Abstract
HIV is an independent risk factor for lung disease, including chronic obstructive pulmonary disease (COPD) and emphysema. Angiotensin receptor blockers may be beneficial in COPD and emphysema through pathways that have been implicated in HIV-related lung disease. We performed a randomized comparison of the effects of losartan versus placebo on the plasma concentrations of the pneumoproteins, surfactant protein D (SPD) and club cell secretory protein (CCSP), in people living with HIV (PLWH). A total of 108 PLWH were included (52 assigned to losartan and 56 assigned to placebo). We found no difference in the change from baseline in log2 concentrations of CCSP or SPD over 1 year of follow-up. For SPD, we found a strong interaction by CD4+ counts, where those with CD4+ counts >350 cells/mm3 treated with losartan had more reduction (improvement) in SPD concentration than those treated with placebo (p value for interaction <.001). In conclusion, we did not find a beneficial effect of losartan on pneumoprotein concentrations in PLWH, but PLWH with higher CD4+ counts may have improvement in SPD when treated with losartan.
Keywords: HIV, biomarkers, lung diseases, angiotensin receptor antagonists, pulmonary surfactant-associated protein D, uteroglobulin
Angiotensin receptor blockers (ARBs) may slow the progression of chronic obstructive pulmonary disease (COPD) and emphysema.1 This has been hypothesized to occur through reduction of oxidative stress via decreased transforming growth factor-beta expression and modulation of inflammatory pathways, T cell function, and epithelial and endothelial cell function.1,2 HIV is an independent risk factor for the development of COPD, potentially through similar mechanisms that might be impacted by ARBs.3,4 Trials of ARBs are ongoing in general COPD populations [NCT02696564], but the effects of ARBs on pulmonary outcomes in HIV have not been investigated. LIFE HIV [NCT02049307] was a randomized (1:1 allocation ratio) double-blind placebo controlled trial of losartan versus placebo in people living with HIV (PLWH).5 Here we present a secondary analysis of randomized comparisons of the effects of losartan versus placebo on the plasma pneumoproteins surfactant protein D (SPD), which is produced in the type II alveolar cells and involved in innate immunity, and club cell secretory protein (CCSP), which is produced in the bronchioles and associated with lower COPD risk and slower lung function decline.6–9 This study was approved by the institutional review board at each study site and all participants gave written informed consent.
Trial participants were PLWH ≥50 years old with CD4+ counts ≤600 cells/mm3 and without an indication for an ARB or angiotensin converting enzyme inhibitor. The primary outcome was the change in plasma interleukin-6, an important marker of inflammation and independent predictor of mortality in HIV, and the trial showed no difference between groups.5 In this secondary analysis, we measured plasma SPD and CCSP at baseline, 3, 6, 9, and 12 months. We compared the randomized arms for changes in log2 pneumoprotein concentrations using generalized linear mixed models adjusted for baseline concentrations, per the parent trial protocol. We also adjusted for current smoking status, age, and race that are associated with differences in pneumoprotein levels.6,10 We additionally adjusted for statin use, which was imbalanced at baseline, and may improve lung function and possibly pneumoprotein concentrations, in PLWH.11 Subgroup analyses were performed for those older than 60 years of age, with hypertension, on lipid-lowering drugs, with CD4+ counts >350 cells/mm3, with nadir CD4+ counts >200 cells/mm3, and by smoking status (current vs. never/former).
A total of 108 PLWH were randomized to losartan (n = 52) or placebo (n = 56). All participants had samples available from at least one follow-up visit. The groups were well balanced with median [interquartile range (IQR)] age 57 (54–62) years, nadir CD4+ count 120 (37–240) cells/mm3, and baseline CD4+ count 408 (310–498) cells/mm3; 3.7% were female, 56.5% were white, and 34.3% were black. In total, 50.9% were ever smokers and 20.4% were current smokers. Fewer participants in the losartan group were on statins (17.3% vs. 26.8%), and fewer were current smokers (14.3% vs. 25.0%). Compared with nonsmokers, smokers had lower baseline concentrations of CCSP [median (IQR) for nonsmokers 22.9 (16.0–30.0) ng/mL and for smokers 15.3 (11.6–22.0) ng/mL], but had similar baseline levels of SPD [nonsmokers 6.1 (4.5–9.5) ng/mL and smokers 5.2 (2.9–9.4) ng/mL].
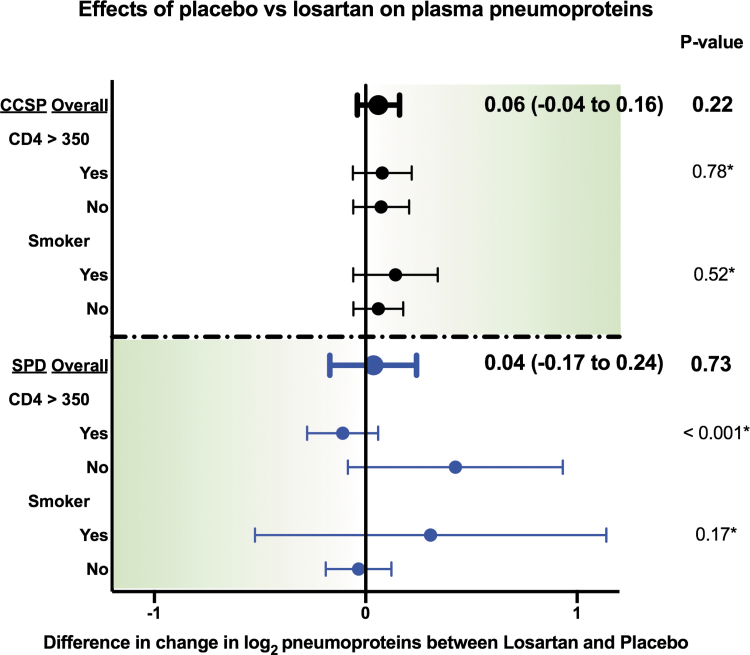
We found no statistically significant differences in change in SPD or CCSP in those assigned to losartan versus placebo (Fig. 1). For SPD, we found a strong interaction by CD4+ counts, where those with CD4+ >350 cells/mm3 treated with losartan had more improvement (reduction) in SPD than those with CD4+ ≤350 cells/mm3 (p value for interaction <.001). In the remainder of the subgroup analyses, including baseline smoking status, there was no significant difference between groups.
FIG. 1.
Randomized effects of losartan on plasma pneumoprotein concentrations (compared with placebo), overall and stratified by baseline CD4+ T cell counts and by baseline smoking status. Shaded direction for each pneumoprotein indicates direction associated with pulmonary benefits in other studies. Point estimates (and their 95% confidence intervals in parentheses) represent differences in the change from baseline in the pneumoprotein of interest, using repeated measures mixed models, adjusted for baseline pneumoprotein concentrations, age, smoking, statin use, and race. *Interaction p value. CCSP, club cell secretory protein; SPD, surfactant protein D.
In this randomized experiment in PLWH, we did not find that losartan had a beneficial effect on change in SPD or CCSP over 1 year of follow-up. We had limited power due to the small sample size and cannot rule out a beneficial effect, but found no major benefit of losartan on plasma CCSP overall, or in interaction analyses. In non-HIV cohorts, higher plasma CCSP is associated with lower rates of COPD and slower decline in forced expiratory volume in 1 second.6 However, a recent small cross-sectional study of PLWH (n = 65) found that higher CCSP was associated with unexpectedly worse diffusing capacity for carbon monoxide and worse spirometry.12 Why the relationship between lung function and CCSP would differ by HIV status is unclear, and CCSP in PLWH requires further investigation.
The strong interaction between losartan and CD4+ counts on changes in SPD suggests there may be a beneficial effect of losartan on SPD among those with higher CD4+ counts. SPD is produced predominantly in response to lung injury. SPD is also involved in innate immunity through interactions with dendritic cells, T cells, and macrophages, all of which can harbor latent HIV.7–9,13,14 In in vitro studies, SPD blocks viral replication and suppresses HIV-induced cytokine expression in T cells.15 One possible explanation for our findings might be that in the setting of low CD4+ counts, there is greater immune dysregulation in the lung leading to activation of multiple pathways leading to increased SPD, thereby preventing losartan from exerting notable benefits. However, at higher CD4+ counts, there may be fewer active pathways to pulmonary inflammation, thus allowing for possible beneficial effects of losartan to become apparent. Similar to CCSP, there is little known about SPD in PLWH. In the same recent cross-sectional study of lung function and pneumoproteins in PLWH, SPD did not associate with any measures of pulmonary function, but that analysis had limited power to analyze differences in CD4+ counts.12 In a larger study (n = 800) comparing pneumoprotein concentrations in PLWH with intravenous drug users, SPD did not vary by HIV status or CD4+ count.16 However, in a small single-arm study of PLWH who were initiated on antiretroviral therapy, SPD declined over a period of 5 months after antiretroviral treatment initiation (median 64.1 to 47.3 ng/mL, p = .01).17 SPD, lung function, and T cells may have unique interactions in PLWH and future studies of SPD in PLWH should investigate this relationship across a range of CD4+ counts.
Our analyses have several limitations. The modest sample size limited our power to detect small differences and limited power of subgroup analyses, including too few women for sex-stratified analyses. The primary outcome of the study was the effect of losartan on general inflammation in HIV and thus we do not have spirometry, diffusing capacity for carbon monoxide (DLCO), chest imaging, or other clinical markers of lung disease. In addition, the relationship between plasma pneumoproteins and lung function in PLWH remains unclear.12 The major strength of our study was the randomized experimental design, rather than typical observational studies wherein ARB use can be confounded by many other factors. Future studies of ARBs in PLWH should consider collecting additional pulmonary parameters and our data suggest that pulmonary benefits from ARBs in PLWH might be limited to those with higher CD4+ counts.
Authors' Contributions
D.M.M. conceived the project, created the initial draft, and conducted revisions and final draft of the article. G.C. and J.W. made substantial contributions to data analysis. C.H.W. made substantial contributions to the conception and design of the study. F.R., S.D., S.A.R., Z.T., C.M., A.P.L., and I.S. made substantial contributions to the acquisition of data. R.P.T. made substantial contributions to laboratory measurements, data collection, and study conduct. J.V.B. made substantial contributions to the conception and design of the study and acquisition of data. K.M.K. made substantial contributions to the conception and design of the study and drafted the article. All authors have approved the submitted version.
Author Disclosure Statement
K.M.K. reports personal fees from Nuvaira for data safety and monitoring board activities and contracted clinical research support from AstraZeneca and Sanofi. The remaining authors have nothing to disclose.
Funding Information
This study was funded by a grant from the NIH National Institute on Aging (NIA/NIH: R01 AG045032). Study drug was provided by Merck Pharmaceuticals. D.M.M. was supported by the University of Minnesota T32 Training in Lung Science training grant (NHLBI: T32 HL007741). I.S. was supported by the intramural research program of NIAID/NIH. K.M.K. was supported by resources and the use of facilities at the Minneapolis VA Health Care System (Minneapolis, MN).
References
- 1. Parikh MA, Aaron CP, Hoffman EA, et al. : Angiotensin-converting inhibitors and angiotensin II receptor blockers and longitudinal change in percent emphysema on computed tomography the multi-ethnic study of atherosclerosis lung study. Ann Am Thorac Soc 2017;14:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vasileiadis IE, Goudis CA, Giannakopoulou PT, Liu T: Angiotensin converting enzyme inhibitors and angiotensin receptor blockers: A promising medication for chronic obstructive pulmonary disease? COPD J Chronic Obstr Pulm Dis 2018;15:148–156. [DOI] [PubMed] [Google Scholar]
- 3. Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT: Prevalence of chronic obstructive pulmonary disease in the global population with HIV: A systematic review and meta-analysis. Lancet Glob Heal 2017;6:e193–e202. [DOI] [PubMed] [Google Scholar]
- 4. Theron AJ, Anderson R, Rossouw TM, Steel HC: The role of transforming growth factor beta-1 in the progression of HIV/AIDS and development of non-AIDS-defining fibrotic disorders. Front Immunol 2017;8:1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker J V., Wolfson J, Collins G, et al. : Losartan to reduce inflammation and fibrosis endpoints in HIV disease (LIFE-HIV). AIDS 2021;35:575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lomas DA, Silverman EK, Edwards LD, Miller BE, Coxson HO, Tal-Singer R: Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax 2008;63:1058–1063. [DOI] [PubMed] [Google Scholar]
- 7. Pastva AM, Wright JR, Williams KL: Immunomodulatory roles of surfactant proteins A and D: Implications in lung disease. Proc Am Thorac Soc 2007;4:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Madsen J, Kliem A, Tornøe I, Skjødt K, Koch C, Holmskov U: Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol 2000;164:5866–5870. [DOI] [PubMed] [Google Scholar]
- 9. Crouch EC: Surfactant protein-D and pulmonary host defense. Respir Res 2000;1:93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhai J, Stern DA, Sherrill DL, et al. : Trajectories and early determinants of circulating CC16 from birth to age 32 years. Am J Respir Crit Care Med 2018;198:267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris A, Fitzpatrick M, Bertolet M, et al. : Use of rosuvastatin in HIV-associated chronic obstructive pulmonary disease: A randomized pilot study. AIDS 2017;31:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeon D, Chang EG, McGing M, et al. : Pneumoproteins are associated with pulmonary function in HIV-infected persons. PLoS One 2019;14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costiniuk CT, Salahuddin S, Farnos O, et al. : HIV persistence in mucosal CD4+ T cells within the lungs of adults receiving long-term suppressive antiretroviral therapy. AIDS 2018;32:2279–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong ME, Jaworowski A, Hearps AC: The HIV reservoir in monocytes and macrophages. Front Immunol 2019;10:1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pandit H, Gopal S, Sonawani A, et al. : Surfactant protein D inhibits HIV-1 infection of target cells via interference with gp120-CD4 interaction and modulates pro-inflammatory cytokine production. PLoS One 2014;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shiels MS, Kirk GD, Drummond MB, et al. : HIV infection and circulating levels of prosurfactant protein B and surfactant protein D. J Infect Dis 2018;217:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kunisaki KM, Quick H, Baker JV: HIV antiretroviral therapy reduces circulating surfactant protein-D levels. HIV Med 2011;12:580–581. [DOI] [PMC free article] [PubMed] [Google Scholar]