Abstract
Introduction:
Postpartum (PP) family planning is critical to reduce maternal-child mortality, abortion, and unintended pregnancy. As in most countries, the majority of PP women in Rwanda have an unmet need for postpartum family planning (PPFP). In particular, increasing use of the highly effective PP long-acting reversible contraceptive (LARC) methods (the intrauterine device [IUD] and implant) is a national priority. We developed a muti-level intervention to increase supply and demand for PPFP services in Kigali, Rwanda.
Methods:
We implemented our intervention (which included PPFP promotional counseling for clients, training for providers, and Ministry of Health stakeholder involvement) in six government health facilities from Aug 2017-Oct 2018. While increasing knowledge and uptake of the IUD was a primary objective, all contraceptive method options were discussed and made available. Here, we report a secondary analysis of PP implant uptake and present already published data on PP IUD uptake for reference.
Results:
Over a 15-month implementation period, 12,068 women received PPFP educational counseling and delivered at a study facility. Of these women, 1252 chose a PP implant (10.4% uptake) and 3372 chose a PPIUD (27.9% uptake). On average providers at our intervention facilities inserted 83.5 PP implants/month and 224.8 PPIUDs/month. Prior to our intervention, 30 PP implants/month and 8 PPIUDs/month were inserted at our selected facilities. Providers reported high ease of LARC insertion, and clients reported minimal insertion anxiety and pain.
Conclusions:
PP implant and PPIUD uptake significantly increased after implementation of our multi-level intervention. PPFP methods were well-received by clients and providers.
Keywords: postpartum family planning, implant, intrauterine device, LARC, Rwanda
INTRODUCTION
Postpartum (PP) family planning is critical to improve birth spacing which reduces maternal-child mortality, abortion, unintended pregnancy, and improves family well-being 1 2. Sixty-one percent of postpartum women aged 15–49 years across 21 low- and middle-income countries, including Rwanda, have an unmet need for postpartum family planning (PPFP) 3. Among the few postpartum women who are contracepting, almost all (79%) use short-acting contraceptive methods, with relatively few using the more effective long-acting reversible contraceptive (LARC) methods 3. In Rwanda, the most densely populated country in continental Africa, the government has made scaling-up PPFP an objective of their Family Planning 2020 Commitment, and LARC methods are prioritized 4 5.
In response to this scale-up, researchers at Projet San Francisco developed and evaluated an evidence-based, multi-level intervention with the goal of increasing supply and demand for PPFP in Kigali, Rwanda. We previously published the effect of our multi-level intervention on PP intrauterine device (IUD) uptake and service delivery outcomes with data reported between August 2017 and July 2018. Briefly, we found that 29% of women who received our PPFP counseling selected a PPIUD (a 2,749% increase in PPIUD insertions comparing pre vs post-intervention). 6
Here, in a secondary analysis, we describe PP implant uptake and service delivery outcomes among women who received our PPFP counseling between August 2017 and October 2018. We present updated PPIUD data (including both the previously reported PPIUD data and additional PPIUD data reported through October 2018) for reference.
METHODS
Informed Consent and Ethics
The Emory University IRB and the Rwanda National Ethic Committee approved the research component of this project (IRB 00001497). Prior to enrollment, written informed consent was obtained from all participants engaging in human subjects research. The Emory University IRB determined the programmatic service delivery component of this project was exempt from review.
Intervention Development and Design
We previously published methods regarding development and implementation of our multi-level PPFP service delivery model targeting supply, demand creation, and sustainability 6 7. Briefly, based on our team’s experience implementing LARC programs in Rwanda 8–10 and formative work conducted from May-August 2017 by Emory University-affiliated, non-governmental organization Projet San Francisco with nurses, providers, clients, community health workers, and government stakeholders informed the development of an educational flipchart on the PPIUD for use in group and one-on-one counseling along with existing flipcharts materials which describe other family planning method options which were already in use in government clinic family planning departments (existing materials did not discuss PPIUD, which has different insertion procedures and side-effects relative to interval IUD insertion, necessitating creation of the PPIUD flipchart). We trained providers in PPIUD insertion/removal. As providers had previously been trained in implant insertion/removal, we offered refresher trainings as needed. We reimbursed providers at a higher rate for the IUD compared to the implant ($1.20 for PPIUD insertion, $0.62 for PP implant insertion [in USD]) because of the additional training and certification required before providing PPIUDs to clients as has been described 11. Finally, we engaged Ministry of Health stakeholders at the outset and throughout the implementation.
Promoting PPFP
The PPIUD flipchart was administered in addition to standard FP counseling materials in two health centers and two high-volume hospitals including their affiliated health centers (6 facilities total). All PP contraceptive method options were discussed and made available. Notably, family planning counseling is not systematically provided in Rwandan antenatal care (ANC), labor and delivery (L&D), and infant vaccination (IV) services, but rather women are referred to separate family planning services. Therefore, our program made efforts to increase uptake of all PPFP methods by integrating counseling into ANC, L&D, and IV services. Study nurses and other trained staff delivered educational promotions on all PPFP methods to expectant mothers and new mothers (≤ 6-weeks postpartum) during their family planning counseling sessions in ANC, L&D and the postpartum ward, and/or during IV appointments occurring within 6 weeks of delivery. In L&D wards in Kigali, we counseled women not yet in active labor in the L&D waiting room (women in active labor are moved to L&D beds and were not counseled). Study nurses delivered educational promotions about the entire contraceptive method mix in group settings first (20 minutes). Then, they held one-on-one promotions with any women from the group sessions who were interested in more information about any PPFP method (an additional 20 minutes of counseling including time for questions and discussion). Partners or fathers were included in these educational promotions, if present. If PPIUD clients did not receive an IUD within 48 hours postpartum, they could not receive an IUD until after 4 weeks postpartum per WHO guidance 2. PP implant clients were eligible to receive an implant anytime within 6 weeks postpartum. All PPFP methods were provided at no cost to the client.
Service Delivery Outcomes
The service delivery outcomes of interest included (1) the number of participants who received the intervention (at least one promotion) and delivered at a study health facility, (2) the number of participants declining a PPFP method, (3) the number of PP implant insertions, and (4) the number of PPIUD (ParaGard TCu 380A) insertions. Only PPIUD clients had a 6-week insertion follow-up to check for IUD expulsion or discomfort (we reimbursed PPIUD clients for travel to and from follow-up appointments). PP implant clients received either levonorgestrel-releasing (Jadelle) or etonogestrel-releasing (Implanon) implants. PPIUDs were the non-hormonal, copper IUD. If a participant received one-on-one promotion(s) but delivered at non-study facility or at home, we assumed no PP LARC uptake unless she returned to a study facility for infant vaccinations and then elected to receive a PP LARC method.
Patient and Provider Experiences
PP implant and PPIUD clients and providers (nurses and midwives) were surveyed about their anxiety and pain during insertion (clients) and ease-of-insertion (providers) using 10-point Likert scale measures.
Data Collection
Study nurses assigned participants unique codes lacking personal information during their first one-on-one promotion. Nurses manually recorded promotions, insertions, and follow-up data, including Likert score data, in government logbooks then subsequently (≤1 week) uploaded that information into tablets using Survey CTO (Dobility, Cambridge, USA). We recorded demographic information only for those receiving a PPFP method.
REQUIREMENT FOR PATIENT AND PUBLIC INVOLVEMENT STATEMENT
Our intervention and outcome measures were developed after extensive formative work with pregnant and postpartum women 6–9. Participants were not formally involved in recruitment. Results will be disseminated to stakeholders to support improving postpartum family planning services.
RESULTS
PPFP Promotions
Study staff delivered a total of 13,694 one-on-one PPFP promotions prioritizing PPFP methods between August 2017 and October 2018. N=12,068 of these women delivered in one of our selected six health facilities. Promotions were distributed over four different intervention timepoints: antenatal care (28.0%), labor and delivery (23.9%), in the postpartum ward (37.3%), and at infant vaccinations ≤ 6-weeks postpartum (10.8%).
Most participants received only one promotion (89%), while 9% received two, and 1% received 3–4 promotions. The average number of promotions per client was the same between the two LARC methods (1.1 promotions/clients, SDPP implant=0.4, SDPPIUD=0.3) (Table 1). Receiving more promotions was associated with client uptake for PP implants (test for trend, X2=65.8, p<0.0001) and PPIUDs (test for trend, X2=26.9, p<0.0001).
Table 1.
Service delivery outcomes and participant characteristics according to postpartum long-acting reversible contraceptive choice; Kigali, Rwanda; August 2017 – October 2018 (among N=12,068 women who received our intervention and delivered at a study health facility)
| PP Implant (n=1252) |
PPIUD (n=3372) |
|
|---|---|---|
| PP LARC Promotions | ||
| Number of promotions received per client (mean, SD)* | 1.1 (0.4) | 1.1 (0.3) |
| Number of insertions per month (mean, SD) | 83.5 (51.9) | 224.8 (75.3) |
| LARC uptake | 10.4% | 27.9% |
| LARC insertion timing | ||
| Before leaving hospital | 53% | 91% |
| ≤ 6 weeks postpartum | 47% | 9% |
| LARC recipients demographics | ||
| Age (mean, SD) | 27.0 (5.6) | 28.3 (6.0) |
| Parity (mean, SD) | 2.3 (1.3) | 2.4 (1.5) |
| Insertions outcomes | ||
| Women’s self-reported pain during insertion (10-point Likert scale where 1=no anxiety/pain and 10=maximum anxiety/pain) (mean, SD) | 2.0 (2.6) | 1.8 (1.0) |
| Women’s self-reported anxiety during insertion (10-point Likert scale where 1=no anxiety/pain and 10=maximum anxiety/pain), (mean, SD) | 2.1 (2.6) | 1.7 (0.9) |
| Provider self-reported ease-of-insertion (10-point Likert scale where 1= least amount of ease-of-insertion and 10= most ease), (mean, SD) | 9.9 (0.7) | 9.2 (1.0) |
Continuous measures were normally distributed.
PP: post-partum
LARC: long-acting reversible contraception
PPIUD: post-partum intrauterine device
SD: standard deviation
Women who had already received a PPFP method were not systematically counseled again (women who had received a method but expressed interest in receiving a different method could receive additional counseling upon request but were not included in this calculation).
LARC Uptake and Insertion Timing
Of the 12,068 women who received our intervention and delivered at a study facility, 1252 chose a PP implant (10.4% uptake), 3372 chose a PPIUD (27.9% uptake), and 7444 declined a postpartum LARC method (61.7% non-uptake). Over the 15-month intervention period, providers at our intervention facilities inserted 83.5 PP implants per month (SD=51.9) and 224.8 PPIUDs per month (SD=75.3). Notably, prior to our intervention, only 30 PP implant insertions per month and 8 PP IUD insertions per month occurred in our selected facilities. Figure 1 details insertions of the two PPFP methods over time.
Figure 1.
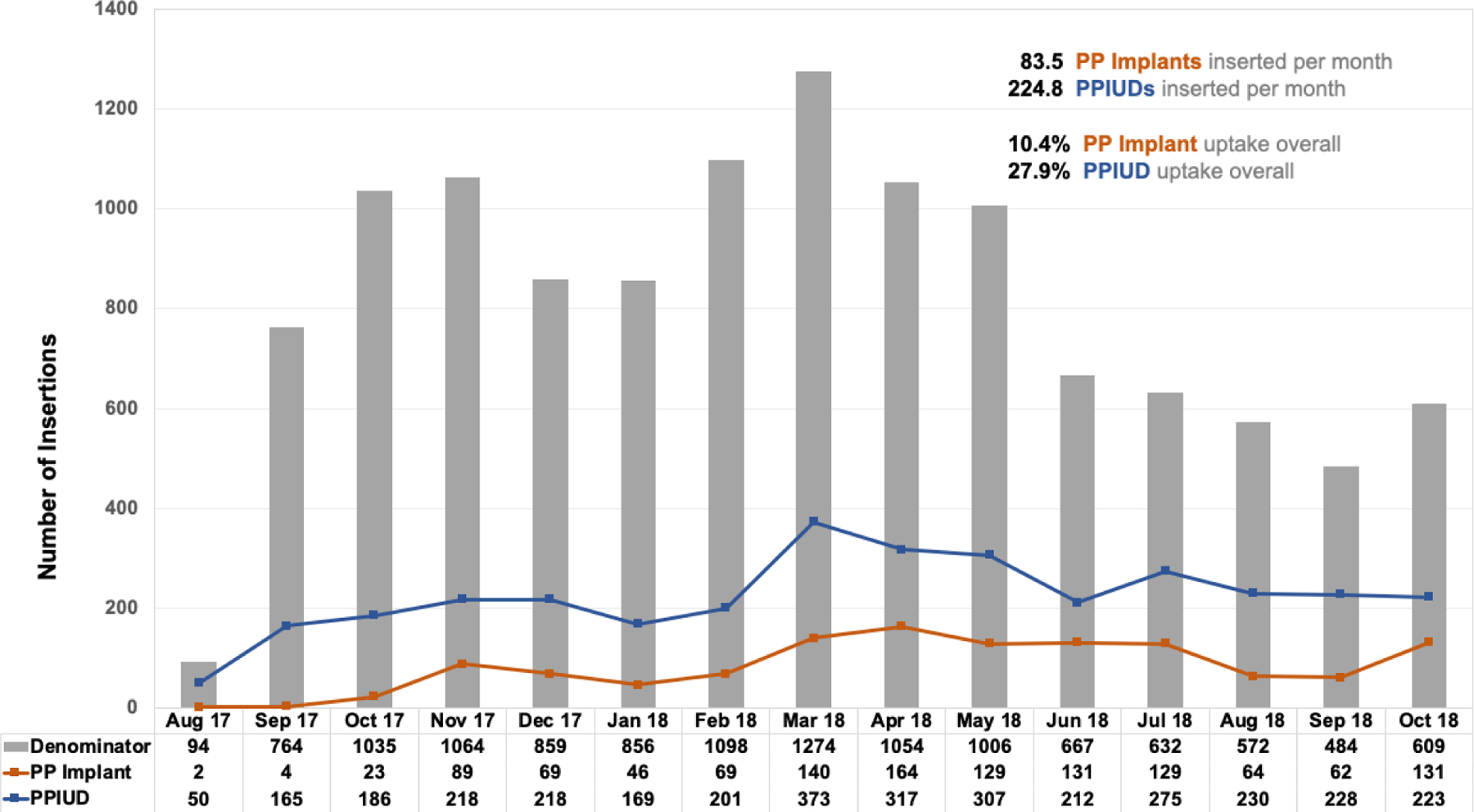
Postpartum Implant (PP Implant) and Postpartum Intrauterine Device (PPIUD) Insertions among by Month. Kigali, Rwanda; August 2017 – October 2018 (among N=12,068 women who received our intervention and delivered at a study health facility)
PP implant insertions occurred more often before leaving the hospital (53%) versus within 6-weeks postpartum (47%) (Table 1). In contrast, ninety-one percent of PPIUDs were inserted before the participant left the hospital while the remaining (9%) were inserted between 4- and 6-weeks postpartum. Promotions given during labor and delivery (versus ANC) were associated with higher LARC uptake before women left the health facility after delivery (p<0.05). We observed a small but not statistically significant association between male presence and higher PP LARC uptake.
PP Implant and PPIUD Client Demographics
On average, PP implant clients were 27.0 years old, and PPIUD clients were 28.3 years old (p <0.0001). PP implant clients reported 2.3 previous live births on average (SD=1.3) while PPIUD clients reported 2.4 live births on average (SD=1.5) (not significantly different).
PP Implant and PPIUD Insertion outcomes
PP implant clients did not report any adverse reactions or discomfort due to their implants, and no PP implant client requested a removal measured up to the 6-week postpartum IV visit. Of the 77 women who experienced a PPIUD expulsion at follow-up, 12% elected to have a PP implant inserted while 60% chose to have an IUD reinserted (data not shown). All PP IUD expulsions were complete expulsions.
Using a Likert scale (where 1=no anxiety/pain and 10=maximum anxiety/pain), PP implant clients reported an average anxiety score of 2.1 (SD=2.6) and an average pain score of 2.0 (SD=2.6). Using the same scale, PPIUD clients reported an average anxiety score of 1.7 (SD=0.9) and an average pain score of 1.8 (SD=1.0). Reported ease-of-insertion by providers (where 1=maximum difficulty insertion and 10=no difficulty insertion) was high for both LARC methods (PP implants=9.9/10, SD=0.7 and PPIUDs=9.2/10, SD=1.0) (Table 1). There were no statistical differences in pain and anxiety score by timing of insertion.
DISCUSSION
This report describes the uptake and service delivery outcomes of PP implants. We also present PPIUD uptake and outcome data with additional follow-up after our previous publication that focused only on the PPIUD 6.
PP implant uptake was lower than PPIUD (10.4% vs. 27.9%, respectively). In settings like ours, it is common for PP implants to be more popular than IUDs often due to concerns and lack of information about the less well-known IUD 12 13. However, our extensive formative work with clients before the intervention found the non-hormonal nature and longevity of the IUD were desirable traits for potential clients and we also identified common concerns such as infection and side-effects 7. In our intervention, we highlighted favorable aspects and discussed common concerns during educational promotion of all LARC and non-LARC methods. It is possible that the combination of evidence-based education about the IUD and access to trained providers certified in PPIUD insertion increased uptake of this method. Additionally, removing implants can be relatively difficult which may concern clients -- it is critical that providers receive quality, specific training on implant removal for programs to be effective.
Though most PP implant and PPIUD clients received an average of 1.1 promotions per person, there was a statistical relationship between having more promotions and electing to receive a LARC method. This finding suggests that repeat promotions may be important for uptake and is in line with a study from Nigeria which found multiple counseling sessions improved the use of modern postpartum contraceptive methods 14. Additionally, repeat promotions increases the opportunity for male engagement. In Rwanda, 80% of first ANC visits are attended by couples. We and others have found that male involvement in postpartum contraception discussions increases intention to uptake and actual uptake of PPFP in studies across sub-Saharan Africa 7 15–18.
Our study demonstrated that PPFP services can be integrated into routine care within ANC, labor and delivery, and infant vaccination services. Other studies have focused on integration of family planning services specifically within infant vaccination and have also found this to be a feasible and acceptable venue for services 19–24. We additionally demonstrate here that ANC and labor and delivery are promising times to promote services, and that most PPFP uptake in our study occurred at labor and delivery.
In previous in-depth interview with key MOH stakeholders, it was generally agreed that reimbursing providers an additional amount for LARC provision was acceptable and ethical (as provision of PP LARC take more training, skill, and time than provision of other reversible methods). Moreover, we reimbursed providers at a higher rate for the IUD compared to the implant because of the additional training and certification required before providing PPIUDs. While this strategy was employed to remove any disincentive that providers may face in getting trained and providing PPIUDs, non-coercive provision of all contraceptive methods based on women’s preferences must always remain imperative and is a cornerstone of rigorous counseling procedures.
The only non-LARC methods available to postpartum (<6 weeks postpartum) breastfeeding women per WHO recommendations are sterilization, condoms, and lactational amenorrhea (LAM) 2. Women who are not breastfeeding may choose progestogen-only methods (e.g., DMPA injectables) immediately postpartum. In our study, all pregnant and postpartum women were given counseling on condoms (with access to free condoms) and LAM. No women in our study selected immediate (before leaving the health facility) post-partum sterilization, and no women stated that they did not intend to breastfeed.
Provision of integrated PPFP services was feasible and acceptable, and relied on ongoing stakeholder involvement for sustainability. The Rwandan Ministry of Health supported clinic staff to participate in PPFP trainings and helped to identify champions who oversaw clinical mentorship along with supportive clinic directors. To further support sustainability, we recently published a cost-effectiveness analysis of our intervention. The largest expense (34% of total) was personnel. Costs per insertion were $25/PPIUDs and $77/PP implant and costs per couple-year of protection were $6/PPIUDs and $21/PP implant 11. Kelly’s placental forceps were used for PPIUD insertion, and future studies may explore the expulsion rates and costs of using different insertion methods such as ringed forceps or dedicated inserters. These analyses are important to inform the cost of scaling up PPFP services and indicate that, when funding is limited, it may be more cost-effective to prioritize promotions and training for the PPIUD.
Limitations
We collected minimal demographic data during this service delivery intervention, and only for PP implant and PPIUD clients. As a result, we are not able to compare demographics between those who declined a LARC method to those who did. We did not collect data on whether IUD expulsions were recognized by women or on use of non-LARC methods (sterilization, self-reported condom use, or self-reported LAM practices) after leaving the health facility which would have been informative. We also did not document reasons why women with a stated desire for a PPFP method did not receive one at a health facility, which is a future area of exploration. Finally, understanding the effect of engaging community health workers in creating demand and reducing misconceptions, which has shown promise in improving uptake of contraceptive methods 25, would be informative.
CONCLUSIONS
PP implant and PPIUD uptake significantly increased after implementation of our multi-level intervention focused on PPFP supply, demand, and sustainability. The PPFP methods were well-received by clients and providers. With renewed interest in post-partum family planning services, our comprehensive multi-level intervention, which is replicable and expandable, is extremely well-timed to make a significant impact on PPFP services in Rwanda and other countries.
Key Messages.
We developed a multi-level intervention to increase postpartum family planning (PPFP) long-acting reversible contraceptive (LARC) method supply, demand, and sustainability in Kigali, Rwanda.
Uptake PP implant and PP intrauterine devices significantly increased pre- versus post-implementation.
Providers reported high ease of PP LARC insertion, and clients reported minimal insertion anxiety or pain.
Funding Source Declaration and Acknowledgements:
This work was supported by the Bill & Melinda Gates Foundation [OPP1160661]. Additional support came from the Emory University Research Council Grant [URCGA16872456], Emory Global Field Experience Award, the Emory Center for AIDS Research [P30AI050409], the National Institutes of Health [NIAID R01 AI51231; NIAID R01 AI64060; NIAID R37 AI51231], and Emory AITRP Fogarty [5D43TW001042].
Footnotes
Competing Interests: The authors have no competing interests to declare.
Contributor Information
Julie Espey, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Rosine Ingabire, Projet San Francisco, Kigali, Rwanda; Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Julien Nyombayire, Projet San Francisco, Kigali, Rwanda; Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Alexandra Hoagland, Projet San Francisco, Kigali, Rwanda; Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Vanessa Da Costa, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Amelia Mazzei, Projet San Francisco, Kigali, Rwanda; Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Lisa B. Haddad, Department of Gynecology and Obstetrics, School of Medicine, Emory University.
Rachel Parker, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Jeannine Mukamuyango, Projet San Francisco, Kigali, Rwanda; Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Victoria Umutoni, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Susan Allen, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Etienne Karita, Projet San Francisco, Kigali, Rwanda; Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Amanda Tichacek, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University.
Kristin M. Wall, Department of Epidemiology, Rollins School of Public Health, Laney Graduate School, Emory University, Atlanta, GA, 30322, USA, Rwanda Zambia HIV Research Group, Department of Pathology & Laboratory Medicine, School of Medicine, Rollins School of Public Health, Emory University, Atlanta, GA, 30322, USA.
References
- 1.Muthal-Rathore A Immediate postpartum insertion for intrauterine devices: RHL commentary Geneva: The WHO Reproductive Health Library; 2010. [Available from: http://cms.kcn.unima.mw:8002/moodle/downloads/Department%20of%20Maternal%20&%20Child%20Health/who%20videos/apps.who.int/rhl/fertility/contraception/cd003036_muthalrathorea_com/en/index.html accessed August 3 2018. [Google Scholar]
- 2.WHO. Medical eligibility criteria for contraceptive use Geneva: WHO Press; 2015. [5th:[Available from: http://www.who.int/reproductivehealth/publications/family_planning/Ex-Summ-MEC-5/en/ accessed August 3 2018. [Google Scholar]
- 3.Moore Z, Pfitzer A, Gubin R, et al. Missed opportunities for family planning: an analysis of pregnancy risk and contraceptive method use among postpartum women in 21 low- and middle-income countries. Contraception 2015;92(1):31–9. doi: 10.1016/j.contraception.2015.03.007 [published Online First: 2015/03/15] [DOI] [PubMed] [Google Scholar]
- 4.Government of Rwanda Ministry of Health. Family Planning 2020 Commitment 2017 [Available from: http://ec2-54-210-230-186.compute-1.amazonaws.com/wp-content/uploads/2018/02/Govt.-of-Rwanda-FP2020-Commitment-2018-Update.pdf accessed 2018 June 15.
- 5.Family Planning 2020. Rwanda 2018 [Available from: http://www.familyplanning2020.org/entities/81 accessed June 11 2018.
- 6.Ingabire R, Nyombayire J, Hoagland A, et al. Evaluation of a multi-level intervention to improve postpartum intrauterine device services in Rwanda. Gates Open Res 2018;2:38. doi: 10.12688/gatesopenres.12854.2 [published Online First: 2018/12/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Costa V, Ingabire R, Sinabamenye R, et al. An Exploratory Analysis of Factors Associated With Interest in Postpartum Intrauterine Device Uptake Among Pregnant Women and Couples in Kigali, Rwanda. Clin Med Insights Reprod Health 2019;13:1179558119886843. doi: 10.1177/1179558119886843 [published Online First: 2019/12/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingabire R, Mukamuyango J, Nyombayire J, et al. Development and Uptake of Long-Acting Reversible Contraception Services in Rwanda, 2009–2016. J Womens Health (Larchmt) 2019;28(12):1640–49. doi: 10.1089/jwh.2018.7423 [published Online First: 2019/07/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukamuyango J, Ingabire R, Parker R, et al. Motivational interviewing to promote long-acting reversible contraception among Rwandan couples wishing to prevent or delay pregnancy. Am J Obstet Gynecol 2020;222(4s):S919.e1–S19.e12. doi: 10.1016/j.ajog.2019.11.1280 [published Online First: 2019/12/16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wall KM, Bayingana R, Ingabire R, et al. Rwandan stakeholder perspectives of integrated family planning and HIV services. Int J Health Plann Manage 2018;33(4):e1037–e49. doi: 10.1002/hpm.2586 [published Online First: 2018/07/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wall K, Ingabire R, Allen S, et al. Cost per insertion and couple year of protection for post-partum intrauterine devices and implants provided during service scale-up in Kigali, Rwanda 2018. [DOI] [PMC free article] [PubMed]
- 12.Callahan RL, Brunie A, Mackenzie ACL, et al. Potential user interest in new long-acting contraceptives: Results from a mixed methods study in Burkina Faso and Uganda. PLoS One 2019;14(5):e0217333. doi: 10.1371/journal.pone.0217333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duvall S, Thurston S, Weinberger M, et al. Scaling up delivery of contraceptive implants in sub-Saharan Africa: operational experiences of Marie Stopes International. 2014;2(1):72–92. doi: 10.9745/GHSP-D-13-00116 %J Global Health: Science and Practice [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adanikin AI, Onwudiegwu U, Loto OM. Influence of multiple antenatal counselling sessions on modern contraceptive uptake in Nigeria. The European Journal of Contraception & Reproductive Health Care 2013;18(5):381–87. doi: 10.3109/13625187.2013.816672 [DOI] [PubMed] [Google Scholar]
- 15.Coomson JI, Manu A. Determinants of modern contraceptive use among postpartum women in two health facilities in urban Ghana: a cross-sectional study. Contracept Reprod Med 2019;4:17. doi: 10.1186/s40834-019-0098-9 [published Online First: 2019/10/28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraha TH, Belay HS, Welay GM. Intentions on contraception use and its associated factors among postpartum women in Aksum town, Tigray region, northern Ethiopia: a community-based cross- sectional study. Reprod Health 2018;15(1):188. doi: 10.1186/s12978-018-0632-2 [published Online First: 2018/11/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berta M, Feleke A, Abate T, et al. Utilization and Associated Factors of Modern Contraceptives During Extended Postpartum Period among Women Who Gave Birth in the Last 12 Months in Gondar Town, Northwest Ethiopia. Ethiop J Health Sci 2018;28(2):207–16. doi: 10.4314/ejhs.v28i2.12 [published Online First: 2018/07/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashebir W, Tadesse T. Associated Factors of Postpartum Modern Contraceptive Use in Burie District, Amhara Region, Ethiopia. J Pregnancy 2020;2020:6174504. doi: 10.1155/2020/6174504 [published Online First: 2020/04/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erhardt-Ohren B, Schroffel H, Rochat R. Integrated Family Planning and Routine Child Immunization Services in Benin: A Process Evaluation. Matern Child Health J 2020. doi: 10.1007/s10995-020-02915-5 [published Online First: 2020/04/19] [DOI] [PubMed] [Google Scholar]
- 20.Cooper CM, Fields R, Mazzeo CI, et al. Successful proof of concept of family planning and immunization integration in Liberia. Glob Health Sci Pract 2015;3(1):71–84. doi: 10.9745/ghsp-d-14-00156 [published Online First: 2015/03/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson AR, Cooper CM, Kamara S, et al. Operationalizing Integrated Immunization and Family Planning Services in Rural Liberia: Lessons Learned From Evaluating Service Quality and Utilization. Glob Health Sci Pract 2019;7(3):418–34. doi: 10.9745/ghsp-d-19-00012 [published Online First: 2019/09/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dulli LS, Eichleay M, Rademacher K, et al. Meeting Postpartum Women’s Family Planning Needs Through Integrated Family Planning and Immunization Services: Results of a Cluster-Randomized Controlled Trial in Rwanda. Glob Health Sci Pract 2016;4(1):73–86. doi: 10.9745/ghsp-d-15-00291 [published Online First: 2016/03/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mody SK, Nair S, Dasgupta A, et al. Postpartum contraception utilization among low-income women seeking immunization for infants in Mumbai, India. Contraception 2014;89(6):516–20. doi: 10.1016/j.contraception.2014.01.001 [published Online First: 2014/02/25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vance G, Janowitz B, Chen M, et al. Integrating family planning messages into immunization services: a cluster-randomized trial in Ghana and Zambia. Health Policy Plan 2014;29(3):359–66. doi: 10.1093/heapol/czt022 [published Online First: 2013/04/11] [DOI] [PubMed] [Google Scholar]
- 25.Mazzei A, Ingabire R, Mukamuyango J, et al. Community health worker promotions increase uptake of long-acting reversible contraception in Rwanda. Reprod Health 2019;16(1):75. doi: 10.1186/s12978-019-0739-0 [published Online First: 2019/06/06] [DOI] [PMC free article] [PubMed] [Google Scholar]


