This cohort study examines data from 15 152 adult patients who had moderate to severe traumatic brain injury to assess risk factors for and outcomes from early posttraumatic seizures.
Key Points
Question
What are the risk factors and what is the effect of early posttraumatic seizures (EPS) after a moderate to severe traumatic brain injury (TBI)?
Findings
This registry cohort study found that risk factors for EPS may be identified via multivariable analysis and a prediction algorithm. Severity of the TBI, prior medical comorbidities, and presence of subdural hematoma or subarachnoid hemorrhage were identified as risk factors, and EPS were associated with poor outcomes, including mortality, after adjustment for confounders.
Meaning
Early posttraumatic seizures after moderate to severe TBI are associated with poorer in-hospital and longer-term outcomes and may be predicted via identified clinical risk factors.
Abstract
Importance
Early posttraumatic seizures (EPS) that may occur following a traumatic brain injury (TBI) are associated with poorer outcomes and development of posttraumatic epilepsy (PTE).
Objective
To evaluate risk factors for EPS, associated morbidity and mortality, and contribution to PTE.
Design, Setting, and Participants
Data were collected from an Australian registry-based cohort study of adults (age ≥18 years) with moderate to severe TBI from January 2005 to December 2019, with 2-year follow-up. The statewide trauma registry, conducted on an opt-out basis in Victoria (population 6.5 million), had 15 152 patients with moderate to severe TBI identified via Abbreviated Injury Scale (AIS) head severity score, with an opt-out rate less than 0.5% (opt-out n = 136).
Main Outcomes and Measures
EPS were identified via International Statistical Classification of Diseases, Tenth Revision, Australian Modification (ICD-10-AM) codes recorded after the acute admission. Outcome measures also included in-hospital metrics, 2-year outcomes including PTE, and post-discharge mortality. Adaptive least absolute shrinkage and selection operator (LASSO) regression was used to build a prediction model for risk factors of EPS.
Results
Among the 15 152 participants (10 457 [69%] male; median [IQR] age, 60 [35-79] y), 416 (2.7%) were identified with EPS, including 27 (0.2%) with status epilepticus. Significant risk factors on multivariable analysis for developing EPS were younger age, higher Charlson Comorbidity Index, TBI sustained from a low fall, subdural hemorrhage, subarachnoid hemorrhage, higher Injury Severity Score, and greater head injury severity, measured using the AIS and Glasgow Coma Score. After adjustment for confounders, EPS were associated with increased ICU admission and ICU length of stay, ventilation and duration, hospital length of stay, and discharge to inpatient rehabilitation rather than home, but not in-hospital mortality. Outcomes in TBI admission survivors at 24 months, including mortality (relative risk [RR] = 2.14; 95% CI, 1.32-3.46; P = .002), development of PTE (RR = 2.91; 95% CI, 2.22-3.81; P < .001), and use of antiseizure medications (RR = 2.44; 95% CI, 1.98-3.02; P < .001), were poorer for cases with EPS after adjustment for confounders. The prediction model for EPS had an area under the receiver operating characteristic curve of 0.72 (95% CI, 0.66-0.79), sensitivity of 66%, and specificity of 73% in the validation set.
Discussion
We identified important risk factors for EPS following moderate to severe TBI. Early posttraumatic seizures were associated with longer ICU and hospital admissions, ICU ventilation, and poorer 24-month outcomes including mortality and development of PTE.
Introduction
Early posttraumatic seizures (EPS) occur during the acute phase following a traumatic brain injury (TBI). The incidence of EPS varies, depending on the population and EPS definition, from 0.4% to 10.5%.1,2,3,4,5,6,7,8 These EPS have been associated with greater morbidity and mortality.6,9
The role of EPS in the subsequent development of recurrent unprovoked seizures, or posttraumatic epilepsy (PTE), is not well understood. Early posttraumatic seizures may increase the risk of PTE.10 Whether treatment of EPS exerts an antiepileptogenic effect on developing PTE is largely unknown. Antiseizure prophylaxis is recommended for the first 7 days after TBI, but has no known longer-term benefits in preventing PTE.11 Once the first late unprovoked posttraumatic seizure occurs, the prevalence of seizures is as high as 86% at 2 years.12 Suppression of EPS may reduce the risk of PTE, which accounts for 20% of acquired epilepsies and 5% of all epilepsies.13 Being able to predict which patients are at higher risk of EPS may allow a precision medicine approach to diagnosis and would inform clinical trials of potential antiepileptogenic therapies.
Studies evaluating risk factors for the development of EPS are relatively few. Older age,6 TBI severity,6,11 medical comorbidities, subdural hemorrhage (SDH),2,6 epidural hematoma,11 brain contusion,2 and chronic alcohol abuse2,11 and other in-hospital complications6 have been identified as risk factors for EPS. Only 1 study developed a model of predicting EPS, but it had poor predictive performance.14
Using an Australian population-based trauma registry, the aims of this study were to (1) identify risk factors for EPS; (2) assess the association between EPS and short-term and long-term morbidity, including PTE, and mortality; and (3) develop a risk assessment prediction model for EPS.
Methods
Study Design
This was a registry-based cohort study. Adult patients (≥18 years) with a date of injury from January 2005 to December 2019 who were registered on the Victorian State Trauma Registry with a moderate to severe TBI were included. Patients with a preexisting diagnosis of epilepsy were excluded from the study. All data were supplied by the Victorian State Trauma Outcomes Registry and Monitoring group following local ethics approval (MUHREC Project ID 18104). The Victorian State Trauma Registry has ethical approval from the Victorian Department of Health and Human Services Human Research Ethics Committee (DHHREC 11/14) and the Monash University Human Research Ethics Committee (CF13/3040–2001000165).
Data Collection
Moderate to severe TBI was defined as an Abbreviated Injury Scale (AIS) head severity score of 3 to 6, indicating moderate to severe intracranial injury or skull fracture.15 The AIS is an anatomical injury severity scoring system, with established correlation with TBI outcomes.15 Anonymized data for these cases were extracted. Data extracted included demographics, admission information, injury event details, operations and procedures, International Statistical Classification of Diseases, Tenth Revision, Australian Modification (ICD-10-AM) diagnosis and comorbidities, and discharge information, including in-hospital mortality.
The ICD-10-AM codes R56.8–unspecified convulsions, G41–status epilepticus, and G40–acute symptomatic seizures were used to identify EPS as new-onset seizures during the acute admission, excluding preexisting seizures and epilepsy, as per previous studies.6,7 Neither nonconvulsive or electrographic seizures were independently identified. Trained registry coders performed the ICD-10-AM coding once the admission was complete and are regularly audited.
Isolated TBI was defined as the absence of any other AIS body region with a severity score greater than 1. The Injury Severity Score was derived from the AIS to provide an overall severity score.15 The initial Glasgow Coma Scale (GCS) score was used as a further measure of TBI severity. Other initial vital sign observations were recorded. The Charlson Comorbidity Index (CCI) score and indicators for preexisting mental health, drug, and alcohol conditions were mapped from the ICD-10-AM codes.16,17 The Accessibility and Remoteness Index of Australia, a measure used for relation to accessible services, including health care, and the Index of Relative Socio-economic Advantage and Disadvantage, for social status, were determined by residential postcode.
All cases on the Victorian State Trauma Registry are routinely followed up by standardized, structured telephone interview at 6, 12, and 24 months postinjury and the Glasgow Outcome Scale–Extended (GOS-E) score collected. Consistent with other studies, diagnosis of PTE was determined by patient responses to the GOS-E question, “Since the injury, has the patient had any epileptic fits?”18 Postdischarge mortality was determined by data linkage with the Victorian Registry of Births, Deaths, and Marriages to July 2019. (The eMethods in the Supplement contain more details about collecting data from the registry.)
Statistical Analysis
Frequencies and percentages for categorical variables, and median and IQR for nonnormally distributed continuous variables, were used to summarize the data. Incidence of EPS was calculated as the ratio of EPS to TBI in each year of the study. Poisson regression with adjustment for age and sex was used to measure the change in the incidence of EPS over the study period. Univariable associations between individual variables and development of EPS were assessed using χ2 statistics for categorical variables and Mann-Whitney U tests for nonnormally distributed continuous variables. Factors with a P value less than .20 were included in the multivariable Poisson regression model with robust variance to measure their association with EPS. Adjusted risk ratios and the corresponding 95% CI were reported.
Multivariable multinomial logistic regression was performed to assess the association between EPS and GOS-E outcomes in survivors at 24 months. A Little test of missing completely at random19 was used to test the missingness of the follow-up outcomes (GOS-E and PTE) at each time point. To assess the association between EPS and long-term mortality in survivors, a univariable log-rank test for categorical variables and Wald test in Cox regression for continuous variables were first performed to screen potential risk factors for long-term mortality. Early posttraumatic seizures and covariates with univariable P < .20 were included in the multivariable Cox regression model. Statistical significance level was set at P < .05. Holm-Bonferroni method was used to correct for post hoc pairwise comparisons in categorical variables with more than 2 groups.
Prediction Model
Adaptive least absolute shrinkage and selection operator (LASSO) regression20 was used to develop a prediction model for EPS. Adaptive LASSO is a multistep version of the conventional cross-validation–based LASSO where adaptive weights are further applied for penalizing different coefficients. Covariates whose estimated coefficients are zero were excluded. Per conventional partition protocol, we randomly selected 80% of patients in the cohort as the training set and the remaining 20% as the test set. The ability of the model to predict EPS was evaluated with a series of metrics, including area under the receiver operating characteristic (ROC) curve, accuracy, sensitivity, and specificity. All statistical tests and adaptive LASSO regression were performed using Stata version 16 (StataCorp).
Results
Incidence of EPS
A total of 15 152 patients met the criteria for inclusion (median length of stay: 7.0 days; IQR, 3.6-13.8); 416 (2.7%) had EPS (median length of stay: 17.0 days; IQR, 9.4-28.6) (eTable 1 in the Supplement). Incidence of EPS decreased each year (incidence rate ratio: 0.94; 95% CI, 0.92-0.96; P < .001) (eFigure in the Supplement). Of patients with EPS, 6.5% experienced status epilepticus (0.2% of all cases).
Demographics and Preexisting Illness
Patients experiencing EPS were older than patients who did not experience EPS (median [IQR] age: 69 [44-81] years vs 60 [35-79] years). There was no association between sex, service accessibility and rurality (Accessibility and Remoteness Index of Australia), preexisting mental health conditions and drug misuse, or socioeconomic status and EPS (eTable 2 in the Supplement). Compared with patients without EPS, a higher CCI score and preexisting alcohol misuse were associated with EPS (eTable 2 in the Supplement).
Injury and Neurosurgical Characteristics
A higher proportion of patients with EPS sustained their injury in a low fall (from standing height or <1 m) than patients without EPS (eTable 3 in the Supplement). The prevalence of isolated TBI, SDH, subarachnoid hemorrhage (SAH), intracranial hemorrhage, epidural hematoma, and base-of-skull fracture was higher in patients with EPS than patients without EPS. Greater severity of head injury (AIS or GCS) and injury overall (Injury Severity Score) were associated with EPS. Any neurosurgical intervention was associated with a higher proportion of EPS compared with patients without EPS.
Hospital Services and Vital Characteristics
A higher proportion of patients who developed EPS were admitted to major trauma services and took longer to arrive at the hospital (eTable 4 in the Supplement). Median pulse rate and respiratory rate did not differ by EPS group; median blood pressure was higher (140 mm Hg vs 147 mm Hg) in patients who developed EPS.
Hospital Morbidity and Mortality
Compared with patients without EPS, a higher proportion of patients with EPS were admitted to the intensive care unit (ICU) (eTable 5 in the Supplement), spent longer in the ICU (median 5 days vs 8 days), were more often ventilated and for longer times (median 4 days vs 6 days), and spent longer in the hospital (median 7 days vs 17 days), and a higher proportion were discharged to rehabilitation rather than home.
Outcomes at 24-Month Follow-up
At 24 months after the TBI, 75% of patients eligible for 24-month follow-up had a completed GOS-E assessment. A higher proportion of patients who developed EPS were severely disabled or deceased, compared with patients who did not develop EPS (eTable 6 in the Supplement). The PTE question was complete in 1665 patients (15%). Higher proportions of patients with EPS had developed PTE (78% [35/45] vs 19% [314/1620], P < .001) or were taking antiseizure medication (68% [46/68] vs 19% [543/2878], P < .001) by 24 months after the injury.
Long-term Mortality
The long-term mortality rate was 14% (n = 1658) for the group of patients without EPS and 24% (n = 76) for patients who had EPS (P < .001).
Multivariable Analysis
The variables included in the multivariable analysis are shown in Table 1. Age showed a negligible (RR = 0.99) association with EPS. Increasing comorbidity severity, indicated by a CCI score of 1 or 2, was associated with 1.76 and 3.87 times the risk of EPS, respectively, when compared with patients without a CCI condition. Alcohol misuse and trauma type were not associated with EPS. Patients injured from low falls had 1.63 times the risk of EPS compared with patients injured in motor vehicle crashes. Presence of an SDH increased EPS risk by 77% and an SAH by 40%. Patients with an AIS head severity score of 5 or 6 had more than 3 times the risk of EPS compared with patients with AIS of 3, and patients with a GCS score of 3 to 8 had 1.47 times the risk of EPS compared with patients with mild head injury. The Injury Severity Score showed an associated modest smaller risk of EPS.
Table 1. Multivariable Analysis of Demographics, Preexisting Conditions, and Injury Characteristics Associated With EPS Developmenta.
| Variable | Adjusted risk ratio (95% CI) | P value |
|---|---|---|
| Age | 0.99 (0.99-1.00) | .045 |
| IRSAD quintile | .23 | |
| 1st (Most disadvantaged) | 1 [Reference] | |
| 2nd | 1.31 (0.93-1.85) | .12 |
| 3rd | 0.98 (0.69-1.40) | .93 |
| 4th | 0.96 (0.68-1.35) | .81 |
| 5th (Least disadvantaged) | 1.19 (0.87-1.63) | .29 |
| Preexisting Charlson Comorbidity Index | <.001 | |
| 0 | 1 [Reference] | |
| 1 | 1.76 (1.34-2.33) | <.001 |
| ≥2 | 3.87 (2.95-5.09) | <.001 |
| History of alcohol misuse | 1.06 (0.78-1.43) | .72 |
| Trauma type | ||
| Isolated TBI | 1 [Reference] | |
| Multitrauma | 0.95 (0.74-1.22) | .69 |
| Cause of injury | .38 | |
| Motor vehicle crash | 1 [Reference] | |
| Motorcycle | 0.88 (0.42-1.84) | .73 |
| Bicycle | 1.51 (0.74-3.11) | .26 |
| Pedestrian | 1.38 (0.79-2.42) | .26 |
| Low fall | 1.63 (1.03-2.56) | .04 |
| High fall | 1.33 (0.79-2.23) | .29 |
| Others | 1.46 (0.90-2.38) | .12 |
| Subdural hematoma | 1.77 (1.32-2.37) | <.001 |
| Subarachnoid hemorrhage | 1.40 (1.13-1.74) | .002 |
| Intraventricular hemorrhage | 1.11 (0.68-1.81) | .67 |
| Epidural hematoma | 0.83 (0.59-1.18) | .31 |
| Base-of-skull fracture | 0.82 (0.63-1.06) | .13 |
| Vault fractures | 0.89 (0.67-1.19) | .44 |
| AIS head severity | <.001 | |
| 3 | 1 [Reference] | |
| 4 | 1.45 (1.01-2.07) | .04 |
| 5-6 | 3.02 (1.88-4.84) | <.001 |
| Injury Severity Score | 0.98 (0.96-1.00) | .04 |
| Glasgow Coma Scale score | .007 | |
| Mild head injury | 1 [Reference] | |
| Moderate | 1.21 (0.88-1.65) | .24 |
| Severe | 1.47 (1.15-1.87) | .002 |
Abbreviations: AIS, Abbreviated Injury Scale; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage; TBI, traumatic brain injury.
Factors were included into multivariable analysis with a cutoff of P = .20.
After adjustment for potential confounders, EPS were associated with in-hospital (Table 2) and longer-term outcomes (Table 3). Patients who developed EPS while in the hospital had an increased risk of ICU admission, ventilation, longer time in the ICU ventilated, and longer stay in the hospital compared with patients who did not develop EPS. Patients with EPS also experienced a higher risk of discharge to inpatient rehabilitation. Early posttraumatic seizures were not associated with in-hospital mortality after adjustment for potential confounders (P = .67).
Table 2. Multivariable Analyses of Associations Between Early Posttraumatic Seizure and Hospital Outcomes.
| Hospital outcome | EPS vs no EPSa | |
|---|---|---|
| Incidence rate ratio or risk ratio (95% CI) | P value | |
| ICU | ||
| Admission | 1.43 (1.31-1.57) | <.001 |
| Ventilated | 1.12 (1.07-1.17) | <.001 |
| Length of stay, d | 1.38 (1.25-1.52) | <.001 |
| Ventilated length of stay, d | 1.33 (1.19-1.49) | <.001 |
| Hospital length of stay, d | 1.89 (1.70-2.10) | <.001 |
| Discharge to home | 0.42 (0.31-0.56) | <.001 |
| Discharge to rehabilitation | 1.44 (1.30-1.58) | <.001 |
| In-hospital mortality | 1.04 (0.86-1.27) | .67 |
Abbreviations: AIS, Abbreviated Injury Scale; EPS, early posttraumatic seizure; ICU, intensive care unit; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage.
Main predictor is EPS, which is adjusted for age, IRSAD quintile, preexisting Charlson Comorbidity Index value, history of alcohol abuse, trauma type, cause of injury, subdural hematoma, subarachnoid hemorrhage, intraventricular hemorrhage, epidural hematoma, base-of-skull fracture, vault fractures, categorized AIS head severity score, Injury Severity Score and categorized Glasgow Coma Scale.
Table 3. Multivariable Analyses of Associations Between Early Posttraumatic Seizure and Follow-up Outcomes Among Patients Who Survived the Initial Admission.
| Follow-up outcomes | EPS vs no EPSa | |
|---|---|---|
| Relative risk ratio, risk ratio, or hazard ratio (95% CI) | P value | |
| Glasgow Outcome Scale at 24 mo, good recovery as base outcome | ||
| Moderate disability | 1.24 (0.78-1.97) | .37 |
| Severe disability | 2.10 (1.35-3.28) | .001 |
| Vegetative state | 3.97 (1.03-15.3) | .046 |
| Dead | 2.14 (1.32-3.46) | .002 |
| Posttraumatic epilepsy within 24 mo | 2.91 (2.22-3.81) | <.001 |
| Use of ASM within 24 mo | 2.44 (1.98-3.02) | <.001 |
Abbreviations: AIS, Abbreviated Injury Scale; ASM, antiseizure medication; EPS, early posttraumatic seizure; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage.
Main predictor is EPS, which is adjusted for age, IRSAD quintile, preexisting Charlson Comorbidity Index value, history of alcohol abuse, trauma type, cause of injury, subdural hematoma, subarachnoid hemorrhage, intraventricular hemorrhage, epidural hematoma, base-of-skull fracture, vault fractures, categorized AIS head score, Injury Severity Score, and categorized Glasgow Coma Scale.
Following adjustment for potential confounders, EPS increased the risk of being severely disabled (RR = 2.10), in a vegetative state (RR = 3.97), and deceased (RR = 2.14) at follow-up (Table 3). Patients with EPS had a higher risk of developing PTE (RR = 2.91) and taking antiseizure medication (RR = 2.44) in the first 24 months after injury. Risk of long-term mortality was similar in patients with EPS and patients without EPS (hazard ratio = 1.22; 95% CI, 0.95-1.55; P = .12). The median follow-up time for the linkage in patients who survived the TBI was 5.5 years (IQR, 1.9-9.6 years).
Prediction Modeling Analysis
Using a risk factor prediction algorithm via LASSO regression, EPS were associated with trauma type (isolated TBI over multitrauma); lower social status (Index of Relative Socio-economic Advantage and Disadvantage); higher medical comorbidity scores; cause of injury, including low falls; the injury specifics of SDH and SAH; and the injury severity indicators AIS head, Injury Severity Score, and GCS score. Preexisting CCI score of 2+ (penalized coefficient = 0.303), SDH (0.333), and AIS head severity score of 5 or 6 (0.333) were the 3 highest contributors toward association with EPS; a lower preexisting CCI of 0 was the strongest negative predictor (−0.270) of EPS in the model (Table 4).
Table 4. Variables With Nonzero Penalized Coefficient in the Adaptive Least Absolute Shrinkage and Selection Operator Regression.
| Variable | Penalized Coefficient |
|---|---|
| IRSAD quintile | |
| 1st (Most disadvantaged) | 0 |
| 2nd | 0.036 |
| 3rd | 0 |
| 4th | −0.059 |
| 5th (Least disadvantaged) | 0 |
| Cause of injury low fall | 0.0406 |
| Preexisting Charlson Comorbidity Index | |
| 0 | −0.270 |
| 1 | 0 |
| ≥2 | 0.303 |
| Trauma type, isolated TBI | 0.081 |
| Subdural hematoma | 0.333 |
| Subarachnoid hemorrhage | 0.165 |
| AIS head severity score | |
| 3 | −0.104 |
| 4 | 0.000 |
| 5-6 | 0.333 |
| Injury severity score | −0.163 |
| Glasgow Coma Scale category | |
| Mild | −0.115 |
| Moderate | 0 |
| Severe | 0 |
Abbreviations: AIS, Abbreviated Injury Scale; IRSAD, Index of Relative Socio-economic Advantage and Disadvantage; TBI, traumatic brain injury.
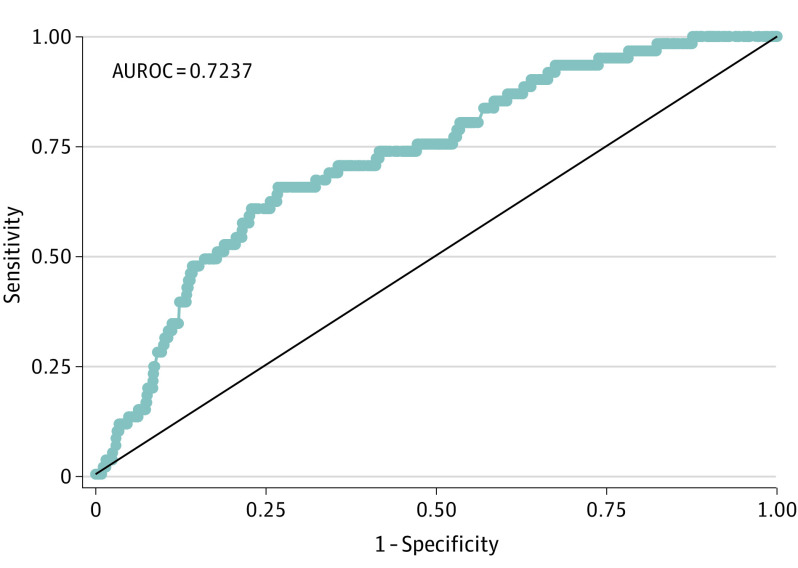
The adaptive LASSO regression prediction model for EPS demonstrated an overall performance of area under the ROC curve of 0.72 (95% CI, 0.66-0.79) in the test set (Figure), with a maximum value of Youden index of 0.39 at total penalized coefficient value of 1.05 (sensitivity = 66% and specificity = 73%).
Figure. Adaptive Least Absolute Shrinkage and Selection Operator (LASSO) Regression Prediction Model for Early Posttraumatic Seizure: Test Set.
AUROC indicates area under the receiver operating characteristic curve.
Discussion
In this population-based study of more than 15 000 patients with moderate to severe TBI, we identified clinically relevant risk factors for the development of EPS and found a higher risk of morbidity, mortality, and subsequent PTE in patients with EPS.
The observed prevalence of EPS (2.7%) was consistent with previous studies, although EPS have been variably defined. Some studies separated “immediate” seizures within 24 hours and “early” seizures up to 7 days after the TBI. Immediate seizures have been associated with higher risk of PTE,5 but are not well defined and may include an antecedent seizure causing TBI. The International League Against Epilepsy classifies acute symptomatic seizures as “occurring at the time of a systemic insult or in close temporal association with a documented brain insult,” of which TBI is included.21 This also suggests use of the arbitrary cutoff of 1 week, based on a cohort study of depressed TBI from 1973.22 There is no evidence that “late” (unprovoked) seizures or PTE should be defined as 1 week or more given any specified increase in risk of recurrence or etiological differences.
We observed a prevalence of PTE in patients who had experienced EPS of 11% at 2 years (3% in patients without EPS), with ICU and hospital mean length of stay being 10.4 days and 24.5 days, respectively, for patients with EPS (compared with 7.7 days and 11.0 days, respectively, for those without EPS). Englander et al1 closely followed up patients from injury to 2 years and reported that a high proportion of posttraumatic seizures occurring in the first month were likely multifactorial, including acute provoking factors such as brain swelling and perioperative and metabolic factors. Considering the associated complications of mechanical ventilation and other in-hospital conditions after moderate to severe TBI,6 any seizure occurring during the acute hospital stay may represent a provoked acute posttraumatic seizure and therefore termed EPS. Accordingly, the role and duration of antiseizure prophylaxis requires further investigation.23
Most studies examining EPS were conducted before the widespread use of continuous electroencephalography (cEEG) in ICU patients after a TBI. The rate of status epilepticus of 0.2% in this study, comparable with that of previous studies,24 likely exclusively represents convulsive status epilepticus, because the incidence is much higher of nonconvulsive seizures and nonconvulsive status epilepticus detected by cEEG in patients after a severe TBI.25 Although long-term outcomes of nonconvulsive seizures and status epilepticus in TBI may be unclear, they are associated with neurophysiological metabolic damage causing secondary injury26,27 and structural hippocampal atrophy.28 Clinically overt seizures therefore represent “the tip of the iceberg” in relation to all EPS, and the incidence rate found in this study is likely a marked underestimate. The use of cEEG may not be beneficial for patients at low risk of acute seizures29; therefore, using risk factors to select patients for cEEG in TBI appears important.
Several risk factors have been identified for EPS following moderate-severe TBI. Pre-injury patient factors such as a history of alcohol misuse and medical comorbidities, previously associated with EPS,6,9,30 may interact with TBI and decompensation during acute admission, contributing to EPS separately. Greater TBI severity, measured by surrogate initial GCS or more accurately via composite severity scores such as AIS, confers a worse acute neurological insult, provoking EPS.15 This correlates with acute blood products causing irritation of the brain, such as SAH and SDH, which are known to independently cause acute seizures. Neurosurgical procedures may also inadvertently cause cerebral irritation and edema and were associated with EPS in this study as well as another study.14 The mechanism of injury seems less important and potentially difficult to interpret, although here low falls was associated with EPS in multivariable analysis, raising the possibility that an unwitnessed seizure caused the fall. In-hospital complications such as sepsis and metabolic derangements, not evaluated in this study, may contribute to EPS risk although are difficult to predict and attribute causality on a group level. Most variables identified by the prediction algorithm are concordant with the multivariable analysis in this study, including medical comorbidities, low falls, SDH, SAH, and injury severity measures (AIS head, Injury Severity Score, and GCS). Identifying patients at high risk of EPS may assist with clinical management, help focus evaluation with cEEG, and potentially aid in clinical trial protocol development, including antiepileptogenic therapies.
In this cohort, EPS were associated with risk of worsened morbidity and mortality, adjusted for injury severity and other factors. As per previous studies, EPS were associated with a longer hospital stay6 but also ICU admission, ventilation and its duration, and discharge to rehabilitation rather than home. In-hospital mortality was increased in the EPS group but not when adjusted for EPS risk factors, including injury severity; yet patients with EPS had an increased risk of poorer outcomes, including mortality, at 24 months as measured by the study follow-up GOS-E. The instantaneous risk of mortality as assessed by hazard ratio using linkage to the death index (median follow-up 5.5 y) was nonsignificant, although the CI only marginally crossed the threshold of nonsignificance (95% CI, 0.95-1.55; P = .12).
The association of EPS with an increased risk of developing PTE has been consistently reported10; however, the causal link or epileptogenic process remains unclear. In this study of moderate to severe TBI inclusive of all seizures during the acute admission, just 11% of patients with EPS developed PTE, although this was significantly greater that the 3% incidence in those without EPS. Many risk factors of EPS are similar for PTE, and some studies do not separate them in their analysis. As mentioned earlier, blurring of time frames for the definitions of EPS and PTE likely contributes to conflicting data in the literature. Posttraumatic epilepsy is associated with significant disability, mortality, and treatment-resistant chronic epilepsy, with the latent phase of PTE or epileptogenesis being the target for novel biomarkers and potential antiepileptogenic treatments of the international multicenter study EpiBioS4Rx in TBI.31 Even though EPS are associated with an increased risk for developing PTE, the overall risk is not high enough for the recommendation of lifelong antiseizure medication. The effect of identification of EPS and its suppression with antiseizure medication on the development of PTE warrants investigation.
Limitations
This study is an observational study based on registry-coded diagnoses, and although they are well managed and regularly audited, that is a limitation in determining causality. While there were some missing data, there was a high rate of follow-up, and the mortality data were strengthened by linkage analysis. Identification of moderate to severe TBI by AIS head severity score is novel in the literature, considered more accurate than other measures correlating well with overall TBI outcomes.15 Association of EPS with other in-hospital risk factors of acute symptomatic seizures, such as systemic infections, etc, as assessed in 1 study,6 was not evaluated in this study because of the difficulty in implying causality to EPS. The diagnosis of PTE was performed via self-report questionnaire, rather than an epileptologist, the latter being difficult to implement on a large population level. Given the open questioning in the GOS-E on PTE, patients may have included EPS in their responses to seizures after injury. Despite these limitations, this study remains significant in its cohort size of EPS; multivariable analysis of risk factors for EPS, including a novel prediction model; and outcomes with matched cohorts based on risk factors of EPS identified in multivariable analysis.
Conclusions
This large cohort study of moderate to severe TBI identified several clinical risk factors that may be used to predict EPS, showing good concordance with a novel machine learning approach, specifically prior medical comorbidities, SAH and SDH, and injury severity. Early posttraumatic seizures are associated with significant in-hospital morbidity, poorer outcomes, and subsequent risk of mortality at 24 months on follow-up GOS-E. Identifying patients at high risk of developing EPS may allow a precision medicine diagnostic approach, focusing management strategies and targeting clinical trials of antiepileptogenic therapies.
eMethods
eTable 1. Prevalence of EPS
eFigure. Incidence of Early Posttraumatic Seizures by Year From 2005 to 2019
eTable 2. Demographics and Preexisting Illness
eTable 3. Injury and Neurosurgery Characteristics
eTable 4. Health Services and Vital Characteristics
eTable 5. Hospital Morbidity and Mortality
eTable 6. Follow-up Outcomes Among Patients Who Survived the Initial TBI Admission and Due for 24-Month Follow-up
References
- 1.Englander J, Bushnik T, Duong TT, et al. Analyzing risk factors for late posttraumatic seizures: a prospective, multicenter investigation. Arch Phys Med Rehabil. 2003;84(3)(suppl 1):365-373. doi: 10.1053/apmr.2003.50022 [DOI] [PubMed] [Google Scholar]
- 2.Thapa A, Chandra SP, Sinha S, Sreenivas V, Sharma BS, Tripathi M. Post-traumatic seizures: a prospective study from a tertiary level trauma center in a developing country. Seizure. 2010;19(4):211-216. doi: 10.1016/j.seizure.2010.02.004 [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Wu H, Wang X, Li J, Zhang S. Clinical epidemiology of posttraumatic epilepsy in a group of Chinese patients. Seizure. 2012;21(5):322-326. doi: 10.1016/j.seizure.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharyya K, Mandal N, Paul UK, Bhattacharyya AK, Sinharay K, Gantait K. Post-traumatic seizure: a multicentric epidemiological study. J Indian Med Assoc. 2014;112(2):93-95. [PubMed] [Google Scholar]
- 5.Ritter AC, Wagner AK, Fabio A, et al. Incidence and risk factors of posttraumatic seizures following traumatic brain injury: a Traumatic Brain Injury Model Systems Study. Epilepsia. 2016;57(12):1968-1977. doi: 10.1111/epi.13582 [DOI] [PubMed] [Google Scholar]
- 6.Majidi S, Makke Y, Ewida A, Sianati B, Qureshi AI, Koubeissi MZ. Prevalence and risk factors for early seizure in patients with traumatic brain injury: analysis from National Trauma Data Bank. Neurocrit Care. 2017;27(1):90-95. doi: 10.1007/s12028-016-0363-6 [DOI] [PubMed] [Google Scholar]
- 7.DeGrauw X, Thurman D, Xu L, Kancherla V, DeGrauw T. Epidemiology of traumatic brain injury-associated epilepsy and early use of anti-epilepsy drugs: an analysis of insurance claims data, 2004-2014. Epilepsy Res. 2018;146:41-49. doi: 10.1016/j.eplepsyres.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z, Chen Q, Chen Z, et al. Clinical analysis on risk factors and prognosis of early post-traumatic epilepsy. Arq Neuropsiquiatr. 2019;77(6):375-380. doi: 10.1590/0004-282x20190071 [DOI] [PubMed] [Google Scholar]
- 9.Wiedemayer H, Triesch K, Schäfer H, Stolke D. Early seizures following non-penetrating traumatic brain injury in adults: risk factors and clinical significance. Brain Inj. 2002;16(4):323-330. doi: 10.1080/02699050110102077 [DOI] [PubMed] [Google Scholar]
- 10.Tubi MA, Lutkenhoff E, Blanco MB, et al. Early seizures and temporal lobe trauma predict post-traumatic epilepsy: a longitudinal study. Neurobiol Dis. 2019;123:115-121. doi: 10.1016/j.nbd.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons . Guidelines for the management of severe traumatic brain injury (4th Edition). September 2016. Accessed September 2, 2021. https://braintrauma.org/uploads/03/12/Guidelines_for_Management_of_Severe_TBI_4th_Edition.pdf
- 12.Haltiner AM, Temkin NR, Dikmen SS. Risk of seizure recurrence after the first late posttraumatic seizure. Arch Phys Med Rehabil. 1997;78(8):835-840. doi: 10.1016/S0003-9993(97)90196-9 [DOI] [PubMed] [Google Scholar]
- 13.Agrawal A, Timothy J, Pandit L, Manju M. Post-traumatic epilepsy: an overview. Clin Neurol Neurosurg. 2006;108(5):433-439. doi: 10.1016/j.clineuro.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 14.Ritter AC, Wagner AK, Szaflarski JP, et al. Prognostic models for predicting posttraumatic seizures during acute hospitalization, and at 1 and 2 years following traumatic brain injury. Epilepsia. 2016;57(9):1503-1514. doi: 10.1111/epi.13470 [DOI] [PubMed] [Google Scholar]
- 15.Foreman BP, Caesar RR, Parks J, et al. Usefulness of the Abbreviated Injury Score and the Injury Severity Score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J Trauma. 2007;62(4):946-950. doi: 10.1097/01.ta.0000229796.14717.3a [DOI] [PubMed] [Google Scholar]
- 16.Gabbe BJ, Magtengaard K, Hannaford AP, Cameron PA. Is the Charlson Comorbidity Index useful for predicting trauma outcomes? Acad Emerg Med. 2005;12(4):318-321. doi: 10.1197/j.aem.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TQ, Simpson PM, Braaf SC, Cameron PA, Judson R, Gabbe BJ. Comparison of the performance of mental health, drug and alcohol comorbidities based on ICD-10-AM and medical records for predicting 12-month outcomes in trauma patients. BMC Health Serv Res. 2018;18(1):408. doi: 10.1186/s12913-018-3248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson JTL, Pettigrew LEL, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573-585. doi: 10.1089/neu.1998.15.573 [DOI] [PubMed] [Google Scholar]
- 19.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83(404):1198-1202. doi: 10.1080/01621459.1988.10478722 [DOI] [Google Scholar]
- 20.Zou H. The adaptive lasso and its oracle properties. J Am Stat Assoc. 2006;101(476):1418-1429. doi: 10.1198/016214506000000735 [DOI] [Google Scholar]
- 21.Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):671-675. doi: 10.1111/j.1528-1167.2009.02285.x [DOI] [PubMed] [Google Scholar]
- 22.Jennett B, Teather D, Bennie S. Epilepsy after head injury: residual risk after varying fit-free intervals since injury. Lancet. 1973;2(7830):652-653. doi: 10.1016/S0140-6736(73)92488-4 [DOI] [PubMed] [Google Scholar]
- 23.Chang BS, Lowenstein DH; Quality Standards Subcommittee of the American Academy of Neurology . Practice parameter: antiepileptic drug prophylaxis in severe traumatic brain injury: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;60(1):10-16. doi: 10.1212/01.WNL.0000031432.05543.14 [DOI] [PubMed] [Google Scholar]
- 24.Dhakar MB, Sivakumar S, Bhattacharya P, Shah A, Basha MM. A retrospective cross-sectional study of the prevalence of generalized convulsive status epilepticus in traumatic brain injury: United States 2002-2010. Seizure. 2015;32:16-22. doi: 10.1016/j.seizure.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 25.Vespa PM, Nenov V, Nuwer MR. Continuous EEG monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol. 1999;16(1):1-13. doi: 10.1097/00004691-199901000-00001 [DOI] [PubMed] [Google Scholar]
- 26.Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830-2836. doi: 10.1097/00003246-200712000-00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witsch J, Frey HP, Schmidt JM, et al. Electroencephalographic periodic discharges and frequency-dependent brain tissue hypoxia in acute brain injury. JAMA Neurol. 2017;74(3):301-309. doi: 10.1001/jamaneurol.2016.5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75(9):792-798. doi: 10.1212/WNL.0b013e3181f07334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossetti AO, Schindler K, Sutter R, et al. Continuous vs routine electroencephalogram in critically ill adults with altered consciousness and no recent seizure: a multicenter randomized clinical trial. JAMA Neurol. 2020;77(10):1225-1232. doi: 10.1001/jamaneurol.2020.2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parmontree P, Tunthanathip T, Doungngern T, Rojpitbulstit M, Kulviwat W, Ratanalert S. Predictive risk factors for early seizures in traumatic brain injury. J Neurosci Rural Pract. 2019;10(4):582-587. doi: 10.1055/s-0039-1700791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vespa PM, Shrestha V, Abend N, et al. The Epilepsy Bioinformatics Study for Anti-epileptogenic Therapy (EpiBioS4Rx) clinical biomarker: study design and protocol. Neurobiol Dis. 2019;123:110-114. doi: 10.1016/j.nbd.2018.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Prevalence of EPS
eFigure. Incidence of Early Posttraumatic Seizures by Year From 2005 to 2019
eTable 2. Demographics and Preexisting Illness
eTable 3. Injury and Neurosurgery Characteristics
eTable 4. Health Services and Vital Characteristics
eTable 5. Hospital Morbidity and Mortality
eTable 6. Follow-up Outcomes Among Patients Who Survived the Initial TBI Admission and Due for 24-Month Follow-up