Abstract
Rationale & Objective
Thrice-weekly hemodialysis can result in adequate urea clearance; however, the morbidity and mortality rates of patients treated with maintenance dialysis remain unacceptably high, partly because of nonadherence. African Americans have a higher prevalence of kidney failure treated with dialysis, greater dialysis nonadherence, and higher odds of hospitalization. We hypothesized that more precise ways of assessing dialysis treatment adherence will reflect the severity of nonadherence, distinguish patterns of nonadherence, and inform the design of personalized behavioral interventions.
Study Design
Retrospective cohort study.
Setting & Participants
African American patients receiving hemodialysis for >90 days.
Exposure
Hemodialysis.
Outcome
Dialysis adherence.
Analytical Approach
Dialysis attendance data were displayed using a dot plot, categorized based on missed and shortened treatments, and examined for patterns. Descriptive characteristics were reported. In an exploratory analysis, associations between dialysis treatment adherence and participant characteristics were evaluated using ordinary least squares regression. An analysis was performed using missed minutes of dialysis and current metrics for measuring dialysis treatment adherence (ie, missed and shortened treatments).
Results
Among 113 African American patients treated with dialysis, 47% were men; the median age was 57 years (interquartile range, 46-70 years), and the median dialysis vintage was 54 months (interquartile range, 22-90 months). With rows ordered based on the total missed minutes of dialysis, the dot plot displayed a decreasing gradient in the severity of nonadherence, with novel dialysis treatment adherence categories termed as follows: consistent underdialysis, inconsistent dialysis, and consistent dialysis. Distinct patterns of nonadherence and heterogeneity emerged within these categories. Older age was consistently associated with better adherence, as determined by the analyses performed using the total missed minutes of dialysis as well as missed and shortened treatments.
Limitations
The study findings, although replicable and paradigm-shifting, might be limited by the short timeline, focus on adherence data specific to African American patients treated with dialysis, and restriction to dialysis units affiliated with 1 academic center.
Conclusions
This study presents more precise and novel ways of measuring and displaying dialysis treatment adherence. The findings introduce a more personalized approach for evaluating actual dialysis uptake. Identification of unique patterns of adherence behavior is important to inform the design of effective behavioral interventions and improve outcomes for vulnerable African American patients treated with dialysis.
Index Words: Adherence, African Americans, dialysis, end-stage kidney disease, hemodialysis
Plain-Language Summary.
African Americans have a higher prevalence of kidney failure treated with dialysis and higher rates of nonadherence to hemodialysis, which are associated with excessive hospitalizations and increased financial costs. It is critical to improve existing knowledge of hemodialysis nonadherence by improving and enhancing the current methods of assessing hemodialysis adherence. To guide intervention development, the assessment of hemodialysis nonadherence should include details of the type of (shortened vs missed hemodialysis treatments), severity of, and variation in patterns of hemodialysis treatment nonadherence. This study fills the existing void by introducing more precise and novel ways of measuring and displaying hemodialysis adherence using missed minutes of dialysis, a continuous measure, and a graphical display of hemodialysis adherence data using a dot plot.
Kidney failure treated with dialysis is a significant public health concern.1, 2, 3 The current evidence-based practice of thrice-weekly hemodialysis treatments provides adequate urea clearance but fails to fully mitigate excessively high morbidity and mortality rates persistent in patients treated with maintenance dialysis.4 Excessive dialysis-related hospitalizations deplete >30% of the Medicare end-stage kidney disease budget of $30 billion, and patients treated with maintenance dialysis undergo twice the number of hospitalizations of other age-matched patients with chronic conditions.1,5,6 Nonadherence to prescribed dialysis treatment schedules is a key modifiable target to reduce the risk of increased hospitalizations.7, 8, 9 This is critically important among African American patients treated with dialysis, who constitute ∼12% of the US population but comprise 32% of patients treated with dialysis and face challenges associated with structural racism and health inequities.10,11 Despite a paradoxical survival benefit seen in these patients compared with non-Hispanic White patients treated with dialysis, African American patients treated with dialysis have a 4-fold higher kidney failure prevalence rate, 4-fold higher odds of hospitalization, and higher dialysis treatment nonadherence rates compared with White patients.6, 7, 8,12, 13, 14, 15, 16, 17
Dialysis treatment adherence data specific to African Americans, or those reported based on race, are limited.13,18 Details related to shortened treatments are limited, and in general, there is wide variation in the adherence levels reported in the literature. For instance, 50% of patients reportedly shortened and/or missed their treatments in a cohort with 41.2% African American patients treated with dialysis, whereas 18.4% and 3.1% of African American patients treated with hemodialysis shortened and missed their dialysis treatments, respectively, in another study.19,20 Existing literature currently defines dialysis treatment nonadherence as missing ≥1 dialysis treatments or shortening dialysis treatments by >10 or 15 minutes per treatment per month.20, 21, 22, 23 Although these definitions are currently accepted, they may benefit from augmentation with more specific details to robustly ascertain and characterize the nature of missing and shortening behaviors among patients treated with dialysis. To improve the understanding of the impact of missing and shortening dialysis treatments on outcomes as well as guide intervention development, it is important to explore more precise ways of assessing dialysis treatment nonadherence. Ideally, this should include information on the type and severity of dialysis treatment nonadherence as well as intraindividual and interindividual variability in patterns of nonadherence. It is also important to provide these details specifically for African American patients treated with dialysis.
The standards for calculating dialysis adequacy (Kt/V) extrapolate the urea clearance measured in a single dialysis and assume that dialysis occurs for the same amount of time thrice weekly, making adherence critical.24 Dialysis treatment adherence data would be more clinically meaningful and precise if the total number of missed minutes of dialysis per month were also reported. Currently, adherence data are reported as completed or missed treatments and/or the number of minutes of dialysis prescribed and delivered per treatment. However, reporting dialysis treatment adherence data as an aggregate sum of the number of missed minutes of dialysis per month will provide an opportunity for a comparison of the differential impact of the various forms of dialysis treatment nonadherence: missed versus shortened treatments. Additionally, there is a gap in our knowledge of patient behaviors specific to dialysis treatment adherence, which can be addressed by a better understanding of patterns of dialysis treatment adherence behavior. Patterns of dialysis treatment adherence behavior will provide a framework for studying and understanding the underlying psychosocial predictors of adherence, offer an opportunity for a deeper appreciation of the potential dynamic nature of these predictors, and provide additional information on the character and severity of dialysis treatment nonadherence, thereby facilitating the design of effective and personalized behavioral interventions.
Developing interventions to change patient behavior is a complex, multistaged process. It starts with an appreciation of the health problem and the underlying target behavior change, an understanding of the underlying determinants of the problematic behavior, the setting of intervention objectives, the selection of behavior change techniques, and the development of a practical intervention delivery method.25 To reach subcultural groups and reduce health disparities, behavioral interventions designed to promote the health of racial or ethnic minorities need to be culturally tailored.26, 27, 28, 29, 30 Cultural tailoring of interventions involves strategies that either have a “surface” or a “deep” structure.30 Although deep structure-based strategies involve an assessment of factors that require a profound understanding of the cultural values of a population, surface structure-based strategies match the components of an intervention with observable characteristics of the population. The former is beyond the scope of this study; however, the latter requires an understanding of the heterogeneity of any population of interest. This study aimed to provide critical information to guide the development of more effective and personalized behavioral interventions to address dialysis treatment nonadherence in African Americans.
Methods
Participant Selection
The institutional review board approved this retrospective cohort study as an exempt study, with waived informed consent. The study was conducted within a 3-month observational period (between February 2017 and April 2017) in 2 hemodialysis clinics affiliated with an urban academic medical center. The study population consisted of all African Americans, at least 18 years old, with kidney failure treated with in-center hemodialysis for >90 days.
Data Collection
The participants’ age, sex, and dialysis treatment information, such as dialysis clinic, dialysis schedule (ie, days of the week and shift), and dialysis vintage (ie, length of time on dialysis), were extracted from their medical records. The details of each dialysis treatment were extracted, including the number of prescribed and delivered dialysis treatments, number of missed and shortened dialysis treatments, number of missed and shortened minutes of dialysis, and details of emergency room visits, hospitalizations, and excused travel that occurred during the study period. Excused travel describes travel with arrangements made for dialysis elsewhere. The patients’ routine dialysis treatments were counted as completed if rescheduled, regardless of whether they occurred on a different day from the patients’ usual dialysis days (Monday, Wednesday, and Friday vs Tuesday, Thursday, and Saturday). Dialysis treatments that did not occur because of hospitalizations or excused travel were not documented as missed treatments. Nonadherence data were captured as the total number of missed minutes of dialysis as well as missed or shortened treatments, which were defined as missing ≥1 dialysis treatments per month and shortening a dialysis treatment by >15 minutes of the prescribed time for an individual treatment, respectively.
Measures
The measures included age (in years), sex (male or female), dialysis clinic (unit A or B), dialysis days (Monday, Wednesday and Friday or Tuesday, Thursday, and Saturday), dialysis shift (first, second, third, fourth), dialysis vintage (months on dialysis), dialysis vintage category (<1 year, 1-5 years, >5 years), total number of missed dialysis minutes (continuous), number of missed treatments (continuous), missed treatments (0, 1-3, 4-6, 7-9, >9), number of shortened treatments (continuous), shortened treatments (0, 1-3, 4-6, 7-9, 10-12, >12), hospitalization (no or yes), and emergency room visits (no or yes). If a treatment was shortened, it was subcategorized into shortened minutes (16-30, 31-60, or >60 minutes).
Statistical Analysis
Statistical analyses were performed using R. Descriptive statistics were reported overall and based on sex using median (interquartile range [IQR]) for continuous variables and percentage (frequency) for categorical variables. The details of every dialysis treatment for each patient were graphically displayed using a dot plot grouped by novel dialysis treatment adherence categories. The dot plot was initially created manually using Microsoft Excel and subsequently created with a combination of R and a vector graphics program. Novel dialysis treatment adherence categories were generated by a clinician and were descriptive based on the visual display of the adherence data in the dot plot over the 3-month study window. The 3-month timeline was deemed reasonable, given the evidence of significant differences in all-cause mortality among patients treated with dialysis with versus without ≥1 missed treatment over a 4-month period.13
An exploratory analysis of the association between demographic or clinical factors and dialysis treatment adherence was performed using the Wilcoxon or Kruskal-Wallis test for continuous variables and the χ2 test for categorical variables. The total number of missed minutes, missed treatments, shortened treatments, and missed + shortened treatments were analyzed using an ordinary least squares regression model. Given the nature of this small cohort and the limited observation period, this analysis was limited to a few covariates: age, sex, dialysis vintage, clinic, dialysis days, and dialysis shift. For all models, age was included with restricted cubic splines to capture potential nonlinear associations with the outcome. The association between each of the covariates and the 5 outcomes were summarized using partial effect plots. Hypotheses related to the model’s covariates were tested using likelihood ratio statistics.
Results
A total of 122 African American patients treated with maintenance dialysis received hemodialysis treatments at the affiliated dialysis clinics during the study period, and 113 were included in the study. Patients were excluded if they had received hemodialysis for <90 days (n=4), transferred care to a different dialysis clinic, or received a kidney transplant (n=5). Table 1 shows the population characteristics overall and stratified by sex. The median age of the patients was 57 years (IQR, 46-70 years), 47% of the patients were men (n=53), and the median dialysis vintage was 54 months (IQR, 22-90 months). More patients underwent dialysis according to the Monday-Wednesday-Friday schedule compared with those who underwent dialysis according to the Tuesday-Thursday-Saturday schedule (61% vs 39%, respectively). Most of the patients underwent dialysis during the first, early morning, shift (55%; n=62), whereas many of the remaining patients underwent dialysis during the second, midday, shift (35%; n=39). Very few patients underwent dialysis during the third, late afternoon (5%; n=6), and fourth, nocturnal (5%; n=6), shifts. The median number of missed minutes of dialysis was 435.5 (IQR, 113.5-1130.8). The median number of missed treatments was 1 (IQR, 0-3) and the median number of shortened treatments was 3 (IQR, 1-7) within the 3-month observation period. The number of patients who missed at least 1 treatment but did not shorten any of their treatments was 7 (6%), whereas the number of patients who shortened but did not miss any treatments was 40 (35%). The proportion of missed minutes of dialysis due to missed and shortened treatments was 57.78% (49,825 minutes) and 42.22% (36,405 minutes), respectively. A total of 55 patients (49%) both missed and shortened their dialysis treatments, whereas 11 patients (10%) had perfect dialysis treatment attendance. The proportion of female patients who missed dialysis treatments was higher than that of male patients (60% vs 49%, respectively). A total of 39 patients (35%) visited the emergency room during the study time frame, whereas 45 patients (40%) were hospitalized.
Table 1.
Dialysis Adherence Characteristics Based on Sex
| Male (n=53) | Female (n=60) | Combined (N=113) | |
|---|---|---|---|
| Age, median (IQR) | 56.0 (47.0-66.0) | 59.0 (45.8-72.0) | 57.0 (46.0-70.0) |
| Clinic | |||
| Dialysis unit A | 32% (17) | 42% (25) | 37% (42) |
| Dialysis unit B | 68% (36) | 58% (35) | 63% (71) |
| Dialysis days | |||
| MWF | 62% (32) | 61% (36) | 61% (68) |
| TTS | 38% (20) | 39% (23) | 39% (43) |
| Dialysis shift | |||
| First | 55% (29) | 55% (33) | 55% (62) |
| Second | 36% (19) | 33% (20) | 35% (39) |
| Third | 4% (2) | 7% (4) | 5% (6) |
| Fourth | 6% (3) | 5% (3) | 5% (6) |
| Vintage, median (IQR) | 56.0 (29.0-86.0) | 46.0 (21.2-91.0) | 54.0 (22.0-90.0) |
| Vintage categories | |||
| <1 y | 21% (11) | 18% (11) | 19% (22) |
| 1-5 y | 32% (17) | 42% (25) | 37% (42) |
| >5 y | 47% (25) | 40% (24) | 43% (49) |
| Number of missed treatments, median (IQR) | 0.00 (0.00-3.00) | 1.00. (0.00-2.25) | 1.00. (0.00-3.00) |
| Number of shortened treatments | 3 (1-7) | 3 (1-7) | 3 (1-7) |
| Missed treatment categories, % (n) | |||
| 0 | 51% (27) | 40% (24) | 45% (51) |
| 1-3 | 28% (15) | 42% (25) | 35% (40) |
| 4-6 | 13% (7) | 13% (8) | 13% (15) |
| 7-9 | 6% (3) | 0% (0) | 3% (3) |
| >9 | 2% (1) | 5% (3) | 4% (4) |
| Shortened treatment categories, % (n) | |||
| 0 | 15% (8) | 17% (10) | 16% (18) |
| 1-3 | 43% (23) | 38% (23) | 41% (46) |
| 4-6 | 15% (8) | 18% (11) | 17% (19) |
| 7-9 | 9% (5) | 8% (5) | 9% (10) |
| 10-12 | 6% (3) | 7% (4) | 6% (7) |
| >12 | 11% (6) | 12% (7) | 12% (13) |
| Missed/shortened categories, % (n) | |||
| Both | 45% (24) | 52% (31) | 49% (55) |
| Missed | 4% (2) | 8% (5) | 6% (7) |
| Perfect | 11% (6) | 8% (5) | 10% (11) |
| Shortened | 40% (21) | 32% (19) | 35% (40) |
| Missed min, median (IQR) | 404 (68.8-1,121.2) | 465 (138.8-1,145.0) | 435.5 (113.5-1,130.8) |
| Hospitalization categories, % (n) | |||
| Hospitalizations: no | 66% (35) | 55% (33) | 60% (68) |
| Hospitalizations: yes | 34% (18) | 45% (27) | 40% (45) |
| ER categories, % (n) | |||
| ER: no | 60% (31) | 70% (42) | 65% (73) |
| ER: yes | 40% (21) | 30% (18) | 35% (39) |
Abbreviations: ER, emergency room; IQR, interquartile range; MWF, Monday, Wednesday, and Friday; TTS, Tuesday, Thursday, and Saturday.
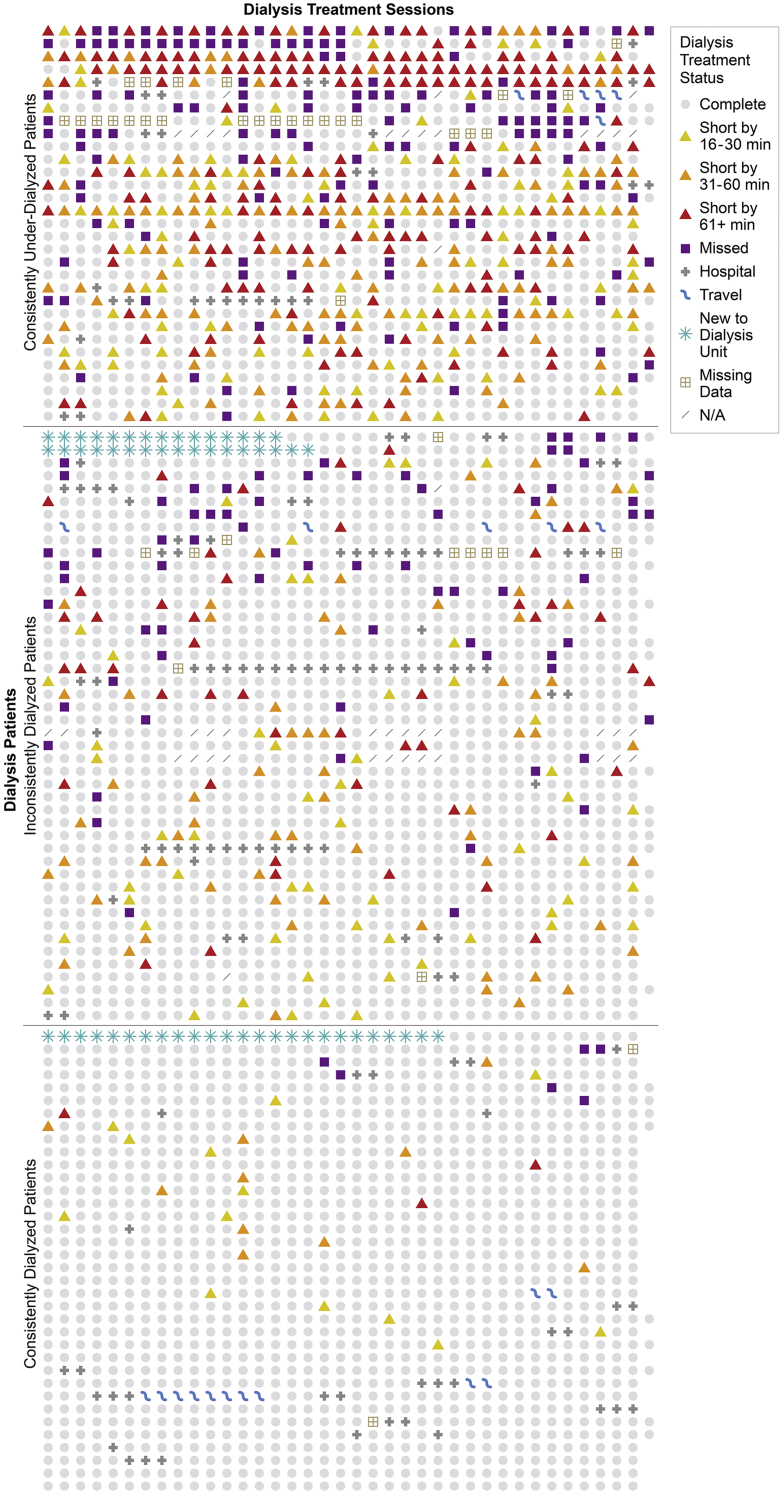
Figure 1 shows a dot plot of the treatment adherence details of each patient across the 3-month study window grouped by novel dialysis treatment adherence category and ordered based on the total missed minutes of dialysis. Because the rows were ordered based on the number of missed minutes, the figure illustrates a decreasing gradient in the severity of nonadherence from the top to the bottom of the dot plot. Three distinct patterns of nonadherence emerged within each of these qualitatively derived categories: a heterogeneous pattern of nonadherence, labeled as consistent underdialysis; a less heterogeneous pattern of nonadherence, labeled as inconsistent dialysis; and a near-homogenous pattern of adherence, labeled as consistent dialysis (Fig 1). The heterogeneous pattern seen in the consistently underdialyzed group highlights marked interindividual variation in adherence behavior, ranging from consecutive shortened treatments to consecutive missed treatments as well as missed treatments interspersed with shortened treatments. The heterogeneity in the pattern was further evident from intraindividual and interindividual variations in the severity and mix of shortened treatments within the underdialyzed group. Some patients consistently shortened their treatments by >60 minutes, others consistently shortened their treatments by either 16-30 minutes or 30-60 minutes, and others exhibited a mix in the range of shortened minutes of dialysis.
Figure 1.
Dot plot of dialysis treatment adherence by treatment days. Abbreviations: min, minutes; N/A, not applicable.
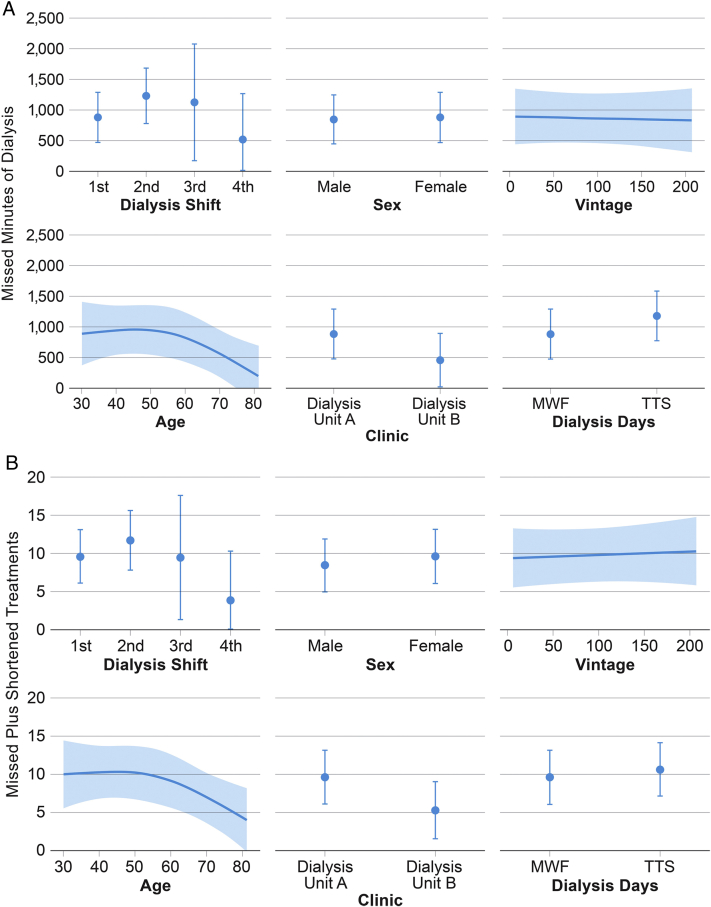
The analyses using ordinary least squares regression model to determine the relationship between dialysis adherence and demographic plus dialysis-specific factors (ie, dialysis days, shifts, and clinic) showed that age is nonlinearly associated with the total number of missed minutes (ie, sum of minutes from missed treatments and shortened treatments) of dialysis treatment. Based on a comparison of the 25th to 75th quartiles of age, a 46-year-old patient, compared with a 70-year-old patient, is estimated to miss an additional 389.32 minutes (95% confidence interval, 113.98-664.65 minutes) of dialysis treatment (P = 0.02), holding other factors constant (Fig 2A). This association held true for the analyses of age and missed plus shortened treatments; when a 46-year-old patient (25th quartile) is compared with a 70-year-old patient (75th quartile), the 46-year-old patient is estimated to miss or shorten an average of 3.33 treatments (95% confidence interval, 0.96-5.70 treatments; P = 0.02), holding other factors constant (Fig 2B).
Figure 2.
(A) Relationship between the missed minutes of dialysis and key factors. (B) Relationship between missed plus shortened treatments and key factors. Abbreviations: MWF, Monday, Wednesday, and Friday; TTS, Tuesday, Thursday, and Saturday.
Discussion
This article describes novel methods of characterizing dialysis treatment adherence. The use of total missed minutes of dialysis to assess dialysis treatment adherence is feasible, adds rigor, provides additional details on adherence, and offers a more precise assessment of adherence patterns than missed and shortened treatment categories. The dot plot is a powerful visual illustration of dialysis treatment adherence data, and its impact is further enhanced when the rows are ordered using available information on the total missed minutes of dialysis. This facilitates the creation of novel dialysis treatment adherence categories that reflect the actual uptake of dialysis. The novel dialysis treatment adherence categories described in this study are descriptive and replicable, and the dot plot provides a detailed snapshot of the severity and complexity of adherence behavior. Variation in the emerging patterns of dialysis treatment adherence within these categories and the interindividual variability in the patterns highlight the need for personalization of interventions, especially among those in the consistently underdialyzed category.
From a statistical standpoint, the measurement of dialysis treatment adherence as a continuous variable (the total minutes of missed dialysis) is more powerful than presenting the same data as a dichotomous or categorical variable because it prevents the loss of information and the underestimation of the extent of variability in outcomes between groups.31 From a clinical perspective, missed minutes of dialysis is a uniform and standardized measure of both missed and shortened treatments that offers an opportunity for a comparison of the differential impact of both forms of nonadherence on clinical outcomes. As a more precise measure of adherence, it can potentially be leveraged as a shared decision-making tool to provide clear and complete information to patients, support autonomy related to the self-care of patients treated with dialysis, and encourage adherence.32 Further, the measurement of adherence in terms of the missed minutes of dialysis aligns very closely with the current metrics of measuring dialysis adequacy (Kt/V), which incorporate the number of delivered minutes of dialysis into its calculation.24 Thus, as a measure of dialysis treatment adherence, reporting the missed minutes of dialysis provides additional clinically meaningful information. From a research vantage point, missed minutes of dialysis provide more precise information than missed or shortened treatments and are useful for evaluating adherence-related outcomes, such as hospitalizations and mortality, and designing tailored interventions.
Reporting adherence rates alone provides limited insight into a patient’s underlying behavior. On the other hand, categorization of patients into groups based on adherence metrics and appreciation of variation in patterns of adherence behavior existent within categories of patients with similar adherence metrics, as illustrated in the dot plot, is extremely useful. It will enhance our understanding of the underlying barriers to successful patient engagement in all prescribed dialysis treatments and provide relevance to clinical practice. Describing and understanding patterns of adherence behavior will facilitate the design of personalized interventions. This has been demonstrated in adherence research, wherein categorization of adherence behavior patterns made up for the wide variation in reported rates of adherence with ocular antihypertensive eye drops and provided additional information about patient behavior, including predictors of adherence.33 Patients who discontinue these eye drops after a short time are different from those who go on consecutive, prolonged “drug holidays”; those who have variable and frequent missed doses; and those who are mostly adherent.33 The recognition and integration of knowledge of these underlying differences in adherence behavior has led to a good agreement between adherence categories and medications or behavioral treatments.34 Research conducted on adherence to antiretroviral medications has provided patient-centered evidence that patients do not view adherence as a single entity, but rather as variable patterns of adherence. In a qualitative study describing patient-led classification of adherence, it was noted that adherence behavioral patterns likely have different causes and health consequences; predictors of adherence may not necessarily be pattern-specific; and patterns of adherence behavior may not be stable over time.35 These have implications in intervention development.
The graphical presentation of adherence data was successfully used to identify and present medication adherence data for travoprost eye drops by plotting interdose intervals against time.36 Such a graphical format made it easy to identify the patterns of medication adherence behavior and provided additional information such as data on treatment holidays and changes in adherence over time.36 To our knowledge, no study of dialysis treatment adherence has presented dialysis treatment adherence data in a graphical format. The use of the dot plot in this study as a powerful graphical illustration of adherence data was motivated by a study that used the dot plot for the illustration of mutation spectra of gene sequences from vector proviral DNA.37 This novel use of the dot plot in this study provides additional useful information to inform research and clinical practice, such as the density of nonadherence; pertinent dialysis treatment adherence-related details, eg, excused travel, emergency room visits, and hospitalizations; and heterogeneity in adherence pattern, especially among those patients who consistently undergo underdialysis.
Importantly, the dot plot provides additional, novel metrics that could be useful for facility-level evaluations. It also provides vital information for quality improvement, which could improve care in facilities by drawing attention to the need for an increase in social work efforts. Although its application can certainly be extended to other patient populations, the use of the dot plot in this study as an excellent analytic tool for enhanced identification of nonadherence in African American patients treated with hemodialysis who are affected by social determinants of health, aligns well with the current goals of the Center for Medicare and Medicaid Services concerning increasing health equity and reducing disparities in care.
In this study, potential bias in the amount of prescribed or delivered dialysis was not measured. Bias in delivered dialysis might have existed if the dialysis staff had taken the patients off dialysis early or encouraged them not to visit for dialysis. Bias in prescribed dialysis might have existed if African American patients treated with dialysis had been prescribed longer dialysis times when their health status might support a shorter time compared with other patients treated with dialysis. Although racial disparities in hemodialysis quality measures have been well described, racial differences specific to dialysis prescription, with a focus on treatment duration, have not been reported.38 There is lack of existing data on racial disparities in measured urine output; however, being Black has been associated with a lower likelihood of self-reported urine output.39 Because residual kidney function is important for hemodialysis prescriptions, a consistent evaluation of self-reported urine output in the clinical setting should be considered because it will likely enhance dialysis patients’ awareness of the importance of residual kidney function on their health outcomes, increase their engagement in their care, and optimize the prescribed hemodialysis time. It is important to acknowledge that a shorter hemodialysis duration may not necessarily lead to improved adherence, and it has actually been associated with a reduced risk of nonadherence to treatment among patients treated with dialysis.40 Further, in a study limited to African American patients treated with hemodialysis, those who underwent dialysis according to the Tuesday-Thursday-Saturday schedule had higher “no-show” rates and shortened hemodialysis times, suggesting that other factors, such as days of the week, influence dialysis uptake.41 Nonetheless, the importance of shared decision making in optimizing dialysis care cannot be overemphasized, and it should extend beyond its application in the selection of kidney replacement therapy and dialysis initiation to include its application in the personalization of dialysis prescriptions.42,43
This study fills a void by proposing an advancement in the method of measuring adherence by presenting dialysis treatment adherence data as a continuous measure of missed minutes of dialysis per month. In addition to increasing precision, it provides a holistic overview of dialysis adherence by integrating data on shortened dialysis treatments, thus augmenting the existing dialysis treatment adherence literature.7,8,18 This study introduces a unique categorization system for dialysis treatment adherence. Further, it pilots a novel visual presentation of adherence data with the categorization of patients into groups based on the severity of nonadherence and the identification of unique patterns of adherence behavior within categories, which is necessary to inform the design of personalized interventions. It is not enough to simply group patients into adherence categories based on rates; it is important to appreciate that there could also be a gradient of actual delivered dialysis within each adherence category because of unique adherence patterns. These could be reflective of different patient behaviors, plausibly driven by a variety of underlying psychosocial factors. This study’s singular focus on the vulnerable population of African American patients treated with maintenance dialysis receiving in-center hemodialysis who are at an increased risk of being nonadherent to dialysis is novel. However, we acknowledge that the measures we introduced in this study help in the identification of opportunities for intervention in all patients treated with hemodialysis, irrespective of their race or ethnicity. Finally, this study’s strategy for better understanding adherence behavioral patterns and enhancing chances of successfully designing effective adherence interventions in the future is unique.
The study findings have some limitations. First, the findings might not be generalizable because this was a 3-month observational study in a limited sample of African American patients treated with dialysis from dialysis centers affiliated with 1 academic medical center. However, this is a representative sample of African American patients treated with dialysis, and these findings are replicable, innovative, and thought-provoking and shift the paradigm in thinking in terms of the current metrics for reporting, describing, and displaying adherence data. Second, we did not review the adherence data for the entire cohort of patients in the dialysis unit at the time; therefore, there might have been unit-level factors contributing to the adherence patterns that we could not explore because we did not have data available for all the patients in the facility at that time. Third, variation in dialysis adherence patterns might be difficult to appreciate if the available data are limited (eg, 1 month of adherence data); hence, the 3-month timeline was selected for this study. Further, an assessment of the impact of missed minutes of dialysis on clinical outcomes, the potential presence of an unmeasured bias in dialysis prescriptions for African American patients treated with dialysis, and the association between shared decision making to determine the optimal dialysis prescription and reasons for missed dialysis treatments are beyond the scope of this study. Future research should include a more extensive study with a longer timeline to provide additional information on the impact of measuring missed minutes of dialysis on clinical practice, stability or changes in the observed dialysis adherence patterns, the definition of quantitative cutoff values for the novel adherence categories over time, and the association between adherence categories and predictors or clinical outcomes. Finally, the data available from this observational cohort did not include pertinent data such as reasons for shortening treatments (eg, late transportation), which might have been outside a patient’s control. However, the recognition of variations in patterns of adherence behavior in patients treated with dialysis will facilitate the development of personalized interventions that involve a deeper investigation of, and a targeted approach toward handling the underlying reasons for adherence.
In summary, dialysis treatment adherence is a challenge in the African American population, and effective interventions remain elusive. The current methods of assessing dialysis treatment adherence are simplistic, fail to reflect the complexity of adherence, and could be improved to facilitate the successful design of adherence interventions. This study presents more precise and novel ways of measuring dialysis treatment adherence, displaying it graphically, grouping it into categories, and incorporating the heterogeneity in patterns of adherence into the intervention’s design. This paradigm shift in dialysis treatment adherence measurement and display is necessary to significantly advance research and clinical practice and inform a personalized approach toward the achievement of consistent dialysis for vulnerable African American patients treated with dialysis. It is a critical step in the path to addressing existing disparities to achieve equity in terms of providing care for patients with kidney disease.
Article Information
Authors’ Full Names and Academic Degrees
Ebele M. Umeukeje, MD, MPH, Deklerk Ngankam, MD, Lauren B. Beach, JD, PhD, Jennifer Morse, MS, Heather L. Prigmore, MPH, Thomas G. Stewart, PhD, Julia B. Lewis, MD, and Kerri L. Cavanaugh, MD, MHS.
Authors’ Contributions
Research idea and study design: EMU, KLC, DN, LBB; data acquisition: EMU, DN; statistical analysis: JM, HLP, TGS; data analysis/interpretation: EMU, DN, LBB, JL, KLC; supervision or mentorship: EMU, KLC. EMU and DN contributed equally to this work. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant 1K23DK114566-01A1 (to Dr Umeukeje); National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant 3 R01 DK 103935-03S1 (to Dr Cavanaugh); and the Vanderbilt University Medical Center Student Research and Training Program. The funders of the study had no role in the study design, collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received June 09, 2021. Evaluated by 3 external peer reviewers, with direct editorial input by a Statistical Editor and the Editor-in-Chief. Accepted in revised form October 6, 2021.
Footnotes
Complete author and article information provided before references.
Deklerk Ngankam is a co-first author.
References
- 1.Chan K.E., Lazarus J.M., Wingard R.L., Hakim R.M. Association between repeat hospitalization and early intervention in dialysis patients following hospital discharge. Kidney Int. 2009;76(3):331–341. doi: 10.1038/ki.2009.199. [DOI] [PubMed] [Google Scholar]
- 2.Flythe J.E., Katsanos S.L., Hu Y., Kshirsagar A.V., Falk R.J., Moore C.R. Predictors of 30-day hospital readmission among maintenance hemodialysis patients: a hospital’s perspective. Clin J Am Soc Nephrol. 2016;11(6):1005–1014. doi: 10.2215/cjn.11611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases 2018 USRDS annual data report: epidemiology of kidney disease in the United States. https://www.usrds.org/2018/view/Default.aspx
- 4.Himmelfarb J. The HEMO study—where do we go from here? Curr Opin Nephrol Hyperten. 2003;12(6):587–591. doi: 10.1097/00041552-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Mathew A.T., Strippoli G.F., Ruospo M., Fishbane S. Reducing hospital readmissions in patients with end-stage kidney disease. Kidney Int. 2015;88(6):1250–1260. doi: 10.1038/ki.2015.307. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. 2015 USRDS annual data report. Atlas of chronic kidney disease in the United States. https://www.usrds.org/media/2293/vol2_usrds_esrd_15.pdf. Accessed January 26, 2016.
- 7.Saran R., Bragg-Gresham J.L., Rayner H.C., et al. Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int. 2003;64(1):254–262. doi: 10.1046/j.1523-1755.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 8.Chan K.E., Thadhani R.I., Maddux F.W. Adherence barriers to chronic dialysis in the United States. J Am Soc Nephrol. 2014;25(11):2642–2648. doi: 10.1681/asn.2013111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaiciuniene R., Kuzminskis V., Ziginskiene E., Skarupskiene I., Bumblyte I.A. Adherence to treatment and hospitalization risk in hemodialysis patients. J Nephrol. 2012;25(5):672–678. doi: 10.5301/jn.5000038. [DOI] [PubMed] [Google Scholar]
- 10.Norton J.M., Moxey-Mims M.M., Eggers P.W., et al. Social determinants of racial disparities in CKD. J Am Soc Nephrol. 2016;27(9):2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crews D.C., Purnell T.S. COVID-19, racism, and racial disparities in kidney disease: galvanizing the kidney community response. J Am Soc Nephrol. 2020;31(8):1–3. doi: 10.1681/asn.2020060809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan G., Norris K.C., Alison J.Y., et al. The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol. 2013;8(6):953–961. doi: 10.2215/CJN.09180912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Salmi I., Larkina M., Wang M., et al. Missed hemodialysis treatments: international variation, predictors, and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2018;72(5):634–643. doi: 10.1053/j.ajkd.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Gerber B.S., Cho Y.I., Arozullah A.M., Lee S.Y. Racial differences in medication adherence: a cross-sectional study of Medicare enrollees. Am J Geriatr Pharmacother. 2010;8(2):136–145. doi: 10.1016/j.amjopharm.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aikens J.E., Piette J.D. Diabetic patients’ medication underuse, illness outcomes, and beliefs about antihyperglycemic and antihypertensive treatments. Diabetes Care. 2009;32(1):19–24. doi: 10.2337/dc08-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel I., Erickson S.R., Caldwell C.H., et al. Predictors of medication adherence and persistence in Medicaid enrollees with developmental disabilities and type 2 diabetes. Res Social Adm Pharm. 2016;12(4):592–603. doi: 10.1016/j.sapharm.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Curtin R.B., Svarstad B.L., Keller T.H. Hemodialysis patients’ noncompliance with oral medications. ANNA J. 1999;26(3):307–316. [PubMed] [Google Scholar]
- 18.Som A., Groenendyk J., An T., et al. Improving dialysis adherence for high risk patients using automated messaging: proof of concept. Sci Rep. 2017;7(1):1–7. doi: 10.1038/s41598-017-03184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman R.A., Cody R.P., Matera J.J., Rogers M.E., Solanchick J.C. Deficiencies in delivered hemodialysis therapy due to missed and shortened treatments. Am J Kidney Dis. 1994;24(6):921–923. doi: 10.1016/s0272-6386(12)81061-4. [DOI] [PubMed] [Google Scholar]
- 20.Obialo C.I., Hunt W.C., Bashir K., Zager P.G. Relationship of missed and shortened hemodialysis treatments to hospitalization and mortality: observations from a US dialysis network. Clin Kidney J. 2012;5(4):315–319. doi: 10.1093/ckj/sfs071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecking E., Bragg-Gresham J.L., Rayner H.C., et al. Haemodialysis prescription, adherence and nutritional indicators in five European countries: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004;19(1):100–107. doi: 10.1093/ndt/gfg418. [DOI] [PubMed] [Google Scholar]
- 22.Tapolyai M., Fülöp T., Uysal A., et al. Regional differences in nonadherence to dialysis among southern dialysis patients: a comparative cross-sectional study to the dialysis outcomes and practice patterns study. Am J Med Sci. 2010;339(6):516–518. doi: 10.1097/MAJ.0b013e3181d94f7a. [DOI] [PubMed] [Google Scholar]
- 23.Tohme F., Mor M.K., Pena-Polanco J., et al. Predictors and outcomes of non-adherence in patients receiving maintenance hemodialysis. Int Urol Nephrol. 2017;49(8):1471–1479. doi: 10.1007/s11255-017-1600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Kidney Foundation KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Heath G., Cooke R., Cameron E. A theory-based approach for developing interventions to change patient behaviours: a medication adherence example from paediatric secondary care. Healthcare (Basel, Switzerland) 2015;3(4):1228–1242. doi: 10.3390/healthcare3041228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey R.D., Afful S.E. Racial typicality, racial identity, and health behaviors: a case for culturally sensitive health interventions. J Black Psychol. 2011;37(2):164–184. doi: 10.1177/0095798410376244. [DOI] [Google Scholar]
- 27.Kreuter M.W., Lukwago S.N., Bucholtz R.D., Clark E.M., Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: targeted and tailored approaches. Health Educ Behav. 2003;30(2):133–146. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- 28.Henderson S., Kendell E., See L. The effectiveness of culturally appropriate interventions to manage or prevent chronic disease in culturally and linguistially diverse communities: a systematic literature review. Health Soc Care Community. 2011;19(3):225–249. doi: 10.1111/j.1365-2524.2010.00972.x. [DOI] [PubMed] [Google Scholar]
- 29.Barrera M., Jr., Castro F.G., Strycker L.A., Toobert D.J. Cultural adaptations of behavioral health interventions: a progress report. J Consult Clin Psychol. 2013;81(2):196–205. doi: 10.1037/a0027085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resnicow K., Baranowski T., Ahluwalia J.S., Braithwaite R.L. Cultural sensitivity in public health: defined and demystified. Ethn Dis. 1999;9(1):10–21. [PubMed] [Google Scholar]
- 31.Altman D.G., Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332(7549):1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorillo A., Barlati S., Bellomo A., et al. The role of shared decision-making in improving adherence to pharmacological treatments in patients with schizophrenia: a clinical review. Ann Gen Psychiatry. 2020;19(1):1–12. doi: 10.1186/s12991-020-00293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cate H., Bhattacharya D., Clark A., Holland R., Broadway D.C. Patterns of adherence behaviour for patients with glaucoma. Eye (Lond) 2013;27(4):545–553. doi: 10.1038/eye.2012.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gueorguieva R., Wu R., Krystal J.H., Donovan D., O'Malley S.S. Temporal patterns of adherence to medications and behavioral treatment and their relationship to patient characteristics and treatment response. Addict Behav. 2013;38(5):2119–2127. doi: 10.1016/j.addbeh.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill Z., Kendall C., Fernandez M. Patterns of adherence to antiretrovirals: why adherence has no simple measure. AIDS Patient Care STDs. 2003;17(10):519–525. doi: 10.1089/108729103322494311. [DOI] [PubMed] [Google Scholar]
- 36.Ajit R.R., Fenerty C.H., Henson D.B. Patterns and rate of adherence to glaucoma therapy using an electronic dosing aid. Eye (Lond) 2010;24(8):1338–1343. doi: 10.1038/eye.2010.27. [DOI] [PubMed] [Google Scholar]
- 37.Dapp M.J., Clouser C.L., Patterson S., Mansky L.M. 5-azacytidine can induce lethal mutagenesis in human immunodeficiency virus type 1. J Virol. 2009;83(22):11950–11958. doi: 10.1128/JVI.01406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris K.C., Williams S.F., Rhee C.M., et al. Hemodialysis disparities in African Americans: the deeply integrated concept of race in the social fabric of our society. Semin Dial. 2017;30(3):213–223. doi: 10.1111/sdi.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.You A.S., Kalantar-Zadeh K., Obi Y., et al. Residual urine output and mortality in a prospective hemodialysis cohort. Kidney Int Rep. 2020;5(5):643–653. doi: 10.1016/j.ekir.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozen N., Cinar F.I., Askin D., Mut D., Turker T. Nonadherence in hemodialysis patients and related factors: a multicenter study. J Nurs Res. 2019;27(4):e36. doi: 10.1097/jnr.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obialo C.I., Bashir K., Goring S., et al. Dialysis “no-shows” on Saturdays: implications of the weekly hemodialysis schedules on nonadherence and outcomes. J Natl Med Assoc. 2008;100(4):412–419. doi: 10.1016/S0027-9684(15)31274-8. [DOI] [PubMed] [Google Scholar]
- 42.Ameling J.M., Auguste P., Ephraim P.L., et al. Development of a decision aid to inform patients’ and families’ renal replacement therapy selection decisions. BMC Med Inform Decis Mak. 2012;12(1):1–14. doi: 10.1186/1472-6947-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheu J., Ephraim P.L., Powe N.R., et al. African American and non-African American patients’ and families’ decision making about renal replacement therapies. Qual Health Res. 2012;22(7):997–1006. doi: 10.1177/1049732312443427. [DOI] [PMC free article] [PubMed] [Google Scholar]