Abstract
Obesity is a major public health problem in the developed world, where it has reached an epidemic status over the last few decades. In parallel with this, the prevalence of chronic kidney disease (CKD) has increased. Although obesity is a risk factor for hypertension and diabetes, it is also independently associated with the development and progression of CKD. Two-third of patients with CKD worldwide will be residents of developing countries by the year 2030. Risk factors for CKD are prevalent in the sub-Saharan Africa region; this review discusses the available data regarding the relationship between obesity and CKD. The prevalence of CKD appears to correlate with increasing adiposity in sub-Saharan Africa; however, limited data are currently available, and the analysis of this association is further complicated by a variety of parameters used to define obesity. (eg, body mass index vs waist circumference). Longer, large-scale studies are needed to inform the prevalence and kidney implications of obesity in sub-Saharan Africa.
Index Words: Obesity, Chronic kidney disease, risk factors, Sub-saharan Africa
Chronic kidney disease (CKD) is an important noncommunicable disease, with numerous causes, that leads to a huge burden of morbidity and mortality worldwide.1 It has shown increasing prevalence in sub-Saharan Africa.2,3 Obesity not only exacerbates major risk factors for CKD, such as hypertension and diabetes mellitus, but has also been recognized as a risk factor for the development and progression of CKD, and this can be independent of other traditional risk factors such as age, blood pressure, and proteinuria.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 Obesity is projected to cause a loss of 80,000,000 years of life by 2040.15 Developing countries, including those in the sub-Saharan Africa region, are experiencing a significant transition with increasing levels of physical inactivity and unhealthy diets, resulting in increasing adiposity and a consequent rise in the burden of associated chronic diseases.16 However, most epidemiologic studies conducted in sub-Saharan Africa failed to emphasize the important contribution of the rising prevalence of obesity to the increasing rates of CKD. In 2005, 80% of global deaths due to chronic diseases occurred in developing nations.17 It has been projected that worldwide, by 2030, around 75% and 80% of people with hypertension and diabetes, respectively, will be residing in developing nations18,19; these conditions are not only risk factors for CKD but also have a strong association with obesity.20,21 CKD leads to a large health and economic burden, and if obesity continues to rise unabated, the consequent rise in the prevalence of CKD alone will pose a colossal challenge, worsening the existing poor outcomes in patients with CKD in sub-Saharan Africa.22 Data examining the association between CKD and obesity in the region are sparse, and we hope that this review will stimulate further interest in elucidating the relationship between CKD and obesity and consequent health consequences in sub-Saharan Africa. Therefore, the objectives of this article were to analyze the burden of obesity and discuss its association with CKD in sub-Saharan Africa.
Geography, Demography, and Health Challenges of Sub-Saharan Africa
Sub-Saharan Africa consists of 48 countries (Fig 1), with a total population of 1.1 billion (out of a total African population of 1.3 billion) and a male-to-female ratio of 1:1.23 The sub-Saharan Africa region is predominantly rural, but urbanization is on the increase. There is a rapid population growth of about 4% per year (compared with 3.4% worldwide), and the number of inhabitants has been projected to double by 2050.24
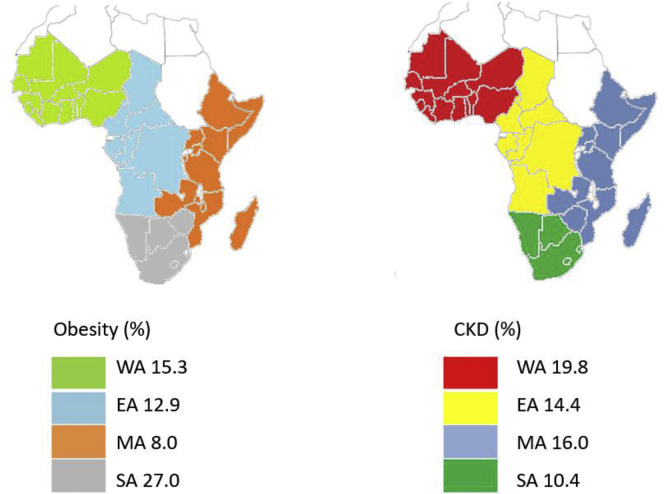
Figure 1.
Map of Africa showing the percentage prevalence of obesity and chronic kidney disease according to region in sub-Saharan Africa. CKD, chronic kidney disease; EA, East Africa; MA, Middle Africa; SA, South Africa; WA, West Africa.
There are major health challenges in sub-Saharan Africa, as reflected in the health outcome indices of the region; in particular, poor availability of funding is associated with suboptimal health infrastructure and service delivery.14,23 Infectious diseases are prevalent in the region, and the rates of noncommunicable diseases, such as hypertension and diabetes, are increasing.2,18,19 These 2 cardiovascular risk factors, which are associated with obesity, are the leading causes of CKD, which has a regional prevalence of 13.9%-17.7%.3,25 Obesity, well known to be common in Western countries, is on the increase in sub-Saharan Africa, with as much as a 70% increase over 25 years.26
High poverty levels predominate much of sub-Saharan Africa. Health care is largely based on out-of-pocket payments; for this reason, many patients do not seek or are denied access to assessment and treatment. Therefore, late presentation of irreversible CKD is a common phenomenon. Facilities and services for lifestyle modification and health promotion are either not available or difficult to come by in most of the region. Additionally, poverty and poor levels of education and awareness about CKD and its associated risks significantly contribute to the development and progression of CKD.27,28
Obesity In Sub-Saharan Africa
An epidemiologic study of obesity has demonstrated significant ethnic differences (Fig 2) influenced by physical factors, social support structures, socioeconomic issues, and cultural beliefs. Rapid urbanization and a drastic change in dietary habits are also contributing to the increasing prevalence of obesity in sub-Saharan Africa.29 There are strong attitudinal and institutional challenges to overcome to reduce the prevalence of obesity and its associated problems in the region.30,31 In some parts of sub-Saharan Africa, a large body habitus is still regarded as a sign of affluence, well-being and the absence of chronic disease such as acquired immunodeficiency syndrome.32,33
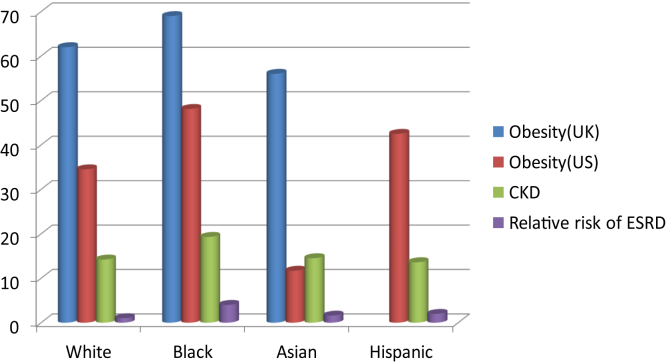
Figure 2.
Prevalence of obesity and chronic kidney disease and the relative risk of end-stage renal disease among US and United Kingdom adults classified by race. Abbreviations: CKD, chronic kidney disease; ESRD, end-stage renal disease; UK, United Kingdom; US, United States.
The report of the Non-Communicable Disease Risk Factor Collaboration showed that sub-Saharan Africa has witnessed a dramatic increase in the prevalence of obesity, more than that in other regions, over recent decades (Fig 3).34 Data pooled from 2,416 studies (including 130 million participants between 1975 and 2016) revealed a higher increase of 3 and 4 kg/m2 in the mean body mass index (BMI) among men and women, respectively, in sub-Saharan Africa compared with 2 and 2.6 kg/m2, respectively, in other parts of the world.34 In Eritrea, obesity (defined as a BMI of ≥30 kg/m2) increased from <5% in 1994 to around 15% in 2004.35 Recent analyses have shown rates of 17.1% in Ghana and 15.1% in Cameroon.36,37 The previous difference in the prevalence of obesity between men and women is gradually getting eroded, and the gap between rural versus urban and between developing versus developed regions is similarly closing as more towns evolve into cities.38 Developing nations are now the predominant drivers of the increase in global obesity, whereas the rates in developed countries seem to have plateaued.39 Disappointingly, governments and health policy managers in sub-Saharan Africa have been devoting limited time and resources to the emerging epidemic of obesity and its related health risk.40 The relatively positive attitude of the society toward obesity in addition to underreporting of its prevalence and heterogeneity of techniques used to measure it (discussed below) have exacerbated the situation, lowering its priority among policymakers.
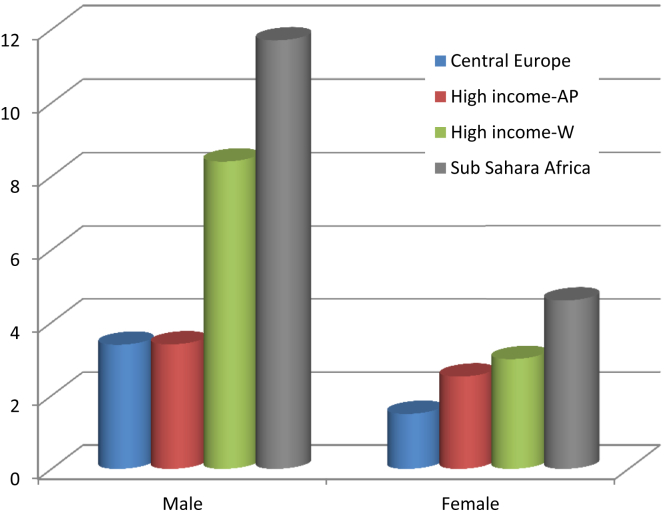
Figure 3.
Increase in the prevalence of obesity between 1975 and 2016 classified by region. Bars show the ratio of obesity prevalence rates in 2016 to those in 1975. Abbreviations: AP, Asian Pacific; W, Western countries.
Obesity and CKD In Sub-Saharan Africa: Choice Between Waist Circumference and BMI?
The reliance of many analyses on the use of BMI alone to define obesity has hampered progress in this area because they exclude valuable data from studies using other parameters.41,42 In a review of 75 studies conducted by Chukwuonye et al,43 only 4 publications used BMI and were, therefore, included. Many of the remaining studies defined obesity based on waist circumference, and it was argued that this had no universally agreed cutoff for the definition of obesity. However, waist circumference is now gaining global acceptance as a robust measure of adiposity and a strong predictor of cardiovascular risk.44 Indeed, both the International Diabetes Federation and World Health Organization have adopted waist circumference as one of the defining criteria for metabolic syndrome, a condition that has a prevalence of up to 50% in sub-Saharan Africa and affects CKD.45,46
It is established that there is variation in body fat distribution in populations from different geographic regions.47, 48, 49 Although used as the sole measure of obesity by almost 60% of researchers, a major limitation of BMI is its inability to differentiate elevated body fat from increased lean body mass. Therefore, overall dependence on BMI as the sole measure will likely lead to underestimation of the true prevalence of obesity and its consequent chronic diseases, including CKD. These differences are exemplified by some studies that were conducted in Nigeria and Ghana. In a report from the southeast region of Nigeria, the prevalence of obesity defined based on BMI was 11.12% compared with 21.75% defined using waist circumference.50 Another study from southwest Nigeria (which also used waist circumference) showed an even higher figure of 33.8%.51 In a semiurban Nigerian community study, the prevalence of obesity defined using BMI and waist circumference was 8.5% and 32%, respectively.52 In a cross-sectional study conducted in Ghana among people >50 years of age, >50% of participants had abdominal obesity, whereas only 15% fit the BMI criteria.53
Overall, the data available from the region examining the relationship between obesity and CKD are limited. There are no large prospective studies, but there are cross-sectional ones with small sample sizes that have often performed secondary analyses to investigate the association between obesity and CKD. Variation in the parameters used to define obesity further compounds the difficulty in drawing robust conclusions. In a Nigerian community study including around 1,500 participants, Olanrewaju et al54 found that obesity defined using the waist-to-hip ratio, not waist circumference or BMI, was associated with CKD (odds ratio, 1.5; 95% confidence interval, 1.10-2.05) (Table 1).54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72 In a cross-sectional study among teachers in South Africa, waist circumference was significantly associated with CKD, and in Senegal, a higher BMI was associated with CKD.55,56 When participants with CKD were compared with those without CKD in a cross-sectional study from the Cape Town Vascular and Metabolic Health study, a significantly higher BMI (30.4 vs 28.2 kg/m2), waist circumference (99 vs 90.8 cm), and visceral adipose tissue (228.4 vs 174.9 cm2) were reported, but there was no significant difference in subcutaneous adipose tissue.20 When adjusted for age, sex, and CKD, only high BMI and waist circumference showed an association with increasing systolic, diastolic, and mean arterial blood pressures. Further analysis of their data showed associations between BMI and waist circumference and a higher blood pressure only in the non-CKD cohort. Therefore, it can be inferred that increased adiposity may promote the initiation of CKD through its effect on increasing blood pressure, but once CKD is established, factors other than obesity might facilitate its progression. The phenomenon of “reverse epidemiology and survival paradox” has been proposed, whereby increased adiposity may have a protective effect in people with CKD.73 Conversely, in a study from Ghana conducted by Aryee et al74 among individuals with hypertension, waist circumference, not BMI, correlated with decreased kidney function. In the US Jackson Heart study, waist circumference, rather than BMI, was associated with incident CKD among African Americans.11 The authors of this report attributed its failure to demonstrate the progression of CKD among obese participants to the inability of BMI to differentiate between lean and nonlean body mass.
Table 1.
Summary of Publications on Obesity and Chronic Kidney Disease
| Author/Country | Study Type/Setting | Sample Size | Prevalence of Obesity/CKD/Proteinuria | Parameters | OR for Association With Obesity | CI | P Value |
|---|---|---|---|---|---|---|---|
| Sumaili et al,57 Democratic Republic of Congo | Cross-sectional community study | 3,018 | Proteinuria 17.1% | Proteinuria and BMI ≥ 25 kg/m2 | 1.2 | 1.02-1.6 | 0.03 |
| Okwuonu et al,58 Nigeria | Cross-sectional study | 304 | CKD 4.6% | CKD and BMI WC |
1.3 3.8 |
1.20-6.21 1.13-12.68 |
<0.001 <0.001 |
| Adeniyi et al,55 South Africa | Cross-sectional study among school teachers | 489 | Obesity (WC) 59.6% CKD 6.4% |
BMI WC |
1.04 2.48 |
1.0-1.07 1.01-5.87 |
0.213 0.046 |
| Matsha et al,59 South Africa | Cross-sectional study | 1,256 | Obesity (BMI) 7.2% CKD 14.8% |
CKD and BMI | 0.81 | 0.77-0.85 | |
| Wachukwu et al,60 Nigeria | Cross-sectional study among university staff | 259 | Obesity (BMI) 12.2% CKD 1.9 % Proteinuria 12.4% |
CKD and BMI | 0.51 | 0.001a | |
| Adebamowo et al,61 4 African countries | Hospital multicenter study among people with T2DM | 4,815 | CKD 13.4% | CKD and BMI | 1.93 | 1.14-3.27 | <0.05 |
| Kaze et al,62 Cameroon | Cross-sectional community study | 500 | Obesity (BMI) 60.4% Low eGFR 11% Albuminuria 7.2% |
CKD and BMI Albuminuria and BMI |
1.09 1.01 |
1.01-1.18 0.94-1.08 |
0.032 0.773 |
| Seck et al,63 Senegal | Cross-sectional community study | 1,037 | Obesity (BMI) 23.4% CKD 4.9% Albuminuria 3.4% |
CKD and BMI | 1.33 | 1.15-3.98 | 0.02 |
| Faye et al,56 Senegal | Cross-sectional study | 1,411 | Obesity (13%) CKD 36% Proteinuria 15% |
CKD and BMI | 0.0017b | ||
| Afolabi et al,64 Nigeria | Hospital-based cross-sectional study | 250 | CKD 10.4% Albuminuria 12.4% |
ACR and ACR | 0.163 | ||
| Lunyera et al,65 Uganda | Cross-sectional community study | 141 | Obesity (BMI) 30% Proteinuria 13% |
Proteinuria and BMI | 0.54 | 0.15-1.93 | NS |
| Egbi et al,66 Nigeria | Cross-sectional study among civil servants | 179 | CKD 7.8% BMI NA |
CKD and BMI | 0.279 | <0.001a | |
| Owiredu et al,67 Ghana | Hospital-based study | 146 | CKD NA Mets 30.1% |
CKD and Mets | 3.4 | 1.2-9.3 | 0.05 |
| Olanrewaju et al,54 Nigeria | Cross-sectional community study | 1,350 | Obesity (BMI) 8.7% CKD 12% |
CKD and waist-to-hip ratio | 1.50 | 1.10-2.05 | 0.01 |
| Gbadegesin et al,68 Nigeria | Hospital study of CKD among patients with hypertension | 250 | Obesity 36.1% CKD 28% Macroalbuminuria 5.6% Microalbuminuria 42% |
CKD and WC CKD and BMI |
0.22 1.12 |
0.02-0.30 0.81-1.44 |
0.03 0.001 |
| Stanifer et al,69 Tanzania | Cluster-design cross-sectional study | 481 | Obesity (BMI) 4.7% CKD 7% |
CKD and BMI | 0.81 | 0.45-1.47 | |
| Babua et al,70 Uganda | Hospital-based cross-sectional study | 217 | Obesity (BMI) 10.1% CKD NA |
CKD and BMI | - | - | 0.797b |
| Oluyombo et al,72 Nigeria | Community cross-sectional study | 454 | Obesity (BMI) 2.6% Waist-to-hip ratio WC 14.1% CKD 12.3% |
0.026b 0.023b |
|||
| Ephraim et al,71 Ghana | Hospital study in high-risk individuals | 382 | CKD 30.2% Obesity (BMI) 28% |
CKD and BMI | 0.13 | 0.01-1.31 | 0.083 |
Abbreviations: ACR, albumin-creatinine ratio; BMI, body mass index; CKD, chronic kidney disease; Mets, metabolic syndrome; NA, not available; T2DM, type 2 diabetes mellitus; WC, waist circumference.
Correlation.
χ2 test.
The US Centre for Disease Control and Prevention has advised that BMI should not be used as a diagnostic tool because it does not measure fat directly.75 A systematic review to understand the diagnostic performance of BMI in about 32,000 individuals showed a sensitivity and specificity of 50% and 90%, respectively, whereas in a Kenyan study, the BMI was reported to have a sensitivity and specificity of 5.3% and 99.4%, respectively, among men and 46.9% and 100%, respectively, among women.76,77 There have been calls to review the thresholds of waist circumference for the definition of obesity in the region because this will likely improve the accuracy of the assessment of the effects of obesity on cardiovascular disease (including CKD).78 Alternative measures that reflect visceral adiposity, such as waist circumference, waist-to-hip ratio, and waist-to-height ratio, are superior to BMI in predicting cardiovascular disease risk.79 This is mainly because increased abdominal adipose tissue is associated with metabolic abnormalities, including decreased glucose tolerance, reduced insulin sensitivity, and adverse lipid profiles.80 In its 2018 guideline on the measurement of obesity, the United Kingdom National Institute for Health and Care Excellence supported the use of BMI but advised that it be interpreted with caution.81 It also suggested that waist circumference be used in addition to BMI in people with a BMI <35 kg/m2. The National Institute for Health and Care Excellence guideline, similarly to a World Health Organization report, highlighted that Black individuals have an increased risk of adverse health conditions at different thresholds of obesity compared with other populations.82 Increasing evidence suggests that abdominal obesity, which is more prevalent in the Black population, is associated with an increased cardiometabolic health risk.83,84
Pathophysiologic Relationships Between Proteinuria, Glomerular Filtration Rate (GFR), and Obesity
Clinical studies in sub-Saharan Africa have demonstrated an important relationship between obesity and proteinuric kidney disease (Fig 4). For example, a report from the Democratic Republic of Congo showed that being overweight increased the risk of proteinuria by 30%.57 In the African American Study of Kidney Disease, for every 2.5-kg/m2 increase in BMI, there were 3.5% and 5.6% increases in the protein-to-creatinine and albumin-to-creatinine ratios, respectively.85 In a study conducted by Ekrikpo et al,86 BMI was a predictor of increased urinary β2 microglobulin levels, which in turn correlated with the urine albumin-to-creatinine ratio and serum creatinine level. In 2 studies from South Africa, leptin levels showed a negative correlation with the estimated GFR.87,88
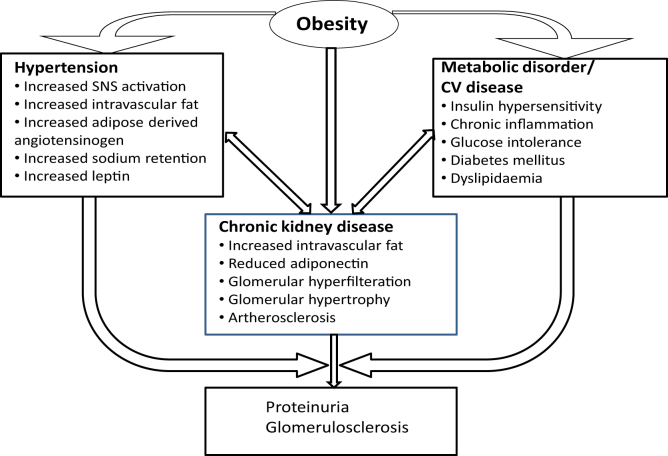
Figure 4.
Relationships between obesity, hypertension, metabolic disorder, and chronic kidney disease. Abbreviations: CV, cardiovascular; SNS, sympathetic nervous system.
Obesity and The Development of CKD In Sub-Saharan Africa
A number of epidemiologic studies have examined the relationship between obesity and the development of CKD or its risk factors in sub-Saharan Africa (Table 1). Sumaili et al89 reported a CKD prevalence of 16% among obese individuals, with only diabetes mellitus and hypertension conferring a higher risk. Both central and generalized obesity were significantly associated with CKD, per a report by Okwuonu et al.58 In 2 South African series, abdominal obesity and high BMI were associated with a reduction in the estimated GFR, and a similar correlation between increasing BMI and CKD was observed in southeastern Nigeria.55,59,60 In a series reported by Nwankwo et al,90 individuals with CKD had a higher average BMI than those without CKD (24.1 ± 3.6 vs 21.2 ± 2.6 kg/m2, respectively, P < 0.0002). Among long-haul drivers in Lagos, Nigeria, 64.7% of those with a waist circumference of >102 cm had an estimated GFR of <60 mL/min compared with 48.2% of those with waist circumference of <102 cm (P < 0.05).91 In a study conducted in 4 countries (Table 1), Adebamowo et al61 found that among patients with type 2 diabetes, obesity was associated with increased rates of CKD. In a Nigerian community study, Oluyombo et al72 demonstrated obesity (based on BMI) to be associated with both albuminuria and a low GFR.
Obesity exacerbates conditions such as hypertension, diabetes mellitus, and other cardiometabolic diseases, which in turn are important risk factors for the development of CKD (discussed further below). It increases the risk of type 2 diabetes mellitus by 7-fold.92 Similarly, it is associated with a 3.5-fold higher incidence of hypertension, and it has been suggested that up to two-third of hypertensive adults are obese.93 Type 2 diabetes mellitus and hypertension are the leading causes of CKD in sub-Saharan Africa.94, 95, 96 In this region, hypertension is common, with a prevalence of up to 46%, and the prevalence of type 2 diabetes mellitus is projected to increase by 141% over the next 2 decades.97, 98, 99 In Nigeria, all 6 geopolitical zones have a high prevalence of obesity (8.1%-22.2%)100, 101, 102; and both Okpechi et al103 and Akpan et al104 have shown BMI to be a significant predictor of hypertension in these populations. In Ethiopian and Tanzanian studies, the risk of hypertension among obese individuals was increased to 3.45 (2.44-4.86) and 2.62 (1.7-4.03), respectively.104,105 Obesity is a risk factor for type 2 diabetes mellitus, and this has been confirmed to be the case in sub-Saharan Africa.106 In addition, obesity plays an important role in the development of metabolic syndrome, a combination of cardiovascular risk factors, with obesity as its cardinal factor, which is associated with kidney disease. In predominantly Black African individuals, Okpechi et al88 demonstrated an inverse relationship between leptin and both metabolic syndrome characteristics and GFR and showed a correlation between metabolic syndrome and increased microalbuminuria. In Nigeria, the prevalence of metabolic syndrome ranges from 2.6% to 26.4% (depending on the criteria used), and this is higher among the obese population.107
Obesity as A Risk Factor For The Progression of CKD In Sub-Saharan Africa
Obesity not only leads to the development of CKD but also independently promotes its progression. In a cohort of nondiabetic and diabetic populations, obesity increased the risk of progression to kidney failure by factors of 7 and 20, respectively.108 There is a vicious cycle relationship between obesity and hypertension: obesity increases arterial blood pressure, leading to kidney hyperfiltration and injury to the glomeruli and a subsequent increase in the blood pressure.109 Proteinuria resulting from obesity (discussed above), if not mitigated, is a significant risk factor for the progression of CKD. Type 2 diabetes mellitus, which is highly prevalent in sub-Saharan Africa, is associated with CKD and its progression to kidney failure: Black Africans with diabetes are 3.5 times more likely to develop kidney failure than Whites.110 Obesity is associated with a chronic inflammatory process and increased cytokine production, and this has also been shown to promote both the development and progression of CKD.111
Genetic variations in lipid metabolism can also affect CKD risk. One such gene is apolipoprotein L1 (APOL-1), and “high risk” variants have been identified, as described by Ulasi et al.112 They found a high prevalence of 23.3% and 66% of such APOL-1 variants among the general population and nondiabetic individuals with CKD, respectively, in southeast Nigeria. These genetic variants were associated with a 5-fold risk of CKD development. APOL-1 polymorphisms are associated with progressive glomerulosclerosis and nephron loss, with consequent rapid progression to kidney failure.
The Burden of Obesity and CKD: Implications For Sub-Saharan Africa
The Global Burden of Disease Study group reported that between 2005 and 2016, deaths due to CKD increased by 31.7%; it was predicted that CKD will be the fifth-most common cause of death by 2040 and that by the same year, obesity will be the leading driver of around 80,000 years of life lost.113 Obesity is an independent predictor of kidney disease, and it promotes other cardiovascular risk factors, such as hypertension, diabetes mellitus, and proteinuria, which are also common in sub-Saharan Africa and are increasing as a result of the remarkable sociocultural, economic, and demographic changes occurring in the region that are leading to urbanization, reduced physical activity, and westernization of diets.57,114,115 The current prevalence of diabetes in sub-Saharan Africa is 2.1%-6.7% and is expected to double by 2040.105
It is estimated that by the year 2030, >70% of patients with kidney failure will be residents of developing nations.116 The current data suggest that only 3%-15% of sub-Saharan Africa inhabitants can afford hemodialysis if required.117, 118, 119 In 2015, the United Nations published a list of 17 sustainable development goals aiming “to promote prosperity while protecting the planet.”120 Among the health-related aspirations was to reduce mortality due to noncommunicable diseases by one-third by the year 2030, and this is likely to gain increasing importance, given the steady progress that has been made in the area of infectious diseases. Left unchecked, the increasing prevalence of obesity in sub-Saharan Africa is likely to hamper these efforts, given its effect on morbidity and mortality due to numerous conditions, including CKD.
Potential Benefits of Reducing Obesity and Effects On CKD
Lifestyle interventions, including education, physical activities, and nutrition, are crucial for curtailing the epidemic of obesity in sub-Saharan Africa. This will require a supportive environment and significant public policy reforms. Additionally, improved engagement of the food industry to support the fight against obesity is vital. Raising awareness about the menace of obesity and CKD is both important and feasible.
Several studies have demonstrated that weight loss in obese patients can lead to improvement in a number of parameters, including kidney function, albuminuria, hypertension, and hyperglycemia, together with metabolic demand on the kidneys. Friedman et al83 demonstrated a significant reduction in serum creatinine and cystatin levels among obese patients with type 2 diabetes mellitus following a 12% reduction in body weight via dietary and lifestyle interventions. A similar effect was seen by Saiki et al82 among obese diabetic patients after a decrease in both BMI and visceral fat, and there was a significant reduction in proteinuria from 3.27 to 1.5 g/d (P < 0.05) over 4 weeks.82 Although Leehey et al84 reported that structured exercise and dietary intervention in obese patients with type 2 diabetes mellitus and CKD simply led to better exercise tolerance but no changes in kidney function or body composition, a meta-analysis of 13 trials by Zhang et al121 found that exercise therapy in individuals with CKD resulted in improvement in a number of parameters, including kidney function, blood pressure, and BMI. Serra et al122 reported that overweight patients with obesity-related glomerular lesions (but normal kidney function) who underwent bariatric surgery demonstrated improvement in albuminuria and blood pressure and maintained normal kidney function in the long term.
Conclusions
Obesity and CKD are both becoming more prevalent in sub-Saharan Africa. Although a few observational reports have suggested an association between the 2, there is a need for more robust studies to investigate this in more detail using validated and standardized criteria to define obesity. The ability to quantify the burden of CKD attributable to obesity (and associated factors such as hypertension and diabetes mellitus) would be extremely valuable. The region should accept that obesity is not only prevalent but also contributes to significant health issues, including CKD. The ability to tackle the challenges of obesity and CKD will require major focus on the accessibility and affordability of health facilities in the region. Allocation of resources should be prioritized toward curtailing the burden of these chronic disease conditions. Governments and health care policymakers should take into account that the effects of obesity on the kidneys come in the form of a 2-pronged attack: the direct effect of obesity itself and the effects of associated conditions occurring indirectly, such as diabetes mellitus, hypertension, and atherosclerosis. Thus, it is likely that timely interventions to promote the achievement of the ideal body weight will have a major impact on amelioration of the growing prevalence of CKD.
Article Information
Authors’ Full Names and Academic Degrees
Rotimi Oluyombo, MBCHB, MSc, Hameed Banjo Oguntade, MBBS, Michael Soje, MBBS, Omotola Obajolowo, MBBS, and Mahzuz Karim, PhD
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgments
Evidence search: Chronic kidney disease and obesity in the sub-Saharan African region. Helen Barrett. HAYWARDS HEATH, UK: Brighton and Sussex Library and Knowledge Service.
Peer Review
Received June 6, 2021. Evaluated by 2 external peer reviewers, with direct editorial input by an Associate Editor and the Editor-in-Chief. Accepted in revised form November 4, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Luyckx V., Tonelli M., Stanifer J.W. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96(6):414–422. doi: 10.2471/BLT.17.206441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George C., Mogueo A., Okpechi I., Echouffo-Tcheugui J.B., Kengne A.P. Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ Glob Health. 2017;2(2) doi: 10.1136/bmjgh-2016-000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanifer J.W., Jing B., Tolan S., et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(3):e174–e181. doi: 10.1016/S2214-109X(14)70002-6. [DOI] [PubMed] [Google Scholar]
- 4.Stenvinkel P., Zoccali C., Ikizler Obesity in CKD—what should nephrologists know? J Am Soc Nephrol. 2013;24(11):1727–1736. doi: 10.1681/ASN.2013040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelber R.P., Kurth T., Kausz A.T., et al. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46(5):871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Minoo F., Mahdavi-Mazdeh M., Abbasi M.R., Sohrabi S. Impact of the severity of obesity on microalbuminuria in obese normotensive non-diabetic individuals. J Renal Inj Prev. 2015;4(2):34–38. doi: 10.12861/jrip.2015.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Chen X., Song Y., Caballero B., Cheskin L.J. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73(1):19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 8.Kramer H., Saranathan A., Luke A., Durazo-Arvizu R., Hou S., Cooper R. Increasing BMI and obesity in the incident end-stage renal disease population. J Am Soc Nephrol. 2006;17(5):1453–1459. doi: 10.1681/ASN.2005111241. [DOI] [PubMed] [Google Scholar]
- 9.Foster M.C., Hwang S.J., Larson M.G., et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52(1):39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao X., Zhou J., Yuan H., Wu L., Chen Z. Chronic kidney disease among overweight and obesity with and without metabolic syndrome in an urban Chinese cohort. BMC Nephrology. 2015;16(1):1–9. doi: 10.1186/s12882-015-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivo R.E., Davenport C.A., Diamantidis C.J., et al. Obesity and synergistic risk factors for chronic kidney disease in African American adults: the Jackson Heart Study. Nephrol Dial Transplant. 2018;33(6):992–1001. doi: 10.1093/ndt/gfx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu C.Y., McCulloch C.E., Iribarren C., Darbinian J., Go A.S. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iseki K., Ikemiya Y., Kinjo K., Inoue T., Iseki C., Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65(5):1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 14.Grubbs V., Lin F., Vittinghoff E., et al. Body mass index and early kidney function decline in young adults: A longitudinal analysis of the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis. 2014;63(4):590–597. doi: 10.1053/j.ajkd.2013.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Findings from the Global Burden of Disease Study 2017. The Lancet. http://www.healthdata.org/sites/default/files/files/policy_report/2019/GBD_2017_Booklet.pdf
- 16.Bhurosy T., Jeewon R. Overweight and obesity epidemic in developing countries: a problem with diet, physical activity, or socioeconomic status? Sci World J. 2014;2014:964236. doi: 10.1155/2014/964236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monteiro C.A., Conde W.L., Lu B., Popkin B.M. Obesity and inequities in health in the developing world. Int J Obes Relat Metab Disord. 2004;28(9):1181–1186. doi: 10.1038/sj.ijo.0802716. [DOI] [PubMed] [Google Scholar]
- 18.International Diabetes Federation . ed 2. International Diabetes Federation; Brussels: 2003. Diabetes Atlas. [Google Scholar]
- 19.Mittal B.V., Singh A.K. Hypertension in the developing world: challenges and opportunities. Am J Kidney Dis. 2010;55(3):590–598. doi: 10.1053/j.ajkd.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 20.George C., Matsha T.E., Davidson F.E., Goedecke J.H., Erasmus R.T., Kengne A.P. Chronic kidney disease modifies the relationship between body fat distribution and blood pressure: a cross-sectional analysis. Int J Nephrol Renovasc Dis. 2020;13:107–118. doi: 10.2147/IJNRD.S247907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noubiap J.J., Naidoo J., Kengne A.P. Diabetic nephropathy in Africa: a systematic review. World J Diabetes. 2015;6(5):759–773. doi: 10.4239/wjd.v6.i5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arogundade F.A., Barsoum R.S. CKD prevention in Sub-Saharan Africa: a call for governmental, nongovernmental, and community support. Am J Kidney Dis. 2008;51(3):515–523. doi: 10.1053/j.ajkd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 23.World Population Prospects 2019 Department of Economic and Social affairs, United Nations. https://population.un.org/wpp/Publications/Files/WPP2019_DataBooklet.pdf
- 24.Population estimates and projections. The World Bank. https://databank.worldbank.org/source/health-nutrition-and-population-statistics:-population-estimates-and-projections
- 25.Kaze A.D., Ilori T., Jaar B.G., Echouffo-Tcheugui J.B. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):1–11. doi: 10.1186/s12882-018-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amugsi D.A., Dimbuene Z.T., Mberu B., Muthuri S., Ezeh A.C. Prevalence and time trends in overweight and obesity among urban women: an analysis of demographic and health surveys data from 24 African countries, 1991–2014. BMJ Open. 2017;7(10) doi: 10.1136/bmjopen-2017-017344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oluyombo R., Ayodele O.E., Akinwusi P.O., et al. Awareness, knowledge and perception of chronic kidney disease in a rural community of South-West Nigeria. Niger J Clin Pract. 2016;19(2):161–169. doi: 10.4103/1119-3077.175960. [DOI] [PubMed] [Google Scholar]
- 28.Young B.A. The interaction of race, poverty and chronic kidney disease. Am J Kidney Dis. 2010;55(6):977–980. doi: 10.1053/j.ajkd.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mbewu A., Mbanya J.C. Disease and Mortality in Sub-Saharan Africa. 2nd ed. World Bank; Washington, D.C: 2006. Cardiovascular disease; pp. 305–327. [Google Scholar]
- 30.Okop K.J., Mukumbang F.C., Mathole T., Levitt N., Puoane T. Perceptions of body size, obesity threat and the willingness to lose weight among black South African adults: a qualitative study. BMC Public Health. 2016;16(1):1–13. doi: 10.1186/s12889-016-3028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simfukwe P., Van Wyk B., Swart C. Perceptions, attitudes and challenges about obesity and adopting a healthy lifestyle among health workers in Pietermaritzburg, KwaZulu-Natal province. Afr J Prim Health Care Fam Med. 2017;9(1):e1–e9. doi: 10.4102/phcfm.v9i1.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matoti-Mvalo T., Puoane T.B. Perceptions of body size and its association with HIV/AIDS. S Afr J Clin Nutr. 2011;24(1):40–45. [Google Scholar]
- 33.Ettarh R., Van de Vijver S., Oti S., Kyobutungi C. Overweight, obesity, and perception of body image among slum residents in Nairobi, Kenya, 2008–2009. Prev Chronic Dis. 2013;10:E212. doi: 10.5888/pcd10.130198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Global obesity observatory. https://data.worldobesity.org/country/eritrea-63/#data_trends
- 36.Ofori-Asenso R., Agyeman A.A., Laar A., Boateng D. Overweight and obesity epidemic in Ghana-a systematic review and meta-analysis. BMC Public Health. 2016;16(1):1239. doi: 10.1186/s12889-016-3901-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nansseu J.R., Noubiap J.J., Bigna J.J. Epidemiology of overweight and obesity in adults living in Cameroon: a systematic review and meta-analysis. Obesity. 2019;27(10):1682–1692. doi: 10.1002/oby.22566. [DOI] [PubMed] [Google Scholar]
- 38.Agyemang C., Meeks K., Beune E., et al. Obesity and type 2 diabetes in sub-Saharan Africans—is the burden in today’s Africa similar to African migrants in Europe? The RODAM study. BMC Med. 2016;14(1):1–12. doi: 10.1186/s12916-016-0709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.NCD Risk Factor Collaboration Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569(7755):260–264. doi: 10.1038/s41586-019-1171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czernichow S., Kengne A.P., Stamatakis E., Hamer M., Batty G.D. Body mass index, waist circumference and waist-hip ratio: which is the better discriminator of cardiovascular disease mortality risk?: evidence from an individual-participant meta-analysis of 82 864 participants from nine cohort studies. Obes Rev. 2011;12(9):680–687. doi: 10.1111/j.1467-789X.2011.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ono T., Guthold R., Strong K. WHO Global Comparable Estimates: Global Infobase data for saving lives 2005. 2012. https://apps.who.int/infobase/Index.aspx
- 42.Adom T., Kegne A.P., De Villiers A., Puoane T. Prevalence of overweight and obesity among African primary school learners: a systemic review and meta-analysis. Obesity Sci Pract. 2019;5(5):487–502. doi: 10.1002/osp4.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chukwuonye I.I., Chuku A., John C., et al. Prevalence of overweight and obesity in adult Nigerians—a systematic review. Diabetes Metab Syndr Obes. 2013;6:43–47. doi: 10.2147/DMSO.S38626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sangachin M.G., Cavuoto L.A., Wang Y. Use of various obesity measurement and classification methods in occupational safety and health research: a systematic review of the literature. BMC Obes. 2018;5(1):1–24. doi: 10.1186/s40608-018-0205-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross R., Berentzen T., Bradshaw A.J., et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes Rev. 2008;9(4):312–325. doi: 10.1111/j.1467-789X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 46.Akintunde A.A., Ayodele O.E., Akinwusi P.O., Opadijo G.O. Metabolic syndrome: comparison of occurrence using three definitions in hypertensive patients. Clin Med Res. 2011;9(1):26–31. doi: 10.3121/cmr.2010.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lear S.A., Birmingham C.L., Chockalingam A., Humphries K.H. Study design of the Multicultural Community Health Assessment Trial (M-CHAT): a comparison of body fat distribution in four distinct populations. Ethn Dis. 2006;16(1):96–100. [PubMed] [Google Scholar]
- 48.Rush E.C., Freitas I., Plank L.D. Body size, body composition and fat distribution: comparative analysis of European, Maori, Pacific Island and Asian Indian adults. Br J Nutr. 2009;102(4):632–641. doi: 10.1017/S0007114508207221. [DOI] [PubMed] [Google Scholar]
- 49.Batsis J.A., Mackenzie T.A., Bartels S.J., Sahakyan K.R., Somers V.K., Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999-2004. Int J Obes (Lond) 2016;40(5):761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iloh G.U.P., Ikwudima A.O. Abdominal obesity in adult Nigerian Africans: prevalence and co-occurrence with cardiometabolic risk factors in a resource poor setting of a rural hospital in Eastern Nigeria. Am J Health Res. 2013;1(3):73–80. [Google Scholar]
- 51.Okafor C.I. The metabolic syndrome in Africa: current trends. Indian J Endocrinol Metab. 2012;16(1):56–66. doi: 10.4103/2230-8210.91191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oluyombo R., Olamoyegun M.A., Olaifa O., Iwuala S.O., Babatunde O.A. Cardiovascular risk factors in semi urban communities in Southwest Nigeria: patterns and prevalence. J Epidemiol Glob Health. 2015;5(2):167–174. doi: 10.1016/j.jegh.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lartey ST, Magnussen CG, Si L, et al. Rapidly increasing prevalence of overweight and obesity in older Ghanaian adults from 2007-2015: evidence from WHO-SAGE Waves 1 & 2. PLoS One. 14(8):e0215045. [DOI] [PMC free article] [PubMed]
- 54.Olanrewaju T.O., Aderibigbe A., Popoola A., et al. Prevalence of chronic kidney disease and risk factors in North-Central Nigeria: a population-based survey. BMC Nephrol. 2020;21(1):1–10. doi: 10.1186/s12882-020-02126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adeniyi A.B., Laurence C.E., Volmink J.A., Davids M.R. Prevalence of chronic kidney disease and association with cardiovascular risk factors among teachers in Cape Town, South Africa. Clin Kidney J. 2017;10(3):363–369. doi: 10.1093/ckj/sfw138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faye M., Lemrabott A.T., Cissé M.M., et al. Prevalence and risk factors of chronic kidney disease in an African semi-urban area: Results from a cross-sectional survey in Gueoul, Senegal. Saudi J Kidney Dis Transpl. 2017;28(6):1389–1396. doi: 10.4103/1319-2442.220878. [DOI] [PubMed] [Google Scholar]
- 57.Sumaili E.K., Nseka N.M., Lepira F.B., et al. Screening for proteinuria and chronic kidney disease risk factors in Kinshasa: a World Kidney Day 2007 study. Nephron Clin Pract. 2008;110(4):c220–c228. doi: 10.1159/000167869. [DOI] [PubMed] [Google Scholar]
- 58.Okwuonu C.G., Chukwuonye I.I., Adejumo O.A., Agaba E.I., Ojogwu L.I. Prevalence of chronic kidney disease and its risk factors among adults in a semi-urban community of South-East Nigeria. Niger Postgrad Med J. 2017;24(2):81–87. doi: 10.4103/npmj.npmj_34_17. [DOI] [PubMed] [Google Scholar]
- 59.Matsha T.E., Yako Y.Y., Rensburg M.A., Hassan M.S., Kengne A.P., Erasmus R.T. Chronic kidney diseases in mixed ancestry South African populations: prevalence, determinants and concordance between kidneys function estimators. BMC Nephrol. 2013;14(1):1–10. doi: 10.1186/1471-2369-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wachukwu C.M., Emem-Chioma P.C., Wokoma F.S., Oko-Jaja R.I. Prevalence of risk factors for chronic kidney disease among adults in a university community in southern Nigeria. Pan Afr Med J. 2015;21(1):120. doi: 10.11604/pamj.2015.21.120.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adebamowo S.N., Adeyemo A.A., Tekola-Ayele F., et al. Impact of type 2 diabetes on impaired kidney function in Sub-Saharan African populations. Front Endocrinol (Lausanne) 2016;7:50. doi: 10.3389/fendo.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaze F.F., Halle M.P., Mopa H.T., et al. Prevalence and risk factors of chronic kidney disease in urban adult Cameroonians according to three common estimators of the glomerular filtration rate: a cross-sectional study. BMC Nephrol. 2015;16(1):1–8. doi: 10.1186/s12882-015-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seck S.M., Doupa D., Gueye L., Dia C.A. Prevalence of chronic kidney disease and associated factors in Senegalese populations: a community-based study in Saint-Louis. Nephrourol Mon. 2014;6(5):e19085. doi: 10.5812/numonthly.19085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Afolabi M.O., EA Abioye-Kuteyi E.A., Arogundade F.A., Bello I.S. Prevalence of chronic kidney disease in a Nigerian family practice population. SA Fam Pract. 2009;51(2):132–137. [Google Scholar]
- 65.Lunyera J., Stanifer J.W., Ingabire P., et al. Prevalence and correlates of proteinuria in Kampala, Uganda: a cross-sectional pilot study. BMC Res Notes. 2016;9(1):1–6. doi: 10.1186/s13104-016-1897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Egbi O.G., Okafor U.H., Miebodei K.E., Kasia B.E., Kunle-Olowu O.E., Unuigbe E.I. Prevalence and correlates of chronic kidney disease among civil servants in Bayelsa state, Nigeria. Niger J Clin Pract. 2014;17(5):602–607. doi: 10.4103/1119-3077.141426. [DOI] [PubMed] [Google Scholar]
- 67.Owiredu W.K., Ephraim R.K., Eghan Jr BA, Amidu N., Laing E.F. Metabolic syndrome among Ghanaian patients presenting with chronic kidney disease. J Medical Biomed Sci. 2012;1(3):1–12. [Google Scholar]
- 68.Gbadegesin A., Okunola O., Ayodele O., Arogundade F., Sanusi A., Akinsola A. Renal risk profiling in newly diagnosed hypertensives in an urban population in Nigeria. Afr Health Sci. 2019;19(4):2863–2873. doi: 10.4314/ahs.v19i4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stanifer J.W., Maro V., Egger J., et al. The epidemiology of chronic kidney disease in Northern Tanzania: a population-based survey. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0124506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Babua C., Kalyesubula R., Okello E., et al. Cardiovascular risk factors among patients with chrnic kidney disease attending a tertiary hospital in Uganda. Cardiovasc J Afr. 2015;26(4):177–180. doi: 10.5830/CVJA-2015-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ephraim R.K., Biekpe S., Sakyi S.A., Adoba P., Agbodjakey H., Antoh E.O. Prevalence of chronic kidney disease among the high risk population in South-Western Ghana: a cross sectional study. Can J Kidney Health Dis. 2015;2:40. doi: 10.1186/s40697-015-0076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oluyombo R., Akinwusi P.O., Olamoyegun M.A., et al. Clustering of cardiovascular risk factors in semi-urban communities in south-western Nigeria. Cardiovasc J Afr. 2016;27(5):322–327. doi: 10.5830/CVJA-2016-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kalantar-Zadeh K., Kovesdy C.P., Derose S.F., Horwich T.B., Fonarow G.C. Racial and survival paradoxes in chronic kidney disease. Nat Rev Nephrol. 2007;3(9):493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 74.Aryee C., Owiredu W.K., Osei-Yeboah J., Owusu-Dabo E., Laing E.F., Owusu I.K. An analysis of anthropometric indicators and modifiable lifestyle parameters associated with hypertensive nephropathy. Int J Hypertens. 2016;2016:6598921. doi: 10.1155/2016/6598921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Safer, Healthier people Body Mass Index: Considerations for Practitioners. https://www.cdc.gov/obesity/downloads/bmiforpactitioners.pdf
- 76.Okorodudu D., Jumean M., Montori V., et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity: a systematic review and meta-analysis. Int J Obes. 2010;34(5):791–799. doi: 10.1038/ijo.2010.5. [DOI] [PubMed] [Google Scholar]
- 77.Sinaga M., Yemane T., Tegene E., Lidstrom D., Belachew T. Performance of newly developed body mass index cut-off diagnosig obesity among Ethiopian adults. J Physiol Anthropol. 2019;38(1):1–9. doi: 10.1186/s40101-019-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ekoru K., Murphy G., Young E., et al. Deriving an optimal threshold of waist circumference for detecting cardiometabolic risk in sub-Saharan Africa. Int J Obesity. 2018;42(3):487–494. doi: 10.1038/ijo.2017.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Amin F., Fatima S.S., Islam N., Gilani A.H. Prevalence of obesity and overweight, its clinical markers and associated factors in a high-risk South-Asian population. BMC Obes. 2015;2(1):1–11. doi: 10.1186/s40608-015-0044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huxley R., Mendis S., Zheleznyakov E., Reddy S., Chan J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—a review of the literature. Eur J Clin Nutr. 2010;64(1):16–22. doi: 10.1038/ejcn.2009.68. [DOI] [PubMed] [Google Scholar]
- 81.Surveillance Report 2018 – Obesity: identification, assessment and management (2014) NICE guideline CG189 and BMI: preventing ill health and premature death in black, Asian and other minority ethnic groups (2013) NICE guideline PH46. https://www.nice.org.uk/guidance/cg189/resources/surveillance-report-2018-obesityidentification-assessment-and-management-2014-nice-guideline-cg189-and-bmi-preventingill-health-and-premature-death-in-black-asian-and-other-minority-ethnic-groups-2-pdf6282437320645 [PubMed]
- 82.Saiki A., Nagayama D., Ohhira M., et al. Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obes (Lond) 2005;29(9):1115–1120. doi: 10.1038/sj.ijo.0803009. [DOI] [PubMed] [Google Scholar]
- 83.Friedman A.N., Chambers M., Kamendulis L.M., Temmerman J. Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol. 2013;8(11):1892–1898. doi: 10.2215/CJN.04010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leehey D.J., Collins E., Kramer H.J., et al. Structured exercise in obese diabetic patients with chronic kidney disease: a randomized controlled trial. Am J Nephrol. 2016;44(1):54–62. doi: 10.1159/000447703. [DOI] [PubMed] [Google Scholar]
- 85.Toto R.D., Greene T., Hebert L.A., et al. Relationship between body mass index and proteinuria in hypertensive nephrosclerosis: results from the African American Study of Kidney Disease and Hypertension (AASK) cohort. Am J Kidney Dis. 2010;56(5):896–906. doi: 10.1053/j.ajkd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ekrikpo U.E., Effa E.E., Akpan E.E., Obot A.S., Kadiri S. Clinical utility of urinary β2-microglobulin in detection of early nephropathy in African diabetes mellitus patients. Int J Nephrol. 2017;2017:4093171. doi: 10.1155/2017/4093171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Okpechi I.G., Pascoe M.D., Swanepoel C.R., Rayner B.L. Microalbuminuria and the metabolic syndrome in non-diabetic black Africans. Diab Vasc Dis Res. 2007;4(4):365–367. doi: 10.3132/dvdr.2007.066. [DOI] [PubMed] [Google Scholar]
- 88.Okpechi I.G., Rayner B.L., Van Der Merwe L. Genetic variation at selected SNPs in the leptin gene and association of alleles with markers of kidney disease in a Xhosa population of South Africa. PLoS One. 2010;5(2):e9086. doi: 10.1371/journal.pone.0009086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sumaili E.K., Cohen E.P., Zinga C.V., Krzesinski J.M., Pakasa N.M., Nseka N.M. High prevalence of undiagnosed chronic kidney disease among at-risk population in Kinshasa, the Democratic Republic of Congo. BMC Nephrol. 2009;10(1):1–12. doi: 10.1186/1471-2369-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nwankwo E.A., Wudiri W.W., Akinsola A. Risk factors for the development of chronic kidney disease among Nigerians with essential hypertension. J Med Sci. 2007;7(4):579–584. [Google Scholar]
- 91.Amadi C.E., Mbakwem A.C., Kushimo O.A., Ajuluchukwu J.N., Akinkunmi M. Prevalence of positive chronic kidney disease screening in professional male long haul drivers at risk of cardiovascular disease in Lagos, Nigeria: a cross-section study. BMC Public Health. 2019;19(1):1–11. doi: 10.1186/s12889-019-7328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schutte A.E., Van Vuuren D., Van Rooyen J.M., et al. Inflammation, obesity and cardiovascular function in African and Caucasian women from South Africa: the POWIRS study. J Hum Hypertens. 2006;20(11):850–859. doi: 10.1038/sj.jhh.1002065. [DOI] [PubMed] [Google Scholar]
- 93.Guwatudde D., Nankya-Mutyoba J., Kalyesubula R., et al. The burden of hypertension in sub-Saharan Africa: a four-country cross sectional study. BMC Public Health. 2015;15(1):1–8. doi: 10.1186/s12889-015-2546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kotchen T.A. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23(11):1170–1178. doi: 10.1038/ajh.2010.172. [DOI] [PubMed] [Google Scholar]
- 95.Hamadou B., Boombhi J., Kamdem F., et al. Prevalence and correlates of chronic kidney disease in a group of patients with hypertension in the Savanah zone of Cameroun: a cross-sectional study in Sub-Saharan Africa. Cardiovasc Diagn Ther. 2017;7(6):581–588. doi: 10.21037/cdt.2017.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oluyombo R. A community study of the prevalence, risk factors and pattern of chronic kidney disease in Osun state, South-West Nigeria. West Afr J Med. 2013;32(2):85–92. [PubMed] [Google Scholar]
- 97.Lokpo S.Y., Osei-Yeboah J., Owiredu W.K., et al. Renal dysfunction among Ghanaians Living with clinically diagnosed hypertension in the Asutifi-South district: a cross-sectional descriptive study at the St. Elizabeth Hospital, Hwidiem. Int J Hypertens. 2018;2018:8428063. doi: 10.1155/2018/8428063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.IDF Diabetes Atlas. 7th ed. International Diabetes Federation; Brussels: 2015. [Google Scholar]
- 99.Cappuccio F.P., Miller M.A. Cardiovascular disease and hypertension in sub-Saharan Africa: burden, risk and interventions. Intern Emerg Med. 2016;11(3):299–305. doi: 10.1007/s11739-016-1423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdullah A., Peeters A., de Courten M., Stoelwinder J. The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies. Diabetes Res Clin Pract. 2010;89(3):309–319. doi: 10.1016/j.diabres.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 101.Oladapo O.O., Salako I., Sodiq O., Shoyinka K., Adedapo K., Falase A.O. A prevalence of cardiometabolic risk factors among a rural Yoruba southwestern Nigerian population: a population-based survey. Cardiovasc J Afr. 2010;21(1):26–31. [PMC free article] [PubMed] [Google Scholar]
- 102.Bakari A.G., Onyemelukwe G.C., Sani B.G., Aliyu I.S., Hassan S.S., Aliyu T.M. Obesity, overweight and underweight in suburban northern Nigeria. Int J Diabetes Metab. 2007;15(2):68–69. [Google Scholar]
- 103.Okpechi I.G., Chukwuonye I.I., Tiffin N., et al. Blood pressure gradients and cardiovascular risk factors in urban and rural populations in Abia State South Eastern Nigeria using the WHO STEPwise approach. PLoS One. 2013;8(9):e73403. doi: 10.1371/journal.pone.0073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akpan E.E., Ekrikpo U.E., Udo A.I., Bassey B.E. Prevalence of hypertension in Akwa Ibom State, South-South Nigeria: rural versus urban communities study. Int J Hypertens. 2015;2015:975819. doi: 10.1155/2015/975819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Demisse A.G., Greffie E.S., Abebe S.M., et al. High burden of hypertension across the age groups among residents of Gondar city in Ethiopia: a population based cross sectional study. BMC Public Health. 2017;17(1):1–9. doi: 10.1186/s12889-017-4646-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mosha N.R., Mahande M., Juma A., et al. Prevalence, awareness and factors associated with hypertension in North West Tanzania. Glob Health Action. 2017;10(1):1321279. doi: 10.1080/16549716.2017.1321279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Raimi T.H., Odusan O., Fasanmade O.A., Odewabi A.O., Ohwovoriole A.E. Metabolic syndrome among apparently healthy nigerians with the harmonized criteria: prevalence and concordance with the International Diabetes Federation (IDF) and third report of the National Cholesterol Education Programme—adult treatment panel III (NCEP-ATP III) criteria. J Cardiovasc Disease Res. 2017;8(4):145–150. [Google Scholar]
- 108.Luyckx V.A., Tuttle K.R., Garcia-Garcia G., et al. Reducing major risk factors for chronic kidney disease. Kidney Int. 2017;7(2):71–87. doi: 10.1016/j.kisu.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hall M.E., do Carmo J.M., da Silva A.A., Juncos L.A., Wang Z., Hall J.E. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. 2014;7:75–88. doi: 10.2147/IJNRD.S39739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Obesity and African Americans—The Office of Minority Health. US Department of Health and Human Services. Accessed March 19, 2020. https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=25
- 111.Sinha S.K., Nicholas S.B., Sung J.H., et al. hs-CRP is associated with incident diabetic nephropathy: findings from the Jackson Heart study. Diabetes Care. 2019;42(11):2083–2089. doi: 10.2337/dc18-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ulasi I.I., Tzur S., Wasser W.G., et al. High population frequencies of APOL1 risk variants are associated with increased prevalence of non-diabetic chronic kidney disease in the Igbo people from south-eastern Nigeria. Nephron Clin Pract. 2013;123(1-2):123–128. doi: 10.1159/000353223. [DOI] [PubMed] [Google Scholar]
- 113.Foreman K.J., Marquez N., Dolgert A., et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi: 10.1016/S0140-6736(18)31694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jiang S.Z., Lu W., Zong X.F., Ruan H.Y., Liu Y. Obesity and hypertension. Exp Ther Med. 2016;12(4):2395–2399. doi: 10.3892/etm.2016.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rosenstock J.L., Pommier M., Stoffels G., Patel S., Michelis M.F. Prevalence of proteinuria and albuminuria in an obese population and associated risk factors. Front Med (Lausanne) 2018;5:122. doi: 10.3389/fmed.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liyanage T., Ninomiya T., Jha V., et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 117.Oluyombo R., Okunola O.O., Olanrewaju T.O., Soje M.O., Obajolowo O.O., Ayorinde M.A. Challenges of hemodialysis in a new renal care center: call for sustainability and improved outcome. Int J Nephrol Renovasc Dis. 2014;7:347–352. doi: 10.2147/IJNRD.S65835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meremo A.J., Ngilangwa D.P., Mwashambwa M.Y., et al. Challenges and outcomes of haemodialysis among patients presenting with kidney diseases in Dodoma, Tanzania. BMC Nephrol. 2017;18(1):1–6. doi: 10.1186/s12882-017-0634-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dada S.A., Ajite A.B., Ibitoba F.A., Thomas A.A., Dada O.E., Deji-Dada O.O. Challenges of haemodialysis: a single centre experience in South West Nigeria. J Clini Nephrol. 2019;3:55–60. [Google Scholar]
- 120.United Nations Millennium Development Goals, United Nations. http://www.un.org/millenniumgoals/
- 121.Zhang L., Wang Y., Xiong L., Luo Y., Huang Z., Yi B. Exercise therapy improves eGFR, and reduces blood pressure and BMI in non-dialysis CKD patients: evidence from a meta-analysis. BMC Nephrol. 2019;20(1):1–12. doi: 10.1186/s12882-019-1586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Serra A., Esteve A., Navarro-Díaz M., López D., Bancu I., Romero R. Long-term normal renal function after drastic weight reduction in patients with obesity-related glomerulopathy. Obes Facts. 2015;8(3):188–199. doi: 10.1159/000431027. [DOI] [PMC free article] [PubMed] [Google Scholar]