Key Points
Question
What is the risk of an immediate severe allergic reaction to a second dose of a SARS-CoV-2 mRNA vaccine among individuals who had an immediate allergic reaction of any severity to their first dose?
Findings
In this systematic review and meta-analysis of 22 studies including 1366 patients revaccinated under the supervision of an allergist, there was a low incidence (0.16%) of immediate severe allergic reactions associated with receiving a second dose of SARS-CoV-2 mRNA vaccine among individuals who had an immediate allergic reaction to their first dose. There were no deaths.
Meaning
This study suggests that there is a low risk of a severe immediate allergic reaction associated with a second SARS-CoV-2 mRNA vaccine dose among persons who had an immediate allergic reaction to their first dose.
Abstract
Importance
Vaccination against SARS-CoV-2 is a highly effective strategy to prevent infection and severe COVID-19 outcomes. The best strategy for a second dose of vaccine among persons who had an immediate allergic reaction to their first SARS CoV-2 vaccination is unclear.
Objective
To assess the risk of severe immediate allergic reactions (eg, anaphylaxis) to a second dose of SARS-CoV-2 mRNA vaccine among persons with immediate allergic reactions to their first vaccine dose.
Data Sources
MEDLINE, Embase, Web of Science, and the World Health Organization Global Coronavirus database were searched from inception through October 4, 2021.
Study Selection
Included studies addressed immediate allergic reactions of any severity to a second SARS-CoV-2 vaccine dose in persons with a known or suspected immediate allergic reaction (<4 hours after vaccination) after their first SARS-CoV-2 vaccine dose. Studies describing a second vaccine dose among persons reporting delayed reactions (>4 hours after vaccination) were excluded.
Data Extraction and Synthesis
Paired reviewers independently selected studies, extracted data, and assessed risk of bias. Random-effects models were used for meta-analysis. The GRADE (Grading of Recommendation, Assessment, Development, and Evaluation) approach evaluated certainty of the evidence.
Main Outcomes and Measures
Risk of severe immediate allergic reaction and repeated severe immediate allergic reactions with a second vaccine dose. Reaction severity was defined by the reporting investigator, using Brighton Collaboration Criteria, Ring and Messmer criteria, World Allergy Organization criteria, or National Institute of Allergy and Infectious Diseases criteria.
Results
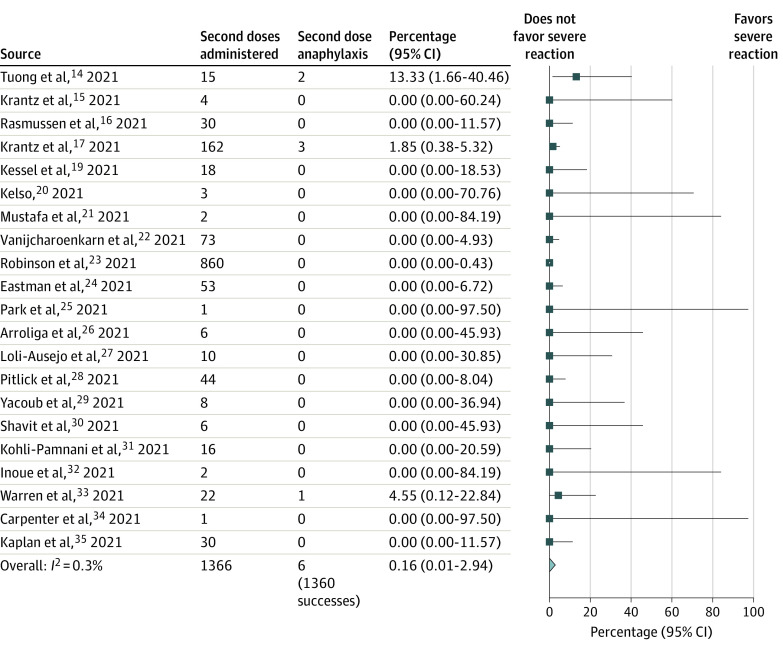
Among 22 studies of SARS-CoV-2 mRNA vaccines, 1366 individuals (87.8% women; mean age, 46.1 years) had immediate allergic reactions to their first vaccination. Analysis using the pooled random-effects model found that 6 patients developed severe immediate allergic reactions after their second vaccination (absolute risk, 0.16% [95% CI, 0.01%-2.94%]), 232 developed mild symptoms (13.65% [95% CI, 7.76%-22.9%]), and, conversely, 1360 tolerated the dose (99.84% [95% CI, 97.09%-99.99%]). Among 78 persons with severe immediate allergic reactions to their first SARS-CoV-2 mRNA vaccination, 4 people (4.94% [95% CI, 0.93%-22.28%]) had a second severe immediate reaction, and 15 had nonsevere symptoms (9.54% [95% CI, 2.18%-33.34%]). There were no deaths. Graded vaccine dosing, skin testing, and premedication as risk-stratification strategies did not alter the findings. Certainty of evidence was moderate for those with any allergic reaction to the first dose and low for those with severe allergic reactions to the first dose.
Conclusions and Relevance
In this systematic review and meta-analysis of case studies and case reports, the risk of immediate allergic reactions and severe immediate reactions or anaphylaxis associated with a second dose of an SARS-CoV-2 mRNA vaccine was low among persons who experienced an immediate allergic reaction to their first dose. These findings suggest that revaccination of individuals with an immediate allergic reaction to a first SARS-CoV-2 mRNA vaccine dose in a supervised setting equipped to manage severe allergic reactions can be safe.
This systematic review and meta-analysis of case studies and case reports assesses the risk of severe immediate allergic reactions to a second dose of SARS-CoV-2 mRNA vaccine among persons with immediate allergic reactions to their first vaccine dose.
Introduction
SARS-CoV-2 vaccination effectively reduces the risk of infection and severe COVID-19 outcomes. With more than 8.6 billion doses administered worldwide, immunization is a global priority to stem the count of the approximately 274 million infected and 5.3 million dead.1 Factors associated with facilitating vaccination include increasing vaccine mandates and supply, whereas factors associated with barriers to vaccination include vaccine inequity, hesitancy, disinformation and misinformation, and rare adverse effects, such as severe allergic reactions (which occur in 7.9 per 1 million vaccinations2). Early in the global vaccine rollout, December 9, 2020,3 rare cases of allergic reactions to mRNA vaccines rapidly led to recommendations stating that persons with an immediate allergic reaction to the first dose of an mRNA COVID-19 vaccine should not receive additional doses of either of the mRNA COVID-19 vaccines.3,4,5 This contraindication is inconsistent with allergy specialist practice parameters, which do not contraindicate readministration of non-COVID vaccines to those with prior vaccine allergic reactions.6
The immunology of allergic reactions is commonly understood to imply that, once an initial reaction occurs, repeated exposures reproducibly lead to acute and potentially life-threatening reactions (eg, anaphylaxis to food in those with a food allergy). Although this paradigm has been assumed regarding COVID vaccination, its evidence base has not been critically appraised. We systematically reviewed the literature on the risk of a second severe allergic reaction after SARS-CoV-2 vaccination.
Methods
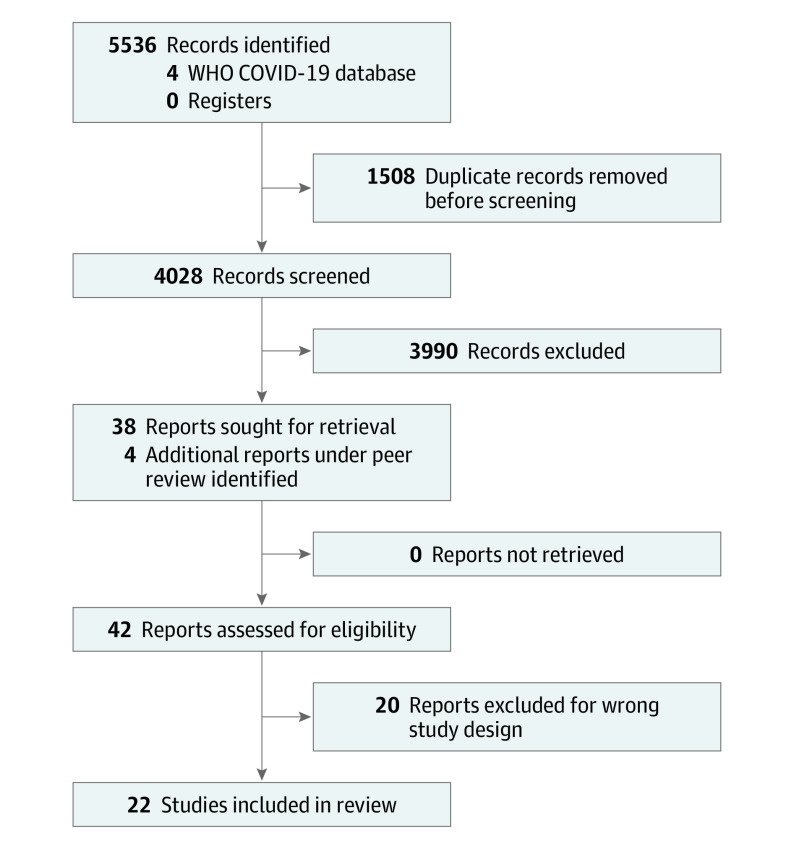
We searched MEDLINE, Embase, and the World Health Organization Global Coronavirus database (a database aggregating published and preprint COVID-19 reports daily from 112 other literature databases), from inception through October 4, 2021, for studies of any design addressing the risk of a second allergic reaction to SARS-CoV-2 vaccines of any severity among individuals who had a prior allergic reaction to a SARS-CoV-2 vaccine (eMethods in the Supplement). We additionally searched Web of Science (all databases) using forward and backward citation analysis to identify any additional relevant records. Studies that detailed delayed (>4 hours after vaccine) reactions or involved SARS-CoV-2 revaccination but did not address individuals with prior allergic reactions were excluded. Three reviewers (D.K.C., M.S., and M.G.) independently and in duplicate screened records using Covidence (Veritas Health Innovation), and 4 reviewers (E.M.A., D.B.K.G., M.S., and M.G.) independently and in duplicate extracted data. Figure 1 details the PRISMA diagram for the literature search and final study selection. Consensus among the reviewers was used to resolve conflicts. We extracted the total number of second dose revaccinations in individuals with an immediate first dose SARS-CoV-2 vaccine allergic reaction, the number of revaccinations tolerated (as indicated by the investigator; this was defined as mild or self-limiting subjective or objective symptoms that either spontaneously resolved or resolved with antihistamine treatment), the number of revaccinations resulting in a severe allergic reaction (eg, described in the studies as either anaphylaxis or as requiring injectable epinephrine administration), and reactions stratified by the severity of initial reaction (anaphylaxis or not). Reaction severity was defined at the study level by the reporting investigator, using Brighton Collaboration criteria,7 classification by Ring and Messmer,8 World Allergy Organization criteria,9 or National Institute of Allergy and Infectious Diseases criteria.10 Study authors were contacted individually to verify final data extraction, if any cases were duplicated, if the author group had multiple included publications, and to clarify any study design questions. Pooled data were analyzed using random-effects generalized linear models (binomial family, logit link) using Stata, version 14.3 (StataCorp LLC). The primary outcome was the incidence of severe allergic reactions (eg, anaphylaxis) after second vaccination, with 95% exact (Clopper-Pearson) CIs. The secondary outcome included the rate of any immediate nonsevere symptoms occurring (defined in the studies as mild or self-limiting symptoms that were either subjective or objective). Sensitivity analyses included modeling the rate of tolerated vaccine administrations, excluding case reports, plausible assumptions to address missing outcome data or potential overlapping studies, using a fixed-effect model with a bayesian framework (mininimally informative priors: main effect [N(0, 10)], between-study variance [inverse gamma (0.001, 0.001)]). Prespecified subgroup analyses were by risk of bias, graded dosing, skin testing, and premedication. The Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) approach11,12 provided assessment of the quality of the body of the evidence, and the Joanna Briggs Institute tool13 provided the framework for assessment of risk of bias (eTable 1 in the Supplement). Publication bias was assessed through GRADE, assessment of the search comprehensiveness, and inspection of funnel plots for small effects. Heterogeneity was assessed using methods recommended by GRADE involving consistency of the point estimates and overlap of 95% CIs given evidence that I2 could be misleading in this type of analysis.11,12 A 2-sided P < .05 was considered statistically significant.
Figure 1. PRISMA Diagram for Study Selection.
Results
Twenty-two studies (single-group cohorts, case series, and case reports) detailing second-dose SARS-CoV-2 mRNA vaccination for 1366 individuals (87.8% women; mean age, 46.1 years) with a known or suspected prior immediate allergic reaction to a SARS-CoV-2 mRNA vaccine, including 78 persons with prior severe immediate allergic reactions (eg, anaphylaxis) to a SARS-CoV-2 mRNA vaccine, were included.14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 Table 114,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 details the study characteristics. All revaccinations were administered to adults under the guidance of an allergy specialist and used mRNA vaccines. A total of 6 severe reactions occurred (absolute risk, 0.16% [95% CI, 0.01%-2.94%]; pooled random-effects model; moderate-certainty evidence; Figure 2); 1360 patients tolerated the dose (99.84% [95% CI, 97.09%-99.99%]; pooled random-effects model). Although 4 of the cases of severe immediate allergic reactions occurred in persons who had severe allergic reactions with their first dose (absolute risk, 4.94% [95% CI, 0.93%-22.28%]; pooled random-effects model; low-certainty evidence; eFigure 1 in the Supplement), none of the other 74 patients with severe immediate allergic reactions to the first dose experienced severe immediate allergic reactions to the second dose. None of the 6 patients with severe immediate allergic reactions died, and 5 recovered rapidly after receiving intramuscular epinephrine (the sixth patient did not seek or receive treatment, despite experiencing a reaction consistent with moderately severe anaphylaxis, and recovered). A total of 232 persons (13.65% [95% CI, 7.76%-22.9%]; pooled random-effects model; moderate-certainty evidence) experienced mild immediate nonsevere symptoms with their second dose (eFigure 2 in the Supplement). Of the 78 persons who had an immediate severe reaction with the first dose, 15 (9.54% [95% CI, 2.18%-33.34%]; pooled random-effects model) experienced mild immediate nonsevere symptoms with their second dose (eFigure 3 in the Supplement). Sensitivity and subgroup analyses, including accounting for studies that permitted the use of graded dosing, premedication, or skin testing, and risk of bias, did not alter the main findings (eTable 2 in the Supplement). Table 2 details the GRADE evidence profile.
Table 1. Characteristics of the Included Studies.
| Source and country | Design | Female, % | Median or mean age, y | Total second doses | Total No. of patients | Included patients with anaphyl to first dose | Graded dosing | Skin testing used | Premedication allowed | Total second doses deferred | Comments | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Administered | With nonsevere symptoms | With anaphyl | Anaphyl after first dose and received a second dose | Anaphyl after both first and second dose | Anaphyl after first dose and deferred second dose | ||||||||||
| Tuong et al,14 2021; US | Case series | 100 | 42.8 (range, 21-64) | 15 | 6 | 2 | 1 | 0 | 0 | Yes | Yes | Yes | No | 0 | Both cases of anaphyl occurred on the last step of a multidose desensitization; 1 of these patients had positive Moderna vaccine testing results indicating allergy, with negative excipient testing results indicating no allergy |
| Krantz et al,15 2021; US and Denmark | Case series | 100 | 44.8 (range, 29-54) | 4a | 0 | 0 | 4 | 0 | 0 | Yes | No | Yes | Yes | 0 | After verification with the authors, the US numbers represented their initial patients, who were included with additional patients who became part of a larger multicenter report in the study by Krantz et al17; therefore, these reflect only the Danish patients |
| Rasmussen et al,16 2021; Denmark | Case series | 88.5 | 46 (range, 18-88) | 30 | 0 | 0 | 4 | 0 | 0 | Yes | No | Yes | No | 1 | 25 Patients received the AstraZeneca vaccine with their first dose and tolerated an mRNA vaccine with their second dose |
| Krantz et al,17 2021; US | Case series | 86 | 43 (SD, 14) | 159 | 32 | 0 | 19 | 0 | 13 | Yes | No | Yes | Yes | 30 | Included 8 patients from the Krantz et al15 study and 11 patients from the Wolfson et al18 study; per discussion with the authors, no adjustments to these totals were made; potentially duplicated cases were adjusted in the data from the other articles |
| Wolfson et al,18 2021; US | Case series | 89 | 40.9 (SD, 13.6) | 58b | 15 | 3 | 3 | 3 | 2 | Yes | No | Yes | Yes | 7 | The 3 patients with anaphyl had anaphyl to the first dose, but had negative skin testing results; 1 of the patients had a grade 2 reaction but did not seek care or treatment; 11 patients had Ring and Messmer grade 2 reactions, but only 6 were treated with epinephrine initially; there were 56 patients in this report, including 8 patients with first dose anaphyl, who were also part of the Krantz et al study17; we accounted for duplication among the patients with anaphyl but not the other patients; we present the primary estimates based on excluding only the unique patients (which were only those with anaphyl) from the Wolfson et al study,18 but did perform sensitivity analysis that included them |
| Kessel et al,19 2021; Israel | Case series | 77.8 | 54.3 (range, 23-75) | 18 | 4 | 0 | 7 | 0 | 0 | Yes | No | Yes | Yes | 0 | NA |
| Kelso,20 2021; US | Case report | 100 | 48.6 (range, 43-56) | 3 | 0 | 0 | 3 | 0 | 1 | Yes | No | Yes | No | 1 | NA |
| Mustafa et al,21 2021; US | Case report | 100 | 64 and 39 | 2 | 0 | 0 | 0 | 0 | 0 | Yes | Yes | Yes | No | 0 | NA |
| Vanijcharoenkarn et al,22 2021; US | Case series | 92 | Not specified | 73 | 5 | 0 | 4 | 0 | 3 | Yes | Mixed | Yes | No | 15 | NA |
| Robinson et al,23 2021; US | Case series | 78 | Mean age range, 41-43 | 860 | 146 | 0 | 3 | 0 | 3 | Yes | No | NS | No | 43 | There were 358 persons receiving a second dose in this study who did not fill out a postvaccination symptom survey; the authors consider the nonresponders to be missing data; the study was not designed to investigate outcomes of a second dose |
| Eastman et al,24 2021; US | Case series | 84.8 | 42 (range, 19-69) | 53 | 17 | 0 | 2 | 0 | 0 | Yes | Mixed | Yes | No | 13 | NA |
| Park et al,25 2021; US | Case report | 100 | 34 | 1 | 0 | 0 | 1 | 0 | 0 | Yes | No | Yes | No | 0 | NA |
| Arroliga et al,26 2021; US | Case series | 86 | 39 (range, 23-61) | 6 | 0 | 0 | 0 | 0 | 2 | No | No | No | No | 13 | There were 8 patients lost to follow-up, and 5 patients deferred receiving a second dose |
| Loli-Ausejo et al,27 2021; Spain | Case series | 81.8 | 39 (range, 29.5-56.5) | 10 | 5 | 0 | 0 | 0 | 0 | No | Yes | Yes | Yes | 1 | NA |
| Pitlick et al,28 2021; US | Case series | 80 | 48 (range, 20-90) | 44 | 7 | 0 | 4 | 0 | 3 | Yes | Yes | Yes | No | 11 | This report includes 8 patients who were initially included as part of an early published case series, but have been included in a single large series of 55 patients |
| Yacoub et al,29 2021; Italy | Case series | 85.7 | 47.6 (range, 38-55) | 8 | 3 | 0 | 0 | 0 | 1 | No | No | No | Yes | 4 | NA |
| Shavit et al,30 2021; Israel | Case series | 70.9 | 52 (SD, 16) | 6 | 3 | 0 | 0 | 0 | 3 | No | No | No | Yes | Not specified | NA |
| Kohli-Pamnani et al,31 2021; US | Case series | 87 | 56 (SD, 16) | 16 | 3 | 0 | 1 | 0 | 0 | Yes | No | Yes | Yes | 2 | 2 Patients had anaphyl to the first dose, one of whom received a second mRNA vaccine dose, but the other opted for the Janssen vaccine for their second dose; both tolerated the second dose |
| Inoue et al,32 2021; Japan | Case report | 67.1 | 20 to ≥60 | 2 | 0 | 0 | 0 | 0 | 0 | No | No | No | No | Not specified | NA |
| Warren et al,33 2021; US | Case series | 91 | 40.9 (SD, 10.3) | 22 | 0 | 1 | 17 | 1 | 0 | Yes | No | Yes | No | 0 | This study was not originally intended to investigate revaccination; at the time of publication, only 1 patient received a second dose, and had repeated anaphyl; after discussion with the authors, they reported that the other 20 persons were revaccinated as part of California’s vaccine mandate, with no further severe reactions |
| Carpenter et al,34 2021; US | Case report | 100 | 60 | 1 | 1 | 0 | 0 | 0 | 0 | No | No | Yes | No | 1 | NA |
| Kaplan et al,35 2021; US | Case series | 86.7 | 48 (range, 19-89) | 30 | 30 | 0 | 5 | 0 | 0 | Yes | Yes | Yes | Yes | 8 | NA |
Abbreviations: Anaphyl, anaphylaxis; NA, not applicable.
This study includes patients who were also reported by Krantz et al.17 All duplicates have been accounted for, and the unique patients are presented here.
This study includes patients who were also reported by Krantz et al.17 All duplicate cases of anaphylaxis have been accounted for, with only the unique patients presented here. After discussion with the authors, we were unable to account for all duplicate patients in the denominator of all second doses administered. Thus, for the primary analysis, only the 3 unique patients with anaphylaxis were included as the study denominator. In the sensitivity analysis, this is reported as shown here, which includes the case number as originally published, including potential duplicates.
Figure 2. Pooled Incidence of Immediate Severe Allergic Reactions to a Second SARS-CoV-2 mRNA Dose Among Persons Who Had an Immediate Allergic Reaction to Their First SARS-CoV-2 mRNA Vaccine Dose.
aFor analysis purposes, this study by Krantz et al17 was combined with the 3 cases (all anaphylaxis) from Wolfson et al18 given that these 2 studies had overlap of cases.
Table 2. GRADE Evidence Profile Table.
| Outcome | No. of studies | Certainty assessment | Effect | Certaintyb | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | No. of events | No. of individualsa | Rate, % (95% CI) | |||
| Incidence of anaphylaxis to a second dose of the SARS-CoV-2 mRNA vaccine in persons who had an allergic reaction to their first dose | 22 | Case studies and case reports | Not seriousc | Not serious | Not serious | Not serious | Large effect of tolerating and residual confounding would suggest an effect of reacting when none was detectedd | 6 | 1366 | 0.16 (0.01-2.94) | +++ (Moderate) |
| Incidence of anaphylaxis to a second dose of the SARS-CoV-2 mRNA vaccine in persons who had anaphylaxis to their first dose | 17 | Case studies and case reports | Not seriousc | Not serious | Not serious | Not seriouse | Large effect of tolerating and residual confounding would suggest an effect of reacting when none was detectedd,e | 4 | 78 | 4.94 (0.93-22.28) | ++ (Low) |
| Incidence of mild allergic symptoms to a second dose of the SARS-CoV-2 mRNA vaccine in persons who had an allergic reaction to their first dose | 22 | Case studies and case reports | Not seriousc | Not serious | Not serious | Not serious | Large effect of tolerating and residual confounding would suggest an effect of reacting when none was detectedd | 232 | 1366 | 13.65 (7.76-22.9) | +++ (Moderate) |
Abbreviation: GRADE, Grading of Recommendation, Assessment, Development, and Evaluation.
Patient or population: patients receiving second mRNA COVID vaccination after a previous allergic reaction to a mRNA COVID vaccine dose.
GRADE Working Group grades of evidence: high certainty, we are very confident that the true effect lies close to that of the estimate of effect; moderate certainty, we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; low certainty, our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of effect; and very low certainty, we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. The number of plus symbols indicates the degree of certainty, where more plus symbols indicate a higher degree of certainty.
Risk of bias addressed in subgroup and sensitivity analyses.
A history of allergic reaction to previous COVID vaccination was a priori thought to guarantee a reaction to repeated doses, but far fewer than all individuals who received the second dose had an allergic reaction or anaphylaxis. Furthermore, those receiving a second dose of the vaccine, after an initial allergic reaction, would be at higher likelihood to be intensely monitored for any possible allergic reaction, whereas those without any history of an allergic reaction would not be intensely monitored.
Imprecision in width of 95% CIs and total sample size sufficient to prevent rating up certainty for considerations of residual confounding, but not to rate down; the qualitative effect of the incidence of repeated anaphylaxis being not very high (eg, 100%) is more certain than the quantitative estimate of a mean of 4.9%.
Discussion
This systematic review and meta-analysis found moderate-certainty evidence of a low incidence of severe immediate allergic reactions associated with the second dose of a SARS-CoV-2 mRNA vaccine among individuals with a history of an allergic reaction of any severity to their first mRNA vaccine dose. Revaccination of such persons led to no repeated reactions in most individuals and to nonsevere immediate symptoms in approximately 13.65% individuals.
These findings contradict the common assumption that a history of immediate reaction, including severe immediate allergic reactions, to a prior SARS-CoV-2 mRNA vaccine guarantees another reaction after revaccination. Immunoglobulin E (IgE) can be responsible for such stereotypically reproducible allergic responses—as in the case of allergic reactions to foods—but anaphylaxis can also occur idiosyncratically and nonspecifically owing to non-IgE–dependent mechanisms. Our findings therefore suggest that SARS-CoV-2 mRNA vaccine–induced anaphylaxis may not occur via an IgE-dependent mechanism, something that is also consistent with mechanistic data,27 the lack of any consistent and verifiable specific allergen within SARS-CoV-2 mRNA vaccines,33 the inability of skin testing of the ingredients of the vaccine to predict immediate allergic reactions to vaccination,18 and the overall very rare baseline incidence of severe immediate allergic reactions to SARS-CoV-2 vaccines.2
These data should prompt reconsideration of a history of allergic reaction to a prior dose of SARS-CoV-2 mRNA vaccine as a contraindication to a second dose of the vaccine.36 Supervision of second vaccination in a medical setting equipped to manage a severe immediate allergic reaction (as opposed to vaccination occurring in a retail pharmacy or nonmedical-based setting) may be appropriate instead.2 Consultation with an allergist prior to the second vaccination, when possible, might be beneficial. Removing barriers to vaccination is paramount to maximizing immunity and thereby protecting individuals and societies against COVID-19.
Limitations
This study has some limitations. First, the data address second-dose SARS-CoV-2 mRNA vaccinations, whereas other vaccine platforms and doses beyond the second dose require further study. Second, there is a risk of imprecision given the limited study numbers and patient numbers detailing these patient outcomes, albeit the absence of reactions in the situation of allergy can be considered as successes, and sensitivity analyses accounting for this led to findings consistent with the main analyses. We speculate that prior work calculating a very low event rate of severe reactions to the first dose (7.9 events per 1 000 000 vaccine doses)2 and a contraindication against provision of additional doses to persons with an immediate allergic reaction to a first dose both may explain why there were not more studies available to include in this systematic review and meta-analysis. However, we hope that our analysis provides reassurance that, when immediate reactions to the first dose do occur, it is safe to give second doses in this context and that this would lead to more published research becoming available because this is planned as a living systematic review. Third, while there may be potential overlap of cases between included reports, we resolved cases through correspondence with primary study authors and use of sensitivity analyses. Fourth, some of the component studies were subject to risk of selection bias, but this was mitigated by the findings being consistent in subgroup and sensitivity analyses and for patients with a history of anaphylaxis, for which this might have been less of a concern. Fifth, severe reactions were partly defined as requiring injectable epinephrine, and while other potential definitions could apply, this severity definition is an accepted standard within the allergy field.37 Sixth, all included studies were conducted with allergy specialist guidance, which could limit generalizability.
Conclusions
In this systematic review and meta-analysis of 22 case studies and case reports, the risk of repeated immediate allergic reactions and severe immediate allergic reactions or anaphylaxis associated with a second SARS-CoV-2 mRNA vaccination was low among persons who experienced an allergic reaction to their first dose, although 1 in 7 may have experienced mild symptoms. Although further research is warranted, these findings support the safe revaccination of individuals with an allergic reaction to a first SARS-CoV-2 mRNA vaccine dose in a setting equipped to manage severe allergic reactions, if they were to occur.
eTable 1. Risk of Bias Ratings for Included Studies
eTable 2. Additional Sensitivity Analyses
eFigure 1. Incidence of Repeat Anaphylaxis to SARS-CoV-2 mRNA Vaccination
eFigure 2. Incidence of Non-Severe Symptoms With 2nd Dose SARS-CoV-2 mRNA Vaccination
eFigure 3. Incidence of Non-Severe Symptoms After Anaphylaxis to mRNA COVID-19 Vaccination
eMethods. Registered Systematic Review Protocol
References
- 1.Johns Hopkins University Coronavirus Resource Center. COVID-19 map. Accessed September 28, 2021. https://coronavirus.jhu.edu/map.html
- 2.Greenhawt M, Abrams EM, Shaker M, et al. The risk of allergic reaction to SARS-CoV-2 vaccines and recommended evaluation and management: a systematic review, meta-analysis, GRADE assessment, and international consensus approach. J Allergy Clin Immunol Pract. 2021;9(10):3546-3567. doi: 10.1016/j.jaip.2021.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medicines and Healthcare Products Regulatory Agency. Confirmation of guidance to vaccination centres on managing allergic reactions following COVID-19 vaccination with the Pfizer/BioNTech vaccine. Accessed December 15, 2020. https://www.gov.uk/government/news/confirmation-of-guidance-to-vaccination-centres-on-managing-allergic-reactions-following-covid-19-vaccination-with-the-pfizer-biontech-vaccine
- 4.CDC COVID-19 Response Team; Food and Drug Administration . Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125-129. doi: 10.15585/mmwr.mm7004e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. What to do if you had an allergic reaction after getting a COVID-19 vaccine. Accessed September 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html
- 6.Kelso JM, Greenhawt MJ, Li JT, et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130(1):25-43. doi: 10.1016/j.jaci.2012.04.003 [DOI] [PubMed] [Google Scholar]
- 7.Rüggeberg JU, Gold MS, Bayas JM, et al. ; Brighton Collaboration Anaphylaxis Working Group . Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675-5684. doi: 10.1016/j.vaccine.2007.02.064 [DOI] [PubMed] [Google Scholar]
- 8.Ring J, Laubenthal H, Messmer K. Incidence and classification of adverse reactions to plasma substitutes. Klin Wochenschr. 1982;60(17):997-1002. doi: 10.1007/BF01716961 [DOI] [PubMed] [Google Scholar]
- 9.Cardona V, Ansotegui IJ, Ebisawa M, et al. World Allergy Organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13(10):100472. doi: 10.1016/j.waojou.2020.100472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampson HA, Muñoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report–Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391-397. doi: 10.1016/j.jaci.2005.12.1303 [DOI] [PubMed] [Google Scholar]
- 11.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870 [DOI] [PubMed] [Google Scholar]
- 12.Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group . GRADE guidelines: 7, rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64(12):1294-1302. doi: 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 13.The University of Adelaide, JBI. Critical appraisal tools. Accessed September 1, 2021. https://jbi.global/critical-appraisal-tools
- 14.Tuong LC, Capucilli P, Staicu M, Ramsey A, Walsh EE, Shahzad Mustafa S. Graded administration of second dose of Moderna and Pfizer-BioNTech COVID-19 mRNA vaccines in patients with hypersensitivity to first dose. Open Forum Infect Dis. 2021;8(12):ofab507. doi: 10.1093/ofid/ofab507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krantz MS, Bruusgaard-Mouritsen MA, Koo G, Phillips EJ, Stone CA Jr, Garvey LH. Anaphylaxis to the first dose of mRNA SARS-CoV-2 vaccines: don’t give up on the second dose! Allergy. 2021;76(9):2916-2920. doi: 10.1111/all.14958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen TH, Mortz CG, Georgsen TK, Rasmussen HM, Kjaer HF, Bindslev-Jensen C. Patients with suspected allergic reactions to COVID-19 vaccines can be safely revaccinated after diagnostic work-up. Clin Transl Allergy. 2021;11(5):e12044. doi: 10.1002/clt2.12044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krantz MS, Kwah JH, Stone CA Jr, et al. Safety evaluation of the second dose of messenger RNA COVID-19 vaccines in patients with immediate reactions to the first dose. JAMA Intern Med. 2021;181(11):1530-1533. doi: 10.1001/jamainternmed.2021.3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolfson AR, Robinson LB, Li L, et al. First-dose mRNA COVID-19 vaccine allergic reactions: limited role for excipient skin testing. J Allergy Clin Immunol Pract. 2021;9(9):3308-3320. doi: 10.1016/j.jaip.2021.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kessel A, Bamberger E, Nachshon L, Rosman Y, Confino-Cohen R, Elizur A. Safe administration of the Pfizer-BioNtTech COVID-19 vaccine following an immediate reaction to the first dose. Allergy. 2021;76(11):3538-3540. doi: 10.1111/all.15038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelso JM. Misdiagnosis of systemic allergic reactions to mRNA COVID-19 vaccines. Ann Allergy Asthma Immunol. 2021;127(1):133-134. doi: 10.1016/j.anai.2021.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mustafa SS, Ramsey A, Staicu ML. Administration of a second dose of the Moderna COVID-19 vaccine after an immediate hypersensitivity reaction with the first dose: two case reports. Ann Intern Med. 2021;174(8):1177-1178. doi: 10.7326/L21-0104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanijcharoenkarn K, Lee FE, Martin L, Shih J, Sexton ME, Kuruvilla ME. Immediate reactions following the first dose of the SARS-CoV2 mRNA vaccines do not preclude second dose administration. Clin Infect Dis. 2021;73(11):2108-2111. doi: 10.1093/cid/ciab448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson LB, Landman AB, Shenoy ES, et al. Allergic symptoms after mRNA COVID-19 vaccination and risk of incomplete vaccination. J Allergy Clin Immunol Pract. 2021;9(8):3200-3202.e1. doi: 10.1016/j.jaip.2021.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eastman J, Holsworth A, Kelbel T, Pebbles T, Hartog N.. Cohort experience of second messenger RNA vaccine dose tolerance after an initial-dose reaction. Ann Allergy Asthma Immunol. Published online October 23, 2021. doi: 10.1016/j.anai.2021.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HJ, Montgomery JR, Boggs NA. Anaphylaxis after the Covid-19 vaccine in a patient with cholinergic urticaria. Mil Med. 2021;usab138. doi: 10.1093/milmed/usab138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arroliga ME, Dhanani K, Arroliga AC, et al. Allergic reactions and adverse events associated with administration of mRNA-based vaccines: a health-care system experience. Allergy Asthma Proc. 2021;42(5):395-399. doi: 10.2500/aap.2021.42.210069 [DOI] [PubMed] [Google Scholar]
- 27.Loli-Ausejo D, González de Abreu JM, Fiandor A, et al. Allergic reactions after administration of Pfizer-BioNTech COVID-19 vaccine to healthcare workers at a tertiary hospital. J Investig Allergol Clin Immunol. 2021;0:0. doi: 10.18176/jiaci.0751 [DOI] [PubMed] [Google Scholar]
- 28.Pitlick MM, Sitek AN, Kinate SA, Joshi AY, Park MA. Polyethylene glycol and polysorbate skin testing in the evaluation of coronavirus disease 2019 vaccine reactions: early report. Ann Allergy Asthma Immunol. 2021;126(6):735-738. doi: 10.1016/j.anai.2021.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yacoub MR, Cucca V, Asperti C, et al. Efficacy of a rational algorithm to assess allergy risk in patients receiving the BNT162b2 vaccine. Vaccine. 2021;39(44):6464-6469. doi: 10.1016/j.vaccine.2021.09.048 [DOI] [PubMed] [Google Scholar]
- 30.Shavit R, Maoz-Segal R, Iancovici-Kidon M, et al. Prevalence of allergic reactions after Pfizer-BioNTech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4(8):e2122255. doi: 10.1001/jamanetworkopen.2021.22255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohli-Pamnani A, Zapata K, Gibson T, Kwittken PL. Coronavirus disease 2019 vaccine hypersensitivity evaluated with vaccine and excipient allergy skin testing. Ann Allergy Asthma Immunol. 2021;128(1):97-98. doi: 10.1016/j.anai.2021.08.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue S, Igarashi A, Morikane K, et al. Adverse reactions to BNT162b2 mRNA COVID-19 vaccine in medical staffs with a history of allergy. medRxiv. Preprint posted September 16, 2021. doi: 10.1101/2021.09.13.21263473 [DOI] [PMC free article] [PubMed]
- 33.Warren CM, Snow TT, Lee AS, et al. Assessment of allergic and anaphylactic reactions to mRNA COVID-19 vaccines with confirmatory testing in a US regional health system. JAMA Netw Open. 2021;4(9):e2125524. doi: 10.1001/jamanetworkopen.2021.25524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carpenter T, Konig J, Hochfelder J, Siegel S, Gans M. Polyethylene glycol and polysorbate testing in 12 patients before or after coronavirus disease 2019 vaccine administration. Ann Allergy Asthma Immunol. 2021;128(1):99-101. doi: 10.1016/j.anai.2021.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan B, Farzan S, Coscia G, et al. Allergic reactions to coronavirus disease 2019 vaccines and addressing vaccine hesitancy: Northwell Health experience. Ann Allergy Asthma Immunol. Published online October 24, 2021. doi: 10.1016/j.anai.2021.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed January 26, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
- 37.Dribin TE, Schnadower D, Spergel JM, et al. Severity grading system for acute allergic reactions: a multidisciplinary Delphi study. J Allergy Clin Immunol. 2021;148(1):173-181. doi: 10.1016/j.jaci.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Risk of Bias Ratings for Included Studies
eTable 2. Additional Sensitivity Analyses
eFigure 1. Incidence of Repeat Anaphylaxis to SARS-CoV-2 mRNA Vaccination
eFigure 2. Incidence of Non-Severe Symptoms With 2nd Dose SARS-CoV-2 mRNA Vaccination
eFigure 3. Incidence of Non-Severe Symptoms After Anaphylaxis to mRNA COVID-19 Vaccination
eMethods. Registered Systematic Review Protocol