Abstract
Diabetes polyneuropathy is an important complication of diabetes polyneuropathy, and its notable sequelae of foot ulceration, autonomic dysfunction, and neuropathic pain are associated with significant morbidity and mortality. Despite the major impact on quality of life and health economic costs, it remains underdiagnosed until late in its natural history, and there is lack of any intervention that can reverse its clinical progress. Assessment of small fiber neuropathy (SFN) in diabetes offers an opportunity to detect abnormalities at an early stage so that both interventional studies and preventative measures can be enacted to prevent progression to the devastating complications of foot ulceration and cardiac dysautonomic death. Over the last two decades, significant advances have been made in understanding the pathophysiology of diabetes neuropathy and its assessment. In this review, we discuss limitations of the screening methods recommended in current clinical guidelines which are based on large nerve fiber assessments. Thereafter, we discuss in detail the various methods currently available to assess small fiber structure and function and examine their individual strength and limitations. Finally, we discuss the reasons why despite the considerable body of evidence available, legislators and global experts have yet to incorporate the assessment of SFN as routine clinical surveillance in diabetes management. We hope that these insights will stimulate further discussion and be instrumental in the early adoption of these methods so as to reduce the burden of complications arising due to diabetes polyneuropathy.
Keywords: diabetes, polyneuropathy, small fibers, diagnosis
Introduction
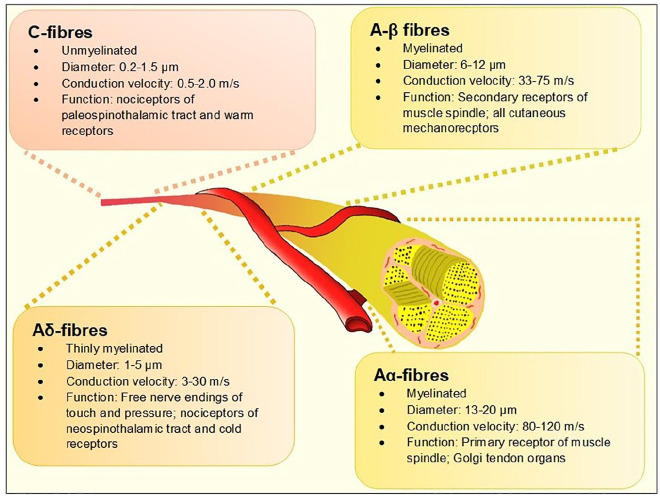
The term diabetes polyneuropathy (DPN) refers to a heterogeneous group of neurological disorders—either clinically evident or subclinical—that occur in the setting of diabetes mellitus after exclusion of other etiologies of peripheral neuropathy. A recent position statement by the American Diabetes Association (ADA) proposed a clinical classification of DPN into typical/diffuse or atypical neuropathy with the former further categorized based on organ-systems affected. 1 Distal symmetrical polyneuropathy (DSPN) is considered the most common form of DPN with estimated prevalence rates reaching up to 75% in some studies. 2 Case definition of DSPN has historically been based either on patient-reporting of neurological symptoms or insensitivity to a 10-gm Semmes-Weinstein mono-filament (SWMF), abnormal vibration sensation using tuning forks, or abnormal ankle reflex during diabetes annual reviews. Electro diagnostic tests like nerve conduction studies (NCS) have been traditionally reserved for atypical presentations such as amyotrophy or entrapment neuropathy3,4 and are not part of routine DPN assessment. All these methodologies assess large fiber neural health. There is a significant body of evidence to suggest that the myelinated Aα and Aβ nerve fibers (Figure 1)—which serve touch, proprioception, position sense, vibration and muscle control and put patients at high risk of foot ulceration and its consequences—are affected late in the natural history of diabetes. 5 However, it is increasingly recognized that small nerve fibers are involved early in the course of DPN, often before large fiber involvement. Therefore, early detection of small fiber neuropathy (SFN) may offer hope for early intervention using disease-modifying strategies such as lifestyle modification, glycemic control, or novel therapeutics, in the same vein as retinal assessments for retinopathy and microalbuminuria for nephropathy have transformed their outcomes.
Figure 1.
The different types of peripheral nerves based on the Erlanger-Gasser classification. 6
Definition of SFN
SFN is a subtype of peripheral neuropathy affecting the thinly myelinated Aδ or the non-myelinated C fibers (Figure 1). These fibers constitute 79.6% to 91.4%7,8 of peripheral nerve fibers and mediate several key functions including temperature and pain perception, sweating, and tissue blood flow, all of which, when impaired, are pathophysiologically related to adverse outcomes associated with foot ulcerations in people with diabetes. 9 Another important function of small fibers is regulation of the autonomic system and when affected, the term “autonomic neuropathy” is often used.10,11
In 2010, the Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes (NEURODIAB) agreed on the diagnostic criteria for SFN in diabetes 12 (Table 1). It is interesting to note that despite significant developments in the detection of SFN in all forms of diabetes including pre-diabetes, type-1 diabetes (T1DM), and type-2 diabetes (T2DM), this definition has yet to be updated and remains based on length-dependent symptoms, intraepidermal nerve fiber density (IEFND), and quantitative sensory testing (QST). Furthermore, the consensus document posited that “at present, it is not possible to suggest criteria to define the severity of SFN in DPN.” This statement is perhaps in need of reappraisal in view of the advancements in tests for SFN, including the establishment of normative values for these tests.
Table 1.
Diagnostic Criteria for SFN in Diabetes Proposed by the Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes (NEURODIAB)—2010. 12
| Grading of SFN | Criterion |
|---|---|
| Possible | The presence of length-dependent symptoms and/or clinical signs of small fiber damage |
| Probable | The presence of length-dependent symptoms, clinical signs of small fiber damage, and normal sural NC study |
| Definite | The presence of length-dependent symptoms, clinical signs of small fiber damage, normal sural NC study, and altered IENF density at the ankle and/or abnormal QST thermal thresholds at the foot |
Abbreviations: IENF, intraepidermal nerve fiber density; NC, nerve conduction; QST, quantitative sensory testing; SFN, small fiber neuropathy.
Methods
Methods for evaluating SFN can be broadly categorized into two groups; first, techniques assessing small fiber function (SFF) mediated by the Aδ or the non-myelinated C fibers; and second, those quantitating small fiber structure (SFS). Table 2 provides a summary of the methods currently available.
Table 2.
A Summary of the Various Methods of Assessing SFF and SFS.
| Tests for SFN | |||||||
|---|---|---|---|---|---|---|---|
| Methods for assessment of SFN function | |||||||
| Test | Type | Technique | Equipment needed | Time to acquire results | Normative data | Operating characteristics for DSPN | Limitations |
| Laser Doppler imager Flare (LDIFLARE) | Noninvasive, quantitative | Measurement of the axon-reflex mediated flare response as a marker of small fiber function | Laser Doppler imager, temperature-controlled room, operator with experience | Image acquisition method since modification needs 30 minutes | One site normative values determined at a single center. Larger data set of normative valves desired | For the newer technique: sensitivity of 70% to 75%, specificity of 66% to 85%, positive predictive value of 74%, and negative predictive value of 86% | Dependent on the microcirculation. Patients need to have no significant macrovascular distal circulatory impairment. |
| Results available immediately | |||||||
| QST | Noninvasive, quantitative | Computerized measurement of thermal thresholds and heat pain thresholds | Computerized assessment device, temperature-controlled laboratory, and a trained technician | Takes about 30 minutes but could take longer, depending on subject concentration | Commercial normative data present from the bigger manufacturers | Normative data from individual manufacturers like Medoc Inc. not available | Psychophysical test-results are dependent on subject compliance and attention. Complex testing protocols present. Varying reproducibility depending on experience of the unit undertaking testing. |
| Sudoscan | Noninvasive, quantitative | Testing is based on stimulation of sweat glands by a low-voltage current (<4 volts) representing an electrochemical reaction between electrodes and chloride ions | Sudoscan device and trained operator | Takes around 5 minutes | Comes with inbuilt normative data. Limited experience at the moment | Increasing literature now available of its use in DSPN. Similar AUC as IENFD (0.761) in one study. For cardiac autonomic neuropathy, sensitivity was 65%, specificity 80%. | Availability is gradually increasing. Validation studies in various ethnicities is still awaited. |
| Neuropad | Simple qualitative indicator of sudomotor dysfunction | Simple sticker which changes color in the presence of sweating | Cheap and easy to avail | Takes around 10 minutes | Qualitative, does not need normative data | Lots of available literature and has been validated against IENFD. In one study, Neuropad had a sensitivity 85% and specificity of 45% for detection of clinical DSPN. | Difficult to interpret when there is partial change in color though. One center has published data on semi-quantification using digital imaging of the Neuropad. |
| Contact heat evoked potentials (CHEPs) | Noninvasive, quantitative | Measure cerebral responses to thermal stimuli mediated by A-delta fibers | Needs thermal threshold testing first. Then small discs are placed on the head to record signals received to the brain from application of 10 to 20 short (a fraction of a second) heat or cold stimuli at a particular point of interest (face, arm, or leg) | Takes 30 minutes but could take longer, depending on subject concentration | Multicenter normative data on 226 adult subjects is available | The AUC for DSPN detection in a small sample has been estimated at 0.778 | Requires active patient co-operation. Like QST and CPT, therefore reproducibility has been a challenge. Not widely available. Also unclear if both A-delta and C-fibers are assessed. |
| Laser evoked potentials (LEPs) | Noninvasive, quantitative | Radiant heat generate by laser selectively excites free nerve endings in the superficial skin layers activating nearby A-delta and C-fiber nociceptors | CO2-laser stimulator, technician with experience, and a temperature-controlled room ideally | May take up to 1 hour to complete the procedure and ensure no artefacts presents in readings gained | Single center normative values available on 100 subjects. No decade specific data reported. | None available. Studies have used age matched control data | Limited availability. May be useful in demonstrating reduced function but unable to detect enhanced transmission as found in hyperalgesia. Small changes in pain sensitivity are not easily detectable with LEP. |
| Microneurography | Minimally invasive, semi-quantitative | Measurement of single fiber recordings from peripheral axons | Skilled operator and extensive equipment list. Preserve of a large neurophysiology lab rather than clinic-based procedure. | May take up to 3 hours to get a satisfactory recording | None available | None available. Considered by EFNS to possess grade A evidence for assessing function of the A-delta fiber pathways in patients with neuropathic pain. | Still primarily a research tool. May have a role in assessment of neuropathic pain rather than early neuropathy. Expensive and needs skill to elicit responses. Patient cooperation is also extensively required. |
| Current perception threshold (CPT) (not described in main text) | Noninvasive, quantitative | Low current intensity stimulation of the small nerve fibers at frequency of 250 Hz for A-delta fibers and the 5 Hz for C-fibers | Neurometer device, temperature-controlled room, and a trained technician | Takes around 30 minutes but could take longer, depending on subject concentration | None available. Most studies have included age matched controls for comparison. | None available | Requires active patient cooperation. Like QST, therefore reproducibility has been a challenge and other methodological challenges persist (such as what frequency to use. Not widely available. |
| Quantitative sudomotor axon reflex test (QSART) | Sudomotor noninvasive, quantitative | Information on skin autonomic function and evaluation of postganglionic sudomotor function using acetylcholine iontophoresis | Purpose-built lab, needs iontophoresis and sudomotor quantification equipment | 45 to 60 minutes to complete | Normative data available from specific centers for QSART. A commercially available device QSWEAT is also available | No specific data available for DSPN but has been widely used, especially in the Rochester Diabetic Neuropathy study | Requires precautions for electrical safety and small risk of minor local injury to the skin |
| Thermo-regulatory sweat test (TST) (not described in main text) | Sudomotor noninvasive, semiquantitative | When core temperature rises beyond a hypothalamic thermoregulatory set point (>38°C), sweating occurs | Needs a laboratory and a digital camera | 90 to 120 minutes to perform correctly. Maximal sweating is achieved within 30 to 65 minutes. | Not available | Helpful data on the TST available in DSPN mainly from the autonomic lab at the Mayo Clinic, Rochester USA. | Patients may not be able to tolerate 60 minutes of warming up |
| Sympathetic skin response (not described in main text) | Sudomotor noninvasive, quantitative | Information on skin autonomic function and evaluation of postganglionic sudomotor function using electrodermal activity | Purpose-built lab, SSR equipment includes electrodes | 45 to 60 minutes to complete | Normative data available from specific centers but usually has been derived from a small normative group | Minimal data only available in DSPN. Some helpful data in diabetes autonomic neuropathy and bladder dysfunction. | Limited availability, needs expertise and experience to test correctly. Popular in Japan. |
| Methods for assessment of SFN structure | |||||||
| Corneal confocal microscopy | Noninvasive, quantitative | Measurement of nerve parameters of the corneal sub-basal layer | Corneal scanning confocal microscope, trained technician | Image acquisition takes 5 to 10 minutes. Results available immediately if automated counting used. | Multicenter normative data present yet to be globally established | Reported sensitivity of 85% and specificity of 84% | Surrogate marker of DSPN rather than a direct indicator. Previously reliant on manual counting but newer automated methods emerging. Emerging evidence suggests CNFL is the preferred longitudinal marker. |
| Skin biopsy | Invasive, quantitative | Measurement of intra-epidermal nerve fiber density | Sterile equipment for biopsy, access to trained personnel, and laboratory | Actual procedure takes 5 to 10 minutes with additional 60 minutes for tissue processing. Needs trained histopathologist to quantify results. | Worldwide normative data present | Published sensitivity doe DSPN is between 60% and 95% and specificity between 90% and 95% | Challenging to use in prospective studies of very large cohorts, infection risk at site of biopsy |
| Sural nerve biopsy | Invasive, quantitative | Ultrastructure and morphometric analysis of sural nerve biopsy specimens | Experienced operator who can perform biopsy and access to pathologist and at times, electron or confocal microscope | Procedure may take up to 45 minutes. Results usually take a few days. | None available | None available | Infection, pain, and hypoesthesia at biopsy site |
Abbreviations: AUC, area under curve; DSPN, distal symmetrical polyneuropathy; EFNS, European Federation of Neurological Societies; IENFD, intraepidermal nerve fiber density; QST, quantitative sensory testing; SFF, small fiber function; SFN, small fiber neuropathy; SFS, small fiber structure.
Methods of Assessing SFF
Laser Doppler imaging flare (LDIFLARE)
The LDIFLARE is a noninvasive technique of measuring C fiber-mediated cutaneous vasodilatation in the foot skin in response to thermal heating. The heating is done by a 1 cm2 probe and the area of induced hyperemia measured by a 610 nm laser probe. Validation studies have suggested a moderate to strong correlation with IEFND) intraepidermal nerve fiber density, 13 and in-vivo corneal confocal microscopy (CCM) 14 for the detection of DPN. The LDIFLARE is progressively reduced with increasing severity of DPN 15 and has also been noted to be impaired in subjects with impaired glucose tolerance (IGT) with normal thermal thresholds and large fiber assessments. 16 In a cohort of newly diagnosed T1DM subjects, following optimization of glycemic control, a significant increase in the LDIFLARE response was noted, suggesting that it is not only a sensitive method of assessing SFN, but importantly also a versatile measure of short-term fluctuation in SFF affected by metabolic milieu, for example, glycemic improvement or deterioration. 17 In a study of young subjects aged 12 to 16, the LDIFLARE method showed significant SFF reduction as compared to healthy and correlation with HbA1c change. 18 However, it takes around 30 minutes per patient for evaluation, which could be a limiting factor in its applicability in the busy clinical setting. 19 More longitudinal data are required to support its use as a routine tool for the assessment of diabetes-related SFN. It has been shown in other non-diabetes studies to be a sensitive investigation to measure SFN in hypothyroidism, 20 chemotherapy-induced peripheral neuropathy, 21 and hypertriglyceridemia. 22
QST for thermal and pain thresholds
Use of QST is a valuable method for assessing SFF and has been extensively used in both research and clinical settings, including assessment of peripheral neuropathy, acute and chronic pain. The various available devices of QST fundamentally determine the sensory and pain thresholds for cold and warm temperatures (thermal thresholds), and some devices also quantitate vibration perception a large fiber function. Examples include the Computer Aided Sensory Evaluator-IV (CASE-IV) and Medoc TSA-II NeuroSensory Analyzer. In a study of 498 T2DM and 434 healthy subjects, Chao et al 23 showed that an elevated warm threshold was the most frequent abnormality in the T2DM group (60.2%), compared with an abnormal cold threshold (39.6%) and an abnormal sural nerve conduction velocity (12.9%); it was also shown to be related to both symptoms and glycemic control. However, other studies have shown that unlike cold perception thresholds and IENFD, warm perception thresholds did not differentiate people with diabetes with and without symptoms. 24 Similarly, in a study of 191 people with diabetes, there was no difference in heat-pain. In a recent prospective T2DM study of 725 patients, 25 Sheen et al 26 showed there was a high prevalence (74.3%) of subclinical abnormal thermal thresholds (both warm and cold detection thresholds) measured by the Q-Sense Thermal Sensory Analyzer. These were significantly associated with peripheral arterial disease and nephropathy in their cohort population.
However, methods of QST have two important limiting factors. First, they need patient cooperation and can be adversely affected by patient-related altered motivation, inattention, and cognitive issues; second, if subjects have neurological abnormalities such as allodynia/hyperpathia, aberrations of temporal latency, or spatial localization, then QST outcomes can be significantly affected. This may explain why QST techniques demonstrate a modest correlation with IEFND. 27 Newer updates like the Medoc TSA-II have been able to mitigate some of these limitations; however, the psychophysical aspect of the tests is a non-modifiable limitation. Furthermore, there are very limited data on whether QSTs are able to detect small to modest degrees of improvement in SFF, a crucial aspect for suitability of technique for use in neuropathy intervention trials.
Assessment of sudomotor function
The assessment of sudomotor function as a marker for SFN is based on the principle that the degeneration of small nerve fibers reduces sweat gland innervation leading to an impairment in sweating. Based on this, several portable devices are now available, which allow rapid evaluation of sudomotor function. The Neuropad, a plaster comprising of an anhydrous cobalt II compound applied on the plantar forefoot, is centered on the principle of color change from blue to pink when in contact with sweat. 28 In a study of 127 subjects of both T1DM and T2DM subjects, Ponirakis et al 29 have shown that the Neuropad has good sensitivity and specificity for the detection of DPN in those with established clinical neuropathy comparing favorably with established modalities including CCM, NCV, and autonomic tests. The test can currently provide qualitative data only. The Sudoscan provides a quantitative measure of chloride conductance (measured as electrochemical skin conductance [ESC]) serving as a biomarker to assess sweat gland function in relation to sweat gland innervation.30,31 Studies have reported that the device has good correlations with DPN clinical scoring systems, large fiber methods like NCS, VPT, and tests for cardiac autonomic nervous system.30,32,33 However, in a study of 63 patients, Duchesne et al 34 showed that ESC measurement has a weak correlation with sweat gland density and IENFD in skin biopsies, prompting the speculation that mechanisms other than loss of innervating fibers may be responsible for sudomotor dysfunction in DPN.
An older method of assessing sudomotor function is the qualitative sudomotor axon reflex testing (QSART), which evaluated sudomotor function by assessing the local sweat response to iontophoresis of acetylcholine 35 and has been shown to be highly sensitive in the detection of distal SFN. 36 It is however time consuming and requires specialist equipment and laboratory access.
Evoked potentials
Nociceptive laser-evoked potentials (LEP) and contact heat-evoked potentials (CHEPs) are electrophysiological methods to detect functional impairment of small fibers in DPN. 37 However, LEP and CHEPs have drawbacks in clinical routine such as the need for expensive equipment and complex procedural algorithms. 38 Furthermore, reproducibility of these techniques is not fully established, 39 and it remains to be determined which of the variables measured—amplitudes, latencies, or areas—robustly differentiate and follow the course of SFN.39,40 The recording of electrically elicited pain-related evoked potentials (PREP) via concentric electrodes is a useful, noninvasive method to detect early SFN. PREP involves delivering a nociceptive electrical stimulus to the skin using a concentric surface electrode, which imparts a high current density with relatively low intensity, limiting the depolarization only to the nociceptive fibers, which is then detected via standard recording electrodes placed at C2. 41 Mueller et al, in a study of 35 patients with and 22 without painful neuropathy, showed that despite normal electrophysiology, PREP could detect increased latency and reduced amplitude in patients with painful neuropathy. 42 In a recent study by La Cesa et al, 43 LEP demonstrated excellent sensitivity and specificity with abnormal pinprick sensitivity and capsaicin-induced epidermal denervation prompting the authors to speculate that unlike LEPs and CHEPs, PREPs are not selectively mediated by nociceptive system. We believe that these techniques are still in their development phase, and further studies are required to outline their use in the assessment of SFN.
Microneurography
Microneurography is a minimally invasive neurophysiological technique allowing direct recording of unmyelinated postganglionic sympathetic or afferent nociceptive fibers function by inserting tungsten needles into a peripheral nerve fascicle.44,45 Orstavik et al 46 proposed that based on microneurographic studies, change in the distribution of C-nociceptive fibers in the skin as shown in the patients with diabetic neuropathy may help to reveal mechanisms responsible for small-fiber dysfunction. This technique has failed to gain traction beyond research uses since it is very time-consuming as well as requires specialist skill. Its strength is in its unique power for exploration of normal neural mechanisms as well as pathophysiological conditions of various neurological disorders. There have also been reports of nerve injury during this procedure. 47
Methods of Assessing SFS
Corneal confocal microscopy (CCM)
In the last two decades, CCM has emerged as the SFN assessment technique with the largest body of evidence to support its use in DPN. The procedure is noninvasive and relatively quick (taking three to five minutes in experienced hands), well tolerated by patients and provides in-vivo quantification of the sub-basal corneal nerve plexus (in-vivo). 48 Its portability makes it an attractive “add-on option” for neuropathy screening when diabetes subjects have their annual retinal surveillance. 49
CCM will be discussed in further detail in another section of this special supplement. It has been studied in most forms of diabetes in both cross-sectional and a few longitudinal studies. CCM has been shown to be predictive of development of future DPN, 50 correlates with severity of DPN, 51 and with autonomic diabetes neuropathy. 52 Finally, in a smaller study of 38 T1DM subjects, Edwards et al 53 showed that CCM demonstrated the greatest, and most sustained degeneration over a four-year period as compared to NCS, hot and cold thermal thresholds, VPT, 10 gm MF and neuropathy scores.
Skin biopsy
Skin biopsy is considered as the “gold-standard” for the diagnosis of SFN, and most methods already mentioned have to be validated against it for accuracy. It is a minimally invasive procedure using a disposable 3-5 mm punch biopsy device to sample the skin and the pan axonal marker PGP-9.5 to identify the small nerve fibers, which can then be quantified and expressed as the number of IENF per length of section (IENF/mm).54,55 It demonstrates good intra and inter-observer validity, 56 but like many SFN measures reduces with age. 57 This led to an international consortium of investigators to compile an age-related normative values for IENFD. 58 The technique has now been further adapted to specifically quantify dermal nerve fibers, autonomic innervation of the sweat glands, and pilomotor muscles. 59 One limitation is that it needs a specialized laboratory with staff trained to use specific neuronal markers. Repeated measures in study subjects may also be challenging, and certainly this aspect makes it less practical for deployment as a routine clinical test for annual DPN assessments. However, it is likely to remain the “gold-standard” for SFN assessment in research practice. 60
Nerve biopsy
This is a highly specialized invasive procedure traditionally used to quantify myelinated nerve fiber density, which is reduced in DPN and correlates with NCS.61,62 It has also been shown to be beneficial for early detection of DPN. 8 However, due to its invasive nature, requirement of neurosurgical expertise especially when fascicular biopsy is necessary, and electron microscopic interpretation, it cannot be advocated to be useful in the routine assessment of SFN in diabetes. Its mention in this section is for completion of the list of methodologies available.
SFF or SFS or Both?
In the past decade, there have been significant refinements of the methodologies for assessing SFN as described. However, there are several key questions that need to be answered by each of these methodologies before their acceptance into a wider clinical practice:
(i) Is the methodology simple enough to be carried out by diabetes-related health care professionals with minimal training and in a busy outpatient environment?
Adoption of a test to be used in a busy clinical setting requires it to be simple and quick to perform with good reproducibility. In this context, point of care devices for sudomotor function like the Neuropad and SUDOSCAN fit the above attributes. Whilst the Neuropad shows good correlation with CCM, NCV, and autonomic tests, it can only provide binary data, 29 and it is unclear how patchy/limited change on the pad should be interpreted. ESC measured by Sudoscan has a weak correlation with sweat gland density and IENFD in skin biopsies. 34 CCM appears to fulfil most criteria, in particular its noninvasive nature, rapid image acquisition, and potential scalability into large screening/assessment programs but costs around $30 000 per instrument, and there is the recurring cost of consumables including $5 per tomocap per patient and other topical medicines. The LDIFLARE and QST have robust clinical evidence to support their use but take longer to perform, require access to a laser doppler imager, and experienced operators, and hence, probably unsuitable for outpatient use. As it stands, there is a lack of an inexpensive, quantitative method to detect SFN in the current clinical settings.
(ii) Is the methodology sensitive enough to detect early changes in neural structure or function when evaluated at relatively short intervals of 12 months or less?
This is a question of fundamental importance, both for research and clinical settings. The two methodologies that are sensitive to detecting changes in small fiber responses to changing metabolic milieu are the LDIFLARE and CCM. Sharma et al 17 have shown that in newly diagnosed people with LDIFLARE T1DM, SFF assessed by the LDIFLARE, and CCM techniques at diagnosis and then at six months after the commencement of insulin and stable glycemic control, there was a significant improvement but nonsignificant change in CCM indices at six months. This study suggests that in people with T1DM, SFF assessed by the LDIFLARE is more sensitive to short-term changes in glycemia than SFS assessed by CCM. 17 Hassler-Hurst et al 18 similarly showed that a change of HbA1c as measure of glycemic control significantly inversely correlated with LDIFLARE size in young diabetes subjects aged 12 to 16. Azmi et al 63 reported that CCM detects SFN in subjects with prediabetes who go on to develop T2DM and found a dynamic worsening or improvement in corneal indices in relation to change in glucose tolerance status. Corneal regeneration evidenced by CCM has also been demonstrated by Tavakoli et al 64 in patients with T1DM undergoing simultaneous pancreas and kidney transplantation corneal nerve regeneration within six months, whereas other parameters including IENFD, QST, and NCS did not improve. There is also good evidence in support of IENFD in tracking relatively short-term changes in small fiber structure; however, the invasive nature of the technique limits its adoption into routine clinical use where repetitive monitoring would be required. Most of the other techniques do not have studies exploring their sensitivity and accuracy in response to diabetes related fluctuations; therefore, it is difficult to comment on their ability to detect meaningful improvements or decrements.
Is There a Strong Argument Now for Routine Use of SFN Techniques in the Early Diagnosis of DPN and Its Follow-Up?
The current ADA position statement on DPN advocates the following:
Assessment for distal symmetric polyneuropathy should include a careful history and assessment of either temperature or pinprick sensation (small-fiber function) and vibration sensation using a 128-Hz tuning fork (for large-fiber function). All patients should have annual 10-g monofilament testing to identify feet at risk for ulceration and amputation.1,65
It is noteworthy that despite the growing body of evidence of early involvement of small fibers in diabetes, the ADA remains reserved on use of modern small fiber techniques to diagnose early neuropathy. The same approach is endorsed by National Institute for Health and Care Excellence (NICE-UK) and European Association for the Study of Diabetes (EASD), which focus on the assessment of loss of protective sensation. 66 In the initial stages, the majority will have normal temperature or pinprick sensation, vibration sensation using a 128-Hz tuning fork, and 10-g monofilament testing. In such an instance, it would seem reasonable to consider the use of tests that will detect neuropathy at its earliest stages. Non-invasive tests like CCM and LDIFLARE have shown this potential but need to be more practical and less expensive for use in clinical envirorment where the focus is on early detection of complications. Annual screening for diabetic retinopathy with digital retinal photography and measurement of urinary microalbuminuria for detection of those at risk of progressing to significant diabetes nephropathy is now an established practice with proven clinical benefit.64,67 Furthermore, the data from such screening have proven important in facilitating the development of therapies that can reduce the decline renal function (eg, Ramipril 68 or more recently the use of sodium-glucose co-transporter-2 [SGLT2] inhibitors).69,70 In contrast, to date, none of the methods for assessing SFS or SFF have been introduced into clinical practice or employed into research for the development of new therapies.
What Are the Challenges That Have Precluded the Adoption of SFN Methods for the Routine Surveillance of DPN?
There may be several reasons why the expert group has not included the assessment of SFN into routine guidelines for the assessment of DPN. First, they have suggested that these methods need further clinical validation before their incorporation.71,72 Second, there are only a few longitudinal studies of the natural history of SFN. A MEDLINE search of this area found 784 citations in the last 10 years of which the majority were cross-sectional. Only six were longitudinal with the longest study duration being 5.3 years. The latter is a multicenter study of 399 T1DM and 191 T2DM patients followed up over 4.4 and 5.3 years, respectively. This important study showed that loss of corneal nerve fiber length (CNFL) of 6% per year occurs in 17% of the study population and “may identify patients at the highest risk of development of DPN.” 73
Another important limiting factor appears to the lack of “established” normative ranges, which would otherwise make its interpretation easier in the clinical world. Unlike IEFND and CCM, which have established worldwide normative ranges,57,74 other SFN methodologies still lack globally agreed normative range for comparison. Furthermore, there is disagreement on which of the variables measured during testing are best suited for clinical adoption and if they vary between the types of diabetes. For example, using pooled data of 998 subjects, Perkins et al 75 have suggested that the optimal threshold for automated CNFL was 12.5 mm/mm2 in T1DM and 12.3 mm/mm2 in T2DM, with a lower and upper threshold values between eight and six and 15.3 mm/mm2 rule out DPN with 88% specificity and 88% sensitivity. However, the authors conclude that “further research must determine to what extent CNFL can be deployed in clinical practice and in clinical trials assessing the efficacy of disease-modifying therapies for DSP,” suggesting that such cutoffs need further validation. Since the publication of this study, more evidence has been published, which supports that normative data set should be age-linked due to age-related decline in SFF and SFS in healthy individuals. 14 So, whist the existing methodologies are effective in smaller cross-sectional studies as biomarkers to improve our understanding of DPN, the lack of widely accepted normative data across might be a major drawback against their widespread use in a clinical environment.
Another important confounder is that protocols have changed over time, making comparisons with previous studies difficult. For example with CCM, although the majority of DPN studies are conducted by investigators based on protocols first established by Malik et al 76 and use the same automated software (Accumetrics) for image interpretation,77-79 these protocols continue to be updated and Petropoulos et al 80 commented that in addition to corneal confocal nerve fiber density (CNFD), nerve branch density (CNBD) and length (CNFL), addition of the inferior whorl length (IWL) may further improve the diagnostic performance of the method. With other investigators, there is considerable heterogeneity on both instruments and protocols involved. 81
Conclusions
The current approach to the assessment of DPN needs urgent reconsideration. Within the minds of many health care practitioners, including most diabetes specialists, determining whether an individual in their clinic has neuropathy involves screening using 10-gm SWMF, or a tuning fork, or even a patellar hammer. These are important as they help the practitioner determine whether the person is “at-risk” of foot ulceration and then to put in place strategies to prevent ulceration and amputation. However, we should be striving to detect the earliest stages of neuropathy during annual screening in line with what is routine for nephropathy and retinopathy. Given that we now have accurate tools to detect the earliest stages of this potentially devastating condition, well before the patient has lost protective sensation, these methods should be refined so that they can be incorporated as part of the annual screening procedure. Adoption of these SFN methodologies might be instrumental to design interventions like aggressive blood pressure or lipid management or even newer drug treatments, which could delay onset or progression of DPN.
We hope that this review will prompt further discussion by global experts on the incorporation of SFN assessment as routine care of DPN.
Acknowledgments
The authors would like to thank the colleagues at the Diabetes Research Unit and staff colleagues at the Diabetes Center, Ipswich Hospital for their continued support and facilitation of research endeavors.
Footnotes
Abbreviations: ADA, American Diabetes Association; CCM, corneal confocal microscopy; CNBD, corneal nerve fibre density; CNFD, corneal nerve fibre density: CNFL, corneal nerve fibre length; DPN, diabetic polyneuropathy; DSP; diabetes symmetrical neuropathy; EFNS, European Federation of the Neurological Societies; HbA1c, glycated hemoglobin A1c; HC, healthy controls; IENFD, intraepidermal nerve fiber density; IGT, impaired glucose tolerance; LDIFLARE, laser doppler imaging flare; NCS, nerve conduction studies; QST, quantitative sensory testing; SFN, small fibre function; SFN, small fibre neuropathy; SFS, small fibre structure; SMWF, Semmes-Weinstein monofilament; T1DM, type-1 diabetes mellitus; T2DM, type-2 diabetes mellitus; VPT, vibration perception threshold.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gerry Rayman  https://orcid.org/0000-0003-3331-7015
https://orcid.org/0000-0003-3331-7015
References
- 1. Pop-Busui R, Boulton AJ, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ziegler D, Papanas N, Vinik AI, Shaw JE. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb Clin Neurol. 2014;126:3-22. [DOI] [PubMed] [Google Scholar]
- 3. Albers JW, Pop-Busui R. Diabetic neuropathy: mechanisms, emerging treatments, and subtypes. Curr Neurol Neurosci Rep. 2014;14(8):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Callaghan BC, Xia R, Reynolds E, et al. Better diagnostic accuracy of neuropathy in obesity: a new challenge for neurologists. Clin Neurophysiol. 2018;129(3):654-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376(24):2367-2375. [DOI] [PubMed] [Google Scholar]
- 6. Erlanger J, Gasser H, Bishop G. The compound nature of the action current of nerve as disclosed by the cathode ray oscillograph. Am J Physiol. 1924;70:624-666. [Google Scholar]
- 7. Said G, Baudoin D, Toyooka K. Sensory loss, pains, motor deficit and axonal regeneration in length-dependent diabetic polyneuropathy. J Neurol. 2008;255(11):1693-1702. [DOI] [PubMed] [Google Scholar]
- 8. Malik RA, Tesfaye S, Newrick PG, et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48(3):578-585. [DOI] [PubMed] [Google Scholar]
- 9. Sharma S, Schaper N, Rayman G. Microangiopathy: is it relevant to wound healing in diabetic foot disease? Diabetes Metab Res Rev. 2020;36(suppl 1):e3244. [DOI] [PubMed] [Google Scholar]
- 10. Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG. Small-fibre neuropathies—advances in diagnosis, pathophysiology and management. Nat Rev Neurol. 2012;8(7):369-379. [DOI] [PubMed] [Google Scholar]
- 11. Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131(pt 7):1912-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tesfaye S, Boulton AJM, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Abnormal LDIflare but normal quantitative sensory testing and dermal nerve fiber density in patients with painful diabetic neuropathy. Diabetes Care. 2009;32(3):451-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sharma S, Tobin V, Vas PRJ, Malik RA, Rayman G. The influence of age, anthropometric and metabolic variables on LDIFLARE and corneal confocal microscopy in healthy individuals. PLoS One. 2018;13(3):e0193452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharma S, Vas PR, Rayman G. Assessment of diabetic neuropathy using a point-of-care nerve conduction device shows significant associations with the LDIFLARE method and clinical neuropathy scoring. J Diabetes Sci Technol. 2015;9(1):123-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green AQ, Krishnan S, Finucane FM, Rayman G. Altered C-fiber function as an indicator of early peripheral neuropathy in individuals with impaired glucose tolerance. Diabetes Care. 2010;33(1):174-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharma S, Tobin V, Rayman G. A prospective study of small fibre structure and function in newly diagnosed type 1 diabetes. Presented at the 76th scientific sessions of the American Diabetes Association (New Orleans, USA. June 10-14, 2016). Diabetes. 2016;65(suppl 1):A101-A169. [Google Scholar]
- 18. Hassler-Hurst J, Sharma S, Rayman G. Abnormal small nerve fibre function demonstrated by the laser Doppler imaging (LDI) flare technique in children and young people with Type 1 diabetes. Presented at the diabetes UK professional conference 2014 (Liverpool, UK, 5-7 March 2014). Diabet Med. 2014;31(suppl 1):73-183. [Google Scholar]
- 19. Vas PR, Rayman G. Validation of the modified LDIFlare technique: a simple and quick method to assess C-fiber function. Muscle Nerve. 2013;47(3):351-356. [DOI] [PubMed] [Google Scholar]
- 20. Sharma S, Tobin V, Vas PRJ, Rayman G. The LDIFLARE and CCM methods demonstrate early nerve fiber abnormalities in untreated hypothyroidism: a prospective study. J Clin Endocrinol Metab. 2018;103(8):3094-3102. [DOI] [PubMed] [Google Scholar]
- 21. Sharma S, Venkitaraman R, Vas PR, Rayman G. Assessment of chemotherapy-induced peripheral neuropathy using the LDIFLARE technique: a novel technique to detect neural small fiber dysfunction. Brain Behav. 2015;5(7):e00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vas PR, Sharma S, Rayman G. LDIflare small fiber function in normal glucose tolerant subjects with and without hypertriglyceridemia. Muscle Nerve. 2015;52(1):113-119. [DOI] [PubMed] [Google Scholar]
- 23. Chao CC, Hsieh SC, Yang WS, et al. Glycemic control is related to the severity of impaired thermal sensations in type 2 diabetes. Diabetes Metab Res Rev. 2007;23(8):612-620. [DOI] [PubMed] [Google Scholar]
- 24. Loseth S, Stalberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008;255(8):1197-1202. [DOI] [PubMed] [Google Scholar]
- 25. Sorensen L, Molyneaux L, Yue DK. The level of small nerve fiber dysfunction does not predict pain in diabetic neuropathy: a study using quantitative sensory testing. Clin J Pain. 2006;22(3):261-265. [DOI] [PubMed] [Google Scholar]
- 26. Sheen YJ, Li TC, Lin JL, et al. Association between thermal threshold abnormalities and peripheral artery disease in patients with type 2 diabetes. Medicine (Baltimore). 2018;97(51):e13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfau DB, Greffrath W, Schilder A, et al. Technical and clinical performance of the thermo-test device “Q-Sense” to assess small fibre function: a head-to-head comparison with the “Thermal Sensory Analyzer” TSA in diabetic patients and healthy volunteers. Eur J Pain. 2019;23(10):1863-1878. [DOI] [PubMed] [Google Scholar]
- 28. Papanas N, Papatheodorou K, Christakidis D, et al. Evaluation of a new indicator test for sudomotor function (Neuropad) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes. 2005;113(4):195-198. [DOI] [PubMed] [Google Scholar]
- 29. Ponirakis G, Petropoulos IN, Fadavi H, et al. The diagnostic accuracy of Neuropad for assessing large and small fibre diabetic neuropathy. Diabet Med. 2014;31(12):1673-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technol Ther. 2013;15(11):948-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith AG, Lessard M, Reyna S, Doudova M, Singleton JR. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications. 2014;28(4):511-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Selvarajah D, Cash T, Davies J, et al. SUDOSCAN: a simple, rapid, and objective method with potential for screening for diabetic peripheral neuropathy. PLoS One. 2015;10(10):e0138224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goel A, Shivaprasad C, Kolly A, Sarathi HAV, Atluri S. Comparison of electrochemical skin conductance and vibration perception threshold measurement in the detection of early diabetic neuropathy. PLoS One. 2017;12(9):e0183973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duchesne M, Richard L, Vallat JM, Magy L. Assessing sudomotor impairment in patients with peripheral neuropathy: comparison between electrochemical skin conductance and skin biopsy. Clin Neurophysiol. 2018;129(7):1341-1348. [DOI] [PubMed] [Google Scholar]
- 35. Sletten DM, Kimpinski K, Weigand SD, Low PA. Comparison of a gel versus solution-based vehicle for the delivery of acetylcholine in QSART. Auton Neurosci. 2010;158(1-2):123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34(1):57-61. [DOI] [PubMed] [Google Scholar]
- 37. Casanova-Molla J, Grau-Junyent JM, Morales M, Valls-Sole J. On the relationship between nociceptive evoked potentials and intraepidermal nerve fiber density in painful sensory polyneuropathies. Pain. 2011;152(2):410-418. [DOI] [PubMed] [Google Scholar]
- 38. Treede RD, Lorenz J, Baumgartner U. Clinical usefulness of laser-evoked potentials. Neurophysiol Clin. 2003;33(6):303-314. [DOI] [PubMed] [Google Scholar]
- 39. Ruscheweyh R, Emptmeyer K, Putzer D, Kropp P, Marziniak M. Reproducibility of contact heat evoked potentials (CHEPs) over a 6 months interval. Clin Neurophysiol. 2013;124(11):2242-2247. [DOI] [PubMed] [Google Scholar]
- 40. Lagerburg V, Bakkers M, Bouwhuis A, et al. Contact heat evoked potentials: normal values and use in small-fiber neuropathy. Muscle Nerve. 2015;51(5):743-749. [DOI] [PubMed] [Google Scholar]
- 41. Kaube H, Katsarava Z, Kaufer T, Diener H, Ellrich J. A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol. 2000;111(3):413-416. [DOI] [PubMed] [Google Scholar]
- 42. Mueller D, Obermann M, Koeppen S, et al. Electrically evoked nociceptive potentials for early detection of diabetic small-fiber neuropathy. European Journal of Neurology 2010;17: 834-841. [DOI] [PubMed] [Google Scholar]
- 43. La Cesa S, Di Stefano G, Leone C, et al. Skin denervation does not alter cortical potentials to surface concentric electrode stimulation: a comparison with laser evoked potentials and contact heat evoked potentials. Eur J Pain. 2018;22(1):161-169. [DOI] [PubMed] [Google Scholar]
- 44. Donadio V, Liguori R. Microneurographic recording from unmyelinated nerve fibers in neurological disorders: an update. Clin Neurophysiol. 2015;126(3):437-445. [DOI] [PubMed] [Google Scholar]
- 45. Serra J. Microneurography: towards a biomarker of spontaneous pain. Pain. 2012;153(10):1989-1990. [DOI] [PubMed] [Google Scholar]
- 46. Orstavik K, Jorum E. Microneurographic findings of relevance to pain in patients with erythromelalgia and patients with diabetic neuropathy. Neurosci Lett. 2010;470(3):180-184. [DOI] [PubMed] [Google Scholar]
- 47. Vallbo AB. Microneurography: how it started and how it works. J Neurophysiol. 2018;120(3):1415-1427. [DOI] [PubMed] [Google Scholar]
- 48. Petropoulos IN, Ponirakis G, Khan A, et al. Corneal confocal microscopy: ready for prime time. Clin Exp Optom. 2020;103(3):265-277. [DOI] [PubMed] [Google Scholar]
- 49. Vas PR, Edmonds ME. Early recognition of diabetic peripheral neuropathy and the need for one-stop microvascular assessment. Lancet Diabetes Endocrinol. 2016;4(9):723-725. [DOI] [PubMed] [Google Scholar]
- 50. Pritchard N, Edwards K, Russell AW, Perkins BA, Malik RA, Efron N. Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. 2015;38(4):671-675. [DOI] [PubMed] [Google Scholar]
- 51. Malik RA, Kallinikos P, Abbott CA, et al. Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683-688. [DOI] [PubMed] [Google Scholar]
- 52. Tavakoli M, Begum P, McLaughlin J, Malik RA. Corneal confocal microscopy for the diagnosis of diabetic autonomic neuropathy. Muscle Nerve. 2015;52(3):363-370. [DOI] [PubMed] [Google Scholar]
- 53. Edwards K, Pritchard N, Dehghani C, et al. Corneal confocal microscopy best identifies the development and progression of neuropathy in patients with type 1 diabetes. J Diabetes Complications. 2017;31(8):1325-1327. [DOI] [PubMed] [Google Scholar]
- 54. Lauria G, Devigili G. Skin biopsy as a diagnostic tool in peripheral neuropathy. Nat Clin Pract Neurol. 2007;3(10):546-557. [DOI] [PubMed] [Google Scholar]
- 55. Lauria G, Lombardi R, Camozzi F, Devigili G. Skin biopsy for the diagnosis of peripheral neuropathy. Histopathology. 2009;54(3):273-285. [DOI] [PubMed] [Google Scholar]
- 56. Smith AG, Howard JR, Kroll R, et al. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005;228(1):65-69. [DOI] [PubMed] [Google Scholar]
- 57. Bakkers M, Merkies IS, Lauria G, et al. Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology. 2009;73(14):1142-1148. [DOI] [PubMed] [Google Scholar]
- 58. Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst. 2010;15(3):202-207. [DOI] [PubMed] [Google Scholar]
- 59. Lauria G, Lombardi R. Small fiber neuropathy: is skin biopsy the holy grail? Curr Diab Rep. 2012;12(4):384-392. [DOI] [PubMed] [Google Scholar]
- 60. Terkelsen AJ, Karlsson P, Lauria G, Freeman R, Finnerup NB, Jensen TS. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol. 2017;16(11):934-944. [DOI] [PubMed] [Google Scholar]
- 61. Vagenas D, Pritchard N, Edwards K, et al. Optimal image sample size for corneal nerve morphometry. Optom Vis Sci. 2012;89(5):812-817. [DOI] [PubMed] [Google Scholar]
- 62. Bril V, Perkins BA. Validation of the Toronto Clinical Scoring System for diabetic polyneuropathy. Diabetes Care. 2002;25(11):2048-2052. [DOI] [PubMed] [Google Scholar]
- 63. Azmi S, Ferdousi M, Petropoulos IN, et al. Corneal confocal microscopy identifies small-fiber neuropathy in subjects with impaired glucose tolerance who develop type 2 diabetes. Diabetes Care. 2015;38(8):1502-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62(1):254-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138. [DOI] [PubMed] [Google Scholar]
- 66. NICE. Diabetic foot problems: prevention and management. 2015. Accessed January 5, 2021 https://wwwniceorguk/guidance/ng19 [PubMed]
- 67. Thomas RL, Luzio SD, North RV, et al. Retrospective analysis of newly recorded certifications of visual impairment due to diabetic retinopathy in Wales during 2007-2015. BMJ Open. 2017;7(7):e015024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355(9200):253-259. [PubMed] [Google Scholar]
- 69. Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28(1):368-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wanner C, Heerspink HJL, Zinman B, et al. Empagliflozin and kidney function decline in patients with type 2 diabetes: a slope analysis from the EMPA-REG OUTCOME trial. J Am Soc Nephrol. 2018;29(11):2755-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- 72. Devigili G, Rinaldo S, Lombardi R, et al. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain. 2019;142(12):3728-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lewis EJH, Lovblom LE, Ferdousi M, et al. Rapid corneal nerve fiber loss: a marker of diabetic neuropathy onset and progression. Diabetes Care. 2020;43(8):1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tavakoli M, Ferdousi M, Petropoulos IN, et al. Normative values for corneal nerve morphology assessed using corneal confocal microscopy: a multinational normative data set. Diabetes Care. 2015;38(5):838-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Perkins BA, Lovblom LE, Bril V, et al. Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018;61(8):1856-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Petropoulos IN, Alam U, Fadavi H, et al. Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55(4):2071-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dabbah MA, Graham J, Petropoulos I, Tavakoli M, Malik RA. Dual-model automatic detection of nerve-fibres in corneal confocal microscopy images. Med Image Comput Comput Assist Interv. 2010;13(pt 1):300-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dabbah MA, Graham J, Petropoulos IN, Tavakoli M, Malik RA. Automatic analysis of diabetic peripheral neuropathy using multi-scale quantitative morphology of nerve fibres in corneal confocal microscopy imaging. Med Image Anal. 2011;15(5):738-747. [DOI] [PubMed] [Google Scholar]
- 79. Chen X, Graham J, Dabbah M, Petropoulos I, Tavakoli M, Malik R. An automatic tool for quantification of nerve fibres in corneal confocal microscopy images. IEEE Trans Biomed Eng. 2017;64(4):786-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Petropoulos IN, Ferdousi M, Marshall A, et al. The inferior whorl for detecting diabetic peripheral neuropathy using corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2015;56(4):2498-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Xiong Q, Lu B, Ye HY, et al. Corneal confocal microscopy as a non-invasive test to assess diabetic peripheral neuropathy. Diabetes Res Clin Pract. 2018;136:85-92. [DOI] [PubMed] [Google Scholar]