Summary
The induction of broadly neutralizing antibodies (bNAbs) that target the hemagglutinin stalk domain is a promising strategy for the development of “universal” influenza virus vaccines. bNAbs can be boosted in adults by sequential exposure to heterosubtypic viruses through natural infection or vaccination. However, little is known about if or how bNAbs are induced by vaccination in more immunologically naive children. Here, we describe the impact of repeated seasonal influenza vaccination and vaccine type on induction of bNAbs against group 1 influenza viruses in a pediatric cohort enrolled in randomized controlled trials of seasonal influenza vaccination. Repeated seasonal vaccination results in significant boosting of a durable bNAb response. Boosting of serological bNAb titers is comparable within inactivated and live attenuated (LAIV) vaccinees and declines with age. These data provide insights into vaccine-elicited bNAb induction in children, which have important implications for the design of universal influenza vaccine modalities in this critical population.
Keywords: influenza virus, vaccines, broadly neutralizing antibodies, children, universal vaccine
Graphical abstract
Highlights
-
•
Repeated inactivated influenza vaccination boosts bNAbs
-
•
Inactivated and live attenuated vaccines are similarly efficient at boosting bNAbs
-
•
The magnitude of IIV and LAIV vaccine-elicited bNAb boosting declines with age
Yegorov et al. evaluate vaccine-elicited broadly neutralizing antibody (bNAb) responses against group 1 influenza A viruses in children. Repeated seasonal immunization of children against influenza boosts bNAb titers. Inactivated and live attenuated vaccine formulations are similarly effective at boosting bNAbs, with the magnitude of boosting inversely correlated with age.
Introduction
Despite decades of study and the accessibility of seasonal vaccination, influenza remains an important public health concern, largely owing to its ability to escape immunity and cause seasonal epidemics and pandemics.1, 2, 3 Seasonal vaccines, the mainstay of influenza prevention, have often shown modest real-world effectiveness due to suboptimal immunogenicity, alongside rapid and unpredictable changes in circulating virus strains.1, 2, 3 Globally, several different seasonal influenza vaccine formulations are approved, including intramuscular injection of inactivated virus (IIV) or mucosal administration of replication-competent live attenuated influenza vaccine (LAIV).1 Although influenza vaccines are often considered equally efficacious, there are insufficient data on the impact of repeated vaccination and vaccine type on the breadth of influenza-virus-directed immunity in specific demographic groups, especially children, who are a major source of influenza transmission and, alongside the elderly, are at heightened risk for influenza-related hospitalizations and death.4,5
The bulk of influenza-vaccine-elicited antibodies (Abs) target the viral membrane surface proteins hemagglutinin (HA) and, to a lesser extent, neuraminidase (NA). Of the HA-reactive Abs, most bind the “immunodominant” globular HA head domain in a strain-specific manner, blocking the interaction between the virus and cell surface sialic acid, while a minority of HA-reactive Abs bind the sub-dominant, but highly conserved HA stalk domain; a subset of these Abs is considered broadly neutralizing (bNAbs). HA stalk-reactive bNAbs have emerged as a promising strategy for the development of a “universal” influenza vaccine.6,7
In adults, we and others have shown that bNAbs are selectively boosted by exposure to pandemic/heterosubtypic HA subtypes (via either infection or vaccination).8, 9, 10 bNAb titers also increase over time owing to an accumulation of lifetime exposures to influenza virus selecting for cross-reactive B cell clone maintenance and expansion.11,12 A recent clinical trial demonstrated that chimeric hemagglutinin vaccines delivered in live attenuated and inactivated formulations could successfully boost bNAb titers in adults.6,7 However, little is known about how bNAbs develop in children and how vaccination impacts bNAb development—knowledge that will have important implications for understanding the nature of vaccine-mediated immunity against influenza, especially in the context of exposure to novel strains.1,13,14
Therefore, we explored the effects of seasonal influenza vaccination and vaccine formulation on bNAb induction in participants of a cluster randomized controlled trial (cRCT) designed to compare the community-level protection mediated by IIV and LAIV vaccination of children. We found that titers of neutralizing bNAbs were enhanced by repeated seasonal vaccination and that bNAb levels were boosted similarly by LAIV and IIV. As expected, LAIV and IIV effects on bNAb titers were more pronounced in the mucosa and blood, respectively, and the magnitude of vaccine-induced bNAb responses in the serum declined with age.
Results
Study participants
The effects of repeated seasonal IIV vaccination on the induction of bNAbs were studied using serum samples from 68 RCT participants (median age 9.0 years) (Table S1), who either received IIV (37 vaccinees) or served as controls (31 controls) for each of the three seasons (2008/09 to 2010/11) captured by the cRCT (Figure 1A and Table 1) and for whom paired samples were available. The effects of vaccine type on bNAb elicitation were subsequently studied using serum and mucosal samples from 72 RCT participants (median age 11.0 years) (Table S1), who received either the inactivated vaccine (IIV, n = 35) or the live attenuated vaccine (LAIV, n = 37) (Figure 1B and Table 1) and for whom paired samples were available.
Figure 1.
Study flowchart
(A) Description of participants in analysis I: effects of repeated seasonal influenza vaccination on bNAb titers in children.
(B) Description of participants in analysis II: effect of vaccine type (IIV versus LAIV) on induction of bNAbs in children. cRCT, cluster randomized controlled trial; bNAb, broadly neutralizing antibody; IIV, inactivated influenza vaccine; LAIV, live attenuated influenza vaccine.
Table 1.
Description of influenza strains used in the standard-of-care trivalent vaccines administered over the study period
| Season | Strain type | Strain Sub-type | Strain name |
|---|---|---|---|
| Analysis I seasons | |||
| 2008/09 | A | H1N1 | Brisbane/59/2007 |
| 2008/09 | A | H3N2 | Brisbane/10/2007 |
| 2008/09 | B | Yamagata | Florida/4/2006 |
| 2009/10 | A | H1N1 | Brisbane/59/2007 |
| 2009/10 | A | H3N2 | Brisbane/10/2007 |
| 2009/10 | B | Victoria | Brisbane/60/2008 |
| 2010/11 | A | H1N1 | California/7/2009 |
| 2010/11 | A | H3N2 | Perth/16/2009 |
| 2010/11 | B | Victoria | Brisbane/60/2008 |
| Analysis II seasons | |||
| 2014/15 | A | H1N1 (pdm09) | California/7/2009 |
| 2014/15 | A | H3N2 | Texas/50/2012 |
| 2014/15 | B | Yamagata | Massachusetts/2/2012 |
Effects of repeated seasonal vaccination on serological bNAb responses
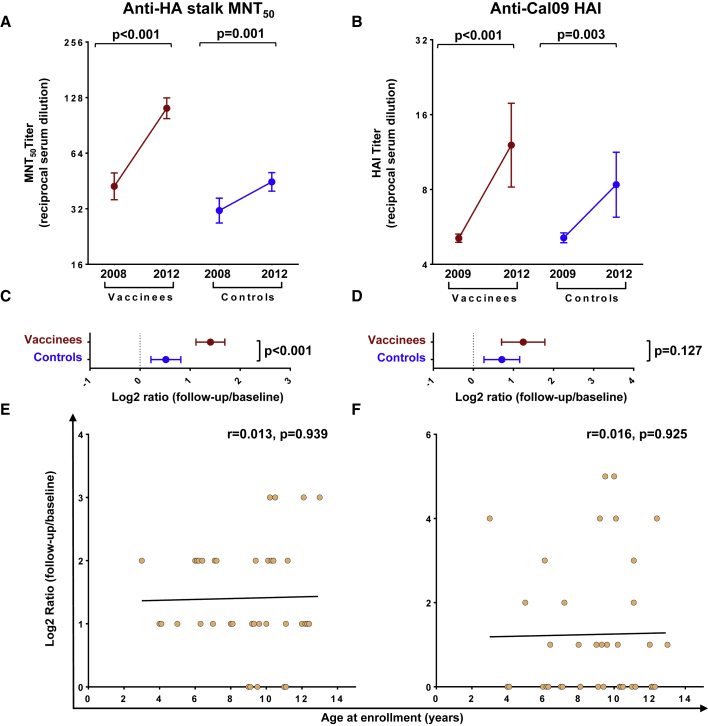
To determine the effect of repeated seasonal IIV vaccination of children on the induction of bNAbs, microneutralization (MNT) assays were performed on the 2008/09 and 2012/13 pre-vaccination sera (corresponding to the beginning of the cRCT and 1 year after its conclusion, respectively) using cH5/1 N3, a chimeric virus previously validated to quantify neutralization mediated by group 1 HA stalk-binding bNAbs.9,10,13,14 After three vaccination seasons, higher MNT50 titers were evident in vaccinees compared with controls (geometric mean fold change (GMFC) = 2.65 versus 1.43, p < 0.001) (Figures 2A, 2C, S1, and S2; Table S2); 43% (16/37) of vaccinees had at least a 4-fold increase of MNT50 compared with the 13% (4/31) seroconversion among controls. bNAbs that bind to the receptor-binding site (RBS) of HA have also been reported.15 To determine whether repeated vaccination boosted titers of this antibody class, we did hemagglutination inhibition (HAI) assays using the cH5/1 N3 virus employed in the MNT assay. No increase in HAI-positive antibodies was observed over time, and there was no difference between the vaccinees and controls (Figure S3). HAI titers against Cal/09, which was the H1N1 component of the seasonal influenza vaccine beginning in 2010/1116 (see Table 1), were slightly elevated after repeated seasonal vaccinations (19/37, 51% had at least a 4-fold increase of HAI), but the post-vaccination HAI titers in most (29/37) vaccinees remained below 40 (the conventional seroprotection threshold), and the change was not significantly different between vaccinees and controls (GMFC = 2.37 versus 1.64, p = 0.127) (Figures 2B and 2D, Table S2). The magnitudes of vaccine-elicited MNT50 or HAI boosting were not correlated with age (Figures 2E and 2F). These results demonstrate that repeated seasonal IIV vaccination induces durable bNAb responses in children.
Figure 2.
Effects of seasonal vaccination of bNAb titers in children
(A) Anti-HA stalk MNT50 titers at baseline (year 2008) and after three cRCT vaccination seasons (year 2012).
(B) Serum HAI activity against the Cal/09 virus assessed at baseline (year 2009) and after three cRCT vaccination seasons (year 2012).
(C) Log2-transformed ratios of MNT50 titers after repeated vaccination (year 2012) versus at baseline (year 2008).
(D) Log2-transformed ratios of HAI titers after repeated vaccination (year 2012) versus at baseline (year 2009).
(E and F) Correlation plots of log2-transformed MNT50 (E) and HAI (F) titer ratios and participant ages at enrollment.
In (A and B), dots and brackets represent geometric means and 95% CIs, respectively; p values indicate the statistical significance of the intra-individual difference between pre- and post-vaccination titers assessed by paired t test. In (C and D), dots and brackets represent means and 95% CIs, respectively; p values indicate the statistical significance of the difference between vaccinees and controls assessed by unpaired t test. In (E and F), the Pearson coefficients (r) and their statistical significance are shown along with lines of best fit for vaccinees.
Effects of vaccine type on serological bNAb responses
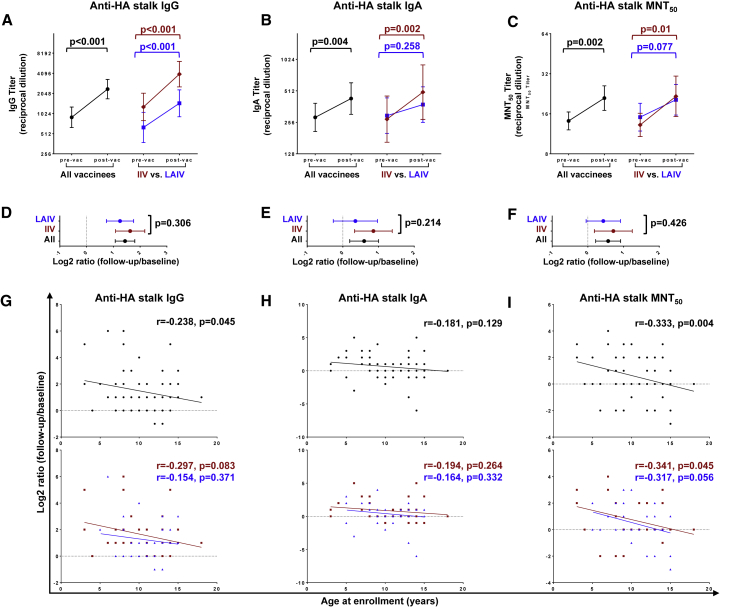
IIV and LAIV vaccines are known to elicit distinct immune responses.17, 18, 19 Thus, we set out to determine whether vaccine formulation (IIV versus LAIV) influences the magnitude of serological vaccine-induced bNAb responses in children. There was a significant elevation of HA stalk-binding (anti-cH6/1) immunoglobulin G (IgG) and IgA and bNAb (anti-cH5/1 N3) MNT50 titers at 1 month after vaccination (GMFC = 2.71, p < 0.001; 1.51, p = 0.004; 1.48, p = 0.002, respectively) (Figures 3A–3C, Table S3); this elevation was more pronounced in IIV vaccinees than in LAIV vaccinees, although the response magnitude was not significantly different between the two groups (Figures 3D and 3F). The magnitude of vaccine-elicited boosting of both anti-HA stalk IgG and MNT50 (r = −0.238, p = 0.045 and r = −0.333, p = 0.004, respectively), but not anti-HA stalk IgA titers, declined with age (Figures 3G–3I). There were no significant differences in vaccination history between the IIV and LAIV groups (Table S4). Altogether, these data show that in young children, both IIV and LAIV boost serum bNAbs in an age-dependent manner.
Figure 3.
Effect of vaccine type on serological bNAb titers
(A–C) Anti-HA stalk IgG (A), IgA (B), and MNT50 (C) titers pre- and post-vaccination.
(D–F) Log2-transformed ratios of anti-HA stalk IgG (D), IgA (E), and MNT50 (F) titers post- versus pre-vaccination.
(G–I) Correlation plots of log2-transformed anti-HA stalk IgA (G), anti-HA stalk IgG (H), and MNT50 (I) titer ratios and participant ages at enrollment.
In (A–C), dots and brackets represent geometric means and 95% CIs, respectively; p values indicate the statistical significance of the intra-individual difference between pre- and post-vaccination titers assessed by paired t test. In (D–F), dots and brackets represent means and 95% CIs, respectively; p values indicate the statistical significance of the difference in the magnitude of immune response between IIV and LAIV vaccinees assessed by unpaired t test. In (G–I), the Pearson coefficients (r) and their statistical significance are shown along with lines of best fit for all participants (black) and IIV (red) versus LAIV (blue) vaccinees.
Effects of vaccine type on strain-specific serological responses
We next asked whether seasonal influenza vaccine type influenced the magnitude of serological responses against strain-specific antigens included in the vaccine. Both IIV and LAIV induced a significant increase of anti-Cal/09 H1N1 IgG, IgA, and HAI titers at 1 month after vaccination (GMFC = 3.0, 1.48, and 2.0, respectively, p < 0.001 for all) (Figures 4A–4C, Table S3). The boost in anti-Cal09 H1N1 IgG and HAI was significantly higher in the IIV vaccinees compared with the LAIV vaccinees (GMFC = 4.59 versus 2.0, p = 0.017 for IgG, and GMFC = 3.03 versus 1.62, p = 0.007 for HAI) (Figures 4D and 4F). There was no difference in boosting magnitude between the two groups for Cal-09 H1N1 IgA responses, though the absolute post-boost HAI titers were higher for IIV vaccinees (Figures 4E and 4F). The magnitude of vaccine-elicited Cal/09 H1N1 serum IgA increase was inversely correlated with age in all vaccinees (r = −0.379, p = 0.001) (Figure 4H). Boosting of Cal/09 H1N1 HAI was also inversely correlated with age for IIV vaccinees (r = −0.359, p = 0.034) (Figure 4I). Thus, the boost in functional antibody responses against vaccine antigen appears more pronounced in IIV and exhibits some age dependence.
Figure 4.
Effect of vaccine type on serological titers of strain-specific antibodies
(A–C) Anti-Cal/09 IgG (A), IgA (B), and HAI (C) titers pre- and post-vaccination.
(D–F) Log2-transformed ratios of anti-Cal09 IgG (D), IgA (E), and HAI (F) titers post- versus pre-vaccination.
(G–I) Correlation plots of log2-transformed anti-Cal09 IgA (G), anti-Cal09 IgG (H), and HAI (I) titer ratios and participant ages.
In (A–C), dots and brackets represent geometric means and 95% CIs, respectively; p values indicate the statistical significance of the intra-individual difference between pre- and post-vaccination titers assessed by paired t test. In (D–F), dots and brackets represent means and 95% CIs, respectively; p values indicate the statistical significance of the difference in the magnitude of immune response between IIV and LAIV vaccinees assessed by unpaired t test. In (G–I), the Pearson coefficients (r) and their statistical significance are shown along with lines of best fit for all participants (black) and IIV (red) versus LAIV (blue) vaccinees.
Effects of vaccine type on mucosal IgG and IgA responses
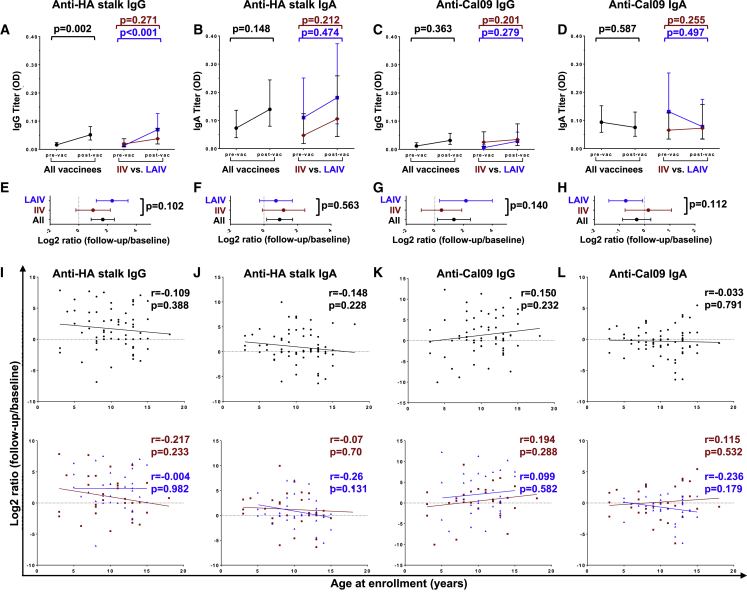
Vaccine-elicited mucosal immune responses are critical for protection against influenza.1 Therefore, we next assessed the antibody profiles in nasal swabs to determine the impact of vaccine type on mucosal immunity. Overall, vaccination tended to increase mucosal bNAb titers, without significant differences between vaccine types (Figures 5A, 5B, 5E, and 5F). Anti-stalk IgG was significantly elevated, with the largest effect size in LAIV vaccinees (GMFC = 3.17, p < 0.001) (Figures 5A and 5E). Notably, alongside an apparent increase in IgG, there was a decrease in IgA titers against the Cal/09 H1N1 vaccine antigen in LAIV vaccinees, as reported previously.17 No significant correlations were observed between vaccine-mediated boosting of mucosal Abs and age (Figures 5I–5L). Altogether, LAIV and IIV boosted mucosal IgG and IgA bNAbs to a similar extent.
Figure 5.
Effect of vaccine type on mucosal bNAb titers
(A–D) Anti-HA stalk IgG (A), IgA (B), and anti-Cal/09 IgG (C) and IgA (D) titers.
(E–H) Log2-transformed ratios of anti-HA stalk IgG (E) and IgA (F), and anti-Cal09 IgG (G) and IgA (H) titers post- versus pre-vaccination.
(I–L) Correlation plots of anti H6/1 IgG (I) and IgA (J) and anti-Cal09 IgG (K) and IgA (L) titer ratios and participant ages at enrollment.
In (A–D), dots and brackets represent geometric means and 95% CIs, respectively; p values indicate the statistical significance of the intra-individual difference between pre- and post-vaccination titers assessed by paired t test. In (E–H), dots and brackets represent means and 95% CIs, respectively; p values indicate the statistical significance of the difference in the magnitude of immune response between IIV and LAIV vaccinees assessed by unpaired t test. In (I–L), the Pearson coefficients (r) and their statistical significance are shown along with lines of best fit for all participants (black) and IIV (red) versus LAIV (blue) vaccinees.
Relationship between vaccine-elicited serological and mucosal antibody response
Whether and how vaccine-elicited immunity correlates between the systemic and mucosal compartments is a topic of debate.20,21 Therefore, we next explored the relationship between the magnitude of vaccine-induced bNAb boost in the serum versus the nasal mucosa. In unstratified analysis, no correlation was observed between the serological and mucosal antibody responses (Figures 6A–6D, left panels). Upon stratification by vaccine type, there was no correlation between serum and mucosal responses for bNAbs (Figures 6A and 6B, right panels). However, the magnitude of change in vaccine Cal/09 H1N1-reactive IgG, but not Cal/09 H1N1-reactive IgA, positively correlated between serum and mucosa in IIV vaccinees only (r = 0.442, p = 0.011) (Figures 6C and 6D, right panels). Thus, bNAb responses appear to be compartment specific.
Figure 6.
Relationship between mucosal and serological antibody titers
(A–D) Correlation plots of anti- HA stalk IgG (A) and IgA (B) and anti-Cal09 IgG (C) and IgA (D) log2-transformed post-/pre-vaccination titer ratios. The Pearson coefficients (r) and their statistical significance are shown along with lines of best fit for all participants (black) and IIV (red) versus LAIV (blue) vaccinees.
Discussion
Notwithstanding the recent global decline in seasonal influenza incidence as a consequence of public health measures,22 influenza remains an important public health concern owing to its inevitable seasonal reemergence as social distancing eases and the ever-present risk of future pandemics. Young children are a major driver of virus transmission, accounting for a significant proportion of influenza-related morbidity and mortality.4,5 Therefore, understanding how seasonal vaccination and varied vaccine formulations shape childhood immunity to influenza A viruses is of critical importance for effective influenza prevention.
Vaccine-elicited immune responses are tightly linked to history of exposure to virus, infection, and/or vaccination,1,3 and >50% of children have encountered influenza virus by 6 years of age.23 Previous studies have shown that vaccination of children with a pandemic H5 vaccine was capable of eliciting bNAbs—similar to what has been reported in adults.8,24,25 A major strength of our study is that it describes the impact of seasonal vaccination on bNAb induction in children whose exposure histories were carefully monitored within a cRCT framework, excluding the confounding effects of symptomatic infection while focusing specifically on the effects of immunization.
After three vaccination seasons, bNAb-mediated virus neutralization in IIV vaccinees increased by >2.5-fold, supporting the notion that seasonal vaccination facilitates sustained expansion of cross-reactive Ab repertoires.10 In contrast, although vaccine-elicited strain-specific HAI activity also rose over three seasons, it was not significantly different between the vaccinated and unvaccinated children at the conclusion of the study.
The magnitude of bNAb expansion induced by repeated IIV vaccination was inversely correlated with age, consistent with preferential boosting of Abs against the HA head domain that is known to occur in adults after seasonal influenza vaccination.9,26 Notably, a very modest bNAb elevation was observed in control subjects between 2008 and 2012, which is consistent with the observation that HA stalk-reactive bNAbs tend to be polyreactive and that polyreactive Abs tend to increase with age.27 The rise in bNAbs in the control group also corresponded to the 2009 H1N1 swine flu pandemic. Circulation of this virus in Canada began in the spring of 2009, outside of the usual influenza season and at a time when active surveillance was not being conducted in the context of this study. Exposure to this virus is well known to boost bNAbs, and we speculate that unrecognized infections may also partially explain this increase.28 In contrast, HAI titer increases observed in vaccinees and controls during the study period were very similar, and overall, these titers remained very low—below the typical seroprotection threshold of 40. Together, these data suggest that seasonally repeated vaccination boosted bNAbs in young children.
Importantly, IIV and LAIV were similarly effective at boosting bNAbs, in line with the equivalent efficacy offered by these two vaccine types in the cRCT.29 Furthermore, vaccine-elicited bNAb boosting was more pronounced in the blood of IIV vaccinees and in the mucosa of LAIV vaccinees, consistent with the respective vaccination routes and the results of previous studies comparing immune responses elicited by IIV and LAIV.30, 31, 32
The role of mucosal immunity in protecting against respiratory virus infections has been somewhat enigmatic. On one hand, people with selective IgA deficiency have relatively modest increases in susceptibility to infections.33 Still, serum IgA has been reported to correlate with protection against influenza, and nasal IgA has been reported to contribute to protection, especially in individuals with low virus-specific HAI titers.34,35 The upper respiratory tract antibody repertoire is dominated by secretory IgA, while in the lower respiratory tract IgG is more abundant.1 We have previously shown that broadly neutralizing IgA is more potent than IgG.13 This is, at least in part, due to the glycosylation of IgA, whereby sialic acids present on the antibody mediate a second mode of binding that inhibits viral entry.36 We have also recently shown that the influenza virus-IgA immune complexes signal through the FcαRI receptor on neutrophils to stimulate NETosis and that neutrophil extracellular traps are capable of inactivating influenza virus.37,38 Therefore, robust mucosal immune response stimulation should be a priority for the development of more broad and efficacious influenza vaccines.
We did not observe a correlation between bNAb responses in the blood and nasal mucosa, suggesting an important contribution from in situ antibody generation at the mucosa. Interestingly, there was an inverse correlation between Cal/09 IgA titers and age at enrollment as well as anti-Cal/09 HAI titers in the IIV group. IIV recipients tended to have higher HAI titers against Cal/09 than LAIV recipients. Preexisting Abs can inhibit vaccine responses.3 Thus, the higher antibody titers in the IIV group might have inhibited IgG+ memory B cell stimulation and reentry into the germinal center and subsequent class switching to IgA. Furthermore, while the serum bNAb and Cal/09-specific IgA titers were similar, the titers of Cal/09-reactive IgG were approximately 2-fold higher compared with HA stalk-reactive IgG, consistent with the immunodominant nature of the HA head domain.1 Notably, mucosal titers of bNAbs and strain-specific Abs were similar, suggesting that mucosal responses may be less focused on immunodominant epitopes.
Conclusions
Our data provide insights into the effects of repeated seasonal influenza vaccination and vaccine type on bNAb induction in children. Seasonal influenza vaccines in children can induce bNAbs against influenza A both in blood and in respiratory mucosa, which has important ramifications for the selection of universal vaccine platforms that could be effectively deployed in this population. Specifically, our data suggest that the threshold for inducing bNAbs in children may be lower than in adults, for whom seasonal vaccines do a poor job at boosting bNAbs owing to immunodominance of the HA head domain.9,39 In addition, our data suggest that IIV and LAIV platforms might be equally suitable for delivery of universal influenza vaccines to pediatric cohorts. Future studies are needed to explore factors responsible for enhanced bNAb generation in the context of seasonal vaccination, how vaccine-elicited bNAbs functionally relate to those induced by natural infection,40 and whether bNAb induction mechanisms can be utilized to improve real-world effectiveness of influenza vaccines.
Limitations of the study
Our analyses were based on a relatively small selection of samples derived from the original cRCTs owing to sample availability. As the objective of the study was to specifically interrogate the impact of vaccination of bNAbs in children, those who were infected during the trial were excluded. Though the attack rates of influenza in these trials were very low (5%–10%), it is possible that the antibody responses of infected individuals might be different from those who were not infected.29,41 For feasibility reasons, we assessed only bNAbs against group 1 HAs. In the future, it would be important to examine group 2 bNAbs as well. Our mucosal analyses were performed using nasal swabs, which precluded conventional antibody titrations owing to the low antibody levels typical for these samples. However, we have shown previously that we are able to reliably measure differences in mucosal IgA using the methods employed herein.17 Finally, the protectiveness of bNAbs has been difficult to directly establish in vivo. Strain-specific Abs are much more potent than bNAbs and likely contribute most directly to protection in many instances.13 The protective effects of bNAbs are likely to be more apparent in seasons wherein there is a substantial mismatch between vaccine strains and circulating strains or in the context of pandemics. Indeed, bNAbs have been reported to correlate with protection against pandemic H1N1 independently of HAI-positive Abs, and the elicitation of bNAbs during pandemics has been postulated as a mechanism for the extinction of seasonal pre-pandemic strains.28,42,43 Rigorous prospective cohort-based studies are needed to shed additional light on bNAb-mediated protection in humans.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-Influenza A Antibody, nucleoprotein, clone A1, biotin-conjugated | EMB Millipore | Cat#MAB8257B |
| Goat Anti-Human IgA-HRP | SouthernBiotech | Cat#2050-05, RRID#AB_2687526 |
| Goat anti-Human IgG (H+L) Secondary Antibody, HRP | Invitrogen/Thermo Fisher | Cat#31410, RRID#AB_228269 |
| Bacterial and virus strains | ||
| cH5/1 N3 virus | Engineered using plasmids encoding 6 wild-type A/Puerto Rico/8/34 (PR8) strain segments and cH5/1 HA and N3 NA from A/Swine/Missouri/4296424/06 (Miss/06) virus9 | N/A |
| A/California/04/2009 (Cal/09) H1N1 | Gift from Dr. Peter Palese, Icahn School of Medicine at Mount Sinai, NY | NCBI taxon ID#641501 |
| MAX Efficiency™ DH10Bac Competent Cells | Gibco/Thermo Fisher | Cat#10361012 |
| Biological samples | ||
| Participant sera | This study | N/A |
| Participant nasal swabs | This study | N/A |
| Chicken erythrocytes in Alsever's solution | Canadian Food Inspection Agency (CFIA) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| cH6/1 hemagglutinin (HA) construct | Engineered using plasmids encoding A/Mallard/Sweden/81/02 H6 head domain and a A/Puerto Rico/8/1934 (PR8) group 1 stalk9 | N/A |
| Dulbecco Modified Eagle Medium (DMEM) | Gibco/Thermo Fisher | Cat# 11965092 |
| Fetal bovine serum | Gibco/Thermo Fisher | Cat#12484028 |
| Penicillin-streptomycin | Gibco/Thermo Fisher | Cat#15140122 |
| HyClone SFX insect cell culture media | Cytiva/Fisher Scientific | Cat#SH3027802 |
| TNM-FH insect cell media | Sigma-Aldrich | Cat#T1032 |
| Trypsin treated with N-tosyl-L-phenylalanine chloromethyl ketone (TPCK) | Sigma-Aldrich | Cat#4370285 |
| L-glutamine | Gibco/Thermo Fisher | Cat#25030149 |
| Critical commercial assays | ||
| SigmaFast OPD Peroxidase Substrate | Sigma-Aldrich | Cat#P9187 |
| pFastBac Dual Expression Vector | Gibco/ThermoFisher | Cat#10712024 |
| Experimental models: Cell lines | ||
| Madin-Darby Canine Kidney (MDCK) cells | American Type Culture Collection (ATCC, VA, USA) | MDCK.2, Cat#CRL-2936 |
| Trichoplusia ni High Five (BTI-TN5B1-4) insect cells | American Type Culture Collection (ATCC, VA, USA) | Cat#CRL-10859 |
| Spodoptera frugiperda Sf9 insect cells | American Type Culture Collection (ATCC, VA, USA) | Cat#CRL-1711 |
| Experimental models: Organisms/strains | ||
| Specific-pathogen-free (SPF) chicken eggs | Canadian Food Inspection Agency (CFIA) | N/A |
| Software and algorithms | ||
| IBM SPSS v. 27 | IBM | https://www.ibm.com/analytics/spss-statistics-software |
| GraphPad Prism v.7 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| Other | ||
| Hutterite Influenza Prevention Study | ClinicalTrials.org | https://clinicaltrials.gov/ct2/show/NCT00877396 |
| Live Versus Inactivated Influenza Vaccine Study in Hutterite Children | ClinicalTrials.org | https://clinicaltrials.gov/ct2/show/NCT01653015 |
Resource availability
Lead contact
Inquiries about any additional information pertaining to the study and requests for resources and reagents should be directed to the lead contact, Matthew S. Miller (mmiller@mcmaster.ca).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Study setting and participant recruitment
This study was nested within the multi-centre cluster randomized controlled trials (cRCT) of annual influenza vaccination in Hutterite communities.29,41,44 Selection of samples from the cRCT was done randomly, ensuring that the comparison groups were age- and gender-matched (see Table S1). Specifically, we included participants with a complete history of repeated vaccinations, who did not receive any external influenza vaccines and never tested PCR-positive for influenza A or B within the study period. Prior to initiation of this study, these communities had very low rates of vaccine uptake. The study also preceded universal recommendation of seasonal influenza vaccination for children in Canada. Therefore, the participants were unlikely to have received an influenza vaccine prior to study enrollment. For analysis of repeated vaccination with IIV (Figure 1A), we used annually collected pre-vaccination samples from the 2008-09, 2009-10 and 2012-13 seasons. The control group for this study included individuals who either received a hepatitis A vaccine, or no vaccine. No differences in immune responses were detected between the hepatitis A-vaccinated participants and the unvaccinated participants, and so they were grouped together for the purposes of this study. To assess the impact of vaccine formulation (IIV vs. LAIV, Figure 1B) we used samples collected at pre-vaccination day 0 and post-vaccination day 28 during the 2014-15 season. Vaccines were administered annually in October; participants' records were screened for a period of 3 months post-vaccination to allow for reporting of any external vaccination and for reporting of PCR-confirmed influenza. The IIV and LAIV vaccinees received a 0.5-mL intramuscular injection of Vaxigrip (Sanofi Pasteur) or a 0.2-mL dose of intranasal FluMist (MedImmune), respectively. The following influenza strains were used in the standard-of-care trivalent vaccines administered over the study period: A/Brisbane/59/2007(H1N1), A/Brisbane/10/2007 (H3N2), B/Florida/4/2006-like virus (2008-09); A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2) and B/Brisbane/60/2008 (2009-10); A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2), B/Brisbane/60/2008 (2010-11) and A/California/7/2009 (H1N1)pdm09, A/Texas/50/2012 (H3N2), B/Massachusetts/2/2012 (2014-15)29,41,44 (Table 1). The control group consisted of participants who received either hepatitis A vaccine (N = 24) or no vaccine (N = 7) during the study period (Figure 1A); no differences in any of the assessed demographic or immune parameters were seen between these two subsets within the control group. All study procedures were approved by the McMaster University Research Ethics Review Board.
Cell lines
Madin-Darby Canine Kidney (MDCK) cells (ATCC, VA, USA) were maintained in Dulbecco Modified Eagle Medium (DMEM, Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco) and 100 U/mL penicillin-streptomycin (Gibco) at 37 °C in 5% CO2. Trichoplusia ni High Five (ATCC) insect cells were maintained in HyClone SFX insect cell culture media (Cytiva/Fisher Scientific) in shaker flasks shaking at 70 rpm at 28 °C.45,46 Spodoptera frugiperda Sf9 insect cells (ATCC) were maintained as adherent cultures in Roux flasks in full TNM-FH insect cell media (Sigma) supplemented with 10% FBS, 1% penicillin-streptomycin and 0.1% Pluronic F68 solution at 27 °C.45,46
Cal/09 H1N1 and cH5/1 N3 viruses
A/California/04/2009 (Cal/09) H1N1 virus was kindly gifted by Dr. Peter Palese (Icahn School of Medicine at Mount Sinai, NY). The cH5/1 N3 virus was engineered to express cH5/1, a fusion of the A/Vietnam/1203/04 H5 head domain with PR8 group 1 stalk and neuraminidase N3.9 The viruses were propagated in 10-day-old embryonated specific-pathogen-free (SPF) chicken eggs sourced from Canadian Food Inspection Agency (CFIA) and maintained at 37C in a dry incubator.
Recombinant viral antigens
All viral antigens were made using the baculovirus expression system45 and constructs kindly gifted by Dr. Peter Palese and Dr. Florian Krammer (Icahn School of Medicine at Mount Sinai, NY). The cH6/1 HA construct was composed of a A/Mallard/Sweden/81/02 H6 head domain and a A/Puerto Rico/8/1934 (PR8) group 1 stalk.9 Briefly, construct sequences were cloned into pFastBac Dual Expression Vector (Gibco/ThermoFisher) plasmids containing a C-terminal trimerization domain and hexahistidine tag, followed by transformation into DH10Bac bacteria (Gibco/ThermoFisher). The resulting bacmids were transfected into Sf9 cells to generate recombinant baculovirus, which was subsequently used to infect High Five insect cells. Viral supernatants were harvested via centrifugation at 5500g for 20 min at 4 °C, and proteins purified by Ni-resin affinity chromatography.45
Method details
Sample collection and processing
Participant blood (5 mL) was collected into gold top serum separator tubes (BD) by venipuncture and centrifuged at 1300 × g for 15 min to extract serum. Nasal flocked swabs were collected into universal transport media (COPAN Diagnostics). All samples were stored at −80C prior to analyses. Serum was inactivated by either heating at 56°C for 30 min prior to ELISA, or by a combination of 56 °C and trypsin-heat-periodate treatment10 prior to MNT and HAI assays.
Microneutralization (MNT) assays
Participant sera were serially diluted 2-fold to create a 40 to 5120-fold dilution series using MEM supplemented with 1× penicillin-streptomycin, 25 nM HEPES, and TPCK-treated trypsin (0.25 - 1 μg/mL) (Sigma-Aldrich). The diluted sera were then incubated with the cH5/1 N3 virus at a concentration of 400 PFU/well at RT for 30 min. The virus-serum mix was then added to MDCK cells plated in a 96-well plate at ∼90% confluency and incubated at 5% CO2 for 1 h at 37°C. The plate was then washed and supplemented with DMEM containing 0.2 M L-glutamine (Gibco), 100U/mL penicillin and 1 μg/μL streptomycin overnight at 5% CO2, 37°C, followed by fixation with 80% acetone. Influenza virus nucleoprotein (NP) was measured using biotin-conjugated anti-NP antibody (EMB Millipore MAB8257B) and HRP-conjugated streptavidin (EMB Millipore). SigmaFast OPD (Sigma-Aldrich) was used as HRP substrate and luminescence was measured using the SpectraMax i3 plate reader at OD490. A MNT50 endpoint titer, serum dilution resulting in at least 50% inhibition of infectivity, was defined as the reciprocal of the lowest serum dilution factor, for which normalized signal remained below the threshold of detection; endpoints outside the assay’s lower limit of detection (<40) were assigned an endpoint titer of 20.
Haemagglutination inhibition (HAI) assays
Serum was mixed with the Cal/09 H1N1 virus and incubated for 30 min at RT. Chicken erythrocytes in Alsever's solution (sourced from CFIA) were then added to the plate and incubated at 4°C for 45 min. An HAI titer was defined as the highest serum dilution factor leading to HAI activity; samples without any detectable HAI activity were assigned a titer of “5”.
Influenza virus antibody ELISA
96-well plates were coated with 2 μg/mL of Cal/09 or cH6/1 HA, followed by 4°C incubation overnight and blocking with 5% non-fat milk in PBS-T. Sera were added to the plate in a 2-fold serial dilution series ranging from 100- to 6400-fold. Mucosal samples were assayed undiluted. Plates were then incubated for 1 h at RT, HRP-conjugated goat anti-human IgG or IgA were added. SigmaFast OPD (Sigma-Aldrich) was used as HRP substrate and luminescence was measured using the SpectraMax i3 plate reader at OD490. A detection threshold was defined as 3 standard deviations above the average background signal. Serum IgA and IgG endpoint titers were defined as the highest serum dilution factor for which normalized OD490 values remained above the background threshold; endpoints outside the assay’s limit of detection (i.e. <100 or >6400) were assigned endpoint titer values of 50 and 25600, respectively. Mucosal ELISA readouts were reported as normalized OD490 values; post-normalization values < 0 were substituted with assay-specific lowest limit of detection values for the geometric mean fold change (GMFC) and log2 calculations.
Statistical analyses
Statistical analyses were performed using IBM SPSS v.27 and GraphPad Prism v.7. Two-sided Mann-Whitney U and Fisher's exact tests were used to assess demographic differences among the participant groups in Tables S1 and S4. Data were scaled via log2 transformation. Geometric means and 95% confidence intervals were calculated for each time point in Figures 2, 3, 4, 5, S2, and S3. GMFC were calculated as ratios of geometric means (i.e. pre-/post-vaccination) for each parameter. Paired analyses were done using the paired t-test in Figures 2, 3, 4, 5, S1, S2, and S3. Differences between the LAIV and IIV groups were assessed using the unpaired t test in Figures 2, 3, 4, 5, and S2. Correlation analyses were performed using the Pearson correlation coefficient test and lines of best fit were derived via linear regression in Figures 2, 3, 4, 5, 6, and S2. Differences and correlations between variables were considered significant at p < 0.05.
Acknowledgments
The authors would like to thank the cRCT participants and study teams. This work was supported, in part, by a CIHR Project Grant (M.S.M.). M.S.M. was supported, in part, by a CIHR New Investigator Award, an Ontario Early Researcher Award, and a Canada Research Chair in Viral Pandemics. H.D.S. was supported, in part, by an Ontario Graduate Scholarship. S.Y. was supported, in part, by an M.G. DeGroote Postdoctoral Fellowship.
Author contributions
Conceptualization, M.S.M. and M.L.; investigation and formal analysis, M.S.M., S.Y., D.B.C., K.B.G., J.C.A., C.V., J.W., K.R., V.T., and H.D.S.; writing – original draft, M.S.M., S.Y., D.B.C., and K.B.G.; writing – review and editing, M.S.M., M.L., S.Y., D.B.C., K.B.G., J.C.A., C.V., J.W., K.R., and V.T.; funding acquisition, M.S.M. and M.L.
Declaration of interests
The authors declare no competing interests.
Published: February 3, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2022.100509.
Supplemental information
Data and code availability
-
•
All raw data used to perform analyses in this article are available in tables found in the supplementary information.
-
•
No original code was generated.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
References
- 1.Krammer F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019;19:383–397. doi: 10.1038/s41577-019-0143-6. [DOI] [PubMed] [Google Scholar]
- 2.Zhang A., Stacey H.D., Mullarkey C.E., Miller M.S. Original antigenic sin: how first exposure shapes lifelong anti-influenza virus immune responses. J. Immunol. 2019;202:335–340. doi: 10.4049/jimmunol.1801149. [DOI] [PubMed] [Google Scholar]
- 3.Miller M.S., Palese P. Peering into the crystal ball: influenza pandemics and vaccine efficacy. Cell. 2014;157:294–299. doi: 10.1016/j.cell.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Li Y., O'Brien K.L., Madhi S.A., Widdowson M.A., Byass P., Omer S.B., Abbas Q., Ali A., Amu A., Azziz-Baumgartner E., Bassat Q., Abdullah Brooks W., Chaves S.S., Chung A., Cohen C., Echavarria M., Fasce R.A., Gentile A., Gordon A., Groome M., Heikkinen T., Hirve S., Jara J.H., Katz M.A., Khuri-Bulos N., Krishnan A., de Leon O., Lucero M.G., McCracken J.P., Mira-Iglesias A., Moïsi J.C., Munywoki P.K., Ourohiré M., Polack F.P., Rahi M., Rasmussen Z.A., Rath B.A., Saha S.K., Simões E.A., Sotomayor V., Thamthitiwat S., Treurnicht F.K., Wamukoya M., Yoshida L.M., Zar H.J., Campbell H., Nair H. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob. Health. 2020;8 doi: 10.1016/S2214-109X(19)30545-5. e497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., Wu P., Kyncl J., Ang L.W., Park M., Redlberger-Fritz M., Yu H., Espenhain L., Krishnan A., Emukule G., van Asten L., Pereira da Silva S., Aungkulanon S., Buchholz U., Widdowson M.A., Bresee J.S. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nachbagauer R., Feser J., Naficy A., Bernstein D.I., Guptill J., Walter E.B., Berlanda-Scorza F., Stadlbauer D., Wilson P.C., Aydillo T., Behzadi M.A., Bhavsar D., Bliss C., Capuano C., Carreño J.M., Chromikova V., Claeys C., Coughlan L., Freyn A.W., Gast C., Javier A., Jiang K., Mariottini C., McMahon M., McNeal M., Solórzano A., Strohmeier S., Sun W., Van der Wielen M., Innis B.L., García-Sastre A., Palese P., Krammer F. A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat. Med. 2021;27:106–114. doi: 10.1038/s41591-020-1118-7. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein D.I., Guptill J., Naficy A., Nachbagauer R., Berlanda-Scorza F., Feser J., Wilson P.C., Solórzano A., Van der Wielen M., Walter E.B., Albrecht R.A., Buschle K.N., Chen Y.Q., Claeys C., Dickey M., Dugan H.L., Ermler M.E., Freeman D., Gao M., Gast C., Guthmiller J.J., Hai R., Henry C., Lan L.Y., McNeal M., Palm A.E., Shaw D.G., Stamper C.T., Sun W., Sutton V., Tepora M.E., Wahid R., Wenzel H., Wohlbold T.J., Innis B.L., García-Sastre A., Palese P., Krammer F. Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: interim results of a randomised, placebo-controlled, phase 1 clinical trial. Lancet Infect. Dis. 2020;20:80–91. doi: 10.1016/S1473-3099(19)30393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellebedy A.H., Krammer F., Li G.M., Miller M.S., Chiu C., Wrammert J., Chang C.Y., Davis C.W., McCausland M., Elbein R., Edupuganti S., Spearman P., Andrews S.F., Wilson P.C., García-Sastre A., Mulligan M.J., Mehta A.K., Palese P., Ahmed R. Induction of broadly cross-reactive antibody responses to the influenza HA stem region following H5N1 vaccination in humans. Proc. Natl. Acad. Sci. U S A. 2014;111:13133–13138. doi: 10.1073/pnas.1414070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller M.S., Tsibane T., Krammer F., Hai R., Rahmat S., Basler C.F., Palese P. 1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans. J. Infect. Dis. 2013;207:98–105. doi: 10.1093/infdis/jis652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller M.S., Gardner T.J., Krammer F., Aguado L.C., Tortorella D., Basler C.F., Palese P. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci. Transl. Med. 2013;5:198ra107. doi: 10.1126/scitranslmed.3006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nachbagauer R., Choi A., Izikson R., Cox M.M., Palese P., Krammer F. Age dependence and isotype specificity of influenza virus hemagglutinin stalk-reactive antibodies in humans. mBio. 2016;7:e01996. doi: 10.1128/mBio.01996-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang N., He J., Weinstein J.A., Penland L., Sasaki S., He X.S., Dekker C.L., Zheng N.Y., Huang M., Sullivan M., Wilson P.C., Greenberg H.B., Davis M.M., Fisher D.S., Quake S.R. Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci. Transl. Med. 2013;5:171ra19. doi: 10.1126/scitranslmed.3004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He W., Mullarkey C.E., Duty J.A., Moran T.M., Palese P., Miller M.S. Broadly neutralizing anti-influenza virus antibodies: enhancement of neutralizing potency in polyclonal mixtures and IgA backbones. J. Virol. 2015;89:3610–3618. doi: 10.1128/JVI.03099-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He W., Mullarkey C.E., Miller M.S. Measuring the neutralization potency of influenza A virus hemagglutinin stalk/stem-binding antibodies in polyclonal preparations by microneutralization assay. Methods. 2015;90:95–100. doi: 10.1016/j.ymeth.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 15.Ekiert D.C., Kashyap A.K., Steel J., Rubrum A., Bhabha G., Khayat R., Lee J.H., Dillon M.A., O’Neil R.E., Faynboym A.M., et al. Neutralization of influenza A viruses by insertion of a single antibody loop into the receptor binding site. Nature. 2012;489:526–532. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broadbent A.J., Subbarao K. Influenza virus vaccines: lessons from the 2009 H1N1 pandemic. Curr. Opin. Virol. 2011;1:254–262. doi: 10.1016/j.coviro.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ang J.C., Wang B., Wang J.J.F., Zeng P.Y.F., Krammer F., Ward B.J., Russell M.L., Loeb M., Miller M.S. Comparative immunogenicity of the 2014–2015 Northern hemisphere trivalent IIV and LAIV against influenza A viruses in children. Vaccines. 2019;7:87. doi: 10.3390/vaccines7030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoft D.F., Lottenbach K.R., Blazevic A., Turan A., Blevins T.P., Pacatte T.P., Yu Y., Mitchell M.C., Hoft S.G., Belshe R.B. Comparisons of the humoral and cellular immune responses induced by live attenuated influenza vaccine and inactivated influenza vaccine in adults. Clin. Vaccin. Immunol. 2017;24:e00414–e00416. doi: 10.1128/CVI.00414-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohn K.G., Smith I., Sjursen H., Cox R.J. Immune responses after live attenuated influenza vaccination. Hum. Vaccin. Immunother. 2018;14:571–578. doi: 10.1080/21645515.2017.1377376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su F., Patel G.B., Hu S., Chen W. Induction of mucosal immunity through systemic immunization: phantom or reality? Hum. Vaccin. Immunother. 2016;12:1070–1079. doi: 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clements J.D., Freytag L.C. Parenteral vaccination can Be an effective means of inducing protective mucosal responses. Clin. Vaccin. Immunol. 2016;23:438–441. doi: 10.1128/CVI.00214-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO | Influenza update - 390. WHO. http://www.who.int/influenza/surveillance_monitoring/updates/latest_update_GIP_surveillance/en/
- 23.Tokars J.I., Olsen S.J., Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin. Infect. Dis. 2018;66:1511–1518. doi: 10.1093/cid/cix1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nachbagauer R., Salaun B., Stadlbauer D., Behzadi M.A., Friel D., Rajabhathor A., Choi A., Albrecht R.A., Debois M., García-Sastre A., Rouxel R.N., Sun W., Palese P., Mallett C.P., Innis B.L., Krammer F., Claeys C. Pandemic influenza virus vaccines boost hemagglutinin stalk-specific antibody responses in primed adult and pediatric cohorts. NPJ Vaccin. 2019;4:51. doi: 10.1038/s41541-019-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosalaraksa P., Jeanfreau R., Frenette L., Drame M., Madariaga M., Innis B.L., Godeaux O., Izurieta P., Vaughn D.W. AS03B-adjuvanted H5N1 influenza vaccine in children 6 months through 17 years of age: a phase 2/3 randomized, placebo-controlled, observer-blinded trial. J. Infect. Dis. 2015;211:801–810. doi: 10.1093/infdis/jiu548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zost S.J., Wu N.C., Hensley S.E., Wilson I.A. Immunodominance and antigenic variation of influenza virus hemagglutinin: implications for design of universal vaccine immunogens. J. Infect. Dis. 2019;219:S38–S45. doi: 10.1093/infdis/jiy696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guthmiller J.J., Lan L.Y., Fernández-Quintero M.L., Han J., Utset H.A., Bitar D.J., Hamel N.J., Stovicek O., Li L., Tepora M., Henry C., Neu K.E., Dugan H.L., Borowska M.T., Chen Y.Q., Liu S.T.H., Stamper C.T., Zheng N.Y., Huang M., Palm A.E., García-Sastre A., Nachbagauer R., Palese P., Coughlan L., Krammer F., Ward A.B., Liedl K.R., Wilson P.C. Polyreactive broadly neutralizing B cells are selected to provide defense against pandemic threat influenza viruses. Immunity. 2020;53:1230–1244.e5. doi: 10.1016/j.immuni.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pica N., Hai R., Krammer F., Wang T.T., Maamary J., Eggink D., Tan G.S., Krause J.C., Moran T., Stein C.R., Banach D., Wrammert J., Belshe R.B., García-Sastre A., Palese P. Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc. Natl. Acad. Sci. U S A. 2012;109:2573–2578. doi: 10.1073/pnas.1200039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeb M., Russell M.L., Manning V., Fonseca K., Earn D.J., Horsman G., Chokani K., Vooght M., Babiuk L., Schwartz L., Neupane B., Singh P., Walter S.D., Pullenayegum E. Live attenuated versus inactivated influenza vaccine in Hutterite children: a cluster randomized blinded trial. Ann. Intern. Med. 2016;165:617–624. doi: 10.7326/M16-0513. [DOI] [PubMed] [Google Scholar]
- 30.Clements M.L., Murphy B.R. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J. Clin. Microbiol. 1986;23:66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy B.R., Nelson D.L., Wright P.F., Tierney E.L., Phelan M.A., Chanock R.M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect. Immun. 1982;36:1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powers D.C., Sears S.D., Murphy B.R., Thumar B., Clements M.L. Systemic and local antibody responses in elderly subjects given live or inactivated influenza A virus vaccines. J. Clin. Microbiol. 1989;27:2666–2671. doi: 10.1128/jcm.27.12.2666-2671.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammarström L., Vorechovsky I., Webster D. Selective IgA deficiency (SIgAD) and common variable immunodeficiency (CVID) Clin. Exp. Immunol. 2000;120:225–231. doi: 10.1046/j.1365-2249.2000.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abreu R.B., Clutter E.F., Attari S., Sautto G.A., Ross T.M. IgA responses following recurrent influenza virus vaccination. Front. Immunol. 2020;11:902. doi: 10.3389/fimmu.2020.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gould V.M.W., Francis J.N., Anderson K.J., Georges B., Cope A.V., Tregoning J.S. Nasal IgA provides protection against human influenza challenge in volunteers with low serum influenza antibody titre. Front. Microbiol. 2017;8:900. doi: 10.3389/fmicb.2017.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer M.A., Meyer L., Bianchi M., Turner H.L., Le N.P.L., Steck M., Wyrzucki A., Orlowski V., Ward A.B., Crispin M., Hangartner L. Glycosylation of human IgA directly inhibits influenza A and other sialic-acid-binding viruses. Cell Rep. 2018;23:90–99. doi: 10.1016/j.celrep.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stacey H.D., Golubeva D., Posca A., Ang J.C., Novakowski K.E., Zahoor M.A., Kaushic C., Cairns E., Bowdish D.M.E., Mullarkey C.E., Miller M.S. IgA potentiates NETosis in response to viral infection. Proc. Natl. Acad. Sci. U S A. 2021;118 doi: 10.1073/pnas.2101497118. e2101497118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George S.T., Lai J., Ma J., Stacey H.D., Miller M.S., Mullarkey C.E. Neutrophils and influenza: a thin line between helpful and harmful. Vaccines (Basel) 2021;9:597. doi: 10.3390/vaccines9060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andrews S.F., Huang Y., Kaur K., Popova L.I., Ho I.Y., Pauli N.T., Henry Dunand C.J., Taylor W.M., Lim S., Huang M., Qu X., Lee J.H., Salgado-Ferrer M., Krammer F., Palese P., Wrammert J., Ahmed R., Wilson P.C. Immune history profoundly affects broadly protective B cell responses to influenza. Sci. Transl. Med. 2015;7:316ra192. doi: 10.1126/scitranslmed.aad0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arevalo C.P., Le Sage V., Bolton M.J., Eilola T., Jones J.E., Kormuth K.A., Nturibi E., Balmaseda A., Gordon A., Lakdawala S.S., Hensley S.E. Original antigenic sin priming of influenza virus hemagglutinin stalk antibodies. Proc. Natl. Acad. Sci. U S A. 2020;117:17221–17227. doi: 10.1073/pnas.1920321117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loeb M., Russell M.L., Moss L., Fonseca K., Fox J., Earn D.J., Aoki F., Horsman G., Van Caeseele P., Chokani K., Vooght M., Babiuk L., Webby R., Walter S.D. Effect of influenza vaccination of children on infection rates in hutterite communities: a randomized trial. JAMA. 2010;303:943. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]
- 42.Palese P., Wang T.T. Why do influenza virus subtypes die out? A hypothesis. mBio. 2011;2 doi: 10.1128/mBio.00150-11. e00150–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng S., Nachbagauer R., Balmaseda A., Stadlbauer D., Ojeda S., Patel M., Rajabhathor A., Lopez R., Guglia A.F., Sanchez N., Amanat F., Gresh L., Kuan G., Krammer F., Gordon A. Novel correlates of protection against pandemic H1N1 influenza A virus infection. Nat. Med. 2019;25:962–967. doi: 10.1038/s41591-019-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang B., Russell M.L., Moss L., Fonseca K., Earn D.J., Aoki F., Horsman G., Caeseele P.V., Chokani K., Vooght M., Babiuk L., Webby R., Walter S.D., Loeb M. Effect of influenza vaccination of children on infection rate in hutterite communities: follow-up study of a randomized trial. PLoS One. 2016;11:e0167281. doi: 10.1371/journal.pone.0167281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Margine I., Palese P., Krammer F. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J. Vis. Exp. 2013:e51112. doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krammer F., Schinko T., Palmberger D., Tauer C., Messner P., Grabherr R. Trichoplusia ni cells (High Five™) are highly efficient for the production of influenza A virus-like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol. Biotechnol. 2010;45:226–234. doi: 10.1007/s12033-010-9268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All raw data used to perform analyses in this article are available in tables found in the supplementary information.
-
•
No original code was generated.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.