Abstract
Viruses constitute a significant part of the human microbiome, so wherever humans go, viruses are brought with them, even on space missions. In this mini review, we focus on the International Space Station (ISS) as the only current human habitat in space that has a diverse range of viral genera that infect microorganisms from bacteria to eukaryotes. Thus, we have reviewed the literature on the physical conditions of space habitats that have an impact on both virus transmissibility and interaction with their host, which include UV radiation, ionizing radiation, humidity, and microgravity. Also, we briefly comment on the practices used on space missions that reduce virus spread, that is, use of antimicrobial surfaces, spacecraft sterilization practices, and air filtration. Finally, we turn our attention to the health threats that viruses pose to space travel. Overall, even though efforts are taken to ensure safe conditions during human space travel, for example, preflight quarantines of astronauts, we reflect on the potential risks humans might be exposed to and how those risks might be aggravated in extraterrestrial habitats.
Key Words: Virology, Space microbiology, Space medicine, Space travel, Decontamination, Virus diversity
1. Introduction: Viruses in the Space Context
Space modules are enclosed, compact environments that harbor various microbial communities. Viruses are a significant part of such enclosed communities (Hjelmsø et al., 2019; Mora et al., 2019), some of which can be pathogenic to humans and pose a threat to individual and public health. However, viruses can also cause a range of other problems. For instance, bacteriophages can carry microbial virulence or antibiotic-resistance genes and spread them throughout bacterial populations on human bodies or in enclosed environments. Another example of the damage that viruses cause on Earth is the significant loss of crops grown for food, which is specific to plant viruses. Since human space missions are planned to mostly provide plant-based food to the astronauts, it is easy to see their threat to space travel. Therefore, plant and microbial viruses, along with human pathogenic viruses, represent a major issue for space travel. Space modules provide exceptional conditions for Earth's microbes to spread and grow (McKernan et al., 2008) due to high radiation doses, microgravity, and compact spaces (Fig. 1).
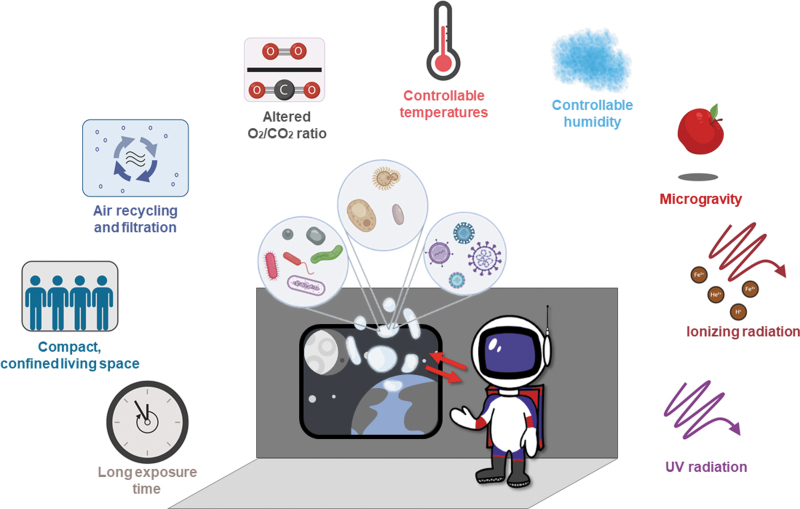
FIG. 1.
The environmental factors acting on microbes (including viruses), humans, and their interaction in space environments. Created with BioRender.com.
Due to the extreme conditions in space, astronauts are especially vulnerable to infections given that cosmic radiation, microgravity, and psychological stress tend to compromise the human immune system (Crucian et al., 2015; Fernandez-Gonzalo et al., 2017; Akiyama et al., 2020). Before departure to the International Space Station (ISS), crew members go through a 7-day isolation known as the “Health Stabilization Program” (NASA, 2010). Crucian and colleagues reported the occurrence of microbial diseases, cold sores, and allergies among 50% of the crew members in 38 six-month missions (Crucian et al., 2016a).
Researching viruses is important in the space industry because the unique conditions of space travel can weaken human immunity. Therefore, viral infections during space travel may have a detrimental impact on the success of human missions. In contrast to the bacterial and fungal microbiomes, research on the virome variability in spacecraft is scarce (Berliner et al., 2018). The study of viruses is challenging due to the need for a host, a low biomass in the environment, and a complex phylogeny. Nevertheless, new methods for high-throughput DNA-sequencing enable the collection of high amounts of environmental sequence data, which illustrates viral diversity (Roux et al., 2017; Berliner et al., 2018; Nooij et al., 2018; Ann Gregory et al., 2019). Metagenomics allows the detection of previously unknown viruses (Delwart, 2007) and has revealed that viruses constitute a major part of most microbiomes on Earth (Rosario and Breitbart, 2011; Mokili et al., 2012). Additionally, other studies that have implemented both metagenomics and culture methods in buildings and transport vehicles have shown that most bacteria originated from the human skin both on surfaces and in the air of enclosed spaces (Tsai and Macher, 2005; Gibbons et al., 2015; Hsu et al., 2016; Stephens, 2016). Yet it is not clear whether the same assumption holds for viruses. Literature reports on viral diversity in closed environments vary, depending on the source of microbes (Prussin and Marr, 2015; Prussin et al., 2019, 2020). Prussin and Marr identified the outside environment as the major source of microbes in an indoor environment (Prussin and Marr, 2015). Also, metagenomic studies on the seasonality of microbial distribution in bioaerosols suggest that humans in the enclosed environments strongly affect the airborne viral communities (Prussin et al., 2019). On a space station, however, the source of microbes is represented by the interchange of astronaut and microbe cross-contamination from humans to equipment and subsequently from equipment to humans. The low numbers of passengers make the ISS a microbiologically controlled environment. Additionally, it allows for thorough microbial monitoring of equipment or the astronauts themselves by the implementation of strict hygiene measures, methods of sterilization, and food monitoring (Pierson et al., 2013). Nevertheless, the ISS might have a very dynamic virome.
To better understand how we can monitor and control viral spread in space travel, the present study addresses four main questions:
What is the abundance and diversity of viruses in the ISS microbiome?
How are viruses and their human hosts influenced by the environmental conditions of space travel?
How can viruses be monitored and, in the case of harmful contaminations, decontaminated during space missions?
Are there any health threats associated with viruses in the space context?
2. Viruses in the Microbiome of the ISS Surfaces
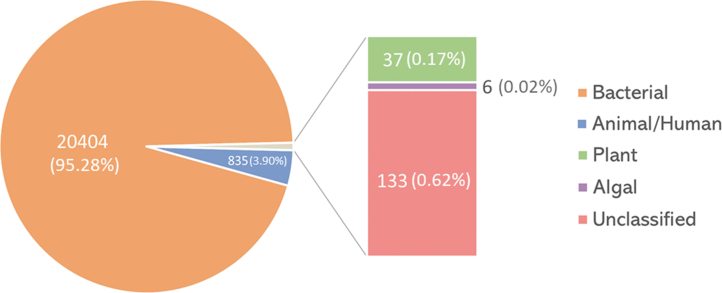
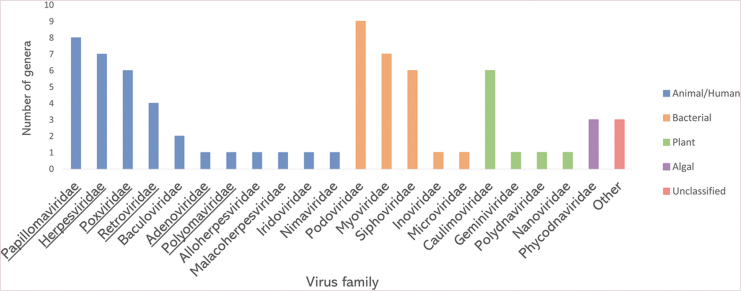
Our understanding of the viral microbiome dynamics on the ISS is sparse, mostly due to limited methodologies with which to study it. So far, there has only been one analysis of the ISS microbiome that included viruses (Mora et al., 2019). Therein, shotgun metagenomic sequencing of environmental surface swabs characterized the microbiome inside the ISS. The sequenced reads were then assigned based on sequence similarity to phylogenetic groups in virus genome databases. The reads similar to virus sequences made up 0.57% (21,415 out of 3,731,403) of all sequence reads. The majority of virus-related reads (∼95%) originated from bacteriophages, while ∼4% were derived from animal/human viruses, including herpesviruses, and the remaining were classified as reads related to plant and algal viruses or remained unclassified (Fig. 2). Among them, the reads similar to viruses from 72 different virus genera were identified to be distributed in 21 families, including the ones that contain human pathogens (Fig. 3). It is also worth noting that the metagenomic analysis was performed only on the pooled subset of environmental samples. The average length of reads was 126 bp. Those are relatively short reads. Therefore, some viruses might have been missed during the analysis.
FIG. 2.
Distribution of viruses by the number of reads detected on ISS surfaces. The total number of detected reads is designated for every category. Data from the work of Mora et al. (2019). Created with MS Excel v2102.
FIG. 3.
Virus families detected on ISS surfaces by shotgun metagenomic analysis. For every family, the number of detected genera is plotted. The families that contain human pathogens are underlined. Data from the work of Mora et al. (2019). Created with MS Excel v2102.
The low abundance of reads similar to virus sequences may be due to the highly sterile conditions on the ISS or caused by the decreased stability of virus samples in comparison to other microbes. Also, the viral genomes are underrepresented in genomic databases used for assigning sequences, so a great portion may remain unidentified. Bacteriophages influence the human microbiome and physiology by altering an organism's microbiome (Navarro and Muniesa, 2017) with potential impacts on the astronauts' health. Reads similar to animal viruses were distributed into 33 genera, 13 of which are known to infect humans and cause diseases of varying severity. They include a range of herpesviruses, which establish latency and can undergo reactivation (Pierson et al., 2005; Mehta et al., 2014, 2017; Rooney et al., 2019; Voorhies et al., 2019). These authors' analysis results indicate that pathogenic viruses were present in low abundance and unlikely to cause significant health problems on short-term space missions, even under conditions unfavorable to a healthy immune system. However, their impact on long-term missions remains unknown.
3. The Influence of Environmental Factors Related to Space Travel on Viruses and Their Hosts
During space travel, humans and their microbiome are exposed to conditions that significantly differ from those in their natural environment. As the ISS orbits Earth at around 420 km above sea level, exposure to cosmic and UV radiation is much higher than on the ground due to the filtration of UV by the ozone layer. There is also the additional stress of microgravity in space. Furthermore, space missions can last 3–6 months, and future missions could last up to a few years. These environmental factors can affect virus integrity directly and influence their stability or indirectly influence the host vulnerability to infection (Foster et al., 2014; Carratalà et al., 2017).
As human space missions are planned more robustly than ever, it is also necessary to consider the effects of the space environment on viral infectivity and environmental stability. This is a highly complex topic given that viruses are influenced by a range of environmental factors related to space travel, depending on the mission. Some examples include extreme and rapid temperature variations during day/night cycles on the Moon, extremely low pressures of the Moon and Mars, or microgravity in deep space. Also, the fine regolith dust of varying chemical composition, present on many rocky celestial bodies, can potentially affect the stability of a viral particle. Since astronauts will spend a long time confined in enclosed habitats, as they currently do on the ISS, the conditions inside them would be the most relevant for human health and virus spread. Therefore, for this mini review, we summarized the effects of physical conditions that affect viruses and their hosts in space habitats, that is, elevated levels of UV and ionizing radiation, humidity since it is an important factor of the enclosed environment that affects viral spread, and microgravity because it is currently impossible to control and has a considerable effect on virus-host interaction. A summary of studies addressing the impact of space-related stresses on virus stability is presented in Table 1. Also, the physiological stresses for humans in such isolated conditions include psychological stress, nutrient availability, close contact with other crew members, artificial light/dark cycles inside the habitat, among others. Even though these are important factors to consider in the future, they are not covered here as their consequences vary among individuals and bear a minimal source of concern in comparison to the physical factors explored in this review.
Table 1.
Summary of the Studies of Individual Simulated Stress Factors Acting on Viruses during Air and Space Travel
| Environmental factor | Effect on viruses/host | Tested viruses | Host | Transmission | References |
|---|---|---|---|---|---|
| UV radiation | reactivation in host | Human papillomavirus | Human | Mucosal contact | Viarisio et al., 2011 |
| Rat cytomegalovirus | Rat | Blood, saliva, transplacental | Garssen et al., 1995 | ||
| Murine herpes simplex virus 1 | Mouse | Mucosal contact, saliva | El-Ghorr and Norval, 1996; Goade et al., 2001 | ||
| genome damage | Poliovirus | Human | Ingestion of food/water, inhalation of aerosols | Simonet and Gantzer, 2006 | |
| Herpes simplex virus | Human | Mucosal contact, saliva | Mirshafiee et al., 2012 | ||
| Mengovirus | Mouse | Inhalation of aerosols | Miller and Plagemann, 1974 | ||
| Murine polyoma virus | Mouse | Inhalation of aerosols | Lytle and Sagripanti, 2005; Huang et al., 2016 | ||
| Encephalomyocarditis virus | Rodents, pig | Ingestion of food/water | Lytle and Sagripanti, 2005 | ||
| Adenovirus | Mammals | Contact-based, inhalation of aerosols | Eischeid and Linden, 2011 | ||
| Vesicular stomatitis virus | Livestock | Contact-based, ingestion of food/water, inhalation of aerosols | Mirshafiee et al., 2012 | ||
| Cowpea mosaic virus | Cowpea plant | Insects, sap inoculation | Rae et al., 2008 | ||
| Bacteriophage T7 | E. coli | Contact-based | Fekete et al., 2008 | ||
| Bacteriophage GA | E. coli | Contact-based | Simonet and Gantzer, 2006 | ||
| Bacteriophage MS2 | E. coli | Contact-based, aerosols | Lytle and Sagripanti, 2005; Simonet and Gantzer, 2006 | ||
| Bacteriophage Qbeta | E. coli | Contact-based, aerosols | Lytle and Sagripanti, 2005; Simonet and Gantzer, 2006 | ||
| Bacteriophage F2 | E. coli | Contact-based, aerosols | Lytle and Sagripanti, 2005 | ||
| viral surface damage | Reovirus | Human | Ingestion of food/water | Subasinghe and Loh, 1972 | |
| Mengovirus | Mouse | Inhalation of aerosols | Miller and Plagemann, 1974 | ||
| Adenovirus | Mammals | Contact-based, inhalation of aerosols | Eischeid and Linden, 2011 | ||
| Bacteriophage MS2 | E. coli | Contact-based | Wigginton et al., 2010, 2012 | ||
| Ionizing radiation | increased illness severity | Herpes simplex virus | Human | Mucosal contact, saliva | Openshaw et al., 1979 |
| Murine herpes simplex virus 1 | Mouse | Mucosal contact, saliva | Wang et al., 1990 | ||
| Theiler's murine encephalitis virus | Mouse | Contact-based, ingestion of food/water | Rodiriguez et al., 1990 | ||
| Rabies virus | Mouse | Contact-based, saliva | Ceccaldi et al., 1996 | ||
| Bacterial prophage | E. coli | Contact-based | Parfenov and Lukin, 1973 | ||
| genome damage | Poliovirus | Human | Ingestion of food/water, inhalation of aerosols | Ward, 1980 | |
| Murine norovirus 1 | Mouse | Ingestion of food/water, inhalation of aerosols | Feng et al., 2011 | ||
| Porcine parvovirus | Pig | Contact-based, ingestion of food/water | Summers and Szybalski, 1967; Ward, 1980; Grieb et al., 2002; Feng et al., 2011 | ||
| Vesicular stomatitis virus | Livestock | Contact-based, inhalation of aerosols, insects | Feng et al., 2011 | ||
| Bacteriophage phi 29 | B. subtilis | Contact-based | Summers and Szybalski, 1967 | ||
| viral surface damage | Poliovirus | Human | Ingestion of food/water, inhalation of aerosols | Ward, 1980 | |
| Human adenovirus | Human | Ingestion of food/water, inhalation of aerosols | Pimenta et al., 2016 | ||
| Murine norovirus 1 | Mouse | Ingestion of food/water, inhalation of aerosols | Feng et al., 2011 | ||
| Vesicular stomatitis virus | Livestock | Contact-based, ingestion of food/water, inhalation of aerosols, insects | Feng et al., 2011 | ||
| Tobacco mosaic virus | Tobacco plant | Direct contact between leaves | Koike et al., 1992 | ||
| Humidity (high) | reduces survivability or transmission of enveloped viruses | Influenza | Human | Contact-based, inhalation of aerosols | Harper, 1961; Schaffer et al., 1976; Noti et al., 2013; Marr et al., 2019 |
| Severe acute respiratory syndrome coronavirus-1 | Human | Inhalation of aerosols | Chan et al., 2011 | ||
| Severe acute respiratory syndrome coronavirus-2 | Human | Inhalation of aerosols | Wang et al., 2020; Haque and Rahman, 2020 | ||
| Human coronavirus-229E | Human | Inhalation of aerosols | Ijaz et al., 1985 | ||
| Langat virus | Human | Insects | Benbough, 1971 | ||
| Respiratory syncytial virus | Human | Contact-based, inhalation of aerosols | Tang, 2009 | ||
| Parainfluenza viruses | Human | Contact-based, inhalation of aerosols | Tang, 2009 | ||
| Measles virus | Human | Contact-based, inhalation of aerosols | Tang, 2009 | ||
| Rubella virus | Human | Inhalation of aerosols, transplacental | Tang, 2009 | ||
| Varicella zoster virus | Human | Contact-based | Tang, 2009 | ||
| Semliki forest virus | Mammals and birds | Inhalation of aerosols, insects | Benbough, 1971 | ||
| Venezuelan equine encephalomyelitis | Horse | Insects | Harper, 1961 | ||
| Vesicular stomatitis virus | Livestock | Contact-based, inhalation of aerosols, vector-based | Songer, 1967 | ||
| Rous sarcoma virus | Chicken | Contact-based | Webb et al., 1963 | ||
| Newcastle disease virus | Birds | Contact-based, ingestion of food/water, inhalation of aerosols | Songer, 1967 | ||
| Humidity (low) | reduces survivability of non-enveloped viruses | Polio virus | Human | Ingestion of food/water, inhalation of aerosols | Harper, 1961 |
| Rhinovirus-14 | Human | Contact-based, inhalation of aerosols | Karim et al., 1985 | ||
| Vesicular exanthema virus | Pig | Ingestion of food/water, inhalation of aerosols | Donaldson and Ferris, 1976 | ||
| Feline calicivirus | Cat | Contact-based of infected mucosa, saliva | Donaldson and Ferris, 1976 | ||
| Bacteriophage T7 | E. coli | Ingestion | Benbough, 1971 | ||
| improved inactivation with UV radiation | Bacteriophage MS2 | E. coli | Ingestion | Tseng and Li, 2005 | |
| Bacteriophage phi X174 | E. coli | Ingestion | Tseng and Li, 2005 | ||
| Bacteriophage phi 6 | Pseudomonas bacteria | Contact-based | Tseng and Li, 2005 | ||
| Bacteriophage T7 | E. coli | Ingestion | Tseng and Li, 2005 | ||
| Microgravity | in vitro inhibits reactivation in host cells | Kaposi's sarcoma-associated herpesvirus | Human | Blood, ingestion of food/water, mucosal contact | Honda et al., 2020 |
| increased illness severity | Herpes simplex virus | Human | Contact-based of infected mucosa, saliva | Fuse and Sato, 2004 | |
| may promote viral spread across an organism | Indirect evidence on FITC-dextran particles | — | — | Alvarez et al., 2019 |
The routes of infection and host for each virus are specified. Herein are included bacteriophages due to the direct impact in bacterial symbionts affecting the host microbiome.
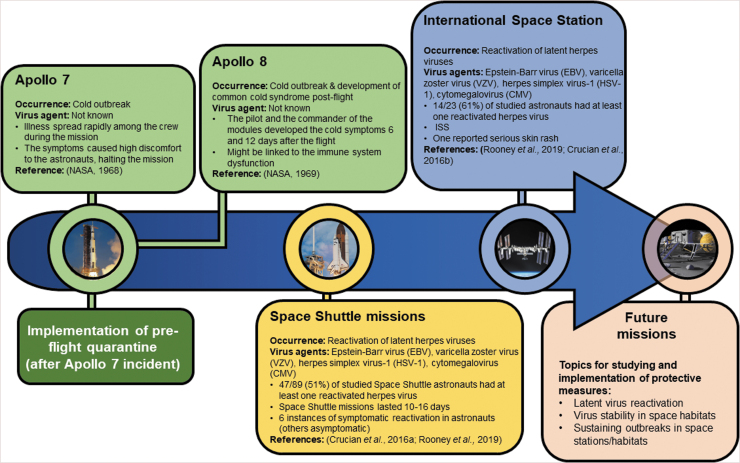
Over the course of spaceflight history, there has only been one reported outbreak in space—the common cold (head cold) outbreak among the three Apollo 7 astronauts, which spread rapidly and reduced the ability of the astronauts to cooperate with the control center (NASA, 1968). The reasons for the lack of reports are mainly pre/flight quarantine, normal mission duration of up to 6 months, but also the confidentiality of the astronaut medical data (Crucian et al., 2016a). However, reactivation of latent viruses has been documented in astronauts on the ISS, which caused skin rash and rhinitis in some exceptional cases (Crucian et al., 2016b). Varicella zoster virus (VZV) is one of the viruses reactivated in astronauts, which is known to cause significant pain and tissue damage in some cases. Therefore, vaccination of astronauts against VZV will be helpful to reduce the symptoms during space missions. Figure 4 illustrates the reported virus-related events over spaceflight history.
FIG. 4.
The occurrence of reported virus-related events over spaceflight history. The 7-day preflight quarantine of the astronauts helped sustain the infectious diseases on space missions as there are no reported outbreaks after its implementation. However, this might also be due to confidentiality of astronaut medical data. Currently, only reactivation of herpes viruses is being reported as a problem on space missions; though this is generally asymptomatic, it causes health issues, especially in the long term.
3.1. UV radiation
Most damaging UV radiation is filtered by Earth's atmosphere; therefore, all species on Earth are protected from most of the UVC, some UVB, and UVA to a lesser extent (De Gruijl and Van der Leun, 2000). UV radiation is one of the most threatening and damaging factors in the space environment for humans and microorganisms, along with ionizing radiation (gamma rays, X-rays, and fast charged particles). Those factors are also of concern on the Moon and Mars (Furukawa et al., 2020). UV has a major impact on viruses as discussed below. Interestingly, it has been proposed that viral populations can contain subgroups that are more resistant to UV inactivation as explained by the two-hit model of inactivation, which postulates that “two hits” of radiation rays are required to inactivate a virus particle (Kowalski et al., 2000; Cutler et al., 2011).
Ultraviolet radiation substantially reduces virus titers on surfaces and in the air (Tseng and Li, 2005, 2007; Sagripanti and Lytle, 2011). UVC radiation at 254 nm wavelength can induce damage to the viral genome and proteins (Beck et al., 2014, 2016, 2018). Overall, DNA viruses tend to be more resistant than RNA viruses, and those with double-stranded genomes are more resistant to UV radiation (Tseng and Li, 2007). Yet, independently of the nucleic acid type, genome damage is the most important factor for viral inactivation (Wang et al., 2004; Ye et al., 2018).
Also, UV radiation can reactivate dormant viruses in rodents (Garssen et al., 1995; El-Ghorr and Norval, 1996; Norval and El-Ghorr, 1996; Goade et al., 2001; Norval, 2006; Viarisio et al., 2011). Epidemiological data on papillomaviruses and herpesviruses suggest the same assumption holds for humans (Chen et al., 2008; Hampras et al., 2014; Uberoi and Lambert, 2017). The mechanism of this reactivation is related to how UV radiation suppresses the immune system (Norval and Halliday, 2011; Schwarz and Schwarz, 2011; Ullrich and Byrne, 2012) by activation of regulatory T cells (thymus cells, a type of lymphocyte) that produce immunosuppressive signals and thus inhibit the immune system (Rana et al., 2008). This paves the way for opportunistic disease-causing viruses to exploit a compromised immune system and provoke disease. Therefore, treatments that influence T cell signaling might impact the astronaut's health and contribute to virus control.
3.2. Ionizing radiation—gamma rays, X-rays, and charged particles
Ionizing radiation is another factor of space travel that influences living organisms, which are shielded from its effects on Earth due to the planet's magnetic field and the ozone layer. Those are gamma and X-rays that originate from the Sun or charged ions (H+, He2+, Fe ions) coming from interstellar space (Horneck et al., 2010; Chancellor et al., 2018). This type of radiation can induce significant damage to biomolecules by causing the formation of reactive oxygen species (ROS) and by breaking the molecular bonds (Reisz et al., 2014; Cortesão et al., 2020) and cause double-stranded breaks in DNA (Vignard et al., 2013).
Long-term exposure to low-intensity ionizing radiation increases the chances of developing cancer, especially of the skin and lungs (Cohen, 2002; WHO, 2016). Rodent models show that both gamma and X-rays have immunosuppressive effects that lead to the reactivation of herpes and rabies viruses and an increase in illness severity (Openshaw et al., 1979; Rodiriguez et al., 1990; Wang et al., 1990; Ceccaldi et al., 1996). One of the most dramatic effects is the apoptosis of dendritic cells, which neutralizes their defensive action that controls B and T cell immune response (Liu et al., 2011).
Ionizing photons also inactivate environmental viruses directly and indirectly (Johnson, 1965; Sullivan et al., 1971; Ward, 1980; Hume et al., 2016) mainly by damaging viral genomes and thus compromising viral replication (Summers and Szybalski, 1967; Ohshima et al., 1996; Lomax et al., 2013). Direct inactivation acts by damaging the viral genome, proteins, and lipids. The indirect mechanism of inactivation acts through the generation of hydroxyl radicals from water, ozone, and oxygen molecules, which originates ROS and which then damages nucleic acids, proteins, and lipids.
Studies suggest that different types of radiation can induce the lytic cycle of herpesviruses—Epstein–Barr virus (EBV) and human herpesviruses (HHV) types 1–3 (Ramirez-Fort et al., 2018; Mehta et al., 2018). Hence, while immunosuppression plays a role in the reactivation of herpesviruses, ionizing radiation can also directly activate lysogenic viruses (Ramirez-Fort et al., 2018).
3.3. Humidity
Humidity is another important environmental factor that can be controlled in space modules and has a known impact on viruses and other microbes (Yamaguchi et al., 2014). It also influences the virus-host interaction. Some studies have shown that relative humidity (RH) can be a predictor of viral stability (Shaman and Kohn, 2009; Tang, 2009). In these studies, stability depended on the presence of a lipid envelope. Viruses with an envelope conveyed more stability at lower RH (20–30%), while those without an envelope and a protein shell are more stable at higher RH (70–90%). However, recent studies on influenza and SARS-CoV-2 suggest that absolute humidity (AH) is a better predictor for stability (Deyle et al., 2016; Marr et al., 2019; Haque and Rahman, 2020; Wang et al., 2020). At higher AH, respiratory viruses show reduced transmission. This is because high AH can be only reached at higher temperatures, so the effect of AH is the combined action of RH and high temperature (Marr et al., 2019). In addition to inactivating airborne infectious viruses, high humidity thickens human mucosa, which acts as the barrier between the organism and environment, therefore reducing the possibility of viral infection from the air (Kudo et al., 2019). In the case of viral outbreaks in space habitats, humidity could be regulated to decelerate viral spread.
Hypothetically, viruses with lipid envelopes accumulate on the surface of water droplets in the air and are afterward inactivated by surface tension (Yang and Marr, 2012). At increased RH, droplets are larger because there is less evaporation that leads to a higher accumulation of lipid-enveloped viruses being inactivated by surface tension. On the other hand, non-enveloped viruses are repelled by water surfaces, which makes inactivation less efficient. Also, higher evaporation rates at lower RH decrease the pH of aerosol droplets, which induces conformational changes on surface proteins. This can make non-enveloped viruses less stable (Yang and Marr, 2012). However, these scenarios are currently only hypotheses, and more mechanistic research is needed to understand virus inactivation by RH and AH. Accordingly, bacteriophages with a protein envelope are better inactivated by UV radiation when exposed to low RH (Tseng and Li, 2005, 2007), but for a porcine reproductive and respiratory syndrome virus (PRRSV), a moderate RH (25–79%) enhances UV inactivation (Cutler et al., 2012) for unknown reasons.
3.4. Microgravity
In space travel, exposure to varying g-forces is very common. Spacecraft experience high g-forces during liftoff and are under the influence of microgravity once they reach orbit. Also, future celestial destinations like the Moon and Mars have lower gravitational force than Earth. Therefore, research of viruses in microgravity-like conditions is more relevant in the context of space travel, as well as hypergravity, which is relevant in the consideration of high g-forces experienced during launching into orbit and orbital transfers.
Research on virus-host interactions in microgravity scenarios indicates that it results in beneficial effects, contrary to other factors that induce viral reactivation (e.g., ionizing radiation), (Honda et al., 2020). Honda and colleagues found that cells infected with Kaposi's sarcoma-associated herpesvirus (KSHV) upregulate cell-intrinsic KSHV-inhibiting restriction factors in microgravity like CCCTC-binding factor (CTCF) or adenosine monophosphate–activated protein kinase (AMPK). This suggests that microgravity alone could partially counteract the damaging or debilitating effects of other space travel stress factors.
However, additional evidence displays a negative impact of microgravity. The intestinal epithelial cells have been shown to experience a decrease in their epithelial barrier function under microgravity (Alvarez et al., 2019). The gut harbors its microbiome, and it prevents viruses and other microbes from entering inside an organism. In this research, ion flux and fluorescein isothiocyanate-dextran (FITC-dextran) permeability of human epithelial barrier were measured in vitro, and it was found that the permeability increased, potentially allowing viral spread.
4. Control of Viruses in Space Travel
Innovative and efficient ways for virus identification, tracking, and inactivation are crucial to tackling the vast spectrum of problems they cause in public health, the economy, and agriculture. This is also important in spaceflight to prevent hindering missions due to viral infections. Various measures are taken to ensure the sanitary conditions aboard spacecraft, from microbial tracking to preflight astronaut isolation (Pierson et al., 2013).
Additional decontamination is applied to spacecraft with a special focus on bacterial and fungal spores, preventing microbial spread to other celestial bodies. Developing novel methods of disinfection and microbial control in space travel poses a challenge because they should efficiently fulfill the healthcare requirements compatible with human exposure without affecting the structural integrity of a spacecraft. For this reason, chemical disinfection is often not suitable for space applications.
4.1. Antimicrobial surfaces
Certain materials and compounds have antimicrobial properties that are exploited to inhibit or reduce microbial growth in environments where strict hygiene standards are necessary, such as airplanes, spaceflight, healthcare, or food production (Page et al., 2009; Mousavi Khaneghah et al., 2018).
Metals with antimicrobial properties are copper, silver, and their respective alloys, but also ions of mercury, iron, lead, zinc, and aluminum (Sreekumari, et al., 2005). Including a minimum of 55% of copper into composite materials (Mehtar et al., 2008) would contribute to ensuring safety during air and space travel as it efficaciously inactivates most viruses, including SARS-CoV-2 and other microbes (Noyce et al., 2007; Warnes et al., 2015; Schmidt et al., 2017; Bryant et al., 2021). There are also efforts to develop alternative antimicrobial surfaces for space travel such as AGXX (Van Loi et al., 2018), a silver/ruthenium surface coating that produces ROS, which inactivates most pathogens, including viruses.
4.2. Sterilization of spacecraft
There is a concern to protect other planets from contamination with Earth's microbes. Spacecraft surfaces are sterilized inside and out by intense treatment at high temperatures (145°C) for several days, which guarantees that no microbes or spores can survive. In recent years, plasma sterilization has been considered an effective alternative due to its more convenient application (Stapelmann et al., 2013). These methods of sterilization are collaterally highly effective against viruses (Bozkurt et al., 2015; Sakudo et al., 2019), making antimicrobial disinfection overly effective in preventing virus contaminations.
Interestingly, full microbial sterility of spacecraft in human missions is hard, if not impossible, to achieve because astronauts themselves represent a reservoir of microbes that can cross-contaminate the environment. Development of simple and easy-to-use detection tests such as SHERLOCK (Gootenberg et al., 2018) or DETECTR (Chen et al., 2018) for specific viral genomes would be helpful in space missions. Such tests utilize CRISPR-Cas9 technology to specifically detect viral sequences within minutes. Also, environmental nucleic acid sequencing techniques like Oxford Nanopore (Quick, 2019) are becoming more robust and easier for application in extreme environments such as space missions. This will vastly aid the detection and characterization of viromes in enclosed environments, not just in space habitats, but also on Earth.
4.3. Air filtration
Inside the ISS, there is constant air circulation and filtration with high-efficiency particulate air (HEPA) filters. They have been reported to efficiently filter out small aerosols (98% efficiency, particles 0.3–10 μm diameter; Mousavi et al., 2020). Even though virus sizes are in nanometer range, they travel in air-suspended droplets and aerosols that are micrometer-sized, being efficiently filtered by HEPA filters. Therefore, they protect the astronauts. Those filters also provide an interesting test sample for researching airborne microbial communities on the ISS. Probably, future space habitats will also include such filters due to the need for constant air recycling.
5. Health Threats Viruses Pose to Space Travel
5.1. Latent infections and viral reactivation
Even though disinfection on space missions is thorough, it is impossible to completely neutralize the disease-inducing factors due to their prevalence in the human hosts, since a large portion of humanity is already latently infected with specific viruses. Latent infections are caused by viruses that, upon a single inoculation, can establish lifelong infections, like herpesviruses. Table 2 lists herpesviruses that cause latent infection in humans regarding global prevalence, route of transmission, possible consequences, and site of persistence. As a result of immune deregulation in space, these viruses can undergo reactivation, potentially with increased severity due to the vulnerability of the host. Their reactivation in astronauts is probably caused by the stress factors discussed above, resulting in the changes in CD8+ T cell (T lymphocytes expressing cluster of differentiation 8 glycoprotein) and regulatory T cell function, which have been described to maintain viral latency (Mehta et al., 2013).
Table 2.
The Global Prevalence, Route of Transmission, Some Possible Consequences of Primary Infection and Reactivation, and Site of Persistence of Herpesviruses Causing Latent Infections in Humans: HSV-1 and HSV-2, VZV, EBV, HCMV, HHV-6 and HHV-7, and KSHV
| Viruses | Global prevalence | Transmission | Possible consequences | Site of persistence | References |
|---|---|---|---|---|---|
| HSV-1 | 67% (age under 50) | Contact-based of infected mucosa | Cold sores, genital ulcers, related skin lesions, keratitis, encephalitis, meningitis | Sensory and cranial nerve ganglia | Grinde, 2013 |
| HSV-2 | 11.3% (age 15–49) the highest burden in Africa | Contact-based of infected mucosa | Cold sores, genital ulcers, keratitis, encephalitis, meningitis, Mollaret's meningitis | Sensory and cranial nerve ganglia | Grinde, 2013; Looker et al., 2015 |
| VZV | >90% (before adolescence, pre-vaccination era, high-income countries) | Contact-based, inhalation of aerosols | Chickenpox, herpes zoster | Sensory and cranial nerve ganglia, spinal cord | Grinde, 2013; WHO, 2014 |
| EBV | <90% (lifetime) | Blood, contact-based of infected mucosa, saliva | Hairy leukoplakia, periodontitis, nasopharyngeal carcinoma, mononucleosis, lymphoma, Hodgkin's lymphoma | Memory B cells | Chang et al., 2009; Grinde, 2013; Ozturk et al., 2020 |
| HCMV | 83% | Blood, mucosal contact during breastfeeding, saliva, urine | Mononucleosis, colitis, esophagitis, retinitis, pneumonia, hepatitis, and encephalitis | Monocytes, lymphocytes, and epithelia | Grinde, 2013; Zuhair et al., 2019; Sezgin et al., 2019 |
| HHV-6 | 70–100% | Contact-based of infected mucosa, saliva | Exanthema subitum, encephalitis, fulminant hepatitis, liver dysfunction, thrombocytopenia, hemophagocytic syndrome | Various leukocytes | De Bolle et al., 2005; Grinde, 2013 |
| HHV-7 | 75–98% except Northern Japan: 44% | Contact-based, saliva | Exanthema subitum, encephalitis | T cells, epithelia | Krueger et al., 1998; Ward, 2005; Grinde, 2013 |
| KSHV | Geographic differences: >1.5% (adults in USA); 55% (Uganda) | Blood, contact-based of infected mucosa, saliva | Kaposi's sarcoma, Castleman disease | B cells | Engels et al., 2007; Biryahwaho et al., 2010; Grinde, 2013; Fajgenbaum and Shilling, 2018 |
B cells = bursa cells, a type of lymphocyte; EBV = Epstein–Barr virus; HCMV = human cytomegalovirus; HHV = human herpesvirus; HSV = herpes simplex virus; KSHV = Kaposi's sarcoma-associated herpesvirus; T cells = thymus cells, a type of lymphocyte; VZV = varicella zoster virus.
Multiple studies have detected reactivation and shedding of viruses in human space and analog missions and environments (Pierson et al., 2005, 2007; Mehta and Pierson, 2007; Mehta et al., 2014, 2017). Due to the prevalence of herpesviruses in the general population, reactivation events cannot be reliably avoided in space either by isolation or by medical treatment. Therefore, developing spaceflight countermeasures to attenuate viral reactivation outcomes such as preflight immunity enhancement to inhibit viruses is a factor to be considered. Though herpesviruses are not the only viruses that latently infect humans, they are the major focus in spaceflight (Rooney et al., 2019). Thus, further studies of latent viral infections are necessary to determine the vulnerability of astronauts to other latent infection viruses besides those belonging to the Herpesviridae family.
The reactivation of these viruses, some of which are associated with increased mortality (Ren et al., 2020), has also been detected in astronauts. Besides the cold-sore-causing HSV-1 (Crucian et al., 2016a), the reactivation of other herpesviruses such as EBV (Payne et al., 1999), human cytomegalovirus (HCMV; Vuong et al., 2000), and VZV has been observed in astronauts before with mild symptoms (Mehta et al., 2004). Though serious consequences have not yet been observed in astronauts, this might be due to the currently short duration of human missions. However, planned long-term missions carry the danger of astronauts developing severe symptoms stemming from latent viral infections. This is especially dangerous due to the limited resources available in space missions to isolate and treat the affected individuals.
5.2. Protecting astronauts from virus infections
In addition to infection by reactivation of latent viruses, there is also a possibility of virus infection on space missions that increases the risk of outbreaks in modules and habitats. Due to the limited possibility of identifying the cause of infection in space, it is challenging to recognize viral infections in addition to treating them. So, how can astronauts protect themselves from viral infections? Currently, most herpesvirus infections cannot be prevented through vaccines, with the exception of VZV, the causative agent of chickenpox and zoster (Papaloukas et al., 2014). A balanced diet that supports a healthy metabolism, boosting the immune response, like probiotics or foods rich in vitamins, minerals, or amino acids could in theory support the fitness of the immune system, though the research in this area is still ongoing (Perdigon et al., 1995; Mora et al., 2008; Crucian et al., 2018). Physical exercise has been found to significantly contribute to the reduced reactivation of viruses in astronauts on the ISS (Agha et al., 2020). A more drastic approach would consist of using immunostimulant drugs such as bacille Calmette–Guérin (BCG), levamisole, isoprinosine, or others (Bascones-Martinez et al., 2014). Anti-herpes products like acyclovir can be used to treat herpesviruses, but these have shown toxicity with prolonged use (WHO, 2013). Current journeys to space are limited in time, but in the case of longer journeys to Mars or further, latent viruses could have a greater impact.
Currently, the standard safety procedure of human spaceflight is the preflight astronauts' quarantine and disinfection of cabins and equipment. However, we must consider the risk that some viral infections might go unnoticed during the quarantine period and cause significant harm once in the space station. Development of optimized methods for virus detection and calculating the impact of the space environment on virus spread will help address this problem and provide the basis for the development of improved protocols to control eventual outbreaks in space.
6. Conclusion and Outlook
Viruses are a diverse biological group that is part of microbial communities in human-inhabited space modules. As such, they can influence astronauts' well-being and may pose a health threat to the crew. Intensive research is required in the field of space virology to improve the current knowledge on the dynamics caused by space stress. The effect of extreme g-forces or microgravity on viruses is underrepresented. It would be, for instance, interesting to test the influence of microgravity on viral spread throughout the body. Also, studies showed that hypergravity encourages the proliferation of healthy cells (Ciofani et al., 2012; Genchi et al., 2016), while the effect on viruses or infected and immune cells is not known. Research of viral UV-stability could be used to develop postflight UV-based sterilization of spacecraft cabins as an easy and efficient method for viral elimination. It would be especially interesting to further investigate the inactivation of human pathogenic viruses by UV radiation in various levels of RH. Developing new antimicrobial materials is another promising method for limiting viral spread during space travel.
Since the plans of future space missions tend to be more prolonged, preflight isolation and a healthy immune system might not be enough to protect astronauts against some viruses due to the overwhelming conditions during space travel. Hence, developing new methods for the detection and treatment of viral infections in space is a relevant topic.
Acknowledgments
We thank Christine Moissl-Eichinger and her entire team for the fruitful discussion and generous exchange of scientific data. We express our gratitude to Melanie Brinkmann and Satish Mehta for their critical reviews and valuable suggestions. We acknowledge Kevin McAlpin's help with the English revision of the manuscript.
Abbreviations Used
- AH
absolute humidity
- B cells
bursa cells, a type of lymphocyte
- EBV
Epstein–Barr virus
- FITC-dextran
fluorescein isothiocyanate-dextran
- HEPA
high-efficiency particulate air
- HHV
human herpesvirus
- HSV
herpes simplex virus
- ISS
International Space Station
- KSHV
Kaposi's sarcoma-associated herpesvirus
- RH
relative humidity
- ROS
reactive oxygen species
- T cells
thymus cells, a type of lymphocyte
- VZV
varicella zoster virus
Funding
The Aerospace Microbiology research group received funding from the following DLR grants: ISS LIFE (Programm RF-FuW, Teilprogramm: 475), AEROMASK, NGT-BIT (DLR V&E: Next Generation Train), and simplAIR (Programm LF (simplAIR ME / KoPa33), Teilbereich: 251). M.C. was supported by the DLR/DAAD Research Fellowship Doctoral Studies in Germany, 2017 (57370122) and B.P. by an ERASMUS+ fellowship (2019-1-HR01-KA103-060250). These results will be included in the PhD thesis of Bruno Pavletić.
Associate Editor: Lewis Dartnell
References
- Agha NH, Mehta SK, Rooney BV, et al. (2020) Exercise as a countermeasure for latent viral reactivation during long duration space flight. FASEB J 34:2869–2881. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Horie K, Hinoi E, et al. (2020) How does spaceflight affect the acquired immune system? npj Microgravity 6, doi: 10.1038/s41526-020-0104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez R, Stork CA, Sayoc-Becerra A, et al. (2019) A simulated microgravity environment causes a sustained defect in epithelial barrier function. Sci Rep 9, doi: 10.1038/s41598-019-53862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ann Gregory AC, Zayed AA, dia Conceiç N, et al. (2019) Marine DNA viral macro-and microdiversity from pole to pole in brief. Cell 177, doi: 10.1016/j.cell.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascones-Martinez A, Mattila R, Gomez-Font R, et al. (2014) Immunomodulatory drugs: oral and systemic adverse effects. Med Oral Patol Oral Cir Bucal 19:e24–e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SE, Rodriguez RA, Linden KG, et al. (2014) Wavelength dependent UV inactivation and DNA damage of adenovirus as measured by cell culture infectivity and long range quantitative PCR. Environ Sci Technol 48:591–598. [DOI] [PubMed] [Google Scholar]
- Beck SE, Rodriguez RA, Hawkins MA, et al. (2016) Comparison of UV-induced inactivation and RNA damage in MS2 phage across the germicidal UV spectrum. Appl Environ Microbiol 82:1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SE, Hull NM, Poepping C, et al. (2018) Wavelength-dependent damage to adenoviral proteins across the germicidal UV spectrum. Environ Sci Technol 52:223–229. [DOI] [PubMed] [Google Scholar]
- Benbough JE (1971) Some factors affecting the survival of airborne viruses. J Gen Virol 10:209–220. [DOI] [PubMed] [Google Scholar]
- Berliner AJ, Mochizuki T, and Stedman KM (2018) Astrovirology: viruses at large in the Universe. Astrobiology 18:207–223. [DOI] [PubMed] [Google Scholar]
- Biryahwaho B, Dollard SC, Pfeiffer RM, et al. (2010) Sex and geographic patterns of human herpesvirus 8 infection in a nationally representative population-based sample in Uganda. J Infect Dis 202:1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt H, D'Souza DH, and Davidson PM (2015). Thermal inactivation of foodborne enteric viruses and their viral surrogates in foods. J Food Prot 78:1597–617. [DOI] [PubMed] [Google Scholar]
- Bryant C, Wilks SA, and Keevil W (2021) Rapid inactivation of SARS-CoV-2 on copper touch surfaces determined using a cell culture infectivity assay. bioRxiv:2021.01.02.424974 [Google Scholar]
- Carratalà A, Shim H, Zhong Q, et al. (2017) Experimental adaptation of human echovirus 11 to ultraviolet radiation leads to resistance to disinfection and ribavirin. Virus Evol 3, doi: 10.1093/ve/vex035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi PE, Marquette C, Weber P, et al. (1996) Ionizing radiation modulates the spread of an apathogenic rabies virus in mouse brain. Int J Radiat Biol 70:69–75. [DOI] [PubMed] [Google Scholar]
- Chan KH, Peiris JSM, Lam SY, et al. (2011) The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol 2011, doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chancellor JC, Blue RS, Cengel KA, et al. (2018) Limitations in predicting the space radiation health risk for exploration astronauts. npj Microgravity 4, doi: 10.1038/s41526-018-0043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CM, Yu KJ, Mbulaiteye SM, et al. (2009) The extent of genetic diversity of Epstein-Barr virus and its geographic and disease patterns: a need for reappraisal. Virus Res 143:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ACH, McMillan NAJ, and Antonsson A (2008) Human papillomavirus type spectrum in normal skin of individuals with or without a history of frequent sun exposure. J Gen Virol 89:2891–2897. [DOI] [PubMed] [Google Scholar]
- Chen JS, Ma E, Harrington LB, et al. (2018) CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360:436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani G, Ricotti L, Rigosa J, et al. (2012) Hypergravity effects on myoblast proliferation and differentiation. J Biosci Bioeng 113:258–261. [DOI] [PubMed] [Google Scholar]
- Cohen BL (2002) Cancer risk from low-level radiation. Am J Roentgenol 179:1137–1143. [DOI] [PubMed] [Google Scholar]
- Cortesão M, de Haas A, Unterbusch R, et al. (2020) Aspergillus niger spores are highly resistant to space radiation. Front Microbiol 11, doi: 10.3389/fmicb.2020.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crucian B, Stowe RP, Mehta S, et al. (2015) Alterations in adaptive immunity persist during long-duration spaceflight. npj Microgravity 1, doi: 10.1038/npjmgrav.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crucian B, Babiak-Vazquez A, Johnston S, et al. (2016a) Incidence of clinical symptoms during long-duration orbital spaceflight. Int J Gen Med 9:383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crucian B, Johnston S, Mehta S, et al. (2016b) A case of persistent skin rash and rhinitis with immune system dysregulation onboard the International Space Station. J Allergy Clin Immunol Pract 4, doi: 10.1016/j.jaip.2015.12.021. [DOI] [PubMed] [Google Scholar]
- Crucian BE, Choukèr A, Simpson RJ, et al. (2018) Immune system dysregulation during spaceflight: potential countermeasures for deep space exploration missions. Front Immunol 9, doi: 10.3389/fimmu.2018.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler T, Wang C, Qin Q, et al. (2011) Kinetics of UV254 inactivation of selected viral pathogens in a static system. J Appl Microbiol 111:389–395. [DOI] [PubMed] [Google Scholar]
- Cutler TD, Wang C, Hoff SJ, et al. (2012) Effect of temperature and relative humidity on ultraviolet (UV254) inactivation of airborne porcine respiratory and reproductive syndrome virus. Vet Microbiol 159:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bolle L, Naesens L, and De Clercq E (2005) Update on human herpesvirus 6 biology, clinical features, and therapy. Clin Microbiol Rev 18:217–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gruijl FR and Van der Leun JC (2000) Environment and health: 3. Ozone depletion and ultraviolet radiation. CMAJ 163:851–855. [PMC free article] [PubMed] [Google Scholar]
- Delwart EL (2007) Viral metagenomics. Rev Med Virol 17:115–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyle ER, Maher MC, Hernandez RD, et al. (2016) Global environmental drivers of influenza. Proc Natl Acad Sci USA 113:13081–13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson AI and Ferris NP (1976) The survival of some air-borne animal viruses in relation to relative humidity. Vet Microbiol 1:413–420. [Google Scholar]
- Eischeid AC and Linden KG (2011) Molecular indications of protein damage in adenoviruses after UV disinfection. Appl Environ Microbiol 77:1145–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghorr AA and Norval M (1996) The effect of UV-B irradiation on secondary epidermal infection of mice with herpes simplex virus type 1. J Gen Virol 77:485–491. [DOI] [PubMed] [Google Scholar]
- Engels EA, Atkinson JO, Graubard BI, et al. (2007) Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for mucosal transmission. J Infect Dis 196:199–207. [DOI] [PubMed] [Google Scholar]
- Fajgenbaum DC and Shilling D (2018) Castleman disease pathogenesis. Hematol Oncol Clin North Am 32:11–21. [DOI] [PubMed] [Google Scholar]
- Fekete A, Kovács G, Hegedüs M, et al. (2008) Biological responses to the simulated martian UV radiation of bacteriophages and isolated DNA. J Photochem Photobiol B Biol 92:110–116. [DOI] [PubMed] [Google Scholar]
- Feng K, Divers E, Ma Y, et al. (2011) Inactivation of a human norovirus surrogate, human norovirus virus-like particles, and vesicular stomatitis virus by gamma irradiation. Appl Environ Microbiol 77:3507–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalo R, Baatout S, and Moreels M (2017) Impact of particle irradiation on the immune system: from the clinic to Mars. Front Immunol 8, doi: 10.3389/fimmu.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JS, Wheeler RM, and Pamphile R (2014) Host-microbe interactions in microgravity: assessment and implications. Life 4:250–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Nagamatsu A, Nenoi M, et al. (2020) Space radiation biology for “living in space.” Biomed Res Int 2020, doi: 10.1155/2020/4703286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse A and Sato T (2004) Effect of microgravity changes on virus infection in mice. J Gravit Physiol 11:65–66. [PubMed] [Google Scholar]
- Garssen J, Van der Vliet H, De Klerk A, et al. (1995) A rat cytomegalovirus infection model as a tool for immunotoxicity testing. Eur J Pharmacol 292:223–231. [DOI] [PubMed] [Google Scholar]
- Genchi GG, Rocca A, Marino A, et al. (2016) Hypergravity as a tool for cell stimulation: implications in biomedicine. Front Astron Space Sci 3, doi: 10.3389/fspas.2016.00026. [DOI] [Google Scholar]
- Gibbons SM, Schwartz T, Fouquier J, et al. (2015) Ecological succession and viability of human-associated microbiota on restroom surfaces. Appl Environ Microbiol 81:765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goade DE, Nofchissey RA, Kusewitt DF, et al. (2001) Ultraviolet light induces reactivation in a murine model of cutaneous herpes simplex virus-1 infection. Photochem Photobiol 74:108–114. [DOI] [PubMed] [Google Scholar]
- Gootenberg JS, Abudayyeh OO, Kellner MJ, et al. (2018) Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieb T, Forng RY, Brown R, et al. (2002) Effective use of gamma irradiation for pathogen inactivation of monoclonal antibody preparations. Biologicals 30:207–216. [DOI] [PubMed] [Google Scholar]
- Grinde B (2013) Herpesviruses: latency and reactivation—viral strategies and host response. J Oral Microbiol 5, doi: 10.3402/jom.v5i0.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampras SS, Giuliano AR, Lin HY, et al. (2014) Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PLoS One 9, doi: 10.1371/journal.pone.0104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque SE and Rahman M (2020) Association between temperature, humidity, and COVID-19 outbreaks in Bangladesh. Environ Sci Policy 114:253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper GJ (1961) Airborne micro-organisms: survival tests with four viruses. Epidemiol Infect 59:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmsø MH, Mollerup S, Jensen RH, et al. (2019) Metagenomic analysis of viruses in toilet waste from long distance flights—a new procedure for global infectious disease surveillance. PLoS One 14, doi: 10.1371/journal.pone.0210368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda T, Nakayama R, Kawahara Y, et al. (2020) Kaposi's sarcoma-associated herpesvirus is cell-intrinsically controlled in latency in microgravity. Virus Res 276, doi: 10.1016/j.virusres.2019.197821. [DOI] [PubMed] [Google Scholar]
- Horneck G, Klaus DM, and Mancinelli RL (2010) Space microbiology. Microbiol Mol Biol Rev 74:121–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T, Joice R, Vallarino J, et al. (2016) Urban transit system microbial communities differ by surface type and interaction with humans and the environment. mSystems 1, doi: 10.1128/mSystems.00018-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Zeng G, Huang Y, et al. (2016) Evaluation of the gastrointestinal tract as potential route of primary polyomavirus infection in mice. PLoS One 11, doi: 10.1371/journal.pone.0150786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume A, Ames J, Rennick L, et al. (2016) Inactivation of RNA viruses by gamma irradiation: a study on mitigating factors. Viruses 8, doi: 10.3390/v8070204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz MK, Brunner AH, Sattar SA, et al. (1985) Survival characteristics of airborne human coronavirus 229E. J Gen Virol 66:2743–2748. [DOI] [PubMed] [Google Scholar]
- Johnson CD (1965) Direct X-ray inactivation of the viruses of Teschen and Talfan diseases, foot-and-mouth disease and vesicular stomatitis. Nature 207:37–39. [DOI] [PubMed] [Google Scholar]
- Karim YG, Ijaz MK, Sattar SA, et al. (1985) Effect of relative humidity on the airborne survival of rhinovirus-14. Can J Microbiol 31:1058–1061. [DOI] [PubMed] [Google Scholar]
- Koike J, Oshima T, Koike KA, et al. (1992) Survival rates of some terrestrial microorganisms under simulated space conditions. Adv Space Res 12:271–274. [DOI] [PubMed] [Google Scholar]
- Kowalski WJ, Bahnfleth WP, Witham DL, et al. (2000) Mathematical modeling of ultraviolet germicidal irradiation for air disinfection. Quant Microbiol 2:249–270. [Google Scholar]
- Krueger GRF, Koch B, Leyssens N, et al. (1998) Comparison of seroprevalences of human herpesvirus-6 and -7 in healthy blood donors from nine countries. Vox Sang 75:193–197. [PubMed] [Google Scholar]
- Kudo E, Song E, Yockey LJ, et al. (2019) Low ambient humidity impairs barrier function and innate resistance against influenza infection. Proc Natl Acad Sci USA 116:10905–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Lin J, Zhao L, et al. (2011) Gamma-ray irradiation impairs dendritic cell migration to CCL19 by down-regulation of CCR7 and induction of cell apoptosis. Int J Biol Sci 7:168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax ME, Folkes LK, and O'Neill P (2013) Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin Oncol 25:578–585. [DOI] [PubMed] [Google Scholar]
- Looker KJ, Magaret AS, Turner KME, et al. (2015) Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 10, doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle CD and Sagripanti J-L (2005) Predicted inactivation of viruses of relevance to biodefense by solar radiation. J Virol 79:14244–14252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr LC, Tang JW, Van Mullekom J, et al. (2019) Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J R Soc Interface 16, doi: 10.1098/rsif.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan LT, Wallingford KM, Hein MJ, et al. (2008) Monitoring microbial populations on wide-body commercial passenger aircraft. Ann Occup Hyg 52:139–149. [DOI] [PubMed] [Google Scholar]
- Mehta S, Bloom D, Plante I, et al. (2018) Reactivation of latent Epstein-Barr virus: a comparison after exposure to gamma, proton, carbon, and iron radiation. Int J Mol Sci 19, doi: 10.3390/ijms19102961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta SK and Pierson DL (2007) Reactivation of latent herpes viruses in cosmonauts during a Soyuz taxi mission. Microgravity Sci Technol 19:215–218. [Google Scholar]
- Mehta SK, Cohrs RJ, Forghani B, et al. (2004) Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol 72:174–179. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Crucian BE, Stowe RP, et al. (2013) Reactivation of latent viruses is associated with increased plasma cytokines in astronauts. Cytokine 61:205–209. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Laudenslager ML, Stowe RP, et al. (2014) Multiple latent viruses reactivate in astronauts during Space Shuttle missions. Brain Behav Immun 41:210–217. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Laudenslager ML, Stowe RP, et al. (2017) Latent virus reactivation in astronauts on the International Space Station. npj Microgravity 3, doi: 10.1038/s41526-017-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehtar S, Wiid I, and Todorov SD (2008) The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: an in vitro study. J Hosp Infect 68:45–51. [DOI] [PubMed] [Google Scholar]
- Miller RL and Plagemann PGW (1974) Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J Virol 13:729–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirshafiee H, Hosseini S, Sharifi Z, et al. (2012) Induction of nucleic acid damage in viral genomes using riboflavin in combination with UV Light. Iran J Virol 6:27–32. [Google Scholar]
- Mokili JL, Rohwer F, and Dutilh BE (2012) Metagenomics and future perspectives in virus discovery. Curr Opin Virol 2:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Iwata M, and von Andrian UH (2008) Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 8:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora M, Wink L, Kögler I, et al. (2019) Space station conditions are selective but do not alter microbial characteristics relevant to human health. Nat Commun 10, doi: 10.1038/s41467-019-11682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi ES, Godri Pollitt KJ, Sherman J, et al. (2020) Performance analysis of portable HEPA filters and temporary plastic anterooms on the spread of surrogate coronavirus. Build Environ 183, doi: 10.1016/j.buildenv.2020.107186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi Khaneghah A, Hashemi SMB, Eş I, et al. (2018) Efficacy of antimicrobial agents for food contact applications: biological activity, incorporation into packaging, and assessment methods: a review. J Food Prot 81:1142–1156. [DOI] [PubMed] [Google Scholar]
- NASA (1968, December) Apollo 7 Mission Report. MSC-PA-R-68-15. Manned Spacecraft Center, Houston. Available online at https://www.hq.nasa.gov/alsj/a410/A07_MissionReport.pdf
- NASA (1969, February) Apollo 8 Mission Report. MSC-PA-R-69-1. Manned Spacecraft Center, Houston. Available online at https://history.nasa.gov/afj/ap08fj/pdf/a08-missionreport.pdf
- NASA (2010) Flight Crew Health Stabilization Program. Document ID 20130000048. Lyndon B. Johnson Space Center, Houston. Available online at https://ntrs.nasa.gov/citations/20130000048
- Navarro F and Muniesa M (2017) Phages in the human body. Front Microbiol 8, doi: 10.3389/fmicb.2017.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooij S, Schmitz D, Vennema H, et al. (2018) Overview of virus metagenomic classification methods and their biological applications. Front Microbiol 9, doi: 10.3389/fmicb.2018.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norval M (2006) The effect of ultraviolet radiation on human viral infections. Photochem Photobiol 82:1495–1504. [DOI] [PubMed] [Google Scholar]
- Norval M and El-Ghorr AA (1996) UV radiation and mouse models of herpes simplex virus infection. Photochem Photobiol 64:242–245. [DOI] [PubMed] [Google Scholar]
- Norval M and Halliday GM (2011) The consequences of UV-induced immunosuppression for human health. Photochem Photobiol 87:965–977. [DOI] [PubMed] [Google Scholar]
- Noti JD, Blachere FM, McMillen CM, et al. (2013) High humidity leads to loss of infectious influenza virus from simulated coughs. PLoS One 8, doi: 10.1371/journal.pone.0057485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyce JO, Michels H, and Keevil CW (2007) Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol 73:2748–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima H, Iida Y, Matsuda A, et al. (1996) Damage induced by hydroxyl radicals generated in the hydration layer of gamma-irradiated frozen aqueous solution of DNA. J Radiat Res 37:199–207. [DOI] [PubMed] [Google Scholar]
- Openshaw H, Shavrina Asher LV, Wohlenberg C, et al. (1979) Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol 44:205–215. [DOI] [PubMed] [Google Scholar]
- Ozturk V, Yikilmaz AS, Kilicarslan A, et al. (2020) The triple positivity for EBV, PD-1, and PD-L1 identifies a very high risk classical Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk 20:e375–e381. [DOI] [PubMed] [Google Scholar]
- Page K, Wilson M, and Parkin IP (2009) Antimicrobial surfaces and their potential in reducing the role of the inanimate environment in the incidence of hospital-acquired infections. J Mater Chem 19, doi: 10.1039/B818698G. [DOI] [Google Scholar]
- Papaloukas O, Giannouli G, and Papaevangelou V (2014) Successes and challenges in varicella vaccine. Ther Adv Vaccines 2:39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenov GP and Lukin AA (1973) Results and prospects of microbiological studies in outer space. Space Life Sci 4:160–179. [DOI] [PubMed] [Google Scholar]
- Payne DA, Mehta SK, Tyring SK, et al. (1999) Incidence of Epstein-Barr virus in astronaut saliva during spaceflight. Aviat Sp Environ Med 70:1211–1213. [PubMed] [Google Scholar]
- Perdigon G, Alvarez S, Rachid M, et al. (1995) Immune system stimulation by probiotics. J Dairy Sci 78:1597–1606. [DOI] [PubMed] [Google Scholar]
- Pierson DL, Stowe RP, Phillips TM, et al. (2005) Epstein–Barr virus shedding by astronauts during space flight. Brain Behav Immun 19:235–242. [DOI] [PubMed] [Google Scholar]
- Pierson DL, Mehta SK, and Stowe RP (2007) Reactivation of latent herpes viruses in astronauts. In Psychoneuroimmunology, 4th ed. Elsevier, Burlington, MA, pp. 851–868. [Google Scholar]
- Pierson DL, Ott CM, Botkin DJ, et al. (2013) Microbial Monitoring of the International Space Station. Document ID 20130013534. Microbiology Laboratory, NASA Johnson Space Center, Houston. Available online at https://ntrs.nasa.gov/citations/20130013534 [Google Scholar]
- Pimenta AI, Guerreiro D, Madureira J, et al. (2016) Tracking human adenovirus inactivation by gamma radiation under different environmental conditions. Appl Environ Microbiol 82:5166–5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin AJ and Marr LC (2015) Sources of airborne microorganisms in the built environment. Microbiome 3, doi: 10.1186/s40168-015-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin AJ, Torres PJ, Shimashita J, et al. (2019) Seasonal dynamics of DNA and RNA viral bioaerosol communities in a daycare center. Microbiome 7, doi: 10.1186/s40168-019-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin AJ, Belser JA, Bischoff W, et al. (2020) Viruses in the Built Environment (VIBE) meeting report. Microbiome 8, doi: 10.1186/s40168-019-0777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick J. (2019) Ultra-long read nanopore sequencing methods for metagenomics. J Biomol Tech 30:S63. [Google Scholar]
- Rae C, Koudelka KJ, Destito G, et al. (2008) Chemical addressability of ultraviolet-inactivated viral nanoparticles (VNPs). PLoS One 3, doi: 10.1371/journal.pone.0003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Fort MK, Zeng J, Feily A, et al. (2018) Radiotherapy-induced reactivation of neurotrophic human herpes viruses: overview and management. J Clin Virol 98:18–27. [DOI] [PubMed] [Google Scholar]
- Rana S, Byrne SN, MacDonald LJ, et al. (2008) Ultraviolet B suppresses immunity by inhibiting effector and memory T cells. Am J Pathol 172:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisz JA, Bansal N, Qian J, et al. (2014) Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 21:260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Wang B, Miao Z, et al. (2020) A correlation analysis of HHV infection and its predictive factors in an HIV-seropositive population in Yunnan, China. J Med Virol 92:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodiriguez M, Patrick A, and Pease L (1990) Abrogation of resistance to Theiler's virus-induced demyelination in C57BL mice by total body irradiation. J Neuroimmunol 26:189–199. [DOI] [PubMed] [Google Scholar]
- Rooney BV, Crucian BE, Pierson DL, et al. (2019) Herpes virus reactivation in astronauts during spaceflight and its application on Earth. Front Microbiol 10, doi: 10.3389/fmicb.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario K and Breitbart M (2011) Exploring the viral world through metagenomics. Curr Opin Virol 1:289–297. [DOI] [PubMed] [Google Scholar]
- Roux S, Emerson JB, Eloe-Fadrosh EA, et al. (2017) Benchmarking viromics: an in silico evaluation of metagenome-enabled estimates of viral community composition and diversity. PeerJ 5, doi: 10.7717/peerj.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagripanti JL and Lytle CD (2011) Sensitivity to ultraviolet radiation of Lassa, vaccinia, and Ebola viruses dried on surfaces. Arch Virol 156:489–494. [DOI] [PubMed] [Google Scholar]
- Sakudo A, Yagyu Y, and Onodera T (2019) Disinfection and sterilization using plasma technology: fundamentals and future perspectives for biological applications. Int J Mol Sci 20, doi: 10.3390/ijms20205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer FL, Soergel ME, and Straube DC (1976) Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch Virol 51:263–273. [DOI] [PubMed] [Google Scholar]
- Schmidt MG, Tuuri RE, Dharsee A, et al. (2017) Antimicrobial copper alloys decreased bacteria on stethoscope surfaces. Am J Infect Control 45:642–647. [DOI] [PubMed] [Google Scholar]
- Schwarz T and Schwarz A (2011) Molecular mechanisms of ultraviolet radiation-induced immunosuppression. Eur J Cell Biol 90:560–564. [DOI] [PubMed] [Google Scholar]
- Sezgin E, An P, and Winkler CA (2019) Host genetics of cytomegalovirus pathogenesis. Front Genet 10, doi: 10.3389/fgene.2019.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J and Kohn M (2009) Absolute humidity modulates influenza survival, transmission, and seasonality. Proc Natl Acad Sci USA 106:3243–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet J and Gantzer C (2006) Inactivation of poliovirus 1 and F-specific RNA phages and degradation of their genomes by UV irradiation at 254 nanometers. Appl Environ Microbiol 72:7671–7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer JR (1967) Influence of relative humidity on the survival of some airborne viruses. Appl Microbiol 15:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreekumari KR, Sato Y, and Kikuchi Y (2005) Antibacterial metals: a viable solution for bacterial attachment and microbiologically influenced corrosion. Mater Trans 46:1636–1645. [Google Scholar]
- Stapelmann K, Fiebrandt M, Raguse M, et al. (2013) Utilization of low-pressure plasma to inactivate bacterial spores on stainless steel screws. Astrobiology 13:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens B (2016) What have we learned about the microbiomes of indoor environments? mSystems 1, doi: 10.1128/mSystems.00083-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subasinghe HA and Loh PC (1972) Reovirus cytotoxicity: some properties of the UV-irradiated reovirus and its capsid proteins. Arch Gesamte Virusforsch 39:172–189. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Fassolitis AC, Larkin EP, et al. (1971) Inactivation of thirty viruses by gamma radiation. Appl Microbiol 22:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers WC and Szybalski W (1967) Gamma-irradiation of deoxyribonucleic acid in dilute solutions. II. Molecular mechanisms responsible for inactivation of phage, its transfecting DNA, and of bacterial transforming activity. J Mol Biol 26:227–235. [DOI] [PubMed] [Google Scholar]
- Tang JW (2009) The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface 6:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai FC and Macher JM. (2005) Concentrations of Airborne culturable bacteria in 100 large US office buildings from the BASE study. Indoor Air Suppl 9:71–81. [DOI] [PubMed] [Google Scholar]
- Tseng CC and Li CS (2005) Inactivation of virus-containing aerosols by ultraviolet germicidal irradiation. Aerosol Sci Technol 39:1136–1142. [Google Scholar]
- Tseng CC and Li CS (2007) Inactivation of viruses on surfaces by ultraviolet germicidal irradiation. J Occup Environ Hyg 4:400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberoi A and Lambert PF (2017) Rodent papillomaviruses. Viruses 9, doi: 10.3390/v9120362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich SE and Byrne SN (2012) The immunologic revolution: photoimmunology. J Invest Dermatol 132:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loi V, Busche T, Preuß T, et al. (2018) The AGXX® antimicrobial coating causes a thiol-specific oxidative stress response and protein s-bacillithiolation in Staphylococcus aureus. Front Microbiol 9, doi: 10.3389/fmicb.2018.03037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viarisio D, Mueller-Decker K, Kloz U, et al. (2011) E6 and E7 from beta Hpv38 cooperate with ultraviolet light in the development of actinic keratosis-like lesions and squamous cell carcinoma in mice. PLoS Pathog 7, doi: 10.1371/journal.ppat.1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignard J, Mirey G, Salles B. (2013) Ionizing-radiation induced DNA double-strand breaks: a direct and indirect lighting up. Radiother Oncol 108:362–369. [DOI] [PubMed] [Google Scholar]
- Voorhies AA, Ott CM, Mehta S, et al. (2019) Study of the impact of long-duration space missions at the International Space Station on the astronaut microbiome. Sci Rep 9, doi: 10.1038/s41598-019-46303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Saenz HL, Götz F, et al. (2000) Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis 182:1761–1764. [DOI] [PubMed] [Google Scholar]
- Wang FI, Stohlman SA, and Fleming JO (1990) Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J Neuroimmunol 30:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mauser A, Chao S-F, et al. (2004) Virus inactivation and protein recovery in a novel ultraviolet-C reactor. Vox Sang 86:230–238. [DOI] [PubMed] [Google Scholar]
- Wang J, Tang K, Feng K, et al. (2020) High temperature and high humidity reduce the transmission of COVID-19. arXiv:2003.05003 [Google Scholar]
- Ward KN (2005) The natural history and laboratory diagnosis of human herpesviruses-6 and -7 infections in the immunocompetent. J Clin Virol 32:183–193. [DOI] [PubMed] [Google Scholar]
- Ward RL (1980) Mechanisms of poliovirus inactivation by the direct and indirect effects of ionizing radiation. Radiat Res 83:330–344. [PubMed] [Google Scholar]
- Warnes SL, Summersgill EN, and Keevil CW (2015) Inactivation of murine norovirus on a range of copper alloy surfaces is accompanied by loss of capsid integrity. Appl Environ Microbiol 81:1085–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Bather R, and Hodges RW (1963) The effect of relative humidity and inositol on air-borne viruses. Can J Microbiol 9:87–92. [Google Scholar]
- WHO (2013) WHO Expert Committee on Biological Standardization. Sixty-First Report. WHO Press, World Health Organization, Geneva, Switzerland. Available online at https://www.who.int/biologicals/expert_committee/TRS_978_61st_report.pdf?ua=1
- WHO (2014) Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec 89:256–287 [PubMed] [Google Scholar]
- WHO (2016) Ionizing Radiation, Health Effects and Protective Measures. World Health Organization, Geneva, Switzerland. Available online at https://www.who.int/news-room/fact-sheets/detail/ionizing-radiation-health-effects-and-protective-measures
- Wigginton KR, Menin L, Montoya JP, et al. (2010) Oxidation of virus proteins during UV254 and singlet oxygen mediated inactivation. Environ Sci Technol 44:5437–5443. [DOI] [PubMed] [Google Scholar]
- Wigginton KR, Menin L, Sigstam T, et al. (2012) UV radiation induces genome-mediated, site-specific cleavage in viral proteins. ChemBioChem 13:837–845. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Roberts M, Castro S, et al. (2014) Microbial monitoring of crewed habitats in space—current status and future perspectives. Microbes Environ 29:250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W and Marr LC (2012) Mechanisms by which ambient humidity may affect viruses in aerosols. Appl Environ Microbiol 78:6781–6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Chang PH, Hartert J, et al. (2018) Reactivity of enveloped virus genome, proteins, and lipids with free chlorine and UV254. Environ Sci Technol 52:7698–7708. [DOI] [PubMed] [Google Scholar]
- Zuhair M, Smit GSA, Wallis G, et al. (2019) Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev Med Virol 29, doi: 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]