This cohort study assesses the risks posed by adolescent asthma, cigarette smoking, obesity, and psychological disorders for chronic disease, disability, and early death in later life.
Key Points
Question
What is the association between adolescent health conditions that are commonly treated by physicians and accelerated biological age at midlife?
Findings
In this cohort study of 910 participants in a longitudinal cohort study in New Zealand, participants who had a daily smoking status, obesity, or a psychological disorder diagnosis during adolescence were found to be biologically older than those without these conditions 3 decades later. Adolescent asthma was not associated with midlife biological age.
Meaning
The findings from this study suggest that adolescent smoking, obesity, and psychological disorders are modifiable health conditions that, if treated, might reduce the risk of accelerated biological aging at midlife.
Abstract
Importance
Biological aging is a distinct construct from health; however, people who age quickly are more likely to experience poor health. Identifying pediatric health conditions associated with accelerated aging could help develop treatment approaches to slow midlife aging and prevent poor health in later life.
Objective
To examine the association between 4 treatable health conditions in adolescence and accelerated aging at midlife.
Design, Setting, and Participants
This cohort study analyzed data from participants in the Dunedin Study, a longitudinal investigation of health and behavior among a birth cohort born between April 1, 1972, and March 31, 1973, in Dunedin, New Zealand, and followed up until age 45 years. Participants underwent an assessment at age 45 years and had data for at least 1 adolescent health condition (asthma, smoking, obesity, and psychological disorders) and outcome measure (pace of aging, gait speed, brain age, and facial age). Data analysis was performed from February 11 to September 27, 2021.
Exposures
Asthma, cigarette smoking, obesity, and psychological disorders were assessed at age 11, 13, and 15 years.
Main Outcomes and Measures
The outcome was a midlife aging factor composite score comprising 4 measures of biological aging: pace of aging, gait speed, brain age (specifically, BrainAGE score), and facial age.
Results
A total of 910 participants (459 men [50.4%]) met the inclusion criteria, including an assessment at age 45 years. Participants who had smoked daily (0.61 [95% CI, 0.43-0.79] SD units), had obesity (0.82 [95% CI, 0.59-1.06] SD units), or had a psychological disorder diagnosis (0.43 [95% CI, 0.29-0.56] SD units) during adolescence were biologically older at midlife compared with participants without these conditions. Participants with asthma were not biologically older at midlife (0.02 [95% CI, −0.14 to 0.19] SD units) compared with those without asthma. These results remained unchanged after adjusting for childhood risk factors such as poor health, socioeconomic disadvantage, and adverse experiences.
Conclusions and Relevance
This study found that adolescent smoking, obesity, and psychological disorder diagnoses were associated with older biological age at midlife. These health conditions could be treated during adolescence to reduce the risk of accelerated biological aging later in life.
Introduction
Childhood experiences, behaviors, and characteristics are associated with trajectories of health across the life span.1 International comparative studies have shown that poor health among adult populations can largely be explained by poor health in early life.1 The risk of poor adult health that accumulates also presents an opportunity for intervention. Intervening to mitigate risk factors before adulthood could prevent poor health as people age.2 Realizing the potential of this opportunity, however, requires identifying the relevant risk factors to target.
One of the factors associated with chronic disease morbidity and mortality is biological aging, which is defined as gradual physiological decline across multiple organ systems over time.3,4,5 Although biological aging is a distinct construct from health, people who age more quickly are more likely to accumulate chronic disease, develop functional disability, and experience early mortality.2,3,4,5,6,7 The wide array of poor health outcomes associated with aging suggests that interventions that slow the aging process could improve health later in life, reducing morbidity, disability, and mortality.8,9,10
We conducted a cohort study to examine the association of 4 health conditions commonly seen in clinical practice by physicians who treat adolescents (ie, asthma,11 cigarette smoking,12 obesity,13 and psychological disorders14,15) with midlife aging. These 4 conditions have high population prevalence, peak onset early in life, high levels of chronicity, and known modifiability.16,17,18,19,20,21,22 The Dunedin Study, a longitudinal investigation of health and behavior of a birth cohort in Dunedin, New Zealand, tracked these conditions in childhood and used multiple validated assessments of biological age during midlife. Identifying the conditions associated with accelerated biological age could help in ascertaining which interventions might slow aging before health consequences accumulate. We hypothesized that adolescent asthma, cigarette smoking, obesity, and psychological disorders would be associated with older biological age at midlife.
Methods
Participants and Study Design
This cohort study analyzed data of the participants in the Dunedin Study. Written informed consent was obtained from all participants. Study protocols were approved by the Southern Health and Disability Ethics Committee at the New Zealand Ministry of Health and Duke University Health System Institutional Review Board. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The full cohort of the Dunedin Study comprised all individuals who were born between April 1, 1972, and March 31, 1973, in Dunedin, New Zealand, and were eligible on the basis of their residency in the province and participation in the first assessment at 3 years of age.23 The cohort represented the full range of socioeconomic status (SES) in the general population of the South Island. As adults, the cohort matched the results of key health indicators from the New Zealand Health Survey and National Nutrition Survey23 as well as the distribution of educational attainment among citizens of the same age from the Census of Population and Dwellings.24
Ninety-three percent of the cohort self-reported as White, consistent with the predominant racial and ethnic group in the South Island. Other self-reported race and ethnicity categories were Asian (<1%) and Maori or Pacific Islander (7%). Assessments were performed at birth; at 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and 38 years of age; and, most recently, at age 45 years (completed in April 2019). In the present study, we included participants who underwent an assessment at age 45 years and had data for at least 1 adolescent health condition (asthma, smoking, obesity, and psychological disorders) and outcome measure (pace of aging, gait speed, brain age, and facial age).
Measures
We used a midlife aging factor comprising 4 continuous measures of aging that were previously identified in this cohort6,25,26,27: pace of aging, gait speed, brain age (specifically, BrainAGE score), and facial age. These measures were given a z score and coded such that the higher scores represented relatively older biological age at age 45 years. Principal component analysis was used to obtain the standardized composite factor score representing midlife biological age. This outcome was used to represent biological age at midlife, as assessed across the 4 measures. Full information maximum likelihood estimation was used to account for participants who were missing data on 1 (n = 41), 2 (n = 2), or 3 (n = 22) of the aging outcome measures.
The pace of aging was measured with repeated assessments of a panel of 19 biomarkers taken at age 26, 32, 38, and 45 years.6 The 19 biomarkers were body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), waist to hip ratio, hemoglobin A1c, leptin, mean arterial pressure, cardiorespiratory fitness, forced expiratory volume in first second of expiration (FEV1), FEV1 to forced vital capacity ratio, total cholesterol, triglycerides, high-density lipoprotein cholesterol, apolipoprotein B100 to A1 ratio, lipoprotein a, creatinine clearance, urea nitrogen, C-reactive protein, white blood cell count, periodontal disease, and caries-affected tooth surfaces. The linear change in each biomarker for each study member was assessed using mixed modeling; the 19 resulting slopes were then summed and scaled so that 1 year of chronological age equated to approximately 1 year of mean change in physiological functioning in the sample.
Gait speed was assessed using a 6-m long electronic walkway (GAITRite; CIR Systems Inc) with 2-m acceleration before and 2-m deceleration after the walkway. Gait speed was assessed under 3 walk conditions: usual gait speed, dual-task gait speed (walking and reciting aloud the alternate letters of the alphabet), and maximum gait speed. These walk conditions were correlated, and the mean speed was used to generate a composite gait speed.25
Brain age at age 45 years was derived from structural magnetic resonance imaging (MRI) data using a 3T scanner equipped with a 64-channel head/neck coil (Siemens Skyra 3T; Siemens Healthcare). BrainAGE score26 was calculated as the difference between a participant’s predicted age (derived from structural MRI data using a publicly available algorithm27 that was trained on vertex-wise cortical thickness and surface area data as well as subcortical volume) and exact chronological age at the date of the MRI scan. We chose the BrainAGE algorithm because of its performance in predicting chronological age in independent samples and its sensitivity to age-related cognitive impairment in older age.27 Test-retest reliability (mean interval, 79 days) of brain age was found to be excellent in 20 participants in the Dunedin Study (intraclass correlation coefficient, 0.81).
Facial age was based on 2 measurements of perceived age28 using ratings of each participant’s facial photograph by an independent panel of 8 raters. First, age range was assessed by an independent panel of 4 raters who were presented with standardized facial photographs of participants and were blinded to their actual age. Raters used a Likert scale to categorize each participant into a 5-year age range such as 20 to 24 years (interrater reliability, 0.77), and mean scores across all raters were calculated. Second, relative age was assessed by a different panel of 4 raters, who were told that all of the people in the photographs were age 45 years. Raters then used a 7-item Likert scale to assign a relative age to each participant (interrater reliability, 0.79). Perceived age at 45 years was obtained by standardizing and calculating the mean age range and relative age scores.
Adolescent Health Conditions
We tested 4 health conditions that were previously examined in this cohort22,29,30,31 at 11, 13, and 15 years of age. At each assessment age, interviewers were masked to the participants' previous health status. Participants were coded as having a health condition if they met the criteria for that condition during at least 1 of the study assessments. The main study findings were unchanged when using a count of visits in which participants had one of the health conditions (eMethods 1 in the Supplement).
Asthma status was assessed using standardized interviews of participants by pulmonary specialists.22,31,32 Participants were required to have a diagnosis of asthma in addition to either showing symptoms (asthma attack or recurrent wheeze; participants who reported ≤2 episodes lasting ≤1 hour were excluded) or undergoing medical treatment over the past 12 months.
Smoking status was assessed using self-reported cigarette smoking from in-person interviews.29
Obesity was assessed using participants’ BMI.30 Height was measured with a portable stadiometer (Harpenden), and weight was measured in light clothing using a calibrated scale. Obesity was defined as BMI greater than or equal to the 95th percentile of the pediatric growth chart, which was in line with recommendations of the Centers for Disease Control and Prevention and similar to the BMI cutoffs of the International Obesity Task Force.33
Psychological disorders were assessed in standardized interviews using the Diagnostic Interview Schedule for Children34 according to the then-current criteria of the Diagnostic and Statistical Manual of Mental Disorders (Third Edition)35 at 11, 13, and 15 years of age.16 Diagnoses included anxiety disorders, depressive disorders, conduct disorder, and attention-deficit/hyperactivity disorder.
Childhood Covariates
Three childhood covariates were assessed between birth and age 15 years: childhood health, adverse childhood experiences (ACEs), and childhood SES. These 3 covariates were correlated with midlife aging (eTable in the Supplement).
Childhood health from birth to age 11 years was assessed using a panel of biomarkers and clinical ratings. Two Dunedin Study staff members rated participants’ overall health at age 3, 5, 7, 9, and 11 years by reviewing birth records and assessment dossiers.36 Documents included assessments by pediatric clinicians and reports of infections, diseases, injuries, hospitalizations, and other health problems collected from participants’ mothers during standardized interviews. Clinical tests of motor development and measures of body mass, triceps and subscapular skinfold thickness, resting blood pressure, FEV1, and the ratio of FEV1 to forced vital capacity were also included. The mean score of these assessments was calculated to create a standardized score of childhood health whereby higher scores represented greater health problems.
Archival study records were reviewed by 4 independent raters to yield a prospective measure comprising 10 ACEs, which were identified in the ACE study,37 from birth to age 15 years. This measure included 5 types of child harm and 5 types of household dysfunction. The 4 raters showed substantial interrater agreement across all ACEs (κ = 0.79). Counts greater than 4 were recoded as 4, which was in line with the ACE study.38
The SES of the childhood families of participants was measured using the 6-point Elley-Irving socioeconomic index for New Zealand.39 Childhood SES represented the mean of the highest SES levels of either parent reported in assessments from birth through age 15 years.
Statistical Analysis
Data analysis was performed from February 11 to September 27, 2021. We used multiple regression models to test the associations between each adolescent health condition (asthma, smoking, obesity, and psychological disorders) and the midlife aging factor composite score (outcome). First, we tested the association between each health condition and aging at midlife in 4 unadjusted models. Second, we tested a multivariable model that included all 4 health conditions. Third, we tested previous models while adjusting for childhood covariates. Fourth, we tested the association between the health conditions and each of the 4 outcome measures (pace of aging, gait speed, brain age [BrainAGE score], and facial age).
All models were run in Mplus, version 8.3,40 using full maximum likelihood estimation to account for missing data and including sex as a covariate. Outcomes were reported in SD units of the aging outcomes associated with each of the childhood health conditions to contextualize the size of the associations between the risk factors and the biological age outcomes at midlife. Analyses were checked for reproducibility by an independent data analyst (R.M.H.) using code that was created from the manuscript and applied to a copy of the original data.
Results
Of the 1037 original participants in the Dunedin Study, 938 were alive and underwent an assessment at age 45 years and 910 (451 women [49.6%] and 459 men [50.4%]) met the inclusion criteria for the present study. The Supplement presents descriptive statistics and a correlation matrix of study variables (eTable), the aging outcome distributions (eFigure 1), the attrition analyses at the age-45-years assessment (eFigure 2), and a conceptual model of the primary analyses (eFigure 3).
Association of Adolescent Asthma, Smoking, Obesity, and Psychological Disorders With Accelerated Aging at Midlife
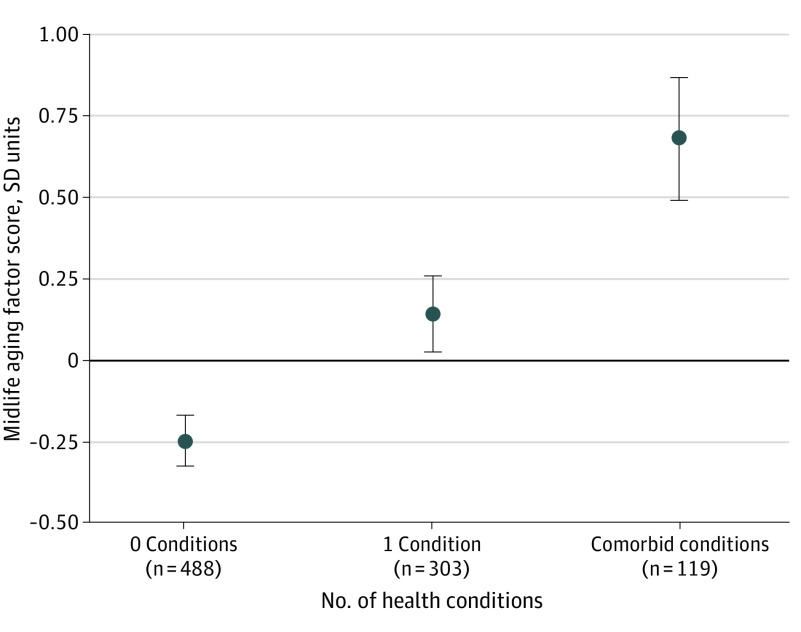
Adolescents who smoked daily (0.61 [95% CI, 0.43-0.79] SD units), had obesity (0.82 [95% CI, 0.59-1.06] SD units), or had a psychological disorder diagnosis (0.43 [95% CI, 0.29-0.56] SD units) were biologically older at midlife according to the aging factor composite score, compared with adolescents without these conditions; however, adolescents with asthma were not biologically older at midlife (0.02 [95% CI, −0.14 to 0.19] SD units) (Table 1). When included in the same model, smoking, obesity, and psychological disorders in adolescence remained associated with older biological age. In summing the results of these conditions, we found that having more health conditions in adolescence was associated with older midlife aging factor scores (0.45 [95% CI, 0.36-0.53] SD units) (Figure 1). Adolescents with 2 or more comorbid conditions were biologically older than adolescents with no health conditions (0.93 [95% CI, 0.76-1.11] SD units) (Figure 1). When adjusting for childhood covariates of health, SES, and ACEs, smoking (0.41 [95% CI, 0.24-0.59] SD units), obesity (0.54 [95% CI, 0.30-0.77] SD units), and psychological disorders (0.28 [95% CI, 0.16-0.41] SD units) remained associated with midlife aging (Table 1). Both internalizing (depression and anxiety; 0.31 [95% CI, 0.14-0.49] SD units) and externalizing (conduct disorder and attention-deficit/hyperactivity disorder; 0.52 [95% CI, 0.36-0.69] SD units) psychological disorders were associated with midlife aging (eMethods 2 in the Supplement).
Table 1. Associations Between 4 Adolescent Health Conditions and Midlife Aging .
| Health condition | Difference in midlife aging factor score, SD units (95% CI)a | |
|---|---|---|
| Unadjusted model | Multivariable model | |
| Adjusted for sex | ||
| Asthma | 0.02 (−0.14 to 0.19) | −0.04 (−0.19 to 0.12) |
| Smoking | 0.61 (0.43 to 0.79) | 0.49 (0.31 to 0.67) |
| Obesity | 0.82 (0.59 to 1.06) | 0.78 (0.55 to 1.01) |
| Psychological disorders | 0.43 (0.29 to 0.56) | 0.30 (0.17 to 0.43) |
| Also adjusted for childhood covariatesb | ||
| Asthma | −0.07 (−0.23 to 0.08) | −0.09 (−0.27 to 0.04) |
| Smoking | 0.41 (0.24 to 0.59) | 0.35 (0.18 to 0.53) |
| Obesity | 0.54 (0.30 to 0.77) | 0.51 (0.28 to 0.75) |
| Psychological disorders | 0.28 (0.16 to 0.41) | 0.22 (0.09 to 0.34) |
Estimates represent the difference in SD units between the Dunedin Study participants with health conditions vs those without health conditions.
Childhood covariates included childhood health, adverse childhood experiences, and childhood socioeconomic status.
Figure 1. Midlife Aging by Number of Adolescent Health Conditions.
Circles indicated the unadjusted mean midlife aging factor scores with 95% CIs (error bars), and comorbid indicated 2 or more conditions. As the number of conditions increased, midlife age advanced (0.45 [95% CI, 0.36-0.53] SD units).
Association of Adolescent Health Conditions With Pace of Aging, Gait Speed, Brain Age, and Facial Age
We conducted secondary analyses to test whether the health conditions were associated with each of the 4 outcome measures of the midlife aging factor composite score (Table 2). Adolescents who smoked daily had a faster pace of aging, slower gait speed, older brain age, and older facial age at midlife than adolescents without a daily smoking status (with associations between 0.31 and 0.54 SD units). Adolescents with obesity had a faster pace of aging, slower gait speed, and older facial age (with associations between 0.29 and 0.79 SD units) but did not have a significantly older brain age compared with adolescents without obesity (association = 0.14 SD units). Adolescents with a psychological disorder had a faster pace of aging, slower gait speed, and older facial age (with associations between 0.25 and 0.35 SD units) but did not have an older brain age (association = 0.09 SD units) compared with adolescents without a psychological disorder diagnosis.
Table 2. Associations Between 4 Adolescent Health Conditions and Aging Outcome Measuresa.
| Health condition | Difference in midlife aging factor composite score, SD units (95% CI)b | |
|---|---|---|
| Unadjusted model | Multivariable model | |
| Association with accelerated pace of aging | ||
| Asthma | 0.06 (−0.10 to 0.23) | 0.01 (−0.15 to 0.17) |
| Smoking | 0.47 (0.29 to 0.65) | 0.37 (0.19 to 0.55) |
| Obesity | 0.79 (0.55 to 1.03) | 0.75 (0.51 to 0.98) |
| Psychological disorders | 0.35 (0.22 to 0.48) | 0.25 (0.12 to 0.38) |
| Association with slower gait speed | ||
| Asthma | 0.03 (−0.14 to 0.20) | 0.01 (−0.16 to 0.17) |
| Smoking | 0.34 (0.15 to 0.52) | 0.26 (0.07 to 0.45) |
| Obesity | 0.64 (0.40 to 0.88) | 0.61 (0.37 to 0.85) |
| Psychological disorders | 0.25 (0.11 to 0.38) | 0.17 (0.04 to 0.31) |
| Association with older brain age | ||
| Asthma | −0.17 (−0.34 to 0.00) | −0.18 (−0.35 to −0.01) |
| Smoking | 0.31 (0.12 to 0.50) | 0.30 (0.10 to 0.50) |
| Obesity | 0.14 (−0.11 to 0.40) | 0.14 (−0.11 to 0.40) |
| Psychological disorders | 0.09 (−0.05 to 0.23) | 0.04 (−0.11 to 0.18) |
| Association with older facial age | ||
| Asthma | −0.01 (−0.18 to 0.16) | −0.05 (−0.21 to 0.12) |
| Smoking | 0.54 (0.36 to 0.73) | 0.44 (0.25 to 0.63) |
| Obesity | 0.29 (0.05 to 0.54) | 0.25 (0.01 to 0.49) |
| Psychological disorders | 0.36 (0.22 to 0.49) | 0.27 (0.13 to 0.41) |
All models were adjusted for sex. All unstandardized outcome variables were standardized and coded to ensure that higher values corresponded to older age for the purpose of comparison.
Estimates represent the difference in SD units between the Dunedin Study participants with health conditions and those without health conditions.
In summing the results of smoking, obesity, and psychological disorders, we found that adolescents with more health conditions had a faster pace of aging (0.38 [95% CI, 0.29-0.47] SD units), slower gait speed (0.27 [95% CI, 0.17-0.36] SD units), older brain age (0.14 [95% CI, 0.04-0.23] SD units), and older facial age (0.33 [95% CI, 0.24-0.42] SD units) at midlife (Figure 2). Compared with participants who had none of these health conditions during adolescence, participants with comorbid conditions were biologically aging at a faster rate by 2.8 (95% CI, 2.1-3.4) months per year, were slower in gait speed by 11.2 (95% CI, 7.5-14.8) cm/s, had an older brain age by 2.5 (95% CI, 0.9-4.1) years, and had an older facial age by 3.9 (95% CI, 2.7-5.1) years at midlife.
Figure 2. Midlife Aging for Each Outcome Measure by Number of Adolescent Health Conditions.
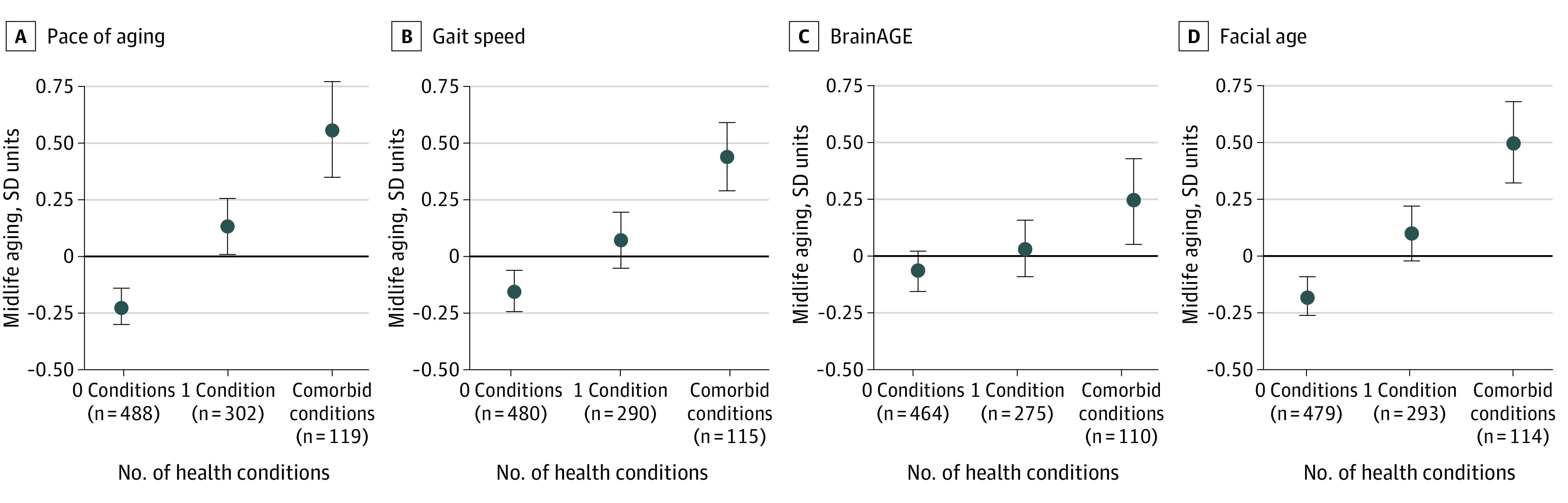
Discrepancies in numbers reflect missing data for each outcome measure. As the number of conditions increased, midlife age advanced, as assessed by pace of aging (0.38 [95% CI, 0.29-0.47] SD units), gait speed (0.27 [95% CI, 0.17-0.36] SD units), BrainAGE score (0.14 [95% CI, 0.04-0.23] SD units), and facial age (0.33 [95% CI, 0.24-0.42] SD units).
Discussion
This cohort study examined the association between 4 adolescent health conditions and accelerated aging at midlife among individuals in the Dunedin Study birth cohort, who underwent assessment from birth up to age 45 years. Adolescents with a daily smoking status, obesity, or a psychological disorder diagnosis were biologically older at midlife. These associations remained after adjusting for 3 childhood covariates and leading risk factors for poor midlife health and accelerated aging: poor health, SES disadvantage, and ACEs. These results aligned with previous empirical evidence that smoking, obesity, and psychological disorders were associated with poor adult health30,32,33,41 and accelerated aging as measured with DNA methylation clocks.42,43,44 The findings of the present study extend this previous work by linking adolescent conditions to direct measures of midlife aging.
There are several reasons that these health conditions may accelerate aging. Smoking can directly alter the physiological functions associated with aging, such as changes in gene expression45 and increased oxidative stress,46 which might explain why the associations between smoking and brain age exceeded those for both obesity and psychological disorders. Smoking and obesity can also play a factor in increased systemic inflammation,47,48 which is associated with accelerated aging.49,50 Moreover, the environments or behaviors of adolescents who smoke or have obesity could also indirectly increase the risk of accelerated aging. Similarly, behavioral or environmental factors,51 such as poor health behaviors, less access to health care, and socioeconomic disadvantage, could be associated with psychopathology and accelerated aging. Alternatively, psychological disorders could be a factor in increased systemic inflammation through greater stress.50,52 Genetic vulnerability for these conditions could also match the vulnerability to accelerated aging.
Studies have proposed that asthma accelerates aging because of increased inflammation53,54; however, adolescent asthma was not associated with accelerated aging in this study. Previous research found that childhood asthma was a factor in accelerated aging and poor health,55 although other studies found mixed evidence22 or no association.55 This discrepancy may reflect the inclusion of adolescent-limited asthma in this study, given that previous research found that chronic asthma persisting into adulthood was most relevant to health.22,31 Alternatively, asthma was more often managed during the 1980s compared with the other health conditions.21,31 For example, no adolescents in this cohort were prescribed stimulants for attention-deficit/hyperactivity disorder,56 and selective serotonin reuptake inhibitors were not yet in use for adolescent depression and anxiety during the study period, whereas 81.1% of the adolescents with asthma received some type of treatment, which could have mitigated the implications for biological aging. Future studies may examine whether asthma treatments, including corticosteroid inhalers, are associated with differences in biological age.
The bivariate associations for smoking (r = 0.22), obesity (r = 0.22), and psychological disorders (r = 0.21) were similar to the associations for established childhood risk factors for accelerated aging such as SES disadvantage (r = 0.29), ACEs (r = 0.22), and poor health (r = 0.25). Although no clinical cutoffs for accelerated biological aging have yet been established, similarities in effect sizes compared with traditional risk factors provide support for the relevance of the observed associations, particularly given the increases in the rates of childhood obesity and psychological disorders over the past several decades.57,58
The study results have clinical implications. Preventing accelerated aging before it occurs is likely the method that is most beneficial to health,2,9 but using preventive interventions requires identifying the risk factors to target. Previous studies have drawn an association between early-life factors, such as socioeconomic disadvantage, low intelligence, or childhood adversity, with accelerated aging,42,43,44 but these factors are difficult to manage. Results of this study suggest that adolescents with a daily smoking status, obesity, or a psychological disorder diagnosis are at risk for accelerated aging later in life and could benefit from existing evidence-based interventions at the individual, family, community, and public health levels.16,17,18,19,20,21,22 For example, adolescent smoking has a modest response to motivational interviewing59 as well as to family-based, community-based, and public health interventions.60 Similarly, diet and weight loss programs have shown some ability to reduce adolescent BMI,61 and behavioral and pharmacological interventions can address adolescent mental health disorders.62,63,64 A combination of public health and individual interventions could slow the rate at which people biologically age2,3,4,5,6,7 from adolescence into adulthood and may improve health at midlife and beyond.8,9,10
Limitations
This study has several limitations. First, the participants self-identified as predominantly White and originated only from New Zealand. Replication of the findings in other cohorts would help support the relevance of these findings to diverse populations, including countries without a national health system and where barriers to health care could affect the observed results. Second, the analyses considered 4 health conditions commonly seen by physicians who treat adolescents, but other health conditions could also be relevant to midlife aging. Third, the Dunedin Study was observational and thus cannot establish whether observed associations were causal. Future studies are necessary to ascertain whether the observed associations with accelerated aging are reversible through treatment.
Conclusions
The findings of this cohort study suggest that daily smoking, obesity, and/or a psychological disorder diagnosis during adolescence are associated with accelerated aging at midlife. Treating these modifiable pediatric health conditions could prevent the accumulation of chronic disease, development of disability, and risk of early death in adulthood by reducing the risk of accelerated biological aging.
eMethods 1. Using Count Variables for Adolescent Health Conditions
eMethods 2. Association of Internalizing and Externalizing Psychological Disorders with Midlife Aging
eTable. Descriptives and Correlations for Study Variables
eFigure 1. Visualization of Aging Outcomes
eFigure 2. Attrition Analyses
eFigure 3. Conceptual Model of Primary Analyses
References
- 1.Power C, Kuh D, Morton S. From developmental origins of adult disease to life course research on adult disease and aging: insights from birth cohort studies. Annu Rev Public Health. 2013;34:7-28. doi: 10.1146/annurev-publhealth-031912-114423 [DOI] [PubMed] [Google Scholar]
- 2.Moffitt TE, Belsky DW, Danese A, Poulton R, Caspi A. The longitudinal study of aging in human young adults: knowledge gaps and research agenda. J Gerontol A Biol Sci Med Sci. 2017;72(2):210-215. doi: 10.1093/gerona/glw191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194-1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaeberlein M. Longevity and aging. F1000Prime Rep. 2013;5:5. doi: 10.12703/P5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709-713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott ML, Caspi A, Houts RM, et al. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat Aging. 2021;1(3):295-308. doi: 10.1038/s43587-021-00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belsky DW, Caspi A, Houts R, et al. Quantification of biological aging in young adults. Proc Natl Acad Sci U S A. 2015;112(30):E4104-E4110. doi: 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strong K, Mathers C, Leeder S, Beaglehole R. Preventing chronic diseases: how many lives can we save? Lancet. 2005;366(9496):1578-1582. doi: 10.1016/S0140-6736(05)67341-2 [DOI] [PubMed] [Google Scholar]
- 9.Barzilai N, Cuervo AM, Austad S. Aging as a biological target for prevention and therapy. JAMA. 2018;320(13):1321-1322. doi: 10.1001/jama.2018.9562 [DOI] [PubMed] [Google Scholar]
- 10.Justice J, Miller JD, Newman JC, et al. Frameworks for proof-of-concept clinical trials of interventions that target fundamental aging processes. J Gerontol A Biol Sci Med Sci. 2016;71(11):1415-1423. doi: 10.1093/gerona/glw126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123(suppl 3):S131-S145. doi: 10.1542/peds.2008-2233C [DOI] [PubMed] [Google Scholar]
- 12.Committee on Environmental HealthHealth; Committee on Substance Abuse; Committee on Adolescence; Committee on Native American Child . From the American Academy of Pediatrics: policy statement--tobacco use: a pediatric disease. Pediatrics. 2009;124(5):1474-1487. doi: 10.1542/peds.2009-2114 [DOI] [PubMed] [Google Scholar]
- 13.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375(9727):1737-1748. doi: 10.1016/S0140-6736(10)60171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitney DG, Peterson MD. US national and state-level prevalence of mental health disorders and disparities of mental health care use in children. JAMA Pediatr. 2019;173(4):389-391. doi: 10.1001/jamapediatrics.2018.5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Committee on Psychosocial Aspects of Child and Family Health and Task Force on Mental Health . Policy statement—the future of pediatrics: mental health competencies for pediatric primary care. Pediatrics. 2009;124(1):410-421. doi: 10.1542/peds.2009-1061 [DOI] [PubMed] [Google Scholar]
- 16.Moffitt TE, Caspi A, Taylor A, et al. How common are common mental disorders? evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med. 2010;40(6):899-909. doi: 10.1017/S0033291709991036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60(7):709-717. doi: 10.1001/archpsyc.60.7.709 [DOI] [PubMed] [Google Scholar]
- 18.Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry. 2014;13(2):153-160. doi: 10.1002/wps.20128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. BMJ. 2013;346:f2539. doi: 10.1136/bmj.f2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huhn M, Tardy M, Spineli LM, et al. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA Psychiatry. 2014;71(6):706-715. doi: 10.1001/jamapsychiatry.2014.112 [DOI] [PubMed] [Google Scholar]
- 21.Preston SH, Stokes A, Mehta NK, Cao B. Projecting the effect of changes in smoking and obesity on future life expectancy in the United States. Demography. 2014;51(1):27-49. doi: 10.1007/s13524-013-0246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belsky DW, Shalev I, Sears MR, et al. Is chronic asthma associated with shorter leukocyte telomere length at midlife? Am J Respir Crit Care Med. 2014;190(4):384-391. doi: 10.1164/rccm.201402-0370OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poulton R, Moffitt TE, Silva PA. The Dunedin Multidisciplinary Health and Development Study: overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 2015;50(5):679-693. doi: 10.1007/s00127-015-1048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richmond-Rakerd LS, D’Souza S, Andersen SH, et al. Clustering of health, crime and social-welfare inequality in 4 million citizens from two nations. Nat Hum Behav. 2020;4(3):255-264. doi: 10.1038/s41562-019-0810-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasmussen LJH, Caspi A, Ambler A, et al. Association of neurocognitive and physical function with gait speed in midlife. JAMA Netw Open. 2019;2(10):e1913123. doi: 10.1001/jamanetworkopen.2019.13123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott ML, Belsky DW, Knodt AR, et al. Brain-age in midlife is associated with accelerated biological aging and cognitive decline in a longitudinal birth cohort. Mol Psychiatry. 2021;26(8):3829-3838. doi: 10.1038/s41380-019-0626-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liem F, Varoquaux G, Kynast J, et al. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179-188. doi: 10.1016/j.neuroimage.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 28.Christensen K, Thinggaard M, McGue M, et al. Perceived age as clinically useful biomarker of ageing: cohort study. BMJ. 2009;339:b5262. doi: 10.1136/bmj.b5262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belsky DW, Moffitt TE, Baker TB, et al. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiatry. 2013;70(5):534-542. doi: 10.1001/jamapsychiatry.2013.736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belsky DW, Moffitt TE, Houts R, et al. Polygenic risk, rapid childhood growth, and the development of obesity: evidence from a 4-decade longitudinal study. Arch Pediatr Adolesc Med. 2012;166(6):515-521. doi: 10.1001/archpediatrics.2012.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belsky DW, Sears MR, Hancox RJ, et al. Polygenic risk and the development and course of asthma: an analysis of data from a four-decade longitudinal study. Lancet Respir Med. 2013;1(6):453-461. doi: 10.1016/S2213-2600(13)70101-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349(15):1414-1422. doi: 10.1056/NEJMoa022363 [DOI] [PubMed] [Google Scholar]
- 33.Janssen I, Katzmarzyk PT, Srinivasan SR, et al. Utility of childhood BMI in the prediction of adulthood disease: comparison of national and international references. Obes Res. 2005;13(6):1106-1115. doi: 10.1038/oby.2005.129 [DOI] [PubMed] [Google Scholar]
- 34.Costello AJ, Edelbrock C, Kalas R, Kessler MD, Klaric SA. The National Institute of Mental Health Diagnostic Interview Schedule for Children. National Institute of Mental Health; 1982. [Google Scholar]
- 35.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. American Psychiatric Association; 1980. [Google Scholar]
- 36.Belsky DW, Caspi A, Israel S, Blumenthal JA, Poulton R, Moffitt TE. Cardiorespiratory fitness and cognitive function in midlife: neuroprotection or neuroselection? Ann Neurol. 2015;77(4):607-617. doi: 10.1002/ana.24356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reuben A, Moffitt TE, Caspi A, et al. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry. 2016;57(10):1103-1112. doi: 10.1111/jcpp.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention . CDC-Kaiser ACE study. Accessed March 15, 2021. https://www.cdc.gov/violenceprevention/aces/about.html
- 39.Elley WB, Irving JC. Revised socioeconomic index for New-Zealand. New Zealand J Educat Stud. 1976;11(1):25-36. [Google Scholar]
- 40.Muthén LK, Muthén BO. Mplus User’s Guide. 7th ed. Muthén & Muthén; 1998-2012.
- 41.Crimmins EM, Thyagarajan B, Levine ME, Weir DR, Faul J. Associations of age, sex, race/ethnicity, and education with 13 epigenetic clocks in a nationally representative U.S. sample: the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2021;76(6):1117-1123. doi: 10.1093/gerona/glab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raffington L, Belsky DW, Kothari M, Malanchini M, Tucker-Drob EM, Harden KP. Socioeconomic disadvantage and the pace of biological aging in children. Pediatrics. 2021;147(6):e2020024406. doi: 10.1542/peds.2020-024406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belsky DW, Caspi A, Arseneault L, et al. Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm. Elife. 2020;9:e54870. doi: 10.7554/eLife.54870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Belsky DW, Caspi A, Cohen HJ, et al. Impact of early personal-history characteristics on the pace of aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell. 2017;16(4):644-651. doi: 10.1111/acel.12591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujii T, Shimada K, Nakai T, Ohbayashi C. MicroRNAs in smoking-related carcinogenesis: biomarkers, functions, and therapy. J Clin Med. 2018;7(5):98. doi: 10.3390/jcm7050098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piperakis SM, Visvardis EE, Sagnou M, Tassiou AM. Effects of smoking and aging on oxidative DNA damage of human lymphocytes. Carcinogenesis. 1998;19(4):695-698. doi: 10.1093/carcin/19.4.695 [DOI] [PubMed] [Google Scholar]
- 47.Gonçalves RB, Coletta RD, Silvério KG, et al. Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res. 2011;60(5):409-424. doi: 10.1007/s00011-011-0308-7 [DOI] [PubMed] [Google Scholar]
- 48.Rodríguez-Hernández H, Simental-Mendía LE, Rodríguez-Ramírez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. 2013;2013:678159. doi: 10.1155/2013/678159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stepanova M, Rodriguez E, Birerdinc A, Baranova A. Age-independent rise of inflammatory scores may contribute to accelerated aging in multi-morbidity. Oncotarget. 2015;6(3):1414-1421. doi: 10.18632/oncotarget.2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4-S9. doi: 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 51.Wertz J, Caspi A, Ambler A, et al. Association of history of psychopathology with accelerated aging at midlife. JAMA Psychiatry. 2021;78(5):530-539. doi: 10.1001/jamapsychiatry.2020.4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bullmore E. The Inflamed Mind: A Radical New Approach to Depression. Picador; 2018. [Google Scholar]
- 53.Barbé-Tuana FM, Grun LK, Pierdoná V, et al. Shorter telomeres in children with severe asthma, an indicative of accelerated aging. Aging (Albany NY). 2021;13(2):1686-1691. doi: 10.18632/aging.202527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng C, Cardenas A, Rifas-Shiman SL, et al. Epigenetic age acceleration is associated with allergy and asthma in children in Project Viva. J Allergy Clin Immunol. 2019;143(6):2263-2270.e14. doi: 10.1016/j.jaci.2019.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casas M, Belén A, Maitre L, et al. Is childhood asthma associated with biological aging markers? Eur Respiratory J. 2019;54:PA5435. doi: 10.1183/13993003.congress-2019.PA5435 [DOI] [Google Scholar]
- 56.Moffitt TE, Houts R, Asherson P, et al. Is adult ADHD a childhood-onset neurodevelopmental disorder? evidence from a four-decade longitudinal cohort study. Am J Psychiatry. 2015;172(10):967-977. doi: 10.1176/appi.ajp.2015.14101266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dehghan M, Akhtar-Danesh N, Merchant AT. Childhood obesity, prevalence and prevention. Nutr J. 2005;4(1):24. doi: 10.1186/1475-2891-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collishaw S, Maughan B, Goodman R, Pickles A. Time trends in adolescent mental health. J Child Psychol Psychiatry. 2004;45(8):1350-1362. doi: 10.1111/j.1469-7610.2004.00335.x [DOI] [PubMed] [Google Scholar]
- 59.Jensen CD, Cushing CC, Aylward BS, Craig JT, Sorell DM, Steele RG. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: a meta-analytic review. J Consult Clin Psychol. 2011;79(4):433-440. doi: 10.1037/a0023992 [DOI] [PubMed] [Google Scholar]
- 60.Das JK, Salam RA, Arshad A, Finkelstein Y, Bhutta ZA. Interventions for adolescent substance abuse: an overview of systematic reviews. J Adolesc Health. 2016;59(4S):S61-S75. doi: 10.1016/j.jadohealth.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho M, Garnett SP, Baur LA, et al. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167(8):759-768. doi: 10.1001/jamapediatrics.2013.1453 [DOI] [PubMed] [Google Scholar]
- 62.Olfson M, Druss BG, Marcus SC. Trends in mental health care among children and adolescents. N Engl J Med. 2015;372(21):2029-2038. doi: 10.1056/NEJMsa1413512 [DOI] [PubMed] [Google Scholar]
- 63.Kieling C, Baker-Henningham H, Belfer M, et al. Child and adolescent mental health worldwide: evidence for action. Lancet. 2011;378(9801):1515-1525. doi: 10.1016/S0140-6736(11)60827-1 [DOI] [PubMed] [Google Scholar]
- 64.Asarnow JR, Rozenman M, Wiblin J, Zeltzer L. Integrated medical-behavioral care compared with usual primary care for child and adolescent behavioral health: a meta-analysis. JAMA Pediatr. 2015;169(10):929-937. doi: 10.1001/jamapediatrics.2015.1141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Using Count Variables for Adolescent Health Conditions
eMethods 2. Association of Internalizing and Externalizing Psychological Disorders with Midlife Aging
eTable. Descriptives and Correlations for Study Variables
eFigure 1. Visualization of Aging Outcomes
eFigure 2. Attrition Analyses
eFigure 3. Conceptual Model of Primary Analyses