Abstract
Introduction
The need to rapidly identify safe and efficacious drug therapies for COVID-19 has resulted in the implementation of multiple clinical trials investigating potential treatment options. These are being undertaken in an unprecedented research environment and at a higher speed than ever before. It is unclear how West African communities perceive such activities and how such perceptions influence participation in COVID-19 clinical trials. This qualitative study was conducted to assess the level of acceptability of a clinical trial on the prevention and treatment of COVID-19 in The Gambia and identify strategies to better engage communities in participating in such a trial.
Methods
Data were collected using digitally recorded semistructured interviews (SSIs) and focus group discussions (FGDs) in Brikama and Kanifing local government areas. These are two of the most densely populated administrative subdivisions in The Gambia, where the clinical trial was to be implemented by the MRC Unit The Gambia. 26 men and 22 women aged between 19 and 70 years, with diverse socioeconomic profiles, participated in 8 FGDs (n=36) and 12 SSIs (n=12). Thematic analysis was used to analyse the data.
Results
Fear of stigmatisation of patients with COVID-19 was a recurring theme in most FGDs and SSIs, with detrimental effects on willingness to accept COVID-19 testing and home visits to follow up patients with COVID-19 and their household contacts. Preserving the privacy of individuals enrolled in the study was key to potentially increase trial participation. Trust in the implementing institution and its acknowledged expertise were facilitators to accepting the administration of investigational products to sick individuals and their close contacts.
Conclusion
COVID-19 is a stigmatising disease. Developing a research–participant collaboration through an ongoing engagement with community members is crucial to a successful enrolment in COVID-19 clinical trials. Trust and acknowledged expertise of the implementing institution are key facilitators to foster such collaboration.
Keywords: COVID-19, public health, health education and promotion, health systems evaluation, prevention strategies
Key questions.
What is already known?
No previous research has addressed how communities in sub-Saharan Africa perceive COVID-19 prophylactic and therapeutic trials and how their perceptions influence their participation in such trials.
What are the new findings?
COVID-19 is perceived as a stigmatising disease with detrimental effects on willingness to accept COVID-19 testing and home visits to follow up patients with COVID-19 and their household contacts.
Social structures are dynamic, particularly in the case of COVID-19, and new community engagement approaches need to be developed.
Trust and expertise of the implementing institution are facilitators to accepting the administration of investigational products of a COVID-19 clinical research to enrolled individuals.
What do the new findings imply?
Developing and implementing a better research–participant collaboration through an ongoing engagement with communities are crucial to tackling COVID-19 widespread misinformation and trial-related rumours in intervention areas and will also increase acceptability and enrolment in COVID-19 clinical trials.
Trust and expertise of the trial implementing institution are assets to be nurtured and harness for better research collaboration between community members and the trial team.
Identifying knowledge gaps about trial procedures and related therapeutic misconceptions before commencing an intervention is essential to providing trialists with well-informed strategy to address them and to ensure a better and appropriate understanding of ethics rules of autonomy and rational and free choice to consent to intervention principles.
Introduction
The current COVID-19 pandemic has caused unforeseen and extreme challenges across the world. Globally (as of 18 September 2021), it continues to spread (nearly 227 million confirmed cases) and the number of deaths continues to increase (over 3.8 million deaths).1 Although there have been some reports on the benefits of a few therapeutic agents, a safe and efficacious treatment against SARS-CoV-2 remains elusive.2 As of 17 September 2021, there are 2848 ongoing clinical trials on potential treatments, 177 (6.2%) of them in Africa, mostly in South Africa and Egypt.3 Although vaccines are showing to be highly effective in flattening the curve of new infections and mortality, their roll-out in Africa is extremely slow—by 15 September 2021, only 3.3% of Africans have been fully vaccinated across the continent.4 Therefore, therapeutic options to reduce disease transmission and severity remain a key solution to tackle this pandemic across African countries.
Clinical trials are essential to provide evidence on the safety and efficacy of a given treatment.5 The search for an efficacious treatment should also consider the context in which it would be potentially used. In countries with limited financial, technical and human resources, as is the case in many sub-Saharan African countries, clinical trials on promising adequate therapeutic agents that are cost-effective and easy to use6 are a necessity. However, clinical trials usually give rise to concerns on potential safety risks. Therefore, exploring uncertainties before rolling out a medical intervention enables investigators to optimise trial feasibility,7 as community members can substantially enable or undermine the trial goals.8
In low-income and middle-income countries, clinical trials are often regarded as a complex and arduous process due to a broad range of stakeholders involved and cultural contexts that have historically been shaped by disadvantage and exploitation.9 Using community hierarchical decision-making structures and nurturing trust through endorsement of the research by local leaders are some facilitators to the conduct of trials.10–12 Furthermore, communities increasingly place greater emphasis on tangible research benefits.9 13–17 In many African settings, participants enrolled in trials do not always make a clear distinction between research and treatment.18 19 Their engagement is usually for both but with the treatment aspect in the foreground.18–20
Therefore, educating participants about the distinction between therapeutic treatment and research and increasing their awareness of the collective benefits as well as potential harms that trials can result in are needed to foster better research cultures in communities.6 17 21 Accordingly, researchers need to engage with communities when planning their activities. Not doing so may result in both ethical and scientific costs.22 Such engagement with communities is particularly needed in the context of COVID-19, which has been and is characterised by misinformation and rumours on the disease, its symptoms and transmission, prevention, and treatments.23 The present research sought to explore such challenges in The Gambia in order to improve the feasibility of a planned trial before its implementation.
The planned trial
The PaTS COVID-19 trial (Prevention and Treatment for COVID-19 associated Severe pneumonia in The Gambia) is an adaptative, single-blinded, placebo-controlled, individually randomised trial evaluating treatments for two different cohorts of patients (https://www.clinicaltrials.gov/ct2/show/NCT04703608). These are (1) mild or moderate COVID-19 cases and their household contacts (cohort 1) and (2) patients with severe COVID-19 (cohort 2). The treatment currently evaluated for cohort 1 is ivermectin (for both individuals and their household contacts), while for cohort 2 the investigational product is currently aspirin. The investigational products may be modified according to international guidelines or external data available. If that is the case, new drugs will be tested but the overall design of the trial will be maintained as described in the next section.
Patients in cohort 1 are randomised to one of three arms; in two of them, patients are treated with ivermectin while their household contacts are either treated with ivermectin or with a placebo. In the third arm, both patients and their household contacts receive a placebo. Patients in cohort 2 are randomised to either aspirin or placebo (ratio of 1 to 1). All patients receive standard of care. On top of it, they receive either the investigational products or the placebo. For cohort 1, biological samples, for example, nasopharyngeal or oropharyngeal swabs and blood samples, are collected at defined times both from the patients and their household contacts. Participants and household contacts are followed up for 2 weeks. If study participants are in quarantine at national treatment centres, the research team visits them there and the same procedures are performed except for recruitment of their household contacts into the trial. For cohort 2 (hospitalised patients), besides the follow-up carried out in the hospital, there are additional visits at days 28 and 90 after recruitment.
The trial is implemented by the Medical Research Council Unit The Gambia (MRCG) at the London School of Hygiene and Tropical Medicine in collaboration with the Gambian government. MRCG is an institution that has been conducting medical research in The Gambia and other West African countries for over 70 years.
Conducting this qualitative study, we aimed to assess the level of acceptability of the planned clinical trial on the prevention and treatment of COVID-19 in The Gambia and identify strategies to better engage the local communities for participation.
Methods
Study setting
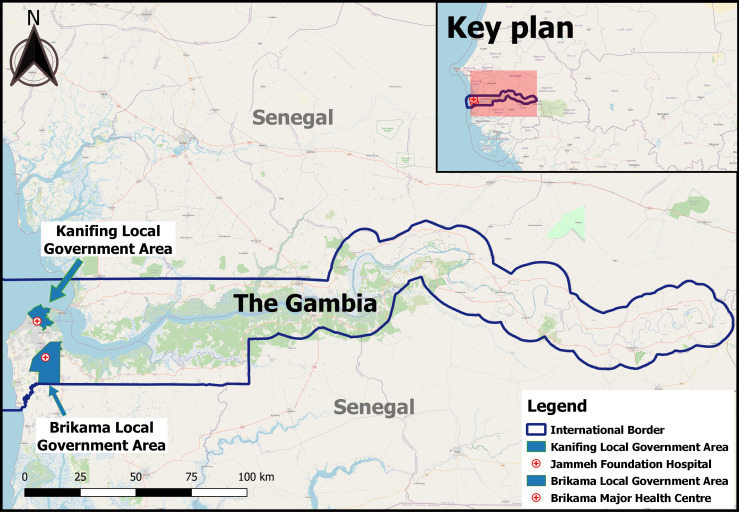
This study was carried out in Brikama and Kanifing local government areas (see figure 1), two densely populated administrative entities representing 37.1% and 20.3% of the total Gambian population, respectively.24 Kanifing is urban, while about three-quarters of the population in Brikama live in urban areas.24
Figure 1.
Map of study locations. Study sites are highlighted in blue. The map was designed using Quantum Geographic Information System (QGIS), a free and open-source Geographic Information System software.
Brikama and Kanifing were selected because they are both in the region with the highest incidence of confirmed COVID-19 cases. Between 15 January and 14 February 2021, the estimated incidence was 102–959 cases per 100 000 in Brikama and 45–101 cases per 100 000 in Kanifing.25 Furthermore, the PaTS trial surveillance activities for the identification of individuals for cohort 1 were to be implemented in health facilities of these two areas, namely Brikama District Hospital and Bundung Maternal and Neonatal Hospital.
The COVID-19 response in The Gambia involved stringent measures following the diagnosis of the first positive case in the country (17 March 2020). These included the declaration of the state of emergency, closure of international land, sea and air borders, and closure of schools, non-essential shops, places of worship and many workplaces.26 Contacts were also traced by the Ministry of Health staff and quarantined for 14 days in hotels during the early stage of the outbreak, April–July 2020, after which self-isolation at home was allowed.26 These activities were frequently conducted under a security escort to provide security to health staff and hospital ambulances transporting people to quarantine centres.27 28 From 4 June 2020, the government eased some restrictions that led to the reopening of markets, mosques and churches, with social distancing and face-mask wearing measures in place.29
Data collection
Fieldwork was carried out between 12 and 27 November 2020. Permission to conduct the data collection was obtained from the Alkalos (chiefs of the areas) and other local authority representatives. Written informed consent was obtained from all participants after having explained the aims and procedures of the study. A total of 8 focus group discussions (FGD) (n=36) and 12 semistructured interviews (SSI) (n=12) were conducted (see details of study participants in table 1). Participants were 26 men and 22 women, aged between 19 and 70 years with diverse professional and marital statuses. No participant dropped out during an FGD or an individual interview.
Table 1.
Target population reached and data collection approach used
| Location | Data collection technique | Sex | Age | Number conducted | Participants (n) |
| Kanifing | FGD | Male | 30–54 | 2 | 8 |
| Female | 38–54 | 2 | 8 | ||
| SSI | Male | 35–54 | 3 | 3 | |
| Female | 20–50 | 3 | 3 | ||
| Brikama | FGD | Female | 22–62 | 2 | 9 |
| Male | 19–70 | 2 | 11 | ||
| SSI | Male | 35–65 | 4 | 4 | |
| Female | 50–55 | 2 | 2 | ||
| Total | 48 | ||||
Source: Fieldwork conducted between 12 and 27 November 2020.
FGD, focus group discussion; SSI, semistructured interview.
The fieldwork started with FGDs in each study site. Each FGD was conducted with a different population group, two with young people (a male group and a female group), one with heads of compounds (male) and one with housewives—four FGDs conducted in each site. To identify group of individuals to be recruited for an FGD, the study team enquired about popular gathering places in each study site. These included compounds, streets, workplaces, specific location around markets and places of worship. Once mobilised to the sites, individuals were approached (purposive sampling) for participation in the data collection. In some instances, participants were asked to help find others with similar characteristics to themselves in order to get a required number for an FGD. Participants of an FGD all knew each other. FGDs included four to seven participants of the same gender to reduce power imbalances between participants and make them comfortable in the discussion settings.
Twelve SSIs (six in each study site) that included seven men and five women were conducted. Participants of SSIs were identified through snowball sampling from the FGDs. They were contacted for SSIs based on their influential roles within the community (representatives of community organisations and local authorities, and other influential young and elderly persons) and their personal experience of COVID-19 (formerly quarantined individuals).
Although communities in Brikama and Kanifing are familiar with the MRCG, prior participation in research projects of the institution was not an inclusion criterion of this formative study and no participant was contacted based on any previous work relations. SSIs and FGDs followed topic guides covering four sections: (1) demographic questions; (2) general knowledge about COVID-19 (perception, prevention, treatment and stigmatisation related to the disease); (3) perception, acceptability and participation in a COVID-19 clinical trial; and (4) information and sensitisation activities for rolling out the PaTS trial activities. In addition, for each SSI, individual experiences of the COVID-19 pandemic and their perceptions and concerns regarding participation in the PaTS trial were explored.
SSIs lasted for 30–45 min and FGDs for 50–60 min. They were conducted in Wolof or Mandinka (the most common local languages) at a time and place convenient to study participants (a participant house, on the street or at a workplace) by OC (male) and two (female) field assistants, supervised by BAD. The data collection team were all staff from the research institution (MRCG), but none of them had direct relation with the trial per se. Both BAD and OC have over 10 years of experience in conducting qualitative work. Fieldnotes were also written down. Participants’ recruitment continued until theoretical saturation was reached. No repeat interview was conducted with any of the participants. SSIs and FGDs were recorded using an encrypted device and transcribed verbatim in English. Transcripts were cross-checked against the audio files by OC.
Analysis
Data were analysed using thematic analysis. Data transcripts were inductively coded. Emerging themes were summarised, and the resulting summaries were cross-checked across cases to identify similarities and differences in COVID-19 perception, acceptance and participation in the PaTS trial. We also looked for an association between themes and respondent characteristics (age, gender).
Data were managed using NVivo (V.12). Data processing and analysis were conducted by BAD and reviewed by MM-A.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting or dissemination plans of our research.
Results
We first discuss respondents’ general perceptions and willingness to participate in the PaTS trial. We later show how such perceptions towards participation change as the trial activities were discussed in more detail. We finish by discussing how the study participants expected the trial team to engage with community members to increase recruitment in the trial.
Drivers of PaTS COVID-19 trial participation
The PaTS trial was the first clinical intervention on COVID-19 in The Gambia. Respondents were aware that no other COVID-19 trial was underway in the country. Participants largely appreciated the project.
If you can cure the disease and prevent people from getting it, this is very good to hear, and it is good work. We should all try to join the trial because as compound heads, it is we who carry the burden of any person that gets sick in the family. (FGD, male, 47 years old, Kanifing)
Although some reservations were made from FGDs and SSIs regarding specific trial activities, which we discuss in the next section, the majority of participants welcomed the PaTS study and reported being willing to participate in it if contacted by the trial team for enrolment. Some of them (both young and elderly respondents) also noted a willingness to convince their friends and/or family members to enrol in the trial. Several factors were mentioned for this willingness to participate.
First, the lack of an efficacious treatment contributed to their positive response, as reported by an FGD participant: “Now we are going to rely our hopes on the drugs you people are talking about” (FGD, male, 68 years old, Brikama).
Second, the expertise of the institution implementing the trial and the trust placed in it were of importance to respondents and contributed to acceptance to participate. This was reported by male and female participants of different age groups, both in FGDs and SSIs. For instance, an SSI participant, despite being somewhat concerned about how the investigators came to realise that the proposed drugs could help with the treatment and prevention of COVID-19, agreed to participate because of the trust he placed in the implementing institution. He contended: “I have trust in it [MRCG] because of the good work they are doing in the country” (SSI, male, 65 years old, Brikama).
Third, the fact that the planned trial includes young and elderly people was perceived as an advantage by both old and young respondents.
[Including people of different ages] will be a very good thing because nowadays we the young ones we believe that only elderly people are at risk of getting this infection. So, since this project is not going to leave any age group behind in terms of treatment to prevent all of us, that is a very good thing. (SSI, female, 20 years old, Kanifing)
Lastly, the fact that the Gambian government had given approval to conduct such an intervention and that Gambian doctors were involved in the trial was also mentioned by elderly respondents as contributing to their willingness to participate.
… I do not think there would be any government that would allow the people of its nation to be killed. […] We also have Gambian medical doctors and I do not think they would want to kill their own people. So, I think you should make people understand very well for them to believe and accept to take the preventive drugs. That is how I see it. (FGD, male, 69 years old, Brikama)
Despite this reported overall willingness to participate in the planned clinical intervention, when discussing its specific components some participants expressed concerns. These are discussed in the following section.
Perceptions on specific trial components
Administering interventional products to healthy and sick individuals
The interventional products tested are to prevent the infection (household contacts of cohort 1 cases) or to treat patients with COVID-19 (index cases for cohort 1 with mild/moderate disease and cohort 2 with severe disease). This means that healthy individuals whose household members test positive for COVID-19 and consent to the study will be offered prophylaxis. Study participants expressed mixed opinions regarding this practice.
Many informants found it acceptable to give drugs to healthy individuals for the sake of prevention. They commonly argued that “prevention is better than cure.” However, two groups of respondents, both male and female across FGDs and SSIs, reported concerns about administering preventive drugs to healthy individuals: those who do not believe that COVID-19 is real and those who do not see the necessity of taking a drug if they are healthy.
For now, it would be very difficult for people to take preventive drugs because many people believe that Corona does not exist. (FGD, female, 54 years old, Kanifing)
A male FGD participant systematically rejected the idea of administering drugs to healthy individuals, contending that “personally am not going to take any of those drugs as am very healthy” (FGD, male, 32 years old, Brikama). The belief that COVID-19 “does not exist” mostly motivated such reaction about administering the investigational products.
Perceptions towards administering a placebo drug
Respondents were asked their perceptions towards being given a placebo drug. Participants in majority accepted this clinical procedure. However, such acceptance was largely based on a misunderstanding of what placebo is meant for in a clinical trial. Most informants found taking a placebo an acceptable practice with the belief that even if the drug is not appropriate to treat COVID-19, being an investigational product, it is expected that it would be beneficial for their health.
If the drug will not be able to treat the disease but will enable to strengthen the body against other diseases, in that case, the drug can be given to the patient, and the patient should be given good care to help fight the disease and good food that would be beneficial for the patient’s body. (FGD, female, 62 years old, Brikama)
A similar argument was made by mainly younger participants. They reported that giving it would be appropriate only in the case of healthy individuals.
For me, the one that has the disease should be given the drugs and the other one who does not have any disease can be given the placebo to boost his/her immune system. (FGD, male, 32 years old, Brikama)
The health condition of the sick individuals was perceived as requiring more care, meaning they should only be offered active treatments.
Testing
The PaTS trial offers testing services to individuals fulfilling the WHO case definition of mild, moderate and severe infections.30 This is a key activity as it enables the trial team to identify and enrol individuals with a confirmed COVID-19 result. However, many respondents across all age groups were reluctant to be tested. Two main reasons were mentioned. First, participants perceived COVID-19 as a stigmatising disease and were therefore scared of the consequences of being diagnosed with it. For example, a female FGD participant reported:
Because of the finger-pointing, if you go for a COVID-19 test, maybe people like me, we are lousy, we will begin to say that Awa went for a Corona test, and she has Corona. So, you must be mindful to not come close to her and to not talk to her. Because of that many people will not go for the test. As I am talking to you right now many people do not go to the hospital because of the Corona test. (FGD, female, 54 years old, Kanifing)
Additionally, the fear of being taken to a treatment or isolation centre was reported as another reason for avoiding a COVID-19 test, as pointed out by a female FGD participant:
The reason why people are not willing to be tested is that there is no drug for the treatment. If you are tested and happen to have the disease, they will keep you in one place and the illness will become worse. (FGD, female, 45 years old, Kanifing)
Although the lack of efficacious treatment was mentioned as contributing to making some reluctant to go to treatment centres, the fear of stigmatisation was the main reason to avoid testing. Therefore, when asked about the way the trial team could adapt their testing strategy, some respondents suggested that health workers could “go to the compound and test the rest of the family and no one would know what happens in that compound” (FGD, female, 32 years old, Kanifing). From both FGDs and SSIs, assigning a health worker to compounds was perceived as being an effective strategy to prevent individuals from being exposed to stigmatisation.
Conducting home visits to take care of patients with COVID-19 and collect samples
Home visits were planned to collect biological samples at defined times both from patients and their household contacts to offer treatment to patients with COVID-19 who have mild/moderate symptoms and to follow them up within 2 weeks. For most respondents, the success of conducting home visit activities relied on how the study team would respect the confidentiality of individuals recruited in the trial.
They should come privately to avoid the notice of the community to prevent the patients and their household members from being stigmatised. (SSI, female, 55 years old, Brikama)
Such a viewpoint was put forth in response to the way the government COVID-19 response team was dealing with positive and/or suspected COVID-19 cases within communities. As described by an FGD participant, the government team would come:
With an ambulance and paramilitary with all the noises because everybody knows that such a car does not go out without anything happening. That will of course alert people and increase the stigma we are talking about. (FGD, female, 35 years old, Brikama)
To avoid stigma, two recommendations were put forward. First, informants suggested that the PaTS team should “enter the houses privately.” Therefore, using a means of transportation that does not raise any suspicion was made a requirement by most of the respondents to ensure individuals’ privacy. Second, the way to dress while visiting a COVID-19 suspected or confirmed case within the community was important. Most FGD and SSI participants reacted against the trial staff wearing personal protective equipment (PPE) when going to houses. For them, PPE should only be worn inside the compounds.
I suggest that the health workers come up to the house and put on their clothes [PPE] inside the compound. This will really help as it goes with respect and confidentiality. Because if people know that in this compound there is a case of COVID-19, everybody will run away from that compound, and this can really affect the relationship between that family and the people in the neighbourhood. (FGD, male, 40 years old, Kanifing)
An FGD participant (elderly person) and another one of an SSI (young girl) were exceptions. They felt that the health staff should rather wear their PPE before coming to houses in compliance with the safety guidelines, for their own health as well as the one of the household members to be visited.
Yeah, for me I would like them to put on their clothes [PPE] when coming to the house because you do not know who is carrying the virus. It can even be those coming to visit your house. So let them put on their clothes [PPE] and come to attend to the sick person. In doing this, we can then stop the spread of the virus among ourselves. (SSI, female, 25 years old, Kanifing)
Regarding keeping patients with COVID-19 who have mild/moderate symptoms at home for treatment, informants provided mixed opinions. For most of them, home treatment was perceived as an alternative to the government treatment centres, especially when the health condition of the sick person allows it.
To me, treating them at home will really help. Because if you are sick and you are at home with your family members, they will give you so much love, support, and care to make you feel so much comfortable. Also, not be lonely will really help in the treatment to recover so quickly. (SSI, female, 50 years old, Kanifing)
Respondents assumed that being kept at home would offer some emotional and psychological support to the patient. However, as the disease was perceived to be highly infectious, some other informants were afraid of providing care to patients with COVID-19 at home. They commented that patients should be kept in a safe place for treatment. Getting a proper isolation room in family settings to effectively control the spread of the disease was seen as a challenge because “patients will not want to be lonely and if they do not see those that they want to see, this will worsen the illness” (FGD, female, 47 years old, Kanifing). For such reason, “by the time that the patient is cured, the disease would be transferred to another family member, and this would increase the work of the health workers” (FGD, male, 70 years old, Brikama).
Engaging with communities for increased participation in PaTS trial
Community engagement is usually undertaken in the conduct of research as a mutually educative process to learn about communities’ cultures and understanding of research-related concepts and to contribute to research literacy by educating the community about key concepts critical to understanding the purpose and procedures of the research.31 When asked about expectations from the trial team, study participants expected the trial team to engage with community members “to explain the trial very well to make them join because it is COVID-19, and everyone may be scared” (FGD, male, 36 years old, Kanifing). The perceived nature of the disease and the related misinformation that prevailed in the country at the time of the study were felt as requiring further engagement with communities.
Because if you do not have an idea about something, someone can come and poison your mind or say negative things about the drug and people would not accept to take it. (FGD, male, 70 years old, Brikama)
When discussing the specific approach to community engagement, participants opined that using the traditional approach of the MRCG, which includes approaching compound heads, may not be sufficient. This was mainly expressed by elderly respondents. According to them, this is because the Gambian society is changing in such a way that the younger generation, being increasingly exposed to the outside world, were less likely to follow orders from the compound head. Therefore, some resistance from family members to join the PaTS study may be expected despite the full commitment of the compound head.
… What was happening before, if a compound head says something or asks his family members to do or join something, everybody will agree to it. But that is not what is happening now. Some of them will never want to accept the wishes of the head of the family. I am going to accept for myself but for the rest of the compound, it depends on them to agree. (SSI, male, 65 years old, Brikama)
Young men shared similar opinions in an FGD. They explained that they followed the advice given by the head of their ‘Strasar’ or ‘Ghetto’ (social group usually composed of agemates) more than the head of their compounds.
In this group, we have a president, and we all give respect to him. Whenever he is talking all of us listen and agree to what he is saying. Some even follow what he advises us to do more than the compounds heads. (FGD, male, 30 years old, Brikama)
In general, respondents, both men and women across FGDs and SSIs, emphasised the need for engaging with the youth (especially young men) with specific health sensitisation messages to increase their awareness and participation in the trial. They described them as the group possibly most reluctant to join the trial “because they [young individuals] believe that if you are not sixty years old and above you would not be infected” (FGD, male, 69 years old, Brikama).
To reach out to the whole community (including the youth), respondents suggested collaborating with trusted community members and involving them in sensitisation activities. The one-on-one sensitisation approach was also the first choice. Key activities to be conducted consisted of organising (face-to-face) meetings with key actors (local authority leaders, community organisation leaders and other influential figures) and compound to compound sensitisation.
You should organize meetings with the heads of youth and women groups, compound heads, and imam of the area. If you have all these persons, the needed information will be spread out to the people. (SSI, female, 25 years old, Kanifing)
Furthermore, organising radio shows, as “anything that is said on radios everybody hears it together” (FGD, male, 70 years, Brikama), was found to be an impactful sensitisation activity by elderly respondents both men and women, especially in the Brikama area. Using a public address system to go around with vehicles within communities to sensitise people was, to a lesser extent, suggested by some respondents.
Discussion
This study assessed community perceptions towards conducting a COVID-19 therapeutic and prophylactic trial in The Gambia, including trial acceptability, barriers to participation and appropriate strategies for engaging with communities to increase their participation in the planned trial.
Most study participants showed enthusiasm for the PaTS project and willingness to participate. The lack of efficacious treatment for COVID-19, the inclusion of different age groups in the intervention, the acknowledged expertise in health research of the institution implementing the trial and the approval given by the Gambian Government were instrumental in making respondents accept PaTS trial activities. However, despite the expressed willingness to participate, some concerns were raised regarding specific trial activities. Many respondents were unwilling to be tested and were also less supportive of home visits to be conducted by the trial team. Although home treatment was perceived as an alternative option to government treatment centres, the fear of being exposed to stigma was perceived as a major barrier. Previous research in The Gambia has shown that participants’ longer-term experiences and perceptions of the MRCG were more important than their knowledge of the particular study being implemented.14 We found that the particularities of the PaTS study rather played a more important role in deciding to enrol in the trial than participants’ long-term experience with the MRCG.
Stigma (or fear of it) related to COVID-19 underlined much of the unwillingness to participate in specific trial activities—participants feared that by taking part in these activities community members might think they have contracted the virus, which would have detrimental consequences to their well-being. The vast literature on stigma underlines the importance of context.32 33 In the context of the COVID-19 pandemic in The Gambia, the drivers of stigma identified through this study included the infectiousness of the disease and the way the national COVID-19 response team managed suspected or confirmed cases within the community, which most study participants perceived as exposing individuals to stigma. The increasing stigmatisation resulting from a societal response at large to COVID-1934 can also be linked to widespread fake news and misinformation.35 As identified elsewhere in the context of Ebola, tackling such issues requires determining and addressing what rumours, concerns, fears or mistrust emerge from dialogues with community members and individuals involved in the trial.13 Giving clear information to the community was suggested by participants as the best means to tackling the widespread misinformation about COVID-19 in PaTS implementation areas. Therefore, developing a participant–researcher relationship aimed to establish a common ground of understanding about COVID-19 between community members and the trial team appeared to be essential. Furthermore, implementing strategies that preserve individuals’ privacy appeared to be key to successfully make individuals accept testing and conduct home visits.
Trust is central for the successful implementation of any clinical intervention, particularly when dealing with a stigmatising disease.13 This was also confirmed by respondents regarding rolling out of the PaTS trial. Trust in the research institution highlighted by respondents was underpinned by its acknowledged expertise in health. It played an important role in respondents’ willingness to participate and to receive active and placebo drugs, both for healthy and sick individuals. However, the understanding of placebo that underlined most informants’ acceptance of this trial procedure revealed a certain therapeutic misconception, which is a widely recognised problem in clinical interventions.17 21 36 Therapeutic misconception occurs when a research participant fails to appreciate the distinction between the imperatives of clinical research and of ordinary treatment, and therefore inaccurately attributes therapeutic intent to research procedures.21 Being provided as an investigational product, our participants viewed the placebo as something that would strengthen the health of individuals enrolled in the trial against COVID-19. Although the issue of therapeutic misconception can be argued, especially in the context of stretched public health services,17 like in The Gambia, the PaTS trial team should clearly communicate with community members when enrolling participants to make them understand appropriately what role the placebo has in a clinical trial.
Moreover, participants’ broad apparent trust elicited further therapeutic misconceptions. First, it contributed to making most respondents rely their hope on the drugs proposed by the intervention—they were aware that an effective treatment for COVID-19 was yet to be made available. Second, it also made some denying any possibilities that there may be disadvantages to participating in the planned trial. They claimed that the trial team would not cause any harm to them. This may create some false hopes in some of them36 as most participants failed to distinguish clinical care that is individualised from research that tends to be standardised.21 Therefore, the primary goal of clinical research, which is to develop treatments that will be beneficial to patients in the future,21 should be clearly explained by PaTS team to individuals when seeking their enrolment in the intervention. Given that the research institution provides clinical care and at the same time carries research, trialists should elicit any nuances between the two practices when engaging with community members to guide a better understanding of ethics rules of autonomy and rational choice to consent to intervention principles.
Additionally, by acknowledging the expertise of the research institution in healthcare to underscore their willingness to participate, participants seemed to see PaTS study as an alternative option to the government treatment centres. The criticisms made regarding the approach used by the government may be taken as a reference point. Respondents’ lack of appreciation showed that they felt some structural constraints accessing COVID-19 care services that meet their needs in their environment. From the perspective of structural coercion,37–39 such contextual factors might be a threat to voluntariness and free choice. Although researchers cannot fully eliminate structural coercion because it operates outside the researcher–participant dyad, they can strive to mitigate its impact by identifying structural constraints and their impact on informed consent.37 In the case of the PaTS trial, prospective participants should be provided with information about alternatives available in terms of accessing COVID-19 care beyond the PaTS study across the country to ensure that their voluntariness and free choice are being safeguarded.
Engaging with communities usually entails a dynamic process and a constantly changing set of negotiated relationships to address expectations and challenges tailored to a given setting.22 40 It is reported elsewhere that the success of such negotiations may rely on the endorsement of the intervention by community leaders and local authority representatives and the respect of hierarchical decision-making structures in the community.10–12 However, we found that these activities alone would not guarantee increased participation. A degree of reluctance to join the trial by the youth, particularly young men, was found. It was also noted that they may be less likely to follow the advice of heads of compounds than usually expected. This dynamic set of relationships and roles in influencing decision-making implied that other groups should also be engaged, including youth social groups (‘Strasar’ or ‘Ghetto’). This is in line with findings of a previous study that recognising that power is not always straightforward is an important foundation for building more nuanced, sensitive and genuine engagement.13 Therefore, engaging with the broader community (beyond compound heads) during sensitisation appeared to be essential for more specific community engagement when rolling out the PaTS trial. Such an engagement was expected to be ongoing and reflexive to adapt to the changing social relations and the dynamic COVID-19 pandemic context within the country. Some activities to be conducted in this regard included one-on-one sensitisation and organisation of meetings with key community actors and other influential figures.
This study has some limitations. The methods of selecting participants may have resulted in selection bias and participants may not be fully representative of the local communities where data were collected. The study findings lack respondents’ validation that may entail some bias on the investigators’ interpretation of the information. However, to minimise our own potential bias, results were reported to show divergent views about trial perception and participation, trial components that respondents had found acceptable and others that they were reluctant about any participation, and how they expected the trialists to engage with communities to increase enrolment. Furthermore, this was a trial-specific study whose data were collected by the staff of the research institution. Therefore, this may have impacted on the depth of the results because respondents may not feel at ease discussing negative views that they may have about the institution in their presence. Nevertheless, the study findings provide a greater understanding of how a COVID-19 clinical intervention is perceived and how barriers to participation could be best addressed to successfully roll out PaTS trial activities. Although the results discussed are specific to our study context, meaning they lack transferability, the approach of conducting such formative research before the trial commences is clearly transferable to other settings; the resulting results can be used to adapt trial strategies to make them more appropriate to the community setting.
Conclusion
To our knowledge, this is the first study reporting insights and opinions on the acceptability and participation in a treatment trial on COVID-19 in sub-Saharan Africa. COVID-19 is a highly stigmatising disease and this may undermine people’s willingness to participate in research. Preserving the privacy of individuals and the acknowledged expertise of the research institution are essential to foster a better collaboration between researchers and communities. Willingness to participate in the planned trial was broadly correlated with misconceptions about research. The data provide insight into developing a more trial-specific engagement strategies with communities to address the prevailing misconceptions, which can be some illegitimate motifs about participation susceptible to discredit a trial; this is essential to ensure a better and appropriate ethical understanding of autonomy and free choice to consent to research. Furthermore, such an engagement with community members should be ongoing to adapt the trial sensitisation strategies to the dynamic social relations and COVID-19 pandemic context.
Acknowledgments
We are grateful to the Gambian health authorities, particularly to Mustapha Bittaye, MD, PhD and Charles Roberts, MD, for their assistance. We thank all the respondents for taking their time to participate in the interviews and focus group discussions. We are also grateful for Bintou Jarju’s and Kady Darboe’s fieldwork.
Footnotes
Handling editor: Seye Abimbola
Twitter: @Diallo_Brahima1, @UmbertoDAlessa2, @AnnaRocaGambia, @Melisa_MarAl
Contributors: BAD, AR, EU, MM-A and OC were involved in the design of this study. OC coordinated the conduct of this study, supervised by BAD. BAD conducted the data analysis. EU, AR, OC, MM-A and UD critically reviewed the paper. BAD is responsible for the overall content as the guarantor. All authors have approved the final draft of the paper.
Funding: This study was funded by the UK Research and Innovation as part of the COVID-19 strategic priorities supplementary funding for the Medical Research Council Unit The Gambia at the London School of Hygiene & Tropical Medicine (grant reference MC_PC_19084).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the Gambian government/MRCG Joint Ethics Committee and the LSHTM Ethics Committee (ref: 22603). Participants gave informed consent to participate in the study before taking part.
References
- 1.WHO . Who coronavirus (COVID-19) Dashboard, 2021. Available: https://covid19.who.int/ [Accessed 17 Sep 2021].
- 2.Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021;19:141–54. 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medicine USNLo . ClinicalTrial.Gov. Recruiting Studies, COVID-19 [Web]. US: National Library of Medicine, 2021. Available: https://clinicaltrials.gov/ct2/results/map?recrs=a&cond=COVID-19&map= [Accessed 17 Sep 2021].
- 4.COVID-19 Vaccination . Latest updates from Africa CDC on progress made in COVID-19 vaccinations on the continent. [Internet]. Centre for Disease Control and Prevention, 2021. Available: https://africacdc.org/covid-19-vaccination/ [Accessed 17 Sep 2021].
- 5.WHO . “Guidelines for Good Clinical Practice (GCP) for Trials on Pharmaceutical Products”[UNAIDS Meeting-Ethical Considerations in Preventative Vaccine Trials Against HIV/AIDS. 1998: Geneva, Switzerland: World Health Organization, 1995. [Google Scholar]
- 6.Franzen SRP, Chandler C, Siribaddana S, et al. Strategies for developing sustainable health research capacity in low and middle-income countries: a prospective, qualitative study investigating the barriers and enablers to locally led clinical trial conduct in Ethiopia, Cameroon and Sri Lanka. BMJ Open 2017;7:e017246. 10.1136/bmjopen-2017-017246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Cathain A, Hoddinott P, Lewin S, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud 2015;1:32. 10.1186/s40814-015-0026-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gikonyo C, Bejon P, Marsh V, et al. Taking social relationships seriously: lessons learned from the informed consent practices of a vaccine trial on the Kenyan coast. Soc Sci Med 2008;67:708–20. 10.1016/j.socscimed.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph PD, Craig JC, Caldwell PHY. Clinical trials in children. Br J Clin Pharmacol 2015;79:357–69. 10.1111/bcp.12305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angwenyi V, Kamuya D, Mwachiro D, et al. Complex realities: community engagement for a paediatric randomized controlled malaria vaccine trial in Kilifi, Kenya. Trials 2014;15:65. 10.1186/1745-6215-15-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okello G, Jones C, Bonareri M, et al. Challenges for consent and community engagement in the conduct of cluster randomized trial among school children in low income settings: experiences from Kenya. Trials 2013;14:142–11. 10.1186/1745-6215-14-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tindana PO, Rozmovits L, Boulanger RF, et al. Aligning community engagement with traditional authority structures in global health research: a case study from Northern Ghana. Am J Public Health 2011;101:1857–67. 10.2105/AJPH.2011.300203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enria L, Lees S, Smout E, et al. Power, fairness and trust: understanding and engaging with vaccine trial participants and communities in the setting up the EBOVAC-Salone vaccine trial in Sierra Leone. BMC Public Health 2016;16:1140. 10.1186/s12889-016-3799-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairhead J, Leach M, Small M. Public engagement with science? Local understandings of a vaccine trial in the Gambia. J Biosoc Sci 2006;38:103–16. 10.1017/S0021932005000945 [DOI] [PubMed] [Google Scholar]
- 15.Geissler PW, Kelly A, Imoukhuede B, et al. 'He is now like a brother, I can even give him some blood'--relational ethics and material exchanges in a malaria vaccine 'trial community' in The Gambia. Soc Sci Med 2008;67:696–707. 10.1016/j.socscimed.2008.02.004 [DOI] [PubMed] [Google Scholar]
- 16.Geissler PW, Pool R. Editorial: Popular concerns about medical research projects in sub-Saharan Africa--a critical voice in debates about medical research ethics. Trop Med Int Health 2006;11:975–82. 10.1111/j.1365-3156.2006.01682.x [DOI] [PubMed] [Google Scholar]
- 17.Molyneux CS, Peshu N, Marsh K. Trust and informed consent: insights from community members on the Kenyan coast. Soc Sci Med 2005;61:1463–73. 10.1016/j.socscimed.2004.11.073 [DOI] [PubMed] [Google Scholar]
- 18.Caldwell PHy, Butow PN, Craig JC. Parents' attitudes to children's participation in randomized controlled trials. J Pediatr 2003;142:554–9. 10.1067/mpd.2003.192 [DOI] [PubMed] [Google Scholar]
- 19.Fairhead J, Leach M, Small M. Where techno-science meets poverty: medical research and the economy of blood in the Gambia, West Africa. Soc Sci Med 2006;63:1109–20. 10.1016/j.socscimed.2006.02.018 [DOI] [PubMed] [Google Scholar]
- 20.Leach A, Hilton S, Greenwood BM, et al. An evaluation of the informed consent procedure used during a trial of a Haemophilus influenzae type B conjugate vaccine undertaken in the Gambia, West Africa. Soc Sci Med 1999;48:139–48. 10.1016/S0277-9536(98)00317-7 [DOI] [PubMed] [Google Scholar]
- 21.Lidz CW, Appelbaum PS. The therapeutic misconception: problems and solutions. Med Care 2002;40:V55–63. 10.1097/01.MLR.0000023956.25813.18 [DOI] [PubMed] [Google Scholar]
- 22.Lavery JV, Tinadana PO, Scott TW, et al. Towards a framework for community engagement in global health research. Trends Parasitol 2010;26:279–83. 10.1016/j.pt.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 23.WHO . Coronavirus disease 2019 (COVID-19). situation report – 86, 2020. Available: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200415-sitrep-86-covid-19.pdf?sfvrsn=c615ea20_6 [Accessed 20 Jun 2021].
- 24.GBoS . Population and housing census 2013. spatial distribution. Gambia: Republic of The Gambia, 2013. [Google Scholar]
- 25.Ministry of Health . The Gambia COVID-19 outbreak situational report # 278. Contract No.: report No. 278. Banjul, 2021. [Google Scholar]
- 26.Abatan B, Agboghoroma O, Akemoke F, et al. Intense and mild first epidemic wave of coronavirus disease, the Gambia. Emerg Infect Dis 2021;27:2064–72. 10.3201/eid2708.204954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NLSC for COVID-19 Response. National security tour to all border posts in the Gambia. Safety and Security Report. Banjul: The Gambia, WHO, 2020. [Google Scholar]
- 28.Jallow M, Sankareh F. Public health response to Covid-19, the Gambian story. The Point, 2020. [Google Scholar]
- 29.Gambia Government . Gambia Government Relaxes Some Covid-19 Restrictions [press release. Banjul, 2020. [Google Scholar]
- 30.WHO . Who COVID-19: case definitions case definitions. updated in public health surveillance for COVID-19. Geneva: WHO, 2020. [Google Scholar]
- 31.CIOMS . International ethical guidelines for health-related research involving humans. Geneva, 2016. [DOI] [PubMed] [Google Scholar]
- 32.Nyblade L, Stangl A, Weiss E, et al. Combating HIV stigma in health care settings: what works? J Int AIDS Soc 2009;12:15–17. 10.1186/1758-2652-12-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nyblade L, Stockton MA, Giger K, et al. Stigma in health facilities: why it matters and how we can change it. BMC Med 2019;17:1–15. 10.1186/s12916-019-1256-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhanot D, Singh T, Verma SK, et al. Stigma and discrimination during COVID-19 pandemic. Front Public Health 2021;8:829. 10.3389/fpubh.2020.577018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Lancet Infectious Diseases . The COVID-19 infodemic. Lancet Infect Dis 2020;20:875. 10.1016/S1473-3099(20)30565-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appelbaum PS, Roth LH, Lidz CW, et al. False hopes and best data: consent to research and the therapeutic misconception. Hastings Cent Rep 1987;17:20–4. 10.2307/3562038 [DOI] [PubMed] [Google Scholar]
- 37.Fisher JA. Expanding the frame of "voluntariness" in informed consent: structural coercion and the power of social and economic context. Kennedy Inst Ethics J 2013;23:355–79. 10.1353/ken.2013.0018 [DOI] [PubMed] [Google Scholar]
- 38.Nyirenda D, Sariola S, Kingori P, et al. Structural coercion in the context of community engagement in global health research conducted in a low resource setting in Africa. BMC Med Ethics 2020;21:90. 10.1186/s12910-020-00530-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kingori P. The 'empty choice': A sociological examination of choosing medical research participation in resource-limited Sub-Saharan Africa. Curr Sociol 2015;63:763–78. 10.1177/0011392115590093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.HIV/AIDS JUNPo . Ethical considerations in biomedical HIV prevention trials. UNAIDS/WHO guidance document. Report No.: 978 92 9173 956 1. Geneva: UNAIDS, 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.