); (2) DSPN screening with attention to guidelines for clinical diagnosis; and (3) well-established diagnostic and more recent, innovative advances in technological screening for DSPN subtypes with attention to performance, reproducibility, and longitudinal outcomes data. Currently, comprehensive, up-to-date reviews on technologies and related advancements for DSPN screening and diagnosis are lacking.
Study inclusion criteria were: (1) examination of technological diagnosis or screening (specifically, nerve conduction study, quantitative sensory testing, contact heat evoked potentials, corneal confocal microscopy, quantitative sudomotor axon reflex testing, and electrochemical skin conductance) approaches for DSPN; (2) related investigation of diagnostic or screening performance, reliability or validity testing, automation procedure, and/or longitudinal outcomes in detecting DSPN; (3) inclusion of adult participants with T1D, T2D, impaired glucose tolerance (IGT), or impaired fasting glucose (IFG); (4) published in English; and (5) published between 2015-April of 2021. Study exclusion criteria were: (1) exclusive use of a descriptive and/or correlational design; (2) investigation of technology in performing the routine foot exam; (3) exclusive examination of DSPN predictors (eg, demographic and clinical factors); (4) intervention studies; (5) conference abstracts; (6) dissertations; and (7) review articles.
We conducted a literature search using the following search engines: PubMed, Scopus, and MEDLINE. The search strategy was conducted by separately entering key search terms (nerve conduction study, quantitative sensory testing, contact heat evoked potentials, corneal confocal microscopy, quantitative sudomotor axon reflex testing, and electrochemical skin conductance) with each term, respectively, accompanied (one at a time) by AND diabetes, AND type 1 diabetes, AND type 2 diabetes, AND prediabetes, AND impaired glucose tolerance, or AND impaired fasting glucose. Then, the key search terms, respectively, were accompanied, individually, by AND peripheral neuropathy AND diabetes; AND peripheral neuropathy AND type 1 diabetes; AND peripheral neuropathy AND type 2 diabetes; AND peripheral neuropathy AND prediabetes; AND peripheral neuropathy AND impaired glucose tolerance; or AND peripheral neuropathy AND impaired fasting glucose. The literature search yielded 66 studies for analysis (see Figure 1).
Figure 1.
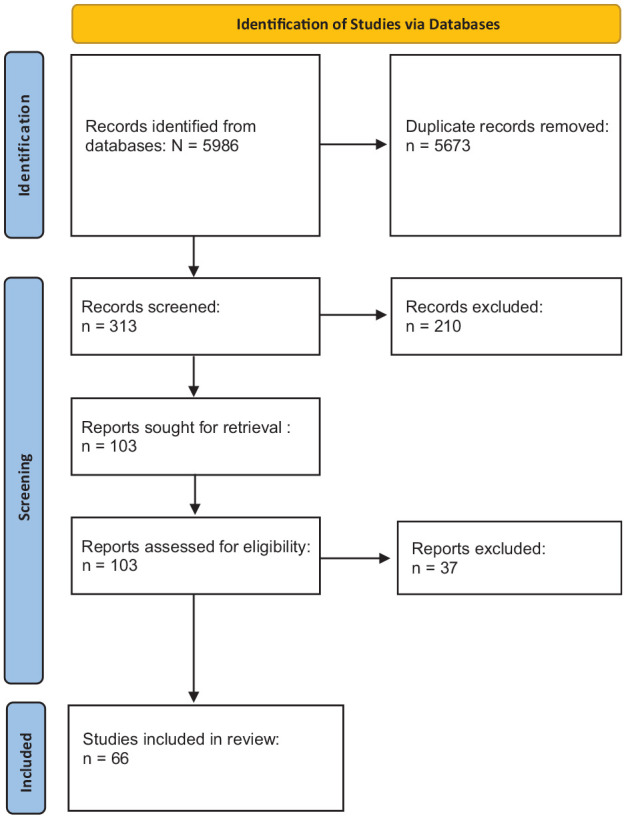
PRISMA Flow Diagram.
DSPN Subtypes
The subtypes of DSPN are small- and large-nerve fiber disease. Small- and large-nerve fiber DSPN may present exclusively or together while each subtype may increase risk for foot ulceration due to reduced sensory function, and thereby heightened risk for lower extremity amputations. 4 Small-fiber DSPN typically precedes large-fiber neuropathy. Small-fiber DSPN impairs functional integrity of the small thinly myelinated Aδ and unmyelinated C fibers. These small, peripheral nerve fibers prominently convey pain to the central nervous system. In DSPN, they may stimulate profound pain. 3 Small-fiber DSPN may also adversely affect local autonomic (eg, decreased sweating, dry skin, impaired vasomotion) and thermoreceptor (cold, warm sensations) functions.3,37,38
Often, pain and other symptoms and signs (see Table 1) first manifest in the feet and progress proximally to the lower extremities and, in some cases, to the hands with a stocking and glove pattern. However, some with small-fiber DSPN may not experience pain.39,40 A proportion of patients with small-fiber neuropathy may present with little evidence of the disease, which may delay DSPN diagnosis.41-42
Table 1.
Symptoms & signs of small & large fiber DSPN. 3
| Type of neuropathy | Subjective symptoms | Objective signs |
|---|---|---|
| Small fiber neuropathy | • Hypersensitivity to pressure or touch • Chronic or transient sensations of paresthesias: ○ tingling ○ burning ○ freezing ○ stabbing ○ aching ○ electrical |
• Sensory loss: 0 – + (thermal
allodynia-cold metallic device or ice in glove)
• Pain: 0 – +++ • Tendon reflex: NL – ↓ • Motor Deficit: 0 • Reduced sensitivity: ○ 1.0 g Semmes Weinstein Monofilament • Reduced prickling pain perception ○ Waardenberg wheel • Abnormal ANS function (feet and/or hands) ○ decreased sweating ○ dry skin ○ cold feet (impaired vasomotion & blood flow) • Normal NCV findings |
| Large fiber neuropathy | • Symptoms may be minimal: ○ sensation of walking on cotton ○ floors feeling “strange” ○ inability turn pages of book or button shirt ○ inability to discriminate among coins • In some cases, with severe distal muscle weakness: ○ inability to stand on toes or heels |
• Sensory loss: 0 – +++ (touch vibration – 128 Hz tuning
fork) • Pain: 0 – +++ • Tendon reflex: 0 – ↓↓↓ • Motor Deficit: 0 – +++ • Impaired light touch &/or joint position perception • Abnormal NCV findings • Sensory ataxia (waddling like a duck) • Wasting of small intrinsic muscles of feet &/or hands ○ hammertoe deformities ○ weakness hands &/or feet • Increased blood flow – hot feet |
Large-fiber disease refers to impairment of Aα and/or Aα/β fibers. These fibers are large myelinated fibers. Aα fibers control motor functions and muscle control while Aα/β fibers are related to sensory functions. 3 In DSPN, damage to Aα fibers may manifest with muscle weakness, painful cramps, among other symptoms. 40 Aα/β fibers are implicated in reduced perception to touch, vibration, balance, and position as well as pain (see Table 1).3,42 Damaged large Aα/β fibers may increase risk for falls and fractures with reduced or absent sensory input for the control of movement.3,43
DSPN Screening and Diagnosis
The American Diabetes Association (ADA) recommends screening for DSPN to promote early interventions (eg, glycemic control and lifestyle modifications), particularly given treatments targeting the underlying nerve damage are lacking. Screening should commence upon the diagnosis of T2D and at least annually thereafter. In the setting of prediabetes, screening may be considered although research suggests 11%-25% of this population may exhibit DSPN and 13%-26% may present with neuropathic pain.4,44 The ADA suggests, after exclusion of other causes, a diagnosis of DSPN may be based on a supportive patient history plus assessment of either gross temperature or pinprick sensation (small-fiber function) and vibration sensation using a 128-Hz tuning fork (large-fiber function). An annual 10-g monofilament test is recommended to assess for risk of foot ulceration and amputation. According to the ADA, the clinical history and physical examination often are sufficient for diagnosis of DSPN. Yet, up to 50% of individuals with DSPN may be asymptomatic. 4 Also, screening methods may not yield valid data to detect early or subclinical stages of DSPN and/or diagnose DSPN in diverse populations.42,45-48 Therefore, additional DSPN testing may be warranted.
Large-Fiber DSPN Diagnostic Testing and Screening
Nerve Conduction Study (NCS)
NCS is considered the gold standard for diagnosing large-fiber neuropathy. NCS has documented sensitivity and specificity, ranging, for example, from 40%-81% and 91%-95%, respectively, for DSPN.49-50 NCS measures the rate at which an electrical impulse travels through nerves, such as the sural sensory, peroneal motor, and tibial nerves. Two electrode patches are placed on the nerve being testing with one electrode stimulating the nerve and the other measuring the electrical impulse. Given NCS findings are complex, data are interpreted by health professionals with requisite advanced training. 3
Conducted from 2015-present, the literature search on NCS yielded 10 studies that revealed technological advances (see Table 2).51-60 In particular, a point-of-care device (POCD), DPN-Check (Neurometrix, Waltham, MA, USA), was identified, which rapidly reproduces part of the NCS (sural nerve conduction).61 Overall, studies reveal DPN-Check may be a highly promising automated technology while allowing increased access to DSPN testing and limited clinician technological expertise in its application.51-60 DPN-Check is a handheld device placed on the lateral aspect of the lower extremity (posterior to the lateral malleolus), following the same measurement principles as standard NCS. 61 Sampling T1D and T2D populations, studies tend to report good to excellent sensitivities (80%-96%) and specificities (80%-97%) for DPN-Check in detecting DSPN and its severity (see Table 2).51,52,55-59 In comparing NCS to DPN-Check, data reveal intraclass correlation coefficients are excellent overall (intrarater reliability: velocity 0.77, amplitude 0.81; interrater reliability: velocity 0.97, amplitude 0.83). 60
Table 2.
Point-of-Care Sural Nerve Conduction.
| Author(s) & study aim | Study population & design | Study outcomes & implications (incorrrect font) |
|---|---|---|
| Binns-Hall et al.
51
-Evaluate the feasibility of a one-stop microvascular screening service for early diagnosis of diabetic DPN, painful DPN, & the at-risk diabetic foot |
N = 236 M = 63.5 ± 14.1 yrs/age n = 231 T2D n = 5 T1D n = 84 +DPN n = 69 −DPN n = 83 unclassified Cross-sectional |
• Area under ROC curve for DPN-Check (Neurometrix, Waltham,
MA, USA) SNAP (threshold ≤ 4.3 µV) & SNCV (threshold ≤
46.3 m/s) was 0.84 & 0.81; Youden Index was 0.52 &
0.52, respectively • DPN-Check SNAP & SNCV had a sensitivity of 84% & 72% & specificity of 68% & 80%, respectively • DPN-Check may be useful screening device for identifying diabetic DPN with respectable performance values |
| Chatzikosma et al.
52
-Evaluate the utility of an automated NCS of the sural nerve with a new portable device for the diagnosis of DPN |
N = 160 n = 114 T2D n = 46 HCs M = 64.6 ± 8.6 yrs/age Cross-sectional |
• DPN-Check exhibited 90% sensitivity, 86% specificity,
& 79% positive predictive value against NDS with a
standard calculated formula • Sural nerve automated NCS with the DPN-Check device exhibited high sensitivity & specificity for diagnosis of DPN in T2D |
| Hamasaki et al.
53
- Investigate associations between DN & clinical parameters related to the development and progression of DN by using DPN-Check |
N = 740,
Japanese n= 18 T1D n = 722 T2D M = 65.6 ± 2.0 yrs/age Retrospective observational study |
• DPN-Check (HDN-1000, Omron, Tokyo, Japan) sensitivity
& specificity, with ankle reflex as reference, were 81%
& 46%, respectively • DPN-Check had high sensitivity & poor specificity in this study, suggesting the POCD may rule out DN if the test is negative but may not be suitable for a definitive diagnosis of DN in Japanese patients |
| Hirayasu et al.
54
- Clarify Japanese normal limits of nerve Amp & CV by DPN-Check (Investigation I); examine validity of DPN-Check to identify DSPN (Investigation II) |
Investigation I: N = 527 USA – data from DPN-Check database M = 48.3 ± 18.5 age/yrs N = 463, Japanese, −DM, −DPN M = 60.8 ± 9.8 age/yrs Investigation II: N = 92, Japanese, +DM M = 65.7 ± 7.0 age/yrs Cross-sectional |
• I. With DPN-Check (HDN-1000, Omron, Tokyo, Japan)
assessments, cut-off values for normal limits of Amp &
CV for Japanese participants identified by JRF; JRF normal
limits higher than those identified for USRF (USRF provided
by Neurometrix) • II. Using DPN-Check, prevalence of NCA1 (1or more abnormal value of Amp & CV) was 25.0% (JFR) & 19.6% (USRF) for Japanese sample with +DM • II. Using DPN-Check, prevalence of NCA2 (2 abnormal values of both Amp & CV) was 6.5% (JFR) & 4.4% (USRF) for Japanese sample with +DM • II. DPN-Check detected ‘probable DSPN’ (NCA1) with sensitivities & specificities of 85% & 86% (JFR) & 71% & 90% (USRF), respectively, for Japanese sample with +DM • II. DPN-Check detected ‘probable DSPN’ (NCA2) with sensitivities & specificities of 43% & 100% (JFR) & 29% & 100% (USRF), respectively, in Japanese sample with +DM • II. AUC of JRF (0.89) was larger than AUC of USRF to detect ‘probable DSPN’ (0.82) in Japanese sample with +DM • A significant difference in normal limits of nerve conduction parameters by DPN-Check between Japanese & USA individuals was observed |
| Kamiya et al.
55
-Validate a novel diagnostic method for DPN using a point-of-care nerve conduction device as an alternative way of diagnosis using a standard EMGS |
N = 375 T2D,
Japanese n = 267 +DPN n = 108 −DPN M = 61.9 ± 14.6 yrs/age Cross-sectional |
• A multiple regression model to predict DPN severity was
generated from the following data: DPN-Check values, age in
yrs, & DPN severity (estimated severity MBC; MBC stage 0
= no DPN, MBC stage 1 = mild DPN, MBC stage 2 =
moderate-to-severe DPN • Using ROC curve analysis, an optimal cutoff value of 1.31of eMBC (categorizing stage 2 vs stage 0 or 1 DPN) was identified with excellent discriminative power (AUROC 0.87); sensitivity was 70.1%, specificity was 87.7%, & positive predictive value was 83.0% • Nerve conduction parameters in the sural nerve acquired by DPN-Check successfully predicted severity of DPN • DPN-Check well predicts DPN & may provide comprehensive, sequential management of diabetic complications in the future |
| Kural et al.
56
-Validate a rapid, accessible method for diagnosing DPN in a large T2D cohort |
N = 168 T2D n = 45 +DPN Med = 71.5 yrs/age IQR: 67.2–75.9 n = 123 −DPN Med = 69.4 yrs/age IQR: 64.9–74.6 Cross-sectional |
• DPN-Check performance (against standard NCS sum-scores),
using ROC curves, revealed a good sensitivity &
specificity for DPN-Check amplitudes with AUCs >0.8,
sural nerve CVs showed moderate to good sensitivity &
specificity with AUCs between 0.7-0.8 • Against NCS, performance of DPN-Check (abnormal or normal), by mean values of amplitudes & CVs of 1 bilateral measure, showed a sensitivity of 82%, specificity of 85%, & PPV of 67%; with up to 3 bilateral measures, a sensitivity of 78%, specificity of 89%, & PPV of 71% was observed against NCS • DPN-Check values were underestimated compared with NCS • DPN-Check, a POCD, is a suitable screening tool for detection of DPN while patients with abnormal or borderline results should undergo conventional NCS |
| Papanas et al.
57
-Examine diagnostic performance of the portable DPN-Check for automated measurement of SNC in diagnosis of DPN |
N = 53, T1D M = 36.9 yrs/age Cross-sectional |
• DPN-Check revealed 96% sensitivity, 93% specificity, &
92% positive predictive value in detecting DPN with NDS
(threshold ≥ 3) as reference • DPN-Check yields high sensitivity, specificity, & positive predictive value for diagnosis of DPN in T1D |
| Scarr et al.
58
Evaluate validity of a POCD as a proxy for standard NCS in older adults (>50 yrs/age) with T1D |
N = 139 n = 68 T1D M = 66 ± 8 yrs/age n = 71 Sex- & age- matched controls M = 65 ± 8 yrs/age Cross-sectional |
• ROC curves generated to obtain threshold values using
DPN-Check (POCD) to identify abnormal age-adjusted standard
NCS values • SN AMPPOCD of ≤6μV had 80% sensitivity & 80% specificity for identifying abnormal SN AMPNCS, while a CVPOCD of ≤44 m/s had 81% sensitivity & 82% specificity • Using the derived AMPPOCD & CVPOCD thresholds, diagnostic POCD performance was evaluated for detection of polyneuropathy based on a modified Toronto consensus. ROC curve analysis showed that abnormality in: (1) either AMPPOCD or CVPOCD, overall, had good sensitivity (86%) & specificity (79%); & (2) both AMPPOCD & CVPOCD had a fairly adequate sensitivity (66%) & excellent specificity (97%) for detecting polyneuropathy • POCD has strong agreement with reference standard NCS values & diagnostic accuracy for identification of polyneuropathy in a high-risk group |
| Sharma et al.
59
-Evaluate a POCD to evaluate the nerve conduction device for detection of DPN & compare with LDIFLARE technique |
N = 242 n = 162 +DM n = 60 −DPN n = 38 mild DPN n = 46 moderate DPN n = 18 severe DPN M= 47.96 ± 13.98 age/yrs n = 80 HCs M= 39.67 ± 15.17 age/yrs Cross-sectional |
• Highly significant relationship between POCD &
LDIFLARE technique in detection of SNCV observed with
similar results shown for SNAP • In HCs & +DM group, DPN-Check or PCOD (SNCV & SNAP) significantly related to LDIFLARE technique; significance also found in all categories of DPN • ROC curves for POCD outcomes revealed SNCV AUC 0.90, 0.74, 0.81, 0.91 for -DPN, mild DPN, moderate DPN, & severe DPN, respectively; & SNAP AUC 0.87, 0.70, 0.80, 0.87 for -DPN, mild DPN, moderate DPN, & severe DPN, respectively • Findings indicate that POCD, irrespective of stage of neuropathy, can detect the presence of neuropathy with good to high sensitivity & specificity |
| Shibata et al.
60
-Examine reliability & validity of nerve conduction parameters acquired by DPN-Check |
N = 57 n = 1 T1D n = 56 T2D n = 16 +DPN M = 63.9 ± 11.0 yrs/age n = 26 −DPN M = 53.2 ± 13.7 yrs/age |
• Bland–Altman plots revealed agreement of values with good
correlations between standard EMGS and DPN-Check parameters
although DPN-Check produced higher values than
EMGS • Using ROC analysis, AUC of amplitudes by DPN-Check (0.70) & standard EMGS (0.72) revealed moderate accuracy • Threshold values with maximized accuracy were ≤6 μV for DPN-Check (sensitivity 86.5%, specificity 43.8%) & ≤3 μV for standard EMGS (sensitivity 96.2%, specificity 40.6%) • Using ROC analysis, the AUC of CV by POCD (0.62) & standard EMGS (0.58) showed low diagnostic accuracy • Threshold value with maximum accuracy was ≤44 m/s (sensitivity 71.2%, specificity 53.6%) for values by EMGS; threshold values for DPN-Check could not be ascertained • Intraclass correlation coefficients were excellent (intrarater: velocity 0.77, amplitude 0.81; interrater: velocity 0.97, amplitude 0.83) • POCD has excellent reproducibility & good agreement with standard EMGS & may be useful to evaluate DPN |
Abbreviations: Amp, amplitude; AMP, amplitude potential; AUC, area under the curve; AUROC, area under the receiver operator characteristic; CV, conduction velocity; DM, diabetes mellitus; DN, diabetic neuropathy; DPN, diabetic peripheral neuropathy or diabetic polyneuropathy; DSPN, diabetic symmetric sensorimotor polyneuropathy; eMBC, estimated severity in modified Baba classification; EMGS, electromyography study; HC, healthy control; IQR, interquartile range; JRF, Japanese regression formulas; LDIFLARE, laser doppler image flare; M, mean; Med, median, m/s, meter per second; MBC, modified Baba classification; NCA, nerve conduction abnormality; NCS, nerve conduction study; NDS, neuropathy disability score; POCD, point-of-care-device; ROC, receiver operator characteristic; SN, sural nerve; SNAP, sensory nerve conduction potential; SNC, sural nerve conduction; SNCV, sensory nerve conduction velocity; T1D, type 1 diabetes; T2D, type 2 diabetes; USRF, regression formulas of individuals from USA; µV, microvolts; yrs, years
Among Japanese and possibly other East Asian populations, review findings indicate US normative ranges for DPN-Check parameters, particularly sural nerve action potentional amplitude, may not be valid. Hirayasu et al. propose a promising Japanense regression formula to promote accurate DSPN assessment with DPN-Check. 54
Small-Fiber DSPN Diagnostic Testing
Intraepidermal Nerve Fiber Density Testing (IENFD)
IENFD testing is the gold standard for diagnosing small-fiber DSPN. IENFD is an invasive procedure wherein a punch biopsy is obtained from the distal leg for quantification of small-fiber densities. Small-fiber DSPN is identified by reduced intraepidermal nerve fiber density or morphological changes.62,63 IENFD has variable sensitivities and specificities, ranging, for example, from 78%-88% and 64%-90%, respectively. 62 Varied diagnostic accuracy may be partially attributed to IENFD cutoff and reference values used in assessing for small-fiber neuropathy as well as specific location of the biopsy site. Normative values require adjustment for age and gender or results may likely be biased.62,64-65 A number of studies suggest IENFD values are affected by ethnicity, which may also bias results as revealed in Asian populations.66-68
Quantitative Sensory Testing (QST)
QST assesses large- and small-fiber function. With respect to small-fiber function, QST technologies assess the thresholds at which small nerve fibers detect pain and thermal sensations. In suspected DSPN, a thermode is placed on the foot and/or hand areas with pain and/or sensory deficits. Patients are instructed to quickly respond to changes in specified sensations, which yield mean peak values or thresholds for pain and thermal sensations. QST may thus be conceived as a psychophysical test wherein sensation stimuli are controlled and stimuli responses are dependent on the active participation of the patient. QST is thus susceptible to bias related to patient motivation, attention, or cognitive impairment. Interpretation of QST findings requires attention to the clinical context while data is best compared to normative data stratified for age, gender, and body site. QST is not recommended as a stand-alone diagnostic test given variable study results and testing methodologies.69-70 Compared to IENFD and clincial examination, published reports document the sensitivity of thermal testing ranges from 36%-100% for small-fiber neuropathy although diagnostic criteria for the condition were not uniform across studies. 70
Since 2015, the literature search identified thirteen studies comparing, for instance, different QST devices and evaluating new methodologies or technological approaches (see Table 3).71-83 NerveCheck (Phi Med Europe SL, Barcelona, Spain), in particular, reveals technological advancement of traditional QST. NerveCheck is a small, portable device, and yields rapid results. NerveCheck assesses vibration (VPT), cold (CPT) and warm (WPT) perception thresholds, and heat pain threshold. NerveCheck has demonstrated an intraclass agreement for VPT (large-fiber vibration testing), CPT (small-fiber thermal testing) and WPT (small-fiber thermal testing) at 0.79, 0.86, and 0.71, respectively. 82 The diagnostic accuracy of NerveCheck in detecting sensory loss, based on the area under the curve (AUC), is reportedly 0.70 for CPT and 0.69 for WPT with IENFD as the reference. 83
Table 3.
Quantitative Sensory Testing (QST).
| Author(s) & study aim | Study population & design | Study outcomes & implications |
|---|---|---|
| Abraham et al.
71
-Explore utility of SFN testing in patients with a clinical presentation suggesting SFN |
N = 123 M = 55 ± 16 yrs/age n = 32 +DM Retrospective study |
• Using the portable TSA-II NeuroSensory Analyzer (Medoc,
Ramat Yishai, Isarel), participants with clinically
suggestive SFN plus DM had significantly elevated vs. normal
cooling thresholds (37%, 19%, respectively) & heat
thresholds (67%, 22%, respectively) • Participants with clinically suggestive SFN plus DM had significantly reduced (37%) vs. normal (16%) LDIFlare (measure of SFN) values • Using Cohen’s kappa coefficient, agreement between the different small-fiber testing modalities were significant; agreement was moderate between LDIFlare & cold testing thresholds (k = 0.52), fair between cooling & heat testing thresholds (k = 0.22), & poor between LDIFlare & heat testing thresholds (k = 0.11) for the entire sample |
| Alam et al.
72
- Compare diagnostic capability of CCM against skin biopsy & QST in patients with DSPN |
N = 88 n = 30 T1D, -DSPN M = 38.8 ± 12.5 n = 31 T1D, +DSPN M = 53.3 ± 11.9 n = 27 HCs M = 41.0 ± 14.9 Cross-sectional |
• ROC curve analyses used to define Wilcoxon estimate of
AUROC & optimal cutoff values with associated
sensitivity & specificity for CST & WST (TSA-II
NeuroSensory Analyzer) • UROC for CST was 0.76 with an optimal cutoff of 25°C; sensitivity of 57% & specificity of 89% for diagnosing DSPN • AUROC for WST was 0.74 with an optimal cutoff of 38°C; sensitivity of 86% & specificity of 64% for diagnosing DSPN • AUROCs revealed moderate accuracy of CST & WST parameters |
| Azmi, et al.
73
-Assess whether baseline and follow-up measures of neuropathy, particularly small-fiber neuropathy, relate to changes in glucose tolerance over 3 yrs |
N = 47 n = 30 IGT M = 60 ± 2.1 n = 17 Controls M = 62.3 ± 1.8 Longitudinal (3-yr FU) |
• FU: CT & WT (TSA-II NeuroSensory Analyzer) values did
not significantly change for participants who reverted to
NGT, remained with IGT, or developed T2D at 3-yr
FU • Findings suggest CT & WT are not responsive to changes in glucose tolerance status or T2D development |
| Courtin, at al.
74
-Investigate the potential of evaluating not only the threshold but also the slope of the psychometric functions for cold & warm detection |
N = 30 n = 15 T2D M = 55 ± 4 age/yrs n = 15 HCs M = 53 ± 4 age/yrs Cross-sectional |
• Using a Laser Stimulator Device (SIFEC, Ferrières,
Belgium), ROC analysis revealed warm detection thresholds
did not well discriminate between T2D participant &
control groups at the wrist (AUC: 0.65) or foot (AUC:
0.67) • ROC analysis showed the spread of psychometric function for warm detection was also uninformative (AUC wrist: 0.59; AUC foot: 0.50) • Using a Thermal Cutaneous Stimulator (QST.Lab, Strasbourg, France), ROC analysis indicated both CDT (AUC wrist: 0.83; AUC foot: 0.80) & spread of psychometric function for cold detection (AUC wrist: 0.82; AUC foot: 0.84) displayed very good discriminative properties • Including both slope & threshold in ROC analysis, cold detection discrimination performance between T2D participants & HCs was further increased (AUC wrist: 0.89; AUC foot: 0.94) • Combining slope & threshold parameters of cold detection performance may yield better discriminative ability than relying solely on thresholds |
| Dhage, et al.
75
-Assess the longitudinal utility of different measures of neuropathy in patients with diabetes |
N = 38 n = 19 +DM M = 52.5 ± 14.7 yrs/age (baseline) n = 19 HCs M = 47.4 ± 14.2 yrs/age (baseline) Longitudinal cohort study (M = 6.5 yrs FU) |
• At baseline, QST (TSA-II NeuroSensory Analyser) measures
of CPT, WPT, CIP, & WIP did not significantly vary
between DM participants & controls • Compared to baseline, significant decreases in CPT were observed in DM participants at FU • CPT may serve as a biomarker of nerve damage in patients with DM |
| Fabry et al.
76
-Determine diagnostic value of skin biopsy, QST, Q-Sweat, LEP, ESC & AVCT for SFN diagnosis |
N = 245 M = 50.4±15.0 yrs/age n = 24, +DM n = 6, IGT n = 102 +SFN n = 90 -SFN Retrospective study |
• Using the Thermotest (Somedic, Sollentuna, Sweden) device
as the measure of QST, no significant difference was found
between +SFN & -SFN groups • QST or Thermotest had a sensitivity of 72%, specificty of 39% & positive predictive value of 57% for SFN diagnosis • QST found to be most sensitive test for SFN diagnosis relative to IENFD, QSART (Q-Sweat, WR Medical Electronics, Minneapolis, USA), ESC (Sudoscan, Impeto Medical, Paris, France), LEP, & AVCT • Combining QST, IENFD, ESC & LEP yielded a sensitivity of 92%, specifity of 88%, & positive predictive value of 90% for diagnosing SFN |
| Farooqi et al.
77
- Validate the performance of CDT to detect DSP in T2D |
N = 220, +DM M = 63 ±11 yrs/age n = 52 Pre-clinical DSP n = 139 +DSP n = 29 Controls Cross-sectional |
• Using the TSA-II NeuroSensory Analyzer to detect clinical
DSP with CDT, AUCCDT was 0.79, significantly
higher than AUCHRV & AUCLDIFLARE
values; CDT (optimal threshold of ≤22.8°C) had a sensitivity
of 64% & specificity of 83% in identifying clinical DSP
with a positive predictive value of 87% • Using the TSA-II NeuroSensory Analyzer to detect pre-clinical DSP with CDT, AUCCDT was 0.80, significantly higher than AUCHRV & AUCLDIFLARE values; CDT (optimal threshold of ≤27.5°C) had a sensitivity of 83% & specificity of 72% in identifying pre-clinical DSP with a positive predictive value of 95% • CDT revealed good diagnostic performance for detection of clinical & pre-clinical DSP in T2D |
| Ferdousi, et al.
78
-Compare the utility of quantifying corneal nerve loss at the inferior whorl & central cornea to QST & NCS in the diagnosis & assessment of DPN severity |
N = 143 n = 93 +DM n = 51 –DPN M = 57.68 ± 1.6 yrs/age n = 47 Mild DPN M = 60.16 ± 1.7 yrs/age n = 45 Moderate to severe DPN M = 64.1 ± 1.48 yrs/age n = 30 Controls M = 54.51 ± 2.3 yrs/age Cross-sectional |
• ROC curve & Youden Index used to define the optimum
cutoff point for WPT & CPT (TSA-II NeuroSensory
Analyzer); WPT AUC 0.67, sensitivity 50%, & specificity
76%; CPT AUC 0.64, sensitivity 80%, & specificity
47% • CPT was significantly lower in patients with mild (19.52±1.47, p=0.02) and moderate to severe (18.99±1.55, p=0.01) neuropathy compared with controls (25.38±2.06) • WPT was significantly higher in patients with no (41.65±0.6, p=0.01), mild (43.47±0.6, p<0.0001) and moderate to severe (43.62±0.7, p<0.0001) neuropathy compared with controls (38.87±0.9) • While CPT & WPT, overall, had suboptimal performance values, progressive abnormalities in CPT & WPT were observed with increasing severity of DPN |
| Løseth et al.
79
- Evaluate progression of DPN & differences in the spectrum & evolution of large- and small-fiber involvement in patients with T1D & T2D over 5 yrs |
N = 59 n = 35 T1D M = 47.4 ± 12.0 yrs/age at 5 yr FU n = 24 T2D M = 57.8 ± 9.0 yrs/age at 5 yr FU Longitudinal |
• Using Thermotest Type 1 (Somedic AB, Sösdala, Sweden)
device for QST measurement, baseline values of CPT were
elevated at baseline for participants with T1D (4.4 ± 4.4)
& T2D (4.8 ± 3.8) • At 5-yr FU, CPT values increased significantly for participants with T2D (6.7 ± 5.3) but not for those with T1D (5.4 ± 5.3) • Yet, CPT z-scores, calculated to adjust for physiologic effects of age, height, & gender, did not reveal significant increases in CPT values for participants with T2D from baseline to 5-yr FU • Further research is indicated to identify if elevated CPT values are a biomarker for DN progression |
| Pfau et al.
80
-Assess the reliability/validity of “Q-Sense” (portable device) by comparing it with TSA II |
N = 204 n = 83 +DM n = 71 +DNP n = 121 HCs M = 32.9 ± 13.7 age/yrs Cross-sectional |
• Agreement between Q-Sense & TSA II
NeuroSensoryAnalyzer (both portable devices) was excellent
for CDT (ICC = 0.89) & WDT (ICC = 0.90), moderate for
HPT (ICC = 0.53), & poor for CPT (ICC =
0.31) • Sensitivity of Q-Sense to detect cold hypoesthesia was reduced in males >60 years • ROC curves for both devices were calculated, using skin biopsy results (“normal” vs. “pathologic”) as reference measure, & resulting AUROCs were compared; statistical comparisons of AUROCs (related to TSA II & Q-Sense measurements, respectively) were non-significant for CDT, WDT, & TSL, revealing the non-inferiority of the Q-Sense, relative to TSA II, for thermal detection • Q-Sense is not advised to use for CPT thresholds & HPT thresholds should be used with caution. Q-Sense suitable for thermal detection thresholds (cutoff lowered to 18° C) |
| Pritchard et al.
81
-Determine if deficits in CNFL assessed using CCM can predict future onset of DPN |
N = 90 T1D,
–DPN (baseline) 4-yr FU n = 16 +DPN M = 51 ± 14 yrs/age (baseline) |
• DPN developed in 16 participants (18%) after 4
yrs • Participants who developed DPN at 4-yr FU had significantly lower baseline values of CST & CPT (TSA-II NeuroSensory Analyzer) & significantly higher baseline values of WST & WPT (TSA-II NeuroSensory Analyzer) relative to those that did not develop DPN • For CST, AUROC was 0.77; sensitivity was 88% & specificity was 55% with a CST cutoff of 29.2°C |
|
n = 64 –DPN M = 42 ± 16 yrs/age (baseline) Longitudinal |
• For CPT, AUROC was 0.68; sensitivity was 50% &
specificity was 86% with a CPT cutoff of 0.2°C • For WST, AUROC was 0.71; sensitivity was 56% & specificity was 82% with a WST cutoff of 39.1°C • For WPT, AUROC was 0.68; sensitivity was 56% & specificity was 80% with a WPT cutoff of 49.5°C |
|
| Ponirakis et al.
82
-Establish the reproducibility & diagnostic validity of NerveCheck for detecting DPN |
N = 186 n = 130 +DM Med = 55.7 yrs/age IQR: 42.9–66.1 n = 74 +DM n = 28 +DPN n = 46 –DPN n = 56 controls Med = 43.6 yrs/age IQR: 35.7–53.1 Longitudinal |
• Controls & DM participants tested 2 times (test-retest
intervals: 1-8 weeks) with identification of intraclass
agreement for NerveCheck (Phi Med Europe SL, Barcelona,
Spain) CPT (0.86) & WPT (0.71) • Using ROC curve analysis, diagnostic accuracy for detecting DPN, against the TSA-II NeuroSensory. Analyzer, revealed AUCs for CPT (0.79) & WPT (0.72) • CPT sensitivity was 89% & specificity was 67%; WPT sensitivity was 75% & specificity was 66% • Findings indicate NerveCheck has good reproducibility & moderate diagnostic accuracy for detecting DPN |
| Ponirakis et al.
83
-Examine diagnostic performance of NerveCheck |
N = 144 n = 74 +DM n = 33 +DPN M = 64.1±1.79 yrs/age n = 41 –DPN M = 44.3 ± 2.19 yrs/age n = 70 Controls M = 41.8 ± 1.63 yrs/age Cross-sectional |
• ROC curve analysis used to compare diagnostic accuracy of
CPT & WPT against IENFD; AUC of CPT was 0.70 & WPT
was 0.69; CPT sensitivity was 53% & specificity was 82%;
WPT sensitivity was 56% & specificity was
81% • Diagnostic accuracy of NerveCheck is poor to good with reference to IENFD |
Abbreviations: ACVT, autonomic cardiovascular tests; AUC, area under the curve; AUROC, area under receiver operator characteristic; CCM, corneal confocal microscopy; CDT, cold detection threshold; CIP, cold induced pain; CPT, cold pain threshold or cold perception threshold; CST, cold sensation threshold; CT, cold threshold; DM, diabetes mellitus; DNP, diabetic neuropathy; DPN, diabetic peripheral neuropathy; DSP, diabetic sensorimotor polyneuropathy; DSPN, diabetic symmetrical peripheral neuropathy; ESC, electrochemical skin conductance; FU, follow-up; HC, healthy control; HPT, heat pain threshold; HRV, heart rate variability; ICC, intraclass correlation coefficient; IENFD, intraepidermal nerve fiber density; IGT, impaired glucose tolerance; IQR, interquartile range; LDIFLARE, laser doppler imager flare; LEP, laser evoked potentials; M, mean; Med, median; NCS, nerve conduction study; NGT, normal glucose tolerance; QST, quantitative sensory testing; ROC, receiver operator characteristic; SFN, small fiber neuropathy; SNAP, sensory nerve action potential; SNCV, sural nerve conduction velocity; T1D, type 1 diabetes; T2D, type 2 diabetes; TSA, Thermal Sensory Analyzer; TSL, thermal sensory limen; VPT, vibration perception threshold; WDT, warm detection threshold; WIP, warm induced pain; WPT, warm pain or perception threshold; WST, warm sensation threshold; WT, warm threshold; yrs, years.
More well established, the TSA NeuroSensory Analyzer (Medoc, Ramat Yishai, Israel) has advanced over the years with development of a precision enhanced, small portable device for QST; i.e., the TSA-II NeuroSensory Analyzer. Using the TSA-II, Farooqi and colleagues advanced the science of QST by validating cooling detection thresholds (CDT) to detect DSPN in a sample of participants with T2D. While not assessed against IENFD, CDT was found to outperform other measures of DSPN. CDT had acceptable discriminatory ability for detecting clinical DSPN (AUC 0.79) with a sensitivity of 64% and specificity of 83%. Good discriminatory performance for pre-clinical DSPN (AUC 0.80), with a sensitivity of 83% and specificity of 72%, was also observed. 77
Contact Heat Evoked Potentials (CHEPs)
CHEPs examine the integrity of Aδ and C fibers by measuring cerebral responses to thermal pain stimuli. A heat evoked potential stimulator is used with a thermode placed on the lower extremity. A heat pulse is delivered from a baseline to increasing safe, warm temperatures. Heat stimuli are repeatedly applied to the same lower extremity area. Concurrently, CHEPs are recorded with electrodes placed on the vertex of the head. An evoked potential system generates waveform tracings reflecting responses in the cerebral cortex.3,84-86
The literature search identified one study meeting eligibility criteria for inclusion in the review of CHEPs. 87 The study sample was comprised of 255 adults with 188 diagnosed with neuropathy (38.5% had diabetes) and 57 controls. Tests of equality were performed to assess the area under the receiver operator characteristic (AUROC) curves with respect to the diagnostic accuracy of CHEPs (Medoc, Ramat Yishai, Israel) versus QST (TSA NeuroSensory Analyzer, Medoc, Ramat Yishai, Israel). Results revealed that CHEP amplitudes (AUC 0.79) had significantly greater accuracy realtive to the QST warm (AUC 0.71) but not cold (AUC 0.72) threshold for diagnosis of small-fiber neuropathy. Using a cutoff of 29.1 mV, CHEPs had a diagnostic sensitivity of 80.7% and specificity of 68.8% for DSPN. 87
Corneal Confocal Microscopy (CCM)
The cornea is abundantly innervated by small thinly myelinated Aδ and unmyelinated C fibers, and thereby uniquely provides indirect assessment and quantification of small-fiber DSPN. CCM has emerged as a noninvasive, valid, reliable technique for assessing small-fiber DSPN.88-90 CCM involves visualization, via image acquisition, of corneal microstructures. Analysis of corneal images allows for detection of small nerve fiber loss with assessment of corneal nerve fiber density (CNFD), nerve branch density (CNBD), and nerve fiber length (CNFL), among other corneal parameters. 91
Since 2015, the literature search identified 32 studies meeting study criteria, including technological advancements of CCM (see Table 4).72-73,75,78,81,92-118 Advancing CCM technology, automated quantification of corneal images was frequently examined with respect to its accuracy, validity, and reliability (reproducibility and repeatability) with very promising results reported.72,93-98,104,105,107,109-110,113-115,118. Compared to traditional manual methods, automated methods were found to be relatively equivalent with overall high levels of agreement.92-94,96-98,107,110,113-115,118 With manual or automated methods for image quantification, CCM parameters tended to have moderate to excellent accuracy (as assessed by AUROC or AUC values) in detecting DSPN, including its levels of severity. However, sensitivies and specifities for CCM parameters tended to be highly variable and not uniformly adequate across studies.72-78,95-98,103,110-112,116-117 Beginning evidence suggests CCM and IENFD may have roughly comparable performance in detecting DSPN although additional research is warranted.72,96
Table 4.
Corneal Confocal Microscopy (CCM)
| Author(s) & study aim | Study population & design | Study outcomes & implications |
|---|---|---|
| Alam et al.
72
- Compare diagnostic capability of CCM against IENFD & QST in patients with DSPN |
N = 88 n = 30 T1D, -DSPN M = 38.8 ± 12.5 n = 31 T1D, +DSPN M = 53.3 ± 11.9 n = 27 HCs M = 41.0 ± 14.9 Cross-sectional |
• AUC for CNFD was 0.81 with an optimal cutoff of 25.0
no/mm2, sensitivity of 77% & specificity
of 79% for diagnosis of DSPN • AUC for CNBD was 0.67 with an optimal cutoff of 36.5 no/mm2, sensitivity of 58% & specificity of 79% for diagnosis of DSPN • AUC for CNFL was 0.74 with an optimal cutoff of 16.8 mm/mm2, sensitivity of 61% & specificity of 80% for diagnosis of DSPN • AUC for IENFD was 0.73 with an optimal cutoff of 4.5 fibers/mm, sensitivity of 61% & specificity of 86% for diagnosis of DSPN • CCM is a non-invasive method, with respectable performance, to identify small nerve fiber pathology & diagnose DSPN |
| Al-Fahdawi et al.
92
-Propose a robust, fast & fully automatic nerve segmentation & morphometric parameter quantification system for CCM images |
N = 20, 498
images n = 12 +DM M = 58 ± 10 yrs/age n = 4 -DPN n = 5 mild DPN n = 3 moderate DPN n = 8 HCs M = 54 ± 7 yrs/age Cross-sectional |
• For manually & automatically traced nerves, average
tortuosity was 8.27 ± 7.18 & 6.7 ± 5.60, respectively,
for controls; 20.11 ± 19.04 & 13.9 ± 12.79,
respectively, for -DPN group; 37.52 ± 36.41 & 29.32 ±
28.36, respectively, for mild DPN group; & 40.45 ± 39.30
& 51.76 ± 50.64, respectively, for moderate DPN group
• For manually & automatically traced nerves, average nerve length was 60.92 mm & 61.22 mm, respectively, for controls; 58.49 mm & 56.87 mm, respectively, for -DPN group; 57.08 mm & 56.87 mm, respectively, for mild DPN group; & 57.08 mm & 56.63 mm, respectively, for moderate DPN group • Across manually & automatically traced nerve methods, comparable results found for average nerve length values although average tortuosity values appear less consistent • Both manually & automatically traced nerve methods reveal increasing nerve tortuosity & decreased nerve length according to severity of DPN |
| Al Rashah et al.
93
-Assess repeatability CNM rate measurement in individuals with or without neuropathy |
N = 14 M = 51.9 ± 13.8 yrs/age n = 5 +DM, +DN n = 5 +DM, -DN n = 4 HCs Longitudinal (3-week FU) |
• Within & between observer repeatability of CNM within
4 different zones & CNM rate of the vertical section of
the wide-field montage was outstanding with ICCs of 0.99
& 0.99, respectively • Repeatability of CNM rate of the vertical section of the wide-field montage within observers, when using semi-automated & fully automated image montaging software, was also outstanding with an ICC of 0.96 • With a laser-scanning CCM, CNM rate measurement shows impressive repeatability for within & between observers & when using manual, semi-automated, & fully automated image montaging |
| Azmi, et al.
73
-Assess whether baseline and follow-up measures of neuropathy, particularly small-fiber neuropathy, relate to changes in glucose tolerance over 3 yrs |
N = 47 n = 30 IGT M = 60 ± 2.1 yrs/age n = 17 Controls M = 62.3 ± 1.8 yrs/age Longitudinal (3-year FU) |
• FU: 10 IGT participants developed T2D & had
significantly lower CNFD, CNBD, & CNFL at baseline
compared to controls; IGT participants who developed T2D
also had a further significant reduction in CNFL, IENFD,
& MDL • FU: 15 participants had no change in IGT status & 5 participants returned to NGT with no significant baseline abnormality on CCM or IENFD • FU: IGT participants (n=15) showed a significant decrease in IENFD but no change in CCM • FU: Participants returning to NGT (n=5) showed a significant increase in CNFD, CNBD, & CNFL, but a significant decrease in IENFD • CCM may be an early marker of small-fiber neuropathy & allow for risk stratification of individuals with IGT likely to progress to T2D |
| Batawi et al.
94
- Study SCNP parameters by IVCCM using a new software technology |
N = 29 M = 46 ± 20 yrs/age n = 22 +DM Cross-sectional |
• Inter-operator reproducibility & intra-operator
reproducibility ICCs, respectively, were: (1) NFLD 0.97
& 0.97; (2) NFL 0.97 & 0.97; (3) NF 0.90 & 0.93;
(4) NT 0.97 & 0.96; (5) NBi 0.83 & 0.87; (6) NBr
0.81 & 0.87; (7) BD 0.92 & 0.95; (8) NBe 0.95 &
0.97; (9) NFT 0.73 & 0.88 • Semi-automated corneal nerve analysis showed excellent precision or reproducibility for evaluation of SCNP parameters with CCM |
| Brines et al.
95
-Using an automated methodology, compare sensitivity & specificity of NFD, NBD, NFL, & NFA for discriminating participants with neuropathy from NCs |
N = 129 n = 21 -DN M = 37.1 ± 16.5 yrs/age n = 21 mild DN M = 55.9 ± 11.0 yrs/age n = 19 moderate DN M = 59.0 ±11.3 yrs/age n = 20 severe DN M = 57.0 ± 14.6 yrs/age n = 48 NCs M = 46.2 ± 16.9 yrs/age Cross-sectional |
• In participants with no or mild DN, relative to NCs,
respectively, AUROC curves for NFD, NFB, NFL, NFA WxL, &
NFA FIJI (≥0.70 – <0.85 range) were fair to
good • In participants with moderate to severe DN, relative to NCs, respectively, AUROC curves for NFD, NBD, NFL, & NFA WxL (>0.80 – <1.0 range) were good to excellent • With DM participants collapsed into 1 group, cutoff points for discrimination of participants having DN from NCs were calculated from ROC curves; DM group cut point for NFD was 23.4 fibers/mm2 with a sensitivity of 90% & specificity of 69%; DM group cut point for NBD was 23.4 branches/mm2 with a sensitivity of 86% & specificity of 71%; DM group cut point for NFL was 12.3 mm/mm2 with a sensitivity of 96% & specificity of 68%; DM group cut point for NFA FIJI was 19,128 μm2/mm2 with a sensitivity of 94% & specificity of 59% • Overall, automated methodology revealed good to excellent diagnostic performance in detecting moderate to severe DN & impressive ability to identify true positives for DN in DM participants |
| Chen, et al.
96
-Determine the diagnostic performance of CCM (manual & automated methods) & IENFD for DSPN |
N = 89 n = 17 T1D, +DSPN M = 59 ± 11 yrs/age n = 46 T1D, -DSPN M = 44 ± 13 yrs/age n = 26 CG M = 44 ± 15 yrs/age Cross-sectional |
• AUCs: CNFD manual 0.82 & automated 0.80; CNFD manual
S/S = 76% at EERP; CNFD manual S/S = 82%/71% at threshold of
24.0 (M ± 2 SDs); CNFD automated S/S = 70% at EERP; CNFD
automated S/S = 60%/83% at threshold of 15.5 (M ± 2
SDs) • AUCs: CNFL manual 0.70 & automated 0.77; CNFL manual S/S = 71% at EERP; CNFL manual S/S = 59%/74% at threshold of 16.5 (M ± 2 SDs); CNFL automated S/S = 70% at EERP; CNFL automated S/S = 59%/80% at threshold of 10.5 (M ± 2 SDs) • AUCs: CNBD manual 0.59 & automated 0.70; CNBD manual S/S = 53% at EERP; CNBD manual S/S = 17%/96% at threshold of 15.0 (M ± 2 SDs); CNBD automated S/S = 59% at EERP; CNBD automated S/S = 29%/98% at threshold of 4.0 (M ± 2 SDs) • AUC of IENFD = 0.66; IENFD S/S = 65% at EERP; IENFD S/S = 53%/76% at threshold of 3.3 (M ± 2 SDs) • Overall, performance between CCM (manual & automated) & IENFD comparable |
| Chen, et al.
97
-Evaluate an automated software tool for nerve-fiber detection & quantification in CCM images, combining sensitive nerve-fiber detection with morphological descriptors |
N = 176; 888
images n = 63 T1D, +DSPN n = 29 T1D, -DSPN n = 84 Controls Cross-sectional |
• AUCs of ACNFD, ACNFL, & ACNBD were 0.76, 0.76, 0.68,
respectively; ACNFD S/S = 65%, ACNFL S/S = 62%, & ACNBD
S/S = 58% at EEP for discriminating +DSPN &
-DSPN • AUCs of MCFND, MCNFL, & ACNBD were 0.79, 0.71, & 0.61, respectively; MCNFD S/S = 72%, MCNFL S/S = 66% & MCNBD S/S = 59% at EEP for discriminating +DSPN & -DSPN • AUCs of combined CCM automated & manual features were 0.74 & 0.78, respectively; CCM automated S/S = 71%; CCM manual S/S = 68% for discriminating +DSPN & -DSPN • Performance of automated quantification compared to manual quantification of corneal nerves in CCM images is relatively equivalent • Automated quantification provides a sensitive tool for identification of DSPN while improving speed & repeatability |
| Chen, et al.
98
-Evaluate the performance of previously established CCM parameters to a novel automated measure of corneal nerve complexity (ACNFrD) |
N = 176 n = 84 AMCs M = 46 ± 15 yrs/age n = 29 T1D, +DSPN M = 63 ± 12 yrs/age n = 63 T1D, -DSPN M = 44 ± 15 yrs/age Cross-sectional |
• AUROC curve for ACNFD was 0.77, ACNFL was 0.74, ACNBD was
0.69, & ACNFrD was 0.74; ACNFD S/S = 65% at EER; S/S =
63%/79% at threshold of 15.1(M ± 2 SDs); ACNFL S/S = 62% at
EER; S/S = 62%/83% at threshold of 10.2 (M ± 2 SDs); ACNBD
S/S = 58% at EER; S/S = 24%/98% at threshold of 3.3 (M ± 2
SDs); ACNFrD S/S = 65% at EER; S/S = 61%/78% at threshold of
1.45 (M ± 2 SDs) • AUROC curves were 0.79 for MCNFD, 0.71 for MCNFL, & 0.61 for MCNBD; MCNFD S/S = 72% at EER; S/S = 79%/71% at threshold of 23.8 (M ± 2 SDs); MCNFL S/S = 65% at EER; S/S = 55%/86% at threshold of 14.9 (M ± 2 SDs); MCNBD S/S = 59% at EER; S/S = 17%/96% at threshold of 13.8 (M ± 2 SDs) • ACNFrD performance comparable to automated & manual CNFD, CNBD, & CNFL in diagnosing patients with +DSPN or -DSPN • Automated & manual methods show good equivalency in accuracy |
| Dehghani et al.
99
- Determine alterations in CSNP over 4 yrs using IVCCM |
N = 108 T1D, -PN (baseline) M = 43.9 ± 15.7 age/yrs (baseline) Longitudinal |
• At 4-yr FU, mean CNFD (no./mm2, 19.6 ± 6.9) was
marginally significantly increased compared to baseline
(18.3 ± 7.1) • At 4-yr FU, mean CNBD (no./mm2, 29.1 ± 19.6) was significantly increased relative to baseline (24.2 ± 17.4) • At 4-yr FU, mean CNFL (mm/mm2, 16.3 ± 3.7) did not significantly change from baseline (16.0 ± 3.8) • IVCCM may be useful for monitoring subclinical alterations in CSNP in T1D |
| Dell’Omo, et al.
100
-Measure the thickness & length of corneal nerves & peri-papillary RNFL thickness in patients recently diagnosed with T2D |
N = 44 n = 22 new onset T2D M = 50.6 ± 6.74 age/yrs n = 22 HCs M = 50.8 ± 4.26 age/yrs Cross-sectional |
• Evaluated by CCM, CNT & CNL were significantly lower
in participants with new onset T2D than
controls • Using SD-OCT, RNFL thickness in temporal quadrant was significantly lower in patients with T2D relative to controls • ICC values for intra-observer and inter-observer repeatability for CNT were 0.76 & 0.78 & for CNL 0.71 & 0.74, respectively • Reductions observed in CNL, CNT, & RNFL thickness suggest that CCM & SD-OCT may detect early markers of neuropathy in patients recently diagnosed with T2D • ICC values revealed good reliability |
| Dhage, et al.
75
-Assess the longitudinal utility of different measures of neuropathy in patients with diabetes |
N = 38 n = 19 +DM M = 52.5 ± 14.7 yrs/age (baseline) n = 19 HCs M = 47.4 ± 14.2 yrs/age (baseline) Longitudinal cohort study (MFU = 6.5 yrs) |
• At baseline, CNFD, CNBD & CNFL significantly reduced
in DM participants vs. controls • Compared to baseline, CNFD, CNBD & CNFL significantly decreased in DM participants at FU • At baseline, NSP & NDS scores significantly higher & IENFD significantly lower in DM participants vs. controls • Compared to baseline, NSP & NDS scores significantly increased & IENFD significantly decreased in DM participants at FU • Change in CNFD, CNBD, & CNFL significantly correlated with change in IENFD • CCM, a rapid, non-invasive & reproducible ophthalmic imaging technique to objectively quantify small-fiber damage in DN, was found to longitudinally identify progressive nerve damage |
| Edwards et al.
101
-Demonstrate DN development & progression in T1D individuals |
N = 38, T1D, -DN (baseline) Longitudinal cohort study (4-yr FU) |
• CNFL identified 7 participants who had unfavorable corneal
nerve changes from baseline to 4-yr FU • CNFL, relative to PNCV, CST, WST, VPT, NDS, & monofilament testing, consistently performed better • CNFL revealed the earliest, most sustained, & highest proportion of abnormal parameters indicative of PN development |
| Ferdousi et al.
102
- Identify longitudinal CNM changes in CC & IW relative to other DN measures |
N = 36 n = 19 age-matched HCs M = 49.47 ± 13.25 yrs/age n = 30 +DM M = 54.08 ± 15.86 yrs/age (baseline) n = 21 T1D n = 9 T2D Longitudinal cohort MFU = 3.6 ± 1.3 |
• In participants with DM, CNBD (mm/mm2) &
CNFL (mm/mm2) were significantly reduced from
baseline (57.72 ± 30.08; 21.77 ± 5.19, respectively) to FU
(44.04 ± 23.69; 15.65 ± 4.7, respectively) • In participants with DM, IWL (mm/mm2) & ANFL (CNFL + IWL/2; mm/mm2) were also significantly reduced from baseline (24.69 ± 8.67; 23.26 ± 5.53, respectively) to FU (14.23 ± 6.13; 15.09 ± 4.48, respectively • Rate of annual decline in CNFL, IWL, & ANFL was significantly higher in patients with DM compared to controls • ICC showed good consistency between the changes per year in CNFL & IWL in participants with DM (ICC = 0.78; 95% confidence interval, 0.56–0.88) |
| Ferdousi, et al.
78
-Compare the utility of quantifying corneal nerve loss at the inferior whorl & central cornea to QST & NCS in the diagnosis & assessment of DPN severity |
N = 143 n = 93 +DM n = 51 -DPN M = 57.68 ± 1.6 yrs/age n = 47 Mild DPN M = 60.16 ± 1.7 yrs/age n = 45 Moderate to severe DPN M = 64.1 ± 1.48 yrs/age |
• ROC curve & Youden index used to define optimum cutoff
points for CNFD, CNBD, CNFL, & IWL; CNFD AUC 0.71,
sensitivity 58%, & specificity 83%; CNBD AUC 0.70,
sensitivity 69%, & specificity 65%; CNFL AUC 0.68,
sensitivity 64%, & specificity 67%; IWL AUC 0.72,
sensitivity 70%, & specificity 65% • CNFD & CNBD were significantly lower in patients with -DPN (26.61 ± 1.05 & 64.07 ± 4.39, respectively) & mild DPN (24.47 ± 1.09 & 58.49 ± 4.76, respectively) compared to controls (33.71 ± 1.3 & 81.52 ± 5.54, respectively); CNFD & CNBD were significantly lower in patients with moderate to severe DPN (22.4 ± 1.14 & 45.60 ± 4.5, respectively) relative to those with -DPN & controls |
|
n = 30 Controls M = 54.51 ± 2.3 yrs/age Cross-sectional |
• CNFL & IWL were significantly lower in patients with
mild (20.84 ± 1.00 & 22.28 ± 1.31, respectively) &
moderate to severe (19.27 ± 1.04 & 19.03 ± 1.36,
respectively) DPN compared to controls (25.07 ± 1.27 &
31.69 ± 1.66, respectively); CNFL & IWL were
significantly lower in patients with moderate to severe DPN
relative to those with -DPN (23.31 ± 0.96 & 24.90 ±
1.26, respectively) • CCM identifies early & progressive corneal nerve loss at the inferior whorl & CC |
|
| Ferdousi, et al.
103
-Assess the diagnostic utility of CCM for DPN & risk factors for corneal nerve loss |
N = 490 n = 149 T1D n = 269 T2D n = 72 HCs Cross-sectional |
• CNFD, CNBD, & CNFL were significantly reduced in
participants with T1D vs. those with T2D • ROC curve analysis for diagnosis DPN showed a very good AUC for CNFD at 0.81 with an optimal cutoff point of 29.40/mm2; sensitivity was 73.5% & specificity was 74.4% • ROC curve analysis for diagnosis DPN showed a reasonable AUC for CNBD at 0.74 with an optimal cutoff point of 64.58/mm2; sensitivity was 66.7% & specificity was 66.7% • ROC curve analysis for diagnosis DPN showed a reasonable AUC for CNFL at 0.73 with an optimal cutoff point of 24.00 mm/mm2; sensitivity was 66.7% & specificity was 66.4% • CCM identified more severe corneal nerve loss in T1D patients relative to those with T2D & shows very reasonable diagnostic accuracy for DPN |
| Guimarães et al.
104
-Describe & validate an automatic approach to nerve tracing |
N = 30 n = 24 pathologic group n = 10 +DM n = 6 HCs Cross-sectional |
• Automatic nerve tracing, following a proposed algorithm,
was assessed with respect to manual grading of CNT (high,
mid, & low tortuosity classes) with a significant
Spearman’s rank correlation coefficient of 0.95
achieved • With setting 2 thresholds to distinguish between the 3 tortuosity classes, correct classification (93.3%) was yielded for the automatic compared to the manual approach • Nerve tracing results reveal the automatic method performed well although larger studies are needed to confirm results |
| Kalteniece et al.
105
-Assess the effect of a standardized protocol for image selection & number of images required for adequate quantification of CN pathology using IVCCM |
N = 35 obese &/or
+DM M = 49.97 ± 12.47 yrs/age Longitudinal |
• In terms of inter-observer variability, ICC values for
CNFD, CNBD, & CNFL were significant at 0.93, 0.96, &
0.95, respectively • With respect to intra-observer variability, ICC values for CNFD, CNBD, & CNFL were significant at 0.95, 0.97, & 0.97, respectively • For sample size variability, ICC values for CNFD, CNBD, & CNFL were significant at 0.94, 0.95, & 0.96, respectively • Bland-Altman plots showed excellent agreement for all parameters • 6 images were found to be adequate for the fully automated analysis • Implementing a standardized protocol to select IVCCM images results in high intra- & inter-observer reproducibility for all corneal nerve parameters |
| Lewis et al.106 -Determine reference distribution of annual CNFL change, prevalence of abnormal change in diabetes, & associated variables |
N = 794 n = 399 T1D M = 39.9 ± 18.7 yrs/age n= 191 T2D M = 60.4 ± 8.2 n = 204 Controls M = 37.9 ± 19.8 yrs/age Secondary analysis of longitudinal observational study |
• Participants with T1D (median FU of 3 visits over 4.4 yrs)
had a mean annual change in CNFL (mm/mm2) of
-0.8%; T1D participants with RCNFL had a significant annual
change in CNFL (-14.67 ± 11.46%) relative to T1D
participants without RCNFL (2.58 ±
9.93%) • Participants with T2D (median FU of 3 visits over 5.3 yrs) had a mean annual change in CNFL of -0.2%; T2D participants with RCNFL had a significant annual change in CNFL (-11.49 ± 6.35%) compared to T2D participants without RCNFL (2.47 ± 7.33%) • RCNFL prevalence was 17% overall & similar by DM type (16.0% T1D, 19.4% T2D) • RNCFL was significantly more frequent in those with baseline DSP (47%) vs. those without baseline DSP (30%) • RCNFL may identify patients at high risk for DSP development & progression |
| Li et al.
107
-Examine & compare fully-automated & manually measured corneal nerve fiber parameters in T2D patients with & without DPN |
N = 152 n = 128 T2D n = 49 -DPN M = 67.12 ± 6.01 yrs/age n = 79 +DPN M = 70.15 ± 7.34 yrs/age n = 24 HCs M = 68.63 ± 5.19 yrs/age Cross sectional |
• CNFLM & CNBDM in +DPN group were
significantly lower than -DPN & HC
groups • Likewise, CNFLFA & CNBDFA in +DPN group were significantly reduced relative to -DPN & HC groups • CNFDFA, but not CNFDM, was significantly reduced in +DPN group vs. HC group • Significant, positive correlations between manual & fully automated CNFL, CNFD, & CNBD were observed • Fully automated method slightly underestimated corneal nerve fiber parameters. • A progressive decrease in manual & fully automated CNFL, CNBD & CNFD accompanied the occurrence of DPN • Fully automated corneal nerve fiber parameter quantification may be a fast, objective way to detect DPN |
| Lovblom et al.
108
-Determine the predictive validity of a baseline IVCCM measure in identifying future DSP onset in patients with T1D |
N = 65 T1D, -DSP M = 34 ± 15 yrs/age Longitudinal (mean 3.5 years FU) |
• At FU, 54 (83%) remained without DSP & 11 (17%)
developed DSP • New-onset cases of DSP had lower baseline CNFL & CNBD but higher baseline CNFT • CNFL: AUC was 0.78; optimal operating threshold of 14.9 mm/mm2 with 82% sensitivity, 69% specificity, & 35% PPV • CNFT: AUC was 0.72; optimal operating threshold of 15.4 (tortuosity coefficient) with 73% sensitivity, 72% specificity, & 35% PPV • CNBD: AUC was 0.71; optimal operating threshold of 36.1 branches/mm2 with 82% sensitivity, 50% specificity, & 25% PPV • CNFL may have applicability in identifying high-risk patients for DSP |
| Ostrovski et al.
109
-Determine inter- & intra-observer reproducibility of a novel automated analysis program vs. manual analysis |
N = 46 n = 26 T1D M = 42.8 ± 16.9 yrs/age n = 20 controls M = 41.4 ± 17.3 yrs/age |
• Inter-observer ICCs for CNFLM,
CNFLSA, & CNFLFA were 0.73,
0.75 & 0.78, respectively, with no significant
differences for 3-way comparisons; intra-observer ICCs were
0.72, 0.74, & 0.84, respectively, with CNFLFA
reproducibility significantly higher than that of
CNFLM & CNFLSA
• Inter-observer & intra-observer ICCs for CNFDM, CNFDSA, CNFDFA, CNBDM, CNBDSA, & CNBDFA were substantially lower compared to those for CNFL • Fully automated analysis preserves CNFL reproducibility despite an apparent measurement bias (underestimation) relative to the manual strategy of image analysis |
| Perkins et al.
110
- Establish concurrent validity & diagnostic thresholds in a large cohort of participants with & without DSP |
N = 998 n = 516 T1D M = 42 ± 19 yrs/age n = 482 T2D M = 62 ± 10 yrs/age Cross-sectional, pooled multi-national consortium study |
• In participants with T1D, CNFLA had an AUC of
0.77 & optimal threshold of 12.5 mm/mm2 (73%
sensitivity & 69% specificity) • In participants with T2D, CNFLA had an AUC of 0.68 & optimal threshold of 12.3 mm/mm2 (69% sensitivity & 63% specificity) • In participants with T1D, AUC for CNBDA & CNFDA were 0.73 & 0.71, respectively • In participants with T2D, AUC for CNBDA & CNFDA were 0.66 & 0.52, respectively • In the total cohort, CNFLA had an AUC of 0.71 & optimal threshold of 12.3 mm/mm2 (67% sensitivity & 66% specificity); AUC of CNFLM (0.70) vs. CNFLA was marginally, yet significantly lower although its optimal threshold value of 16.3 mm/mm2 had similar operating characteristics • A lower CNFLA threshold value of <8.6 mm/mm2 to rule in DSP & upper CNFLA threshold value of 15.3 mm/mm2 to rule out DSP was associated with 88% specificity & 88% sensitivity • In the largest cohort to date, diagnostic validity & common diagnostic thresholds for CNFL in T1D & T2D established |
| Petropoulos et al.
111
- Compare ability of CNFD, CNFL, & IWL alone & in combination for diagnosis of DPN |
N = 68 n = 53 +DM (T1D & T2D) n = 25 +DPN M = 60.1 ± 10.2 yrs/age n = 28 –DPN M = 42.4 ± 14.7 yrs/age n = 15 AMCs Cross-sectional |
• For diagnosis of DPN, ROC curve analysis showed CNFL =
21.9 mm/mm2 had an AUC of 0.75, sensitivity of
76%, & specificity of 65%; CNFD = 28.4
fibers/mm2 had an AUC of 0.74, sensitivity of
68%, & specificity of 61%; IWL = 20.0 mm/mm2
had an AUC of 0.70, sensitivity of 68%, & specificity of
67% • Combination of CNFL & IWL achieved an AUC of 0.75, sensitivity of 80%, & specificity of 71% for DPN • For inter & intra-observer agreement for IWL estimation, no significant difference between 2 separate observers (17.8 ± 8.5 vs. 17.7 ± 9.1 mm/mm2) & no significant difference (17.8 ± 8.5 vs. 17.2 ± 8.0 mm/mm2) when same observer assessed & reassessed IW center images from 1 dataset on 2 separate occasions • CNFD, CNFL, & IWL have comparable ability to diagnose DPN; combining IWL & CNFD may improve CCM diagnostic performance • Inter- & intra-observer agreement for IWL estimation was excellent |
| Pritchard et al.
81
-Determine if deficits in CNFL assessed using CCM can predict future onset of DPN |
N = 90 T1D, -DPN (baseline) 4-yr FU n = 16 +DPN M = 51 ± 14 yrs/age (baseline) n = 64 -DPN M = 42 ± 16 yrs/age (baseline) Longitudinal |
• DPN developed in 16 participants (18%) after 4
yrs • Baseline CNFL (mm/mm2) predicted incident DPN at 4-yr FU • Participants who developed DPN at 4-yr FU had significantly lower baseline CNFL (14.0 ± 4.1) relative to participants (16.2 ± 3.5) who did not develop DPN • For CNFL, AUROC was 0.6 with a threshold cutoff of 14.1 mm/mm2; sensitivity was 63% & specificity was 74% to predict DPN • CCM may predict DPN in T1D |
| Pritchard et al.
112
-Compare SNF damage in central cornea & whorl area in participants with DPN & examine accuracy of evaluating these 2 sites for DPN diagnosis |
N = 187 n = 107 T1D M = 48.3 ± 15.1 yrs/age n = 25 +DPN n = 82 -DPN n = 80 Controls M = 37.0 ± 17.8 yrs/age Cross-sectional |
• Participants with & without DPN had significantly
lower CNFLcenter compared to controls (14.2 ± 3.5
& 16.7 ± 3.5 mm/mm2 vs. 19.3 ± 3.0
mm/mm2, respectively) • Participants with & without DPN had significantly reduced CNFLwhorl relative to controls (15.4 ± 4.4 & 18.2 ± 3.9 mm/mm2 vs. 22.1 ± 3.9 mm/mm2, respectively) • For CNFLcenter, AUC was 0.76; Youden Index cutoff point of <17.9 with sensitivity of 90% & specificity of 50% for detecting DPN • For CNFLwhorl, AUC was 0.77; Youden Index cutoff point of <18.6 with sensitivity of 80% & specificity of 60% for detecting DPN • SNF pathology comparable at corneal central & whorl anatomical sites • Quantification of CNFL from the corneal center is as accurate as CNFL quantification of whorl area for diagnosis of DPN |
| Scarpa et al.
113
- Investigate whether CNN can be successfully used for corneal nerve multiple-image analysis |
N = 100 participants, 600 confocal
images M = 53 ± 13 yrs/age n = 50 +DM (T1D & T2D), +DN n = 50 AMHCs Cross-sectional |
• Cross-validation used to evaluate a proposed algorithm for
binary classification (healthy or pathological) of 100
participants (CNN training on 80 subjects & evaluation
on the other 20, repeated 5 times) with 97% mean accuracy
for CNN correct classification • Also, with a final classification derived for each participant (properly classified if both right & left eyes correctly classified), CNN revealed validity with a mean accuracy of 96% • With outstanding classification accuracy, the CNN is a highly promising, fully automated method of corneal confocal image analysis with strong potential to yield improved results obtained from traditional methods |
| Scarr et al.
114
-Evaluate agreement between manual & automated analysis protocols in controls & T1D & T2D participants with & without DSP |
N = 456 M = 53 ± 18 yrs/age n = 139 T1D n = 249 T2D n = 68 Controls Cross-sectional |
• For the study population, mean CNFLM (15.1 ±
4.9 mm/mm2) was greater than mean
CNFLA (10.5 ± 3.7 mm/mm2) although
values were highly correlated; absolute mean difference
between CNFLA & CNFLM for the
study population was -4.6 ± 2.6 mm/mm2 &
percentage difference was -29 ± 17%, representing
underestimation bias by CNFLA
• A similar pattern of correlations & underestimation bias was observed for CNFLM vs. CNFLA for T1D, T2D, & control groups • In participants with T1D, percentage difference between CNFLA & CNFLM for those with DSP was -27 ± 27%, & for those without DSP the percentage difference was -32 ± 13% although non-significantly • In participants with T2D, percentage difference between CNFLA & CNFLM for those with DSP was -28 ± 16%, & for those without DSP was -27 ± 16% although non-significantly • Weighted kappa statistic for agreement between tertiles of CNFLA & CNFLM was 0.62, indicating moderate to substantial agreement • CNFLA & CNFLM were significantly lower in T1D participants with DSP relative to those without DSP • CNFLA & CNFLM were not significantly lower in T2D participants with DSP compared to those without DSP • Although CNFLA underestimated CNFLM, its bias was non-differential between participant groups & its relationship with DSP status was preserved • Determination of diagnostic thresholds specific to CNFLA requires further investigation |
| Schaldemose et al.
115
-Compare a new sampling method & AAC with established methods of corneal nerve quantification in patients with & without DSPN & HCs |
N = 88 n = 62 T1D n = 17 +DSPN M = 59 ± 11 yrs/age n = 45 -DSPN M = 44 ± 13 yrs/age n = 26 HCs M = 44 ± 15 yrs/age Cross-sectional |
• Using randomized & area adjusted method,
CNFDM & CNFLM were
significantly reduced in +DSPN group compared to both HC
& -DSPN groups; CNFLM values were larger in
-DSPN group relative to HCs • Using a randomized & area adjusted method, CNFDA, CNFLA &, CNBDA were reduced significantly in +DSPN & -DSPN groups compared to HCs & lowest in the +DSPN group • Randomized sampling & adjusted area method with automated analysis showed that, among +DSPN participants, CNFDA (no./mm2; 17.3 ± 12) had a higher mean than standard automated procedures (13.5 ± 9.1) with a significant difference of 28.1%; CNFLA (mm/mm2; 12.3 ± 6.8) had a higher mean than standard automated procedures (8.8 ± 4.7) with a significant difference of 39.8%%; CNBDA (no./mm2; 19.1 ± 14) had a higher mean than standard automated procedures (15.4 ± 12) with a significant difference of 24.0% • Randomized sampling & adjusted area method with automated analysis showed that, among -DSPN participants, CNFDA (no./mm2; 28.2 ± 9.3) had a higher mean than standard automated procedures (22.6 ± 7.3) with a significant difference of 24.8%; CNFLA (mm/mm2; 17.0 ± 4.2) had a higher mean than standard automated procedures (13.4 ± 3.3) with a significant difference of 26.9%%; CNBDA (no./mm2; 31.1 ± 18) had a higher mean than standard automated procedures (26.2 ± 15) with a significant difference of 18.7% • Interobserver reliability testing revealed no significant difference in means for CNFLM, CNFDM, & CNBDM between the investigator & a blinded second observer • Randomized sampling method & area-dependent analysis objectively shows a reduction in corneal nerve parameters in diabetic patients with & without DSPN while providing unbiased corneal nerve quantification |
| Tummanapalli et al.
116
- Determine utility of CCM & tear neuromediator analysis in diagnosis of DPN |
N = 70 n = 38 T1D n = 19 +DPN M = 44 ± 12 age/yrs n = 19 -DPN M = 37 ± 10 age/yrs n = 32 T2D n = 16 +DPN M = 58 ± 6 age/yrs n = 16 -DPN M = 52 ± 12 age/yrs Prospective, cross- sectional |
• For DPN diagnosis in T1D, IWL had the best diagnostic
performance (AUC 0.91, optimal diagnostic threshold of
≤12.93 mm/mm2, 89% sensitivity, & 83%
specificity), followed by CNFrD (AUC 0.89; diagnostic
threshold ≤1.50, 89% sensitivity, & 72% specificity),
CNFD (AUC 0.88, diagnostic threshold ≤22.46
no./mm2, 83% sensitivity, & 83%
specificity), IWNFrD (AUC 0.88, diagnostic threshold ≤1.46,
83% sensitivity, & 89% specificity), CNFL (AUC 0.87,
diagnostic threshold ≤14.24 mm/mm2, 89%
sensitivity, & 78% specificity), CNBD (AUC 0.76,
diagnostic threshold ≤26.17 no./mm2, 72%
sensitivity, & 72% specificity), & CTBD (AUC 0.75,
diagnostic threshold ≤40.40 no./mm2, 72%
sensitivity, & 72% specificity) • For DPN diagnosis in T2D, CNFL had the best diagnostic performance (AUC 0.81, optimal diagnostic threshold ≤13.64 mm/mm2, 81% sensitivity, & 81% specificity), followed by CNFrD (AUC 0.78, diagnostic threshold ≤1.48, 75% sensitivity, & 63% specificity), CNBD (AUC 0.76, diagnostic threshold ≤29.17 no./mm2, 81% sensitivity, & 75% specificity) CNFD (AUC 0.76, diagnostic threshold ≤22.90 no./mm2, 75% sensitivity, & 69% specificity), CTBD (AUC 0.76, diagnostic threshold ≤43.10 no./mm2, 81% sensitivity, & 81% specificity), & IWNFrD (AUC 0.73, diagnostic threshold ≤1.47, 69% sensitivity, & 62% specificity) • In T1D & T2D, different corneal nerve parameters were identified, suggesting different disease processes across conditions |
| Wang et al.
117
-Explore the application of CCM in DPN & other T2D chronic complications |
N = 220 n = 100 T2D +DPN M= 56.2 ± 12.4 age/yrs n = 72 -DPN M = 54.9 ± 11.1 age/yrs n = 37 T2D +DN n = 89 T2D +DR n = 48 HCs M = 51.9 ± 14.9 yrs/age Cross-sectional |
• In participants with T2D, CNFL & CNBD were
significantly lower than HCs, & similar in +DPN group
compared to -DPN group • Using CCM to identify DPN, AUC for CNFD was 0.67 & optimal cutoff was 24.68 no./mm2 (78% sensitivity, 53% specificity); AUC for CNFL was 0.70 & optimal cutoff was 15.32 mm/mm2 (80% sensitivity, 60% specificity); AUC for CNBD was 0.68 & optimal cutoff was 39.0 no./mm2 (85% sensitivity, 47% specificity) • This study provides more support for clinical use of CCM to diagnose DPN |
| Williams et al.
118
-Validate a DLA for corneal nerve segmentation in CCM images & compare to the widely used, validated automated image analysis software, ACCMetrics |
N = 3865 confocal images of corneal SBP
obtained from +DM individuals & HCs n = 120 confocal images from Italy n = 1578 confocal images from China n = 2137 confocal images from UK |
• DLA, employing CNN with data augmentation, trained using a
high-end graphics processor unit on 1698 images for
automated quantification of the corneal SBP for DN
diagnosis • DLA further tested on 2137 images for external validation with DLA ICCs superior to those for ACCMetrics for total CNFL (0.93 vs. 0.83), mean length per segment (0.66 vs. 0.33), number of branch points (0.89 vs 0.57), number of tail points (0.62 vs. 0.26), number of nerve segments (0.88 vs. 0.50) & fractals (0.93 vs 0.76) • DLA achieved an AUC of 0.83, specificity of 0.87, & sensitivity of 0.68 for the classification of participants without (n = 90) or with (n = 132) neuropathy • Results reveal DLA provides rapid & excellent localization performance for quantification of corneal nerve biomarkers • DLA model has potential for adoption into clinical screening programs for DN |
Abbreviations: AAC, adjusted area calculation; ACNBD, automated corneal nerve branch density; ACNFD, automated corneal nerve fiber density; ACNFL, automated corneal nerve fiber length; ACNFrD, automated corneal nerve fiber fractal dimension; AMC, age-matched controls; AMHCs, age-matched healthy controls; ANFL, average nerve fiber length; AUC, area under the curve; AUROC, area under the receiver operator characteristic; BD, beading density; CC, central cornea; CCM, corneal confocal microscopy; CG, control group; CN, corneal nerve; CNBD, corneal nerve branch density; CNBDA, corneal nerve branch density with automated analysis; CNBDFA, corneal nerve branch density with fully automated analysis; CNBDM, corneal nerve branch density with manual analysis; CNBDSA, corneal nerve branch density with semi-automated analysis; CNFD, corneal nerve fiber density; CNFDA, corneal nerve fiber density with automated analysis; CNFDFA, corneal nerve fiber density with fully automated analysis; CNFDM, corneal nerve fiber density with manual analysis; CNFDSA, corneal nerve fiber density with semi-automated analysis; CNFL, corneal nerve fiber length; CNFLA, corneal nerve fiber length with automated analysis; CNFLcenter, corneal nerve fiber length at the central cornea; CNFLFA, corneal nerve fiber length with fully automated analysis; CNFLM, corneal nerve fiber length with manual analysis; CNFLSA, corneal nerve fiber length with semi-automated analysis; CNFLwhorl, corneal nerve fiber length at the whorl; CNFrD, corneal nerve fractal dimension; CNFT, corneal nerve fiber tortuosity; CNL, corneal nerve length; CNM, corneal nerve migration; CNN, convolutional neural network; CNT, corneal nerve thickness or corneal nerve tortuosity; CSNP, corneal sub-basal nerve plexus; CTBD, corneal total branch density; DLA, deep learning algorithm; DM, diabetes mellitus; DN, diabetes neuropathy; DPN, diabetes peripheral neuropathy; DSP, diabetic sensorimotor polyneuropathy or distal symmetric polyneuropathy; DSPN, diabetes symmetrical peripheral neuropathy or diabetic sensorimotor polyneuropathy; EEP, equal error point; EER, equal error rate; EERP, equal error rate point; FU, follow-up; HCs, healthy controls; ICC, interclass correlation coefficient; IENFD, intraepidermal nerve fiber density; IGT, impaired glucose tolerance; IVCCM, in vivo corneal confocal microscopy; IWL, inferior whorl length; IWNFrD, inferior whorl nerve fractal dimension; M, mean; MCNBD, manual corneal nerve branch density; MCNFD, manual corneal nerve fiber density; MCNFL, manual corneal nerve fiber length; MDL, mean dendric length; MFU, mean follow-up; mm, millimeter; mm/mm2, millimeter/square millimeter; mm2, millimeters squared; NBD, nerve branch density; NBe, number of beadings; NBi, number of bifurcations; NBr, number of branches; NCs, normal controls; NDS, Neuropathy Disability Score; NFA FIJI, nerve fiber area determined by total number of pixels within the nerve plexus; NFA W×L, nerve fiber area determined by length times average width; NFD, nerve fiber density; NFL, nerve fiber length; NFLD, nerve fiber length density; NFT, nerve fiber tortuosity; NGT, normal glucose tolerance; no., number; no./mm2, number/square millimeter; NSP, Neuropathy Symptom Profile,; NT, number of trunks; PN, peripheral neuropathy; PNCV, peroneal nerve conduction velocity; PPV, positive predictive value; QST, quantitative sensory testing; RCNFL, rapid corneal nerve fiber loss; RNFL, retinal nerve fiber layer; ROC, receiver operator characteristic; S/S, sensitivity/specificity; SBP, sub-basal nerve plexus; SCNP, sub-basal corneal nerve plexus; SD, standard deviation; SD-OCT, spectral domain optical coherence tomography; SNF, small nerve fiber; T1D, type 1 diabetes; T2D, type 2 diabetes; VPT, vibration perception threshold; vs., versus; WST, warm sensation threshold; yr, year; yrs, years; μm2, square micrometer.
Further, the current longitudinal literature suggests CCM may predict incident DSPN, identify patients at high-risk for DSPN, and monitor progressive nerve damage over time.73,75,81,101,106,108 Sampling adults with T1D, Pritchard et al. found, for example, that baseline CNFL predicted incident DSPN at 4-year follow-up. Participants who developed DSPN had significantly lower baseline CNFL (14.0 ± 4.1) relative to participants (16.2 ± 3.5) who did not develop small-fiber neuropathy. 81 Additional longitudinal research, reviewed in this study, support these findings, revealing reduced CNFL may be an important biomarker for future development of DSPN.101,106,108
Sudomotor Function Testing (SFT)
SFT is used to assess small-fiber DSPN. 3 SFT, a measure of autonomic function, may be assessed with quantitative sudomotor axon reflex testing (QSART), and newer approaches, including electrochemical skin conductance (ESC).3,119-121 SFT non-invasively assesses C fiber function, specifically innervation of sweat glands.
QSART
QSART measures C fiber induced sweat production in predetermined sites (forearm, distal and proximal leg, and foot) with application of iontophoresis and measurement of sweat responses with iontophoretic stimulators.119-120 Abnormal QSART test findings reveal reduced sweat volume or latency. 120 The literature search identified 2 studies specific to QSART technology and one related, novel technological advancement (see Table 5)122-124 The Q-Sweat System (WR Medical Electronics, Stillwater, MN, USA) was found to have suboptimal sensitivity (58%), optimal specificity (100%) in one study while another documented lack of discriminatory power in identifying participants with and without DSPN and controls.122-123
Table 5.
Quantitative Sudomotor Axon Reflex Testing (QSART).
| Author(s) & Study Aim | Study Population & Design | Study Outcomes |
|---|---|---|
| Kamel et al.
122
-Assess the clinical utility of CSP, QSART, SSR & AFT in detection of SFN related to glycemic dysregulation |
N = 52 n = 26 +SFN n = 9 T1D, n = 9 T2D n = 8 IGT M = 50.8 ± 2.6 yrs/age n = 26 Controls M = 48.9 ± 2.6 age/yrs Cross-sectional |
• QSART (Q-Sweat System; WR Medical Electronics, Stillwater,
MN, USA),) results showed a sensitivity of 58% &
specificity of 100% • Sensitivity of the Q-Sweat System may have been improved by limiting the sample to T1D & T2D participants & more rigorous evaluation of sensitivity • Q-Sweat System revealed an optimal specificty (100%) although suboptimal sensitivity (58%) |
| Krieger et al.
123
-Evaluate performance of Sudoscan against QSART (Q-Sweat System) in diagnosing DPN |
N = 63 n = 27 T2D, +DPN M = 69 ± 4.8 yrs/age n = 20 T2D, -DPN M = 66 ± 5.8 yrs/age n = 16 MCs M = 64 ± 5.1 yrs/age Cross-sectional |
• Q-Sweat System could not differentiate between the 3
groups (+DPN, -DPN, MCs) • Q-Sweat System showed poor discriminatory performance |
| Loavenbruck et al.
124
-Report a method that quantifies axon reflex sweating from individual SGs with nanoliter precision |
N = 72 n = 20 +Neuropathy (n = 11 +DM) Aged 45–82 yrs n = 52 AACs Aged 21–88 yrs Cross-sectional |
• Comparing +neuropathy to AAC groups, AUC of 0.90 achieved
for directly & indirectly stimulated rate/SG & total
sweat at the calf • AUC of 0.80 & 0.84, respectively, achieved for directly stimulated rate/SG & total sweat at the feet • AUC of 0.58 & 0.74, respectively, achieved for indirectly stimulated rate/SG & total sweat at the feet • Overall, SST shows impressive performance for quantifying axon reflex sweating from individual SGs with nanoliter precision |
Abbreviations: AAC, age-adjusted control; AFT, autonomic function testing; AUC, area under the curve; AUROC, area under receiver operator characteristic; CSP, cutaneous silent periods; DM, diabetes mellitus; DPN, diabetic polyneuropathy; IGT, impaired glucose tolerance; M, mean; MCs, matched controls; QSART, quantitative sudomotor axon reflex testing; SFN, small fiber neuropathy; SG, sweat gland; SST, sensitive sweat test; T1D, type 1 diabetes; T2D, type 2 diabetes; yrs, years.
Distinct, although related to traditional QSART, Loavenbruck et al. developed a brief, sensitive sweat test (SST) with nanoliter precision, using high-definition videography, to assess C fiber innervation in the foot, calf, thigh, and hand. In SST, the anatomical site being assessed has a small, handheld camera (with starch tape across its lens) pressed firmly, face down. Acetylcholine (ACh) gel, loaded in an anode iontophoresis capsule, is applied directly adjacent and lateral to the camera. Directly stimulated by ACh gel, affected sweat gland (SG) ducts secrete sweat, which is absorbed by the starch tape as small dark spots. Recorded dark spots accurately correspond with sweat volume. In comparing participants with neuropathy to controls, ROC analyses showed the greatest distinction between groups at the calf (directly stimulated rate/SG and total sweat had an AUC of 0.90 and 0.90, respectively) and foot (directly stimulated rate/SG and total sweat had an AUC of 0.80 and 0.84, respectively). 124
ESC
ESC assesses the electrical potential of sweat glands to evaluate sudomotor function. ESC directly evaluates C fiber stimulated sweat gland function through measurement of sweat chloride concentrations using reverse iontophoresis and chronoamperometry.125-127
Since 2015, the literature search identified 14 studies evaluating ESC, namely Sudoscan (Impeto Medical, Paris, France; see Table 6).51,76,123,128-139 Advancing earlier EZSCAN technology, Sudoscan is a POCD wherein testing requires that the patient place his/her hands and feet, respectively, on 2 stainless steal electrodes.125-126 While the patient’s hands and feet remain on the electrodes for 2 minutes, a computer records ESC values. 125
Table 6.
Electrochemical Skin Conductance (ESC).
| Author(s) & Study Aim | Study Population & Design | Study Outcomes & Implications |
|---|---|---|
| Ang et al.
128
- Evaluate ESC as a reliable surrogate for early CAN |
N = 77 n = 37 T1D M = 39 ± 8 yrs/age n = 40 HCs M = 38 ± 13 yrs/age Longitudinal |
• Using Sudoscan (Impeto Medical, Paris, France) to measure
ESC, no significant differences were observed in hands or
feet of T1D participants relative to HCs at
baseline • In T1D participants, mean ESC, as measured by Sudoscan, declined significantly from baseline to 12 months • While both hands & feet ESC declined over time, the significance of this finding is unclear and warrants further reliability testing |
| Binns-Hall et al.
51
-Evaluate feasibility one-stop microvascular screening service for early diagnosis of DSPN, painful DSPN, & at-risk diabetic foot Bordier et al. 129 -Assess repeatability & reproducibility of Sudoscan in HVs & diabetic patients with a range of glycemic control |
N = 236 M = 63.5 ± 14.1 yrs/age n = 231 T2D n = 5 T1D n = 84 +DPN n = 69 -DPN n = 83 unclassified N = 32 n = 14 T2D M = 62 ± 9 yrs/age n = 18 HVs M = 37 ± 13 yrs/age Cross-sectional |
• Using Sudoscan, AUROC curve was 0.75 with an ESC threshold
of ≤58.5 μS; sensitivity was 77.4% & specificity was
68.3% for detecting DSPN • Sudscan may be useful screening device with respectable performance values • On 3 different Sudoscan devices, 2 measurements were performed under usual testing conditions on T2D & HV participants • For hands ESC, in particpants with T2D, the mean repeatability SD was 4.3 μS (mean coefficient of variation was 7.1 ± 5.9%) & mean reproducibility SD was 4.5 μS (mean coefficient of variation was 7.4 ± 6.1%) • For hands ESC, in HVs, the mean repeatability SD was 3.1 μS (mean coefficient of variation was 4.2 ± 2.7%) & mean reproducibility SD was 3.2 μS (mean coefficient of variation was 4.3 ± 2.7%) • For feet ESC, in particpants with T2D, the mean repeatability SD was 4.3 μS (mean coefficient of variation was 6.9 ± 6.3%) & mean reproducibility SD was 4.3 μS (mean coefficient of variation was 6.9 ± 6.3%) • For feet ESC, in HVs, the mean repeatability SD was 2.1 μS (mean coefficient of variation was 2.8 ± 1.6%) & mean reproducibility SD was 2.3 μS (mean coefficient of variation was 3.1 ± 1.5%) • In participants with T2D, ICCs used to compare the 3 devices were 0.95 (0.89–0.98) & 0.88 (0.74–0.96) & for HVs were 0.87 (0.74–0.94) & 0.85 (0.71–0.93) for feet & hands, respectively • Findings establish that repeatability & reproducibility of ESC measurements are respectable in participants with T2D & HVs |
|
Carbajal-Ramírez et al. 130 -Assess the accuracy of Sudoscan (feet & hands) compared to MNSI in a cross-sectional study of Mexicans with T2D |
N = 221, Mexican n = 170 T2D < 5 yrs M = 58.6 ± 12.6 age/yrs n = 51 T2D >5 yrs M = 63.8 ± 11.8 age/yrs Cross-sectional |
• Evaluating the diagnostic accuracy of Sudoscan, in participants with T2D >5 years, AUROC curve of hands & feet ESC were 0.84 & 0.78, respectively, with MINSI B as the reference; sensitivity of abnormal hands or feet ESC for detection of neuropathy was 97% • In participants with T2D <5 years, AUROC curve of hands & feet ESC were 0.66 & 0.72 respectively; sensitivity of abnormal hands or feet ESC for detection of neuropathy was 91% • Sudoscan, which does not require any preparation, is noninvasive, easy & rapid to use in detecting P • Sudoscan may be useful in the early diagnosis of PN in T2D |
| Chae et al.
131
-Determine if Sudoscan can complement NCS & EMG in patients with LSR & PPN |
N = 73 LE
dysesthesia n = 34 Controls n = 18 LSR n = 21 PPN (57% +DM) M = 63.1 ± 10.8 yrs/age for +DM group Cross-sectional |
• AUC was 0.78 for feet ESC; cutoff at 48 µS; sensitivity
was 57.1% & specificity was 94.2% to detect
PPN • At a 55 µS cutoff, hands ESC had a sensitivity of 71.4% & specificity of 78.8% to detect PPN • Sudoscan was found to have highly acceptable diagnostic accuracy for feet ESC with impressive specificity |
| D’Amato et al.
132
-Determine diagnostic value of the combined scores of composite autonomic symptom score 31, validated questionnaire for autonomic symptoms, CAN, & ESC |
N = 102 DM; 65%
T2D M = 57.1 ± 13.7 yrs/age Cross-sectional |
• In assessing the diagnostic accuracy of Sudoscan, AUC of
ESC feet was 0.69 for DPN diagnosis • Among participants with DPN, ESC had a sensitivity of 62% specificity of 67%, & positive predictive value of 67% • Findings reveal fair Sudoscan test performance |
| Fabry et al.
76
-Compare several methods of evaluating small sensory & autonomic nerve fibers |
N = 245 M = 50.4 ± 15.0 yrs/age n = 24 +DM n = 6 +IGT n = 102 “Definite SFN” n = 90 “No SFN” Retrospective study |
• Diagnostic performance of ESC (Sudoscan) & IENFD was
evaluated by studying the normality or abnormality of each
test according to the diagnosis of “Definite SFN” or “No
SFN,” respectively • ESC sensitivity was 60%, specificity was 89%, & positive predictive value was 86% for detecting SFN • IENFD sensitivity was 58%, specificity was 91%, & positive predictive value was 88% for detecting SFN • ESC or Sudoscan revealed significant differences between the “Definite SFN” & “No SFN” groups both in hands (60.2 ± 16.7 vs. 75.0 ± 8.9 μS) & feet (70.2 ± 16.5 vs. 81.6 ± 7.0 μS) • ESC & IENFD had comparable sensitivity, specificity, & positive predictive values |
| Gandecka et al.
133
-Evaluate sudomotor function & its relationship to metabolic control & diabetic complications |
N = 485 Median = 41 yrs/age, IQR = 32–51 n = 404 T1D n = 84 Controls Case Control Study |
• Participants with T1D had a significantly lower ESC (as
measured by Sudoscan+) relative to
controls • Discriminative value of feet ESC to identify patients with PN was slightly better than that of ESC in the hands: AUC 0.77 vs. AUC 0.72 • With a cutoff point of 79 μS or less for feet ESC (optimal Youden index), sensitivity was 72%, specificity was 68%, & Youden index was 0.4 • Reproducibility of Sudoscan (feet & hands ESC) was confirmed with a cutoff value ratio not significantly different from 0 & slope ratio close to unity |
| Goel et al.
134
-Determine efficacy of ECS in diagnosing early DPN when compared to VPT & DNS score |
N = 523 T2D n = 110 +DPN M = 54.4 ± 11.9 yrs/age n = 413 -DPN M = 48.1 ± 11.4 yrs/age Cross-sectional |
• AUC of the ROC plot for feet ESC (Sudoscan) was 0.88 &
for VPT was 0.84 • Feet ESC, with a cutoff of <60 μS, had a sensitivity of 85% & specificity of 85% for classifying DPN • VPT, with a cut-off of >15 V, had a sensitivity of sensitivity of 72% & specificity of 90% for classifying DPN • Feet ESC measurement was superior to VPT testing for identifying patients with early DPN |
| Jin et al.
135
-Evaluate whether SUDOSCAN has good diagnostic ability in DSPN & CAN |
N = 180 T2D,
Chinese n = 60 -DSPN M = 54.4 ± 11.3 yrs/age n = 120 +DSPN M = 59.8 ± 8.0 yrs/age Cross-sectional |
• AUROC was 0.61, sensitivity was 89.8% & specificity
was 41.2% to diagnose DSPN • Sudoscan is a sensitive test to detect DSPN in China & may be an effective screening tool in primary health care settings |
| Krieger et al.
123
-Evaluate performance of Sudoscan against QSART in diagnosing DPN |
N = 63 n = 27 T2D, +DPN M = 69 ± 4.8 yrs/age n = 20 T2D, -DPN M = 66 ± 5.8 yrs/age n = 16 MCs M = 64 ± 5.1 yrs/age Cross-sectional |
• For feet ESC (Sudoscan), AUROC curve was 0.71; cutoff ≤
80.0 μS (optimal Youden index) with a sensitivity of 70%
& specificity of 53% • For hand ECS, AUROC curve was 0.71; cutoff of ≤ 75.0 μS (optimal Youden index) with a sensitivity of 85% & specificity of 50% • Feet & hand ESC significantly lower in patients with +DPN as compared to MCs • Patients with +DPN also had lower hand ESC than patients with -DPN • Sudoscan shows poor to good performance in detecting DPN • Sudoscan has high potential as a DPN screening tool in patients with T2D |
| Novak
136
-Determine the relationship between ECS measurements & loss of small fibers in the skin |
N = 81 +SFN M = 53.3 ± 17.3 yrs/age n = 48 SFN-I n = 33 SFN-AD n = 9 DM n = 2 IGT Prospective, blinded |
• ESC (Sudoscan) of feet (M = 0.88 ± 0.35 μS/kg), among
participants with abnormal IENFD, was significantly reduced
relative to participants with normal IENFD (M = 1.17 ± 0.27
μS/kg) • ESC significantly correlated with IENFD but not symptom scores • AUROC ESC feet was 0.74, with IENFD as reference, while adjusting for weight • ESC shows acceptable performance with the gold standard as the reference, revealing it may be useful in detecting SFN |
| Porubcin et al.
137
- Evaluate diagnostic accuracy of ESC to detect abnormal SGNFD & IENFD |
N = 210 M = 45.5 ± 16.1 yrs/age n = 132 SFN-I n = 78 SFN-AD n = 2 IGT Retrospective, blinded |
• ESC (Sudoscan), adjusted for weight (ESC/kg), was
significantly reduced in participants with abnormally low
IENFD (normal/abnormal ESC/kg 1.20 ± 0.37/1.04 ± 0.33
μS/kg) • AUROC curve was 0.63 for ESC/kg in predicting abnormal IENFD; sensitivity was 69% & specificity was 55% • ESC/kg showed modest performance & accuracy to detect SFN in the diverse sample |
| Selvarajah et al.
138
-Assess if Sudoscan is a reliable screen for DPN in clinics |
N = 70 n = 24 T1D, +DPN M = 52.1 ± 9.7 yrs/age n = 21 T1D, -DPN M = 40.6 ± 9.8 yrs/age n = 25 HVs M = 48.1 ± 16.4 yrs/age Cross-sectional |
• Foot ESC (Sudoscan) was significantly lower in
participants with +DPN compared to those with -DPN &
HVs • AUROC curve for foot ESC was 0.85; foot ESC cutoff point ≤ 77.0 μS (optimal Youden index); sensitivity & specificity were 88% & 76%, respectively, for classifying DPN • Sudoscan, a non-invasive & quick test, may be used as an objective screening test for DPN in busy diabetic clinics |
| Sheshah et al.
139
-Evaluate if ESC at foot can detect DPN & risk of foot ulceration as compared to traditional methods |
N = 296, Saudi
Arabians M = 46.7 ± 11.2 age/yrs n = 272 T2D n = 24 T1D Cross-sectional |
• Feet ESC (Sudoscan; threshold <50 μS for severe SMD)
AUC was 0.73 & 0.73 to detect severe DPN & FU,
respectively, with NDS as the reference; sensitivity (61%,
64%) & specificity (85%, 82%) for DPN & FU,
respectively • Feet ESC (Sudoscan; threshold <70 μS) AUC was 0.66 & 0.65 to detect DPN & FU, respectively, with NDS as the reference; sensitivity 81% & 81% & specificity 51% & 49% for DPN & FU, respectively • Sudoscan, a simple & objective tool, may be used to detect DPN & risk of FU in patients with DM |
Abbreviations: AUC, area under the curve; AUROC, area under receiver operator characteristic; CAN, cardiovascular autonomic neuropathy; DM, diabetes mellitus; DNS, Diabetic Neuropathy Symptom Scale; DPN, diabetic peripheral neuropathy or diabetic polyneuropathy; DSPN, diabetic distal symmetric peripheral neuropathy or distal symmetrical polyneuropathy; EMG, electromyography; ESC, electrochemical skin conductance; FU, foot ulceration; HC, healthy control; HVs, healthy volunteers; IENFD, intraepidermal nerve fiber density; IGT, impaired glucose tolerance; IQR, interquartile range; kg, kilogram; LE, lower extremity; LSR, lumbosacral radiculopathy; M, mean; MC, matched controls; MNSI B, Michigan Neuropathy Screening Instrument B; NCS, nerve conduction study; NDS, Neuropathy Disability Score; PN, peripheral neuropathy; PPN, peripheral polyneuropathy; QSART, quantitative sudomotor axon reflex testing; ROC, receiver operator characteristic; SFN, small fiber neuropathy; SFN-AD, small fiber neuropathy classified according to associated disorders; SFN-I, small fiber neuropathy classified as idiopathic; SGNFD, sweat gland nerve fiber density; T1D, type 1 diabetes; T2D, type 2 diabetes; VPT, vibration perception threshold; yrs, years; μS, microSiemens.
Most studies revealed overall adequate to good performance of Sudoscan.51,76,123,130-131,133-134,136,138-139 For example, sampling Mexicans with T2D, one study examined the performance of Sudoscan in predicting DSPN among 2 groups: (1) Mexicans with T2D for greater than 5 years; and (2) Mexicans with T2D for less than 5 years. In the first group, the AUROC curves for hands and feet ESC were 0.84 and 0.78, respectively, with the Michigan Neuropathy Screening Instrument as the reference. The sensitivity of abnormal hands or feet ESC for detection of neuropathy was 97%, while the positive predictive value was 87%. In the second group, AUROC curves of hands and feet ESC were 0.66 and 0.72, respectively. The sensitivity of abnormal hands or feet ESC for detection of neuropathy was 91%, while the positive predictive value was 88%. 130 These findings, coupled with the work of Goel et al, 134 suggest Sudoscan is a technology that may detect early DSPN. Sudoscan has also demonstrated respectable repeatability and reproducibility. Bordier and colleagues reported that, for feet, the ESC mean coefficient of variation for repeatability was 2.8 ± 1.6% in healthy controls and 6.9 ± 6.3% in participants with T2D, while the coefficients of variation for reproducibility were 3.1 ± 1.5 and 6.9 ± 6.3%, respectively. 129
Discussion
The diagnosis of DSPN is typically informed by a complete patient history and clinical exam. This approach is often sufficient to diagnose DSPN but not in its early asymptomatic and subclinical stages. Also, more advanced DSPN small- and large-fiber disease may present exclusively or together and sometimes without signs or symptoms.4,39-42 Given DSPN may not present with clinical signs and symptoms, utilization of highly sensitive screening technologies may be warranted to promote early detection of DSPN. Also, when signs or symptoms are present, but the distinction between small- and large-fiber disease remains unclear, confirmation of the DSPN subtype may require diagnostic testing. NCS is recommended for confirming large-fiber DSPN. 3
Advances in NCS technologies have yielded a very promising POCD, DPN-Check. DPN-Check assesses sural nerve conduction. Research suggests that DPN-Check, overall, has very respectable accuracy in detecting the presence and severity of DSPN.51-52,56-59 Also, DPN-Check has strong reliability and good agreement with standard electromyography. 60 However, a few studies indicate the POCD may have measurement bias compared to standard NCS.56,140 Yet, DPN-Check, approved by the Food and Drug Administration (FDA), is sufficiently accurate and reliable for use as a screening technology. The POCD is inexpensive ($500), provides rapid results, and does not require highly specialized training. DPN-Check has the potential to widen access to nerve conduction testing in primary care, internal medicine, and endocrinology practices although patients with abnormal or borderline results should undergo standard NCS. 56
Typically preceding large-fiber DSPN, small-fiber DSPN has negative findings upon NCS testing. 3 The gold standard for small-fiber diagnostic testing is IENFD. IENFD may identify early DSPN.141-142 Yet, this diagnostic technology is invasive and may be painful for patients. Serial skin biopsies for detecting early changes in IENFD may not be practical. More recent technological advances for small-fiber DSPN screening provide alternative, more rapid, non-invasive approaches – some of which show promise for early or asymptomatic, subclinical detection of small-fiber disease.
NerveCheck, a POCD, is an inexpensive ($500) QST device. It has demonstrated adequate to good reproducibility but mixed results with respect to diagnostic accuracy.82-83 The TSA-II NeuroSensory Analyzer, while also having varying results with respect to performance, may discriminate between clinical and subclinical DSPN in line with prior QST research.77,143-146 CHEPs has not advanced technologically and lacks practicality given its complex testing approach.
CCM technology has advanced, including automated image assessment. Prior and more current CCM research reveals its high promise to detect incident and early risk for DSPN 73,75,81,101,106,108,147-151 In the present study, CCM, compared to IENFD, was found to have fairly comparable performance.72,96 A limitation of CCM is that it is currently considered investigational for the purposes of detecting DSPN, and thus, is an out-of-pocket patient expense although smartphone CCM may be on the horizon, which may reduce costs in tandem with automated image evaluation.
In terms of sudomotor function testing, technological advancements are also impressive. A novel POCD, using high-definition videography, assesses C fiber innervation in the foot, calf, thigh, and hand with a brief SST. Beginning data indicate that the POCD has respectable accuracy in detecting small-fiber DSPN. 124 Yet, evidence is lacking in SST’s ability to detect asymptomatic or subclinical DSPN. Further rigorous research is needed prior to clinical adoption.
When considered with respect to past and more recent research, Sudoscan has a substantial evidence revealing it may potentially identify early DSPN (although this not its main use) and monitor DSPN progression over time.51,76,123,130-131,133-134,136,138-139,152-154 Sudoscan also has adequate reproducibility and repeatability.129,155-156 Sudoscan is approved by the FDA and may be reimbursed through proper billing. Sudoscan is thus a PCOD worthy of clinical adoption to detect and monitor DSPN in clinical settings, which may be accompanied by initial, confirmatory IENFD results.
While established and more recent technological advances may detect DSPN, it must be underscored that early detection of the disease is critical. At this time, IENFD (gold standard) and CCM have the strongest evidence for early detection of small-fiber disease, a precursor to large-fiber disease. The small-fiber technological screening devices reviewed may build on their respective research bases to further examine performance measures in detecting small-fiber DSPN in asymptomatic, subclinical populations. Furthermore, it appears that additional examination of normative values may be required, as indicated, for ethnic/racial, gender, and age groups to minimize the risk for bias in DSPN device outcomes data.
Conclusion
There is no cure for DSPN. Hence, its early detection is critical. Early, regular screening for DSPN is essential, particularly for small-fiber disease. Although IENFD is the gold standard for assessing small-fiber disease, innovative, non-invasive technologies, such as Sudoscan and particularly CCM, for early disease detection may be useful alternatives. Regardless of whether screening reveals small-fiber disease or not, healthy lifestyle interventions are warranted, if not ideally implemented earlier. Healthy lifestyle behaviors (weight loss, increased physical activity, and smoking cessation), may prevent, modify the progression, or mitigate symptoms of DSPN.9,21,33,157-161 In T1D populations, tight glycemic control tends to delay or prevent DSPN whereas, in T2D populations, tight control, for some, may slow DSPN progression.15,30,162-164 Optimization of blood pressure and lipid levels is also warranted.22-25
Footnotes
Abbreviations: ADA, American Diabetes Association; CCM, corneal confocal microscopy; CHEPs, contact heat evoked potentials; DSPN, distal symmetrical peripheral neuropathy; DTRs, deep tendon reflexes; ESC, electrochemical skin conductance; IENF, intra-epidermal nerve fiber density testing; NCS, nerve conduction study; QST, quantitative sensory testing; QSART, quantitative sudomotor axon reflex testing; SFT, sudomotor function testing; T1D, type 1 diabetes; T2D, type 2 diabetes.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Kelley Newlin Lew  https://orcid.org/0000-0001-5258-4085
https://orcid.org/0000-0001-5258-4085
Tracey Arnold  https://orcid.org/0000-0002-0312-0599
https://orcid.org/0000-0002-0312-0599
Hugo Posada-Quintero  https://orcid.org/0000-0003-4514-4772
https://orcid.org/0000-0003-4514-4772
References
- 1. Tesfaye S, Selvarajah D. Advances in the epidemiology, pathogenesis and management of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2012;28(Suppl 1):8-14. [DOI] [PubMed] [Google Scholar]
- 2. Vinik AI, Mitchell BD, Leichter SB, Wagner AL, O’Brian JT, Georges LP. Epidemiology of the complications of diabetes. In: Leslie RDG, Robbins DC. (eds) Diabetes: Clinical Science in Practice. Cambridge University Press; 1995;221-287. [Google Scholar]
- 3. Vinik A, Casellini C, Nevoret ML. Diabetic Neuropathies. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; February 5, 2018. Accessed September 27, 2021. [Google Scholar]
- 4. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Callaghan B, Kerber K, Langa KM, et al. Longitudinal patient-oriented outcomes in neuropathy: importance of early detection and falls. Neurology. 2015;85(1):71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. International Diabetes Federation. Care and prevention: the diabetic foot. 2020. https://idf.org/our-activities/care-prevention/diabetic-foot.html Accessed September 27, 2021.
- 7. Huang YY, Lin CW, Yang HM, Hung SY, Chen IW. Survival and associated risk factors in patients with diabetes and amputations caused by infectious foot gangrene. J Foot Ankle Res. 2018;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for medicare and private insurers [published correction appears in Diabetes Care. 2014 Sep;37(9):2660]. Diabetes Care. 2014;37(3):651-658. [DOI] [PubMed] [Google Scholar]
- 9. Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29(6):1294-1299. [DOI] [PubMed] [Google Scholar]
- 10. Polemiti E, Baudry J, Kuxhaus O, et al. BMI and BMI change following incident type 2 diabetes and risk of microvascular and macrovascular complications: the EPIC-Potsdam study. Diabetologia. 2021;64:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghavami H, Radfar M, Soheily S, Shamsi SA, Khalkhali HR. Effect of lifestyle interventions on diabetic peripheral neuropathy in patients with type 2 diabetes, result of a randomized clinical trial. Agri. 2018;30(4):165-170. [DOI] [PubMed] [Google Scholar]
- 12. The Look AHEAD Research Group. Effects of a long-term lifestyle modification programme on peripheral neuropathy in overweight or obese adults with type 2 diabetes: the Look AHEAD study. Diabetologia. 2017;60(6):980-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wake N, Hisashige A, Katayama T, et al. Cost-effectiveness of intensive insulin therapy for type 2 diabetes: a 10-year follow-up of the Kumamoto study. Diabetes Res Clin Pract. 2000;48(3):201-210. [DOI] [PubMed] [Google Scholar]
- 14. Streckmann F, Zopf EM, Lehmann HC, et al. Exercise intervention studies in patients with peripheral neuropathy: a systematic review. Sports Med. 2014;44(9):1289-1304. [DOI] [PubMed] [Google Scholar]
- 15. Martin CL, Albers JW, Pop-Busui R; DCCT/EDIC Research Group. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loprinzi PD, Hager KK, Ramulu PY. Physical activity, glycemic control, and diabetic peripheral neuropathy: a national sample. J Diabetes Complications. 2014;28(1):17-21. doi: 10.1016/j.jdiacomp.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 17. Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist. 2008;14(1):23-29. [DOI] [PubMed] [Google Scholar]
- 18. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- 19. Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14(8):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nukada H. Ischemia and diabetic neuropathy. Handb Clin Neurol. 2014;126:469-487. [DOI] [PubMed] [Google Scholar]
- 21. Clair C, Cohen MJ, Eichler F, Selby KJ, Rigotti NA. The effect of cigarette smoking on diabetic peripheral neuropathy: a systematic review and meta-analysis. J Gen Intern Med. 2015;30:1193-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forrest KY, Maser RE, Pambianco G, Becker DJ, Orchard TJ. Hypertension as a risk factor for diabetic neuropathy: a prospective study. Diabetes. 1997;46(4):665-670. [DOI] [PubMed] [Google Scholar]
- 23. Karvestedt L, Martensson E, Grill V, et al. Peripheral sensory neuropathy associates with micro- or macroangiopathy: results from a population-based study of type 2 diabetic patients in Sweden. Diabetes Care. 2009;32(2):317-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tesfaye S, Chaturvedi N, Eaton SE, et al. ; EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. New Engl J Med. 2005;352:341-350. [DOI] [PubMed] [Google Scholar]
- 25. Eid S, Sas KM, Abcouwer SF, et al. New insights into the mechanisms of diabetic complications: role of lipids and lipid metabolism. Diabetologia. 2019;62(9):1539-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirthi V, Perumbalath A, Brown E, et al. Prevalence of peripheral neuropathy in pre-diabetes: A systematic review. BMJ Open Diabetes Res Care. 2021;9(1):e002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonora E, Trombetta M, Dauriz M, et al. Chronic complications in patients with newly diagnosed type 2 diabetes: prevalence and related metabolic and clinical features: the Verona Newly Diagnosed Type 2 Diabetes Study (VNDS) 9. BMJ Open Diabetes Res Care. 2020;8(1):e001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albers JW, Herman WH, Pop-Busui R, et al. ; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ang L, Jaiswal M, Martin C, Pop-Busui R. Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. American Diabetes Association. Neuropathy: steps to prevent or delay nerve damage. Accessed March 29, 2021. [Google Scholar]
- 32. Davis TME, Yeap BB, Davis WA, Bruce DG. Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2008;51(4):562-566. [DOI] [PubMed] [Google Scholar]
- 33. Zilliox LA, Russell JW. Physical activity and dietary interventions in diabetic neuropathy: a systematic review. Clin Auton Res. 2019;29(4):443-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herman WH, Kennedy L. Underdiagnosis of peripheral neuropathy in type 2 diabetes. Diabetes Care. 2005;28(6):1480-1481. [DOI] [PubMed] [Google Scholar]
- 35. Sadosky A, Hopper J, Parsons B. Painful diabetic peripheral neuropathy: results of a survey characterizing the perspectives and misperceptions of patients and healthcare practitioners. Patient. 2014;7(1):107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adams AS, Callaghan B, Grant RW. Overcoming barriers to diabetic polyneuropathy management in primary care. Healthcare. 2017;5(4):171-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Themistocleous AC, Ramirez JD, Serra J, Bennett DLH. The clinical approach to small fibre neuropathy and painful channelopathy. Pract Neurol. 2014;14(6):368-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Glatte P, Buchmann SJ, Hijazi MM, Illigens BMW, Siepmann T. Architecture of the cutaneous autonomic nervous system. Front Neurol. 2019;10:970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russell JW, Zilliox LA. Diabetic neuropathies. Continuum (Minneap Minn). 2014;20(5):1226-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vinik A, Ullal J, Parson HK, Casellini CM. Diabetic neuropathies: clinical manifestations and current treatment options. Nat Clin Pract Endocrinol Metab. 2006;2(5):269-281. [DOI] [PubMed] [Google Scholar]
- 41. Nagaraja BS, Manjula J. A study to estimate the occurrence of peripheral neuropathy among asymptomatic type 2 diabetic patients. Int J Adv Med. 2020;7(5):835-839. https://www.ijmedicine.com/index.php/ijam/article/view/2297. Accessed August 3,2021. [Google Scholar]
- 42. Casellini CM, Vinik AI. Clinical manifestations and current treatment options for diabetic neuropathies. Endocr Pract. 2007;13(5):550-566. [DOI] [PubMed] [Google Scholar]
- 43. Rinkel WD, van Nieuwkasteele S, Castro Cabezas M, van Neck JW, Birnie E, Coert JH. Balance, risk of falls, risk factors and fall-related costs in individuals with diabetes. Diabetes Res Clin Pract. 2019;158:107930. [DOI] [PubMed] [Google Scholar]
- 44. Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3-22. [DOI] [PubMed] [Google Scholar]
- 45. Burgess J, Frank B, Marshall A, et al. Early detection of diabetic peripheral neuropathy: a focus on small nerve fibres. Diagnostics (Basel). 2021;11(165):1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eichholz M, Alexander AH, Cappelleri JC, et al. Perspectives on the impact of painful diabetic peripheral neuropathy in a multicultural population. Clin Diabetes Endocrinol. 2017;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang F, Zhang J, Yu J, et al. Diagnostic accuracy of monofilament tests for detecting diabetic peripheral neuropathy: a systematic review and meta-analysis. J Diabetes Res. 2017;2017:8787261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hirschfeld G, von Glischinski M, Blankenburg M, Zernikow B. Screening for peripheral neuropathies in children with diabetes: a systematic review. Pediatrics. 2014;133(5):e1324-e1330. [DOI] [PubMed] [Google Scholar]
- 49. Smith A, Lessard M, Singleton J. The diagnostic utility of nerve conduction studies and skin biopsy for diabetic neuropathy (DPN): a Bayesian analysis. Neurology.2015;84 (supp14) S17.008. [Google Scholar]
- 50. Dyck PJ, Davies J, Litchy W, O’Brien P. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology.1997;49(1): 229-239. [DOI] [PubMed] [Google Scholar]
- 51. Binns-Hall O, Selvarajah D, Sanger D, Walker J, Scott A, Tesfaye S. One-stop microvascular screening service: an effective model for the early detection of diabetic peripheral neuropathy and the high-risk foot. Diabet Med. 2018;35(7):887-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chatzikosma G, Pafili K, Demetriou M, et al. Evaluation of sural nerve automated nerve conduction study in the diagnosis of peripheral neuropathy in patients with type 2 diabetes mellitus. Arch Med Sci. 2016;12(2):390-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hamasaki H, Hamasaki Y. Diabetic neuropathy evaluated by a novel device: sural nerve conduction is associated with glycemic control and ankle–brachial pressure index in Japanese patients with diabetes. Front Endocrinol (Lausanne). 2017;8:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hirayasu K, Sasaki H, Kishimoto S, et al. Difference in normal limit values of nerve conduction parameters between Westerners and Japanese people might need to be considered when diagnosing diabetic polyneuropathy using a point-of-care sural nerve conduction device (NC-stat®/DPNCheck™). J Diabetes Invest. 2018;9(5):1173-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kamiya H, Shibata Y, Himeno T, et al. Point-of-care nerve conduction device predicts the severity of diabetic polyneuropathy: a quantitative, but easy-to-use, prediction model. J Diabetes Invest. 2021;12(4):583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kural MA, Andersen ST, Andersen NT, et al. The utility of a point-of-care sural nerve conduction device for detection of diabetic polyneuropathy: a cross-sectional study. Muscle Nerve. 2019;59(2):187-193. [DOI] [PubMed] [Google Scholar]
- 57. Papanas N, Pafili K, Demetriou M, Chatzikosma G, Papachristou S, Papazoglou D. Automated measurement of sural nerve conduction is a useful screening tool for peripheral neuropathy in type 1 diabetes mellitus. Rev Diabet Stud. 2019;15:58-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scarr D, Lovblom LE, Cardinez N, et al. Validity of a point-of-care nerve conduction device for polyneuropathy identification in older adults with diabetes: results from the Canadian Study of Longevity in Type 1 Diabetes. PLoS One. 2018;13(4):e0196647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sharma S, Vas PR, Rayman G. Assessment of diabetic neuropathy using a point-of-care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol. 2015;9(1):123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shibata Y, Himeno T, Kamiya T, et al. Validity and reliability of a point-of-care nerve conduction device in diabetes patients. J Diabetes Invest. 2019;10(5):1291-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pafili K, Maltezos E, Papanas N. NC-stat for the diagnosis of diabetic polyneuropathy. Expert Rev Med Devices. 2017;14(4):251-254. [DOI] [PubMed] [Google Scholar]
- 62. Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17(7):903-912. [DOI] [PubMed] [Google Scholar]
- 63. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131(7):1912-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15(3):202-207. [DOI] [PubMed] [Google Scholar]
- 65. Provitera V, Gibbons CH, Wendelschafer-Crabb G, et al. A multi-center, multinational age- and gender-adjusted normative dataset for immunofluorescent intraepidermal nerve fiber density at the distal leg. Eur J Neurol. 2016;23(2):333-338. [DOI] [PubMed] [Google Scholar]
- 66. Shikuma CM, McArthur JC, Ebenezer GJ, et al. Ethnic differences in epidermal nerve fiber density. Muscle Nerve. 2013;48(3):462-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liu Y, Fan X, Wei Y, Piao Z, Jiang X. Intraepidermal nerve fiber density of healthy human. Neurol Res. 2014;36(10):911-914. [DOI] [PubMed] [Google Scholar]
- 68. Jin P, Cheng L, Chen M, Zhou L. Low sensitivity of skin biopsy in diagnosing small fiber neuropathy in Chinese Americans. J Clin Neuromuscul Dis. 2018;20(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shy ME, Frohman EM, So YT, et al. Quantitative sensory testing: report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. 2003;60(6):898-904. [DOI] [PubMed] [Google Scholar]
- 70. Backonja MM, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus [published correction appears in Pain. 2014;155(1):205]. Pain. 2013;154(9):1807-1819. [DOI] [PubMed] [Google Scholar]
- 71. Abraham A, Alabdali M, Alsulaiman A, et al. Laser doppler flare imaging and quantitative thermal thresholds testing performance in small and mixed fiber neuropathies. PLoS One. 2016;11(11):e0165731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Alam U, Jeziorska M, Petropoulos IN, et al. Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS One. 2017;12(7):e0180175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care. 2015;38(8):1502-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Courtin AS, Maldonado Slootjes S, Caty G, Hermans MP, Plaghki L, Mouraux A. Assessing thermal sensitivity using transient heat and cold stimuli combined with a Bayesian adaptive method in a clinical setting: a proof of concept study. Eur J Pain. 2020;24(9):1812-1821. [DOI] [PubMed] [Google Scholar]
- 75. Dhage S, Ferdousi M, Adam S, et al. Corneal confocal microscopy identifies small fibre damage and progression of diabetic neuropathy. Sci Rep. 2021;11(1):1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fabry V, Gerdelat A, Acket B, et al. Which method for diagnosing small fiber neuropathy? Front Neurol. 2020;11:342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Farooqi MA, Lovblom LE, Lysy Z, et al. Validation of cooling detection threshold as a marker of sensorimotor polyneuropathy in type 2 diabetes. J Diabetes Complications. 2016;30(4):716-722. [DOI] [PubMed] [Google Scholar]
- 78. Ferdousi M, Kalteniece A, Azmi S, et al. Corneal confocal microscopy compared with quantitative sensory testing and nerve conduction for diagnosing and stratifying the severity of diabetic peripheral neuropathy. BMJ Open Diabetes Res Care. 2020;8(2):e001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Løseth S, Stålberg EV, Lindal S, Olsen E, Jorde R, Mellgren SI. Small and large fiber neuropathy in those with type 1 and type 2 diabetes: a 5-year follow-up study. J Peripher Nerv Syst. 2016;21(1):15-21. [DOI] [PubMed] [Google Scholar]
- 80. Pfau DB, Greffrath W, Schilder A, et al. Technical and clinical performance of the thermo-test device “Q-Sense” to assess small fibre function: a head-to-head comparison with the “Thermal Sensory Analyzer” TSA in diabetic patients and healthy volunteers. Eur J Pain. 2019;23(10):1863-1878. [DOI] [PubMed] [Google Scholar]
- 81. Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. 2015;38(4):dc142114-dc142675. [DOI] [PubMed] [Google Scholar]
- 82. Ponirakis G, Odriozola MN, Odriozola S, et al. NerveCheck: an inexpensive quantitative sensory testing device for patients with diabetic neuropathy. Diabetes Res Clin Pract. 2016;113:101-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ponirakis G, Odriozola MN, Odriozola S, et al. NerveCheck for the detection of sensory loss and neuropathic pain in diabetes. Diabetes Technol Ther. 2016;18(12):800-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Parson HK, Nguyen VT, Boyd AL, Vinik A. CHEPS detects neuropathic changes earlier than traditional clinical measures. Diabetes. 2009;58(Suppl):829P.19136654 [Google Scholar]
- 85. Warbrick T, Derbyshire SWG, Bagshaw AP. Optimizing the measurement of contact heat evoked potentials. J Clin Neurophysiol. 2009;26(2):117-122. [DOI] [PubMed] [Google Scholar]
- 86. Atherton DD, Facer P, Roberts KM, et al. Use of the novel contact heat evoked potential stimulator (CHEPS) for the assessment of small fibre neuropathy: correlations with skin flare responses and intra-epidermal nerve fibre counts. BMC Neurol. 2007;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu SW, Wang YC, Hsieh PC, et al. Biomarkers of neuropathic pain in skin nerve degeneration neuropathy: contact heat-evoked potentials as a physiological signature. Pain. 2017;158(3):516-525. [DOI] [PubMed] [Google Scholar]
- 88. Hertz P, Bril V, Orszag A, et al. Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabetic Med. 2011;28(10):1253-1260. [DOI] [PubMed] [Google Scholar]
- 89. Halpern EM, Lovblom LE, Orlov S, Ahmed A, Bril V, Perkins BA. The impact of common variation in the definition of diabetic sensorimotor polyneuropathy on the validity of corneal in vivo confocal microscopy in patients with type 1 diabetes: a brief report. J Diabetes Complications. 2013;27(3):240-242. [DOI] [PubMed] [Google Scholar]
- 90. Papanas N, Ziegler D. Corneal confocal microscopy: a new technique for early detection of diabetic neuropathy. Curr Diab Rep. 2013;13(4):488-499. [DOI] [PubMed] [Google Scholar]
- 91. Tavakoli M, Petropoulos IN, Malik RA. Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. J Diabetes Sci Technol. 2013;7(5):1179-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Al-Fahdawi S, Qahwaji R, Al-Waisy AS, et al. A fully automatic nerve segmentation and morphometric parameter quantification system for early diagnosis of diabetic neuropathy in corneal images. Comput Methods Programs Biomed. 2016;135:151-166. [DOI] [PubMed] [Google Scholar]
- 93. Al Rashah K, Pritchard N, Dehghani C, et al. Repeatability of measuring corneal nerve migration rate in individuals with and without diabetes. Cornea. 2016;35(10):1355-1361. [DOI] [PubMed] [Google Scholar]
- 94. Batawi H, Shalabi N, Joag M, et al. Sub-basal corneal nerve plexus analysis using a new software technology. Eye Contact Lens. 2018;44(Suppl 1):S199-S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Brines M, Culver DA, Ferdousi M, et al. Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci Rep. 2018;8(1):4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chen X, Graham J, Dabbah MA, et al. Small nerve fiber quantification in the diagnosis of diabetic sensorimotor polyneuropathy: comparing corneal confocal microscopy with intraepidermal nerve fiber density. Diabetes Care. 2015;38(6):1138-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen X, Graham J, Dabbah MA, Petropoulos IN, Tavakoli M, Malik RA. An automatic tool for quantification of nerve fibers in corneal confocal microscopy images. IEEE Trans Biomed Eng. 2017;64(4):786-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen X, Graham J, Petropoulos IN, et al. Corneal nerve fractal dimension: a novel corneal nerve metric for the diagnosis of diabetic sensorimotor polyneuropathy. Investig Ophthalmol Vis Sci. 2018;59(2):1113-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dehghani C, Pritchard N, Edwards K, Russell AW, Malik RA, Efron N. Risk factors associated with corneal nerve alteration in type 1 diabetes in the absence of neuropathy: a longitudinal in vivo corneal confocal microscopy study. Cornea. 2016;35(6):847-852. [DOI] [PubMed] [Google Scholar]
- 100. dell’Omo R, Cifariello F, De Turris S, et al. Confocal microscopy of corneal nerve plexus as an early marker of eye involvement in patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;142:393-400. [DOI] [PubMed] [Google Scholar]
- 101. Edwards K, Pritchard N, Dehghani C, et al. Corneal confocal microscopy best identifies the development and progression of neuropathy in patients with type 1 diabetes. J Diabetes Complications. 2017;31(8):1325-1327. [DOI] [PubMed] [Google Scholar]
- 102. Ferdousi M, Kalteniece A, Petropoulos I, et al. Diabetic neuropathy is characterized by progressive corneal nerve fiber loss in the central and inferior whorl regions. Invest Ophthalmol Vis Sci. 2020;61(3):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ferdousi M, Kalteniece A, Azmi S, et al. Diagnosis of neuropathy and risk factors for corneal nerve loss in type 1 and type 2 diabetes: a corneal confocal microscopy study. Diabetes Care. 2021;44(1):150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Guimarães P, Wigdahl J, Ruggeri A. A fast and efficient technique for the automatic tracing of corneal nerves in confocal microscopy. Transl Vis Sci Technol. 2016;5(5):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kalteniece A, Ferdousi M, Adam S, et al. Corneal confocal microscopy is a rapid reproducible ophthalmic technique for quantifying corneal nerve abnormalities. PLoS One. 2017;12(8):e0183040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lewis EJH, Lovblom LE, Ferdousi M, et al. Rapid corneal nerve fiber loss: a marker of diabetic neuropathy onset and progression. Diabetes Care. 2020;43(8):1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li Q, Zhong Y, Zhang T, et al. Quantitative analysis of corneal nerve fibers in type 2 diabetics with and without diabetic peripheral neuropathy: comparison of manual and automated assessments. Diabetes Res Clin Pract. 2019;151:33-38. [DOI] [PubMed] [Google Scholar]
- 108. Lovblom LE, Halpern EM, Wu T, et al. In vivo corneal confocal microscopy and prediction of future-incident neuropathy in type 1 diabetes: a preliminary longitudinal analysis. Can J Diabetes. 2015;39(5):390-397. [DOI] [PubMed] [Google Scholar]
- 109. Ostrovski I, Lovblom LE, Farooqi MA, et al. Reproducibility of in vivo corneal confocal microscopy using an automated analysis program for detection of diabetic sensorimotor polyneuropathy. PLoS One. 2015;10(11):e0142309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018;61(8):1856-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Petropoulos IN, Ferdousi M, Marshall A, et al. The inferior whorl for detecting diabetic peripheral neuropathy using corneal confocal microscopy. Investig Ophthalmol Vis Sci. 2015;56(4):2498-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pritchard N, Dehghani C, Edwards K, et al. Utility of assessing nerve morphology in central cornea versus whorl area for diagnosing diabetic peripheral neuropathy. Cornea. 2015;34(7):756-761. [DOI] [PubMed] [Google Scholar]
- 113. Scarpa F, Colonna A, Ruggeri A. Multiple-image deep learning analysis for neuropathy detection in corneal nerve images. Cornea. 2020;39(3):342-347. [DOI] [PubMed] [Google Scholar]
- 114. Scarr D, Lovblom LE, Ostrovski I, et al. Agreement between automated and manual quantification of corneal nerve fiber length: implications for diabetic neuropathy research. J Diabetes Complications. 2017;31(6):1066-1073. [DOI] [PubMed] [Google Scholar]
- 115. Schaldemose EL, Hammer RE, Ferdousi M, Malik RA, Nyengaard JR, Karlsson P. An unbiased stereological method for corneal confocal microscopy in patients with diabetic polyneuropathy. Sci Rep. 2020;10(1):12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tummanapalli SS, Issar T, Kwai N, et al. A comparative study on the diagnostic utility of corneal confocal microscopy and tear neuromediator levels in diabetic peripheral neuropathy. Curr Eye Res. 2020;45(8):921-930. [DOI] [PubMed] [Google Scholar]
- 117. Wang M, Zhang C, Zuo A, Li L, Chen L, Hou X. Diagnostic utility of corneal confocal microscopy in type 2 diabetic peripheral neuropathy. J Diabetes Investig. 2020. published online August 3, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Williams BM, Borroni D, Liu R, et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: a development and validation study. Diabetologia. 2020;63(2):419-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Illigens BMW, Gibbons CH. Sweat testing to evaluate autonomic function. Clin Auton Res. 2009;19(2):79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Gwathmey KG, Pearson KT. Diagnosis and management of sensory polyneuropathy. BMJ. 2019;365:1108. [DOI] [PubMed] [Google Scholar]
- 121. Novak P. Electrochemical skin conductance: a systematic review. Clin Auton Res. 2019;29(1):17-29. [DOI] [PubMed] [Google Scholar]
- 122. Kamel JT, Vogrin SJ, Knight-Sadler RJ, et al. Combining cutaneous silent periods with quantitative sudomotor axon reflex testing in the assessment of diabetic small fiber neuropathy. Clin Neurophysiol. 2015;126(5):1047-1053. [DOI] [PubMed] [Google Scholar]
- 123. Krieger SM, Reimann M, Haase R, Henkel E, Hanefeld M, Ziemssen T. Sudomotor testing of diabetes polyneuropathy. Front Neurol. 2018;9:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Loavenbruck A, Sit N, Provitera V, Kennedy W. High-resolution axon reflex sweat testing for diagnosis of neuropathy. Clin Auton Res. 2019;29(1):55-62. [DOI] [PubMed] [Google Scholar]
- 125. Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther. 2013;15:948-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mayaudon H, Miloche PO, Bauduceau B. A new simple method for assessing sudomotor function: relevance in type 2 diabetes. Diabetes Metab. 2010;36(6 Pt 1):450-454. [DOI] [PubMed] [Google Scholar]
- 127. Vinik AI, Nevoret M, Casellini C, Parson H. Neurovascular function and sudorimetry in health and disease. Curr Diab Rep. 2013;13(4):517-532. [DOI] [PubMed] [Google Scholar]
- 128. Ang L, Jaiswal M, Callaghan B, Raffel D, Brown MB, Pop-Busui R. Sudomotor dysfunction as a measure of small fiber neuropathy in type 1 diabetes. Auton Neurosci. 2017;205:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bordier L, Dolz M, Monteiro L, Névoret ML, Calvet JH, Bauduceau B. Accuracy of a rapid and non-invasive method for the assessment of small fiber neuropathy based on measurement of electrochemical skin conductances. Front Endocrinol (Lausanne). 2016;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Carbajal-Ramírez A, Hernández-Domínguez JA, Molina-Ayala MA, Rojas-Uribe MM, Chávez-Negrete A. Early identification of peripheral neuropathy based on sudomotor dysfunction in Mexican patients with type 2 diabetes. BMC Neurol. 2019;19(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chae CS, Park GY, Choi YM, et al. Rapid, objective and non-invasive diagnosis of sudomotor dysfunction in patients with lower extremity dysesthesia: a cross-sectional study. Ann Rehabil Med. 2017;41(6):1028-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. D’Amato C, Greco C, Lombardo G, et al. The diagnostic usefulness of the combined COMPASS 31 questionnaire and electrochemical skin conductance for diabetic cardiovascular autonomic neuropathy and diabetic polyneuropathy. J Peripher Nerv Syst. 2020;25(1):44-53. [DOI] [PubMed] [Google Scholar]
- 133. Gandecka A, Araszkiewicz A, Piłaciński S, Wierusz-Wysocka B, Zozulińska-Ziółkiewicz D. Evaluation of sudomotor function in adult patients with long lasting type 1 diabetes. Pol Arch Intern Med. 2017;127(1):16-24. [DOI] [PubMed] [Google Scholar]
- 134. Goel A, Shivaprasad C, Kolly A, Sarathi H A V, Atluri S. Comparison of electrochemical skin conductance and vibration perception threshold measurement in the detection of early diabetic neuropathy. PLoS One. 2017;12(9):e0183973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jin J, Wang W, Gu T, et al. The application of SUDOSCAN for screening diabetic peripheral neuropathy in Chinese population. Exp Clin Endocrinol Diabetes. 2018;126(08):472-477. [DOI] [PubMed] [Google Scholar]
- 136. Novak P. Electrochemical skin conductance correlates with skin nerve fiber density. Front Aging Neurosci. 2016;8:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Porubcin MG, Novak P. Diagnostic accuracy of electrochemical skin conductance in the detection of sudomotor fiber loss. Front Neurol. 2020;11:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Selvarajah D, Cash T, Davies J, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One. 2015;10(10):e0138224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sheshah E, Madanat A, Al-Greesheh F, et al. Electrochemical skin conductance to detect sudomotor dysfunction, peripheral neuropathy and the risk of foot ulceration among Saudi patients with diabetes mellitus. J Diabetes Metab Disord. 2015;15(29):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lee JA, Halpern EM, Lovblom LE, Yeung E, Bril V, Perkins BA. Reliability and validity of a point-of-care sural nerve conduction device for identification of diabetic neuropathy [published correction appears in PLoS One. 2014;9(8):e106981]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Umapathi T, Tan WL, Loke SC, Soon PC, Tavintharan S, Chan YH. Intraepidermal nerve fiber density as a marker of early diabetic neuropathy. Muscle Nerve. 2007;35(5):591-598. [DOI] [PubMed] [Google Scholar]
- 142. Khoshnoodi MA, Truelove S, Burakgazi A, Hoke A, Mammen AL, Polydefkis M. Longitudinal assessment of small fiber neuropathy: evidence of a non-length-dependent distal axonopathy. JAMA Neurol. 2016;73(6):684-690. [DOI] [PubMed] [Google Scholar]
- 143. Ragé M, Van Acker N, Knaapen MW, et al. Asymptomatic small fiber neuropathy in diabetes mellitus: investigations with intraepidermal nerve fiber density, quantitative sensory testing and laser-evoked potentials. J Neurol. 2011;258(10):1852-1864. [DOI] [PubMed] [Google Scholar]
- 144. Alahmar AT. Quantitative sensory testing in type-1 diabetic patients with mild to severe diabetic neuropathy. JRMDS.2016;4(2):105-114. [Google Scholar]
- 145. Kopf S, Groener JB, Kender Z, et al. Deep phenotyping neuropathy: an underestimated complication in patients with pre-diabetes and type 2 diabetes associated with albuminuria. Diabetes Res Clin Pract. 2018;146:191-201. [DOI] [PubMed] [Google Scholar]
- 146. Jimenez-Cohl P, Grekin C, Leyton C, Vargas C, Villaseca R. Thermal threshold: research study on small fiber dysfunction in distal diabetic polyneuropathy. J Diabetes Sci Technol. 2012;6(1):177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148-2154. [DOI] [PubMed] [Google Scholar]
- 148. Ziegler D, Papanas N, Zhivov A, et al. Early detection of nerve fiber loss by corneal confocal microscopy and skin biopsy in recently diagnosed type 2 diabetes. Diabetes. 2014;63(7):2454-2463. [DOI] [PubMed] [Google Scholar]
- 149. Pritchard N, Edwards K, Dehghani C, Fadavi H, Jeziorska M, Marshall A, Petropoulos IN, Ponirakis G, Russell AW, Sampson GP, Shahidi AM, Srinivasan S, Tavakoli M, Vagenas D, Malik RA, Efron N. Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristics. Diabetes Res Clin Pract. 2014;104(2):248-56. [DOI] [PubMed] [Google Scholar]
- 150. Edwards K, Pritchard N, Vagenas D, Russell A, Malik RA, Efron N. Utility of corneal confocal microscopy for assessing mild diabetic neuropathy: baseline findings of the LANDMark study. Clin Exp Optom. 2012;95(3):348-354. [DOI] [PubMed] [Google Scholar]
- 151. Asghar O, Petropoulos IN, Alam U, et al. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care. 2014;37(9):2643-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther. 2013;15(11):948-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Smith AG, Lessard M, Reyna S, Doudova M, Singleton JR. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications. 2014;28(4):511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yajnik CS, Kantikar VV, Pande AJ, Deslypere JP. Quick and simple evaluation of sudomotor function for screening of diabetic neuropathy. ISRN Endocrinol. 2012;2012:103714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Calvet J, Dupin J, Winiecki H, Schwarz PE. Assessment of small fiber neuropathy through a quick, simple and non invasive method in a German diabetes outpatient clinic. Exp Clin Endocrinol Diabetes. 2012;121(02):80-83. [DOI] [PubMed] [Google Scholar]
- 156. Gin H, Baudoin R, Raffaitin CH, Rigalleau V, Gonzalez C. Non-invasive and quantitative assessment of sudomotor function for peripheral diabetic neuropathy evaluation. Diabetes Metab. 2011;37(6):527-532. [DOI] [PubMed] [Google Scholar]
- 157. Hozumi J, Sumitani M, Matsubayashi Y, et al. Relationship between neuropathic pain and obesity. Pain Res Manag. 2016;2016:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Invest. 2017;8(5):646-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Oh TJ, Lee JE, Choi SH, Jang HC. Association between body fat and diabetic peripheral neuropathy in middle-aged adults with type 2 diabetes mellitus: a preliminary report. J Obes Metab Syndr. 2019;28(2):112-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Balducci S, Iacobellis G, Parisi L, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complications. 2006;20(4):216-223. [DOI] [PubMed] [Google Scholar]
- 161. Kluding PM, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. New Engl J Med. 2009;360:129-139. [DOI] [PubMed] [Google Scholar]
- 164. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837. [PubMed] [Google Scholar]


