Abstract
Rationale & Objective
Recent evidence suggests a role for magnesium as a calcification inhibitor. Increased magnesium abundance may attenuate vascular calcification and promote bone formation.
Study Design
Parallel-group, 1:1-allocation-ratio, quasi-experimental study.
Setting & Participants
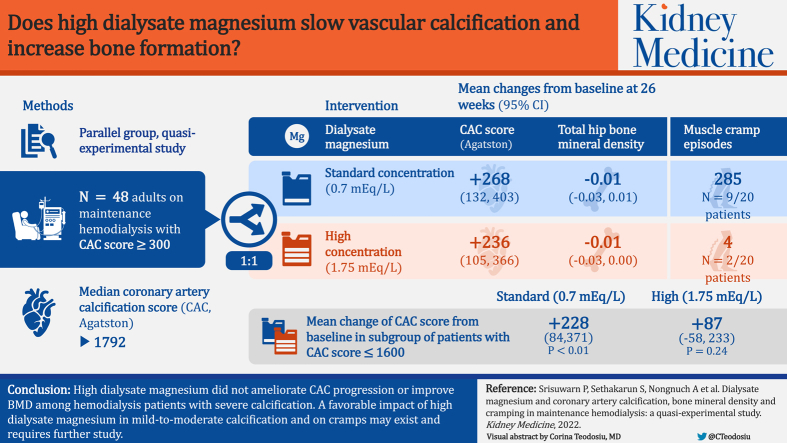
The study was conducted at hemodialysis centers in Bangkok, Thailand. Patients receiving maintenance hemodialysis were screened for coronary artery calcification (CAC) and bone mineral density (BMD), and those with a CAC score of ≥300 were included and matched according to the initial CAC score. The intervention and control groups consisted of 20 patients in each arm.
Interventions
A high (1.75 mEq/L) or standard (0.7 mEq/L) dialysate magnesium concentration was delivered for 26 weeks.
Outcomes
Changes in the CAC score and BMD and the progression of CAC. The safety outcomes included occurrence of cramps recorded as per usual care.
Results
The median CAC score of all patients was 1,792. Serum and ionized magnesium concentrations increased substantially in the high dialysate magnesium group. At the end of the study, the CAC score increased significantly in both the groups, with no significant difference between the groups. The number of participants with CAC progression was comparable between the 2 groups. In exploratory subgroup analyses stratified by the median CAC score, a significant decline in CAC and fewer participants with CAC progression were observed in the subgroup with lower CAC scores that received the high dialysis magnesium concentration. Bone mineral density was largely unchanged in both groups. The number of participants experiencing cramps and the number of episodes of muscle cramps were markedly lower among patients who received the high dialysis magnesium concentration.
Limitations
The participants had severe vascular calcification at baseline; therefore, the findings might not apply to those with less-established calcification. Moreover, cramps were not systematically ascertained.
Conclusions
The high dialysis magnesium concentration did not alleviate the progression of CAC or improve BMD in patients with severe calcification receiving hemodialysis; however, muscle cramps were less frequent among those treated with high dialysate magnesium. Further study is required to determine a possible favorable effect of high dialysis magnesium concentration in individuals with mild-to-moderate calcification.
Index Words: cardiovascular disease, CKD, cramp, dialysis, ESRD, kidney, osteoporosis
Graphical abstract
Plain-Language Summary.
This study observed the effect of increasing the concentration of magnesium in hemodialysis fluid on lowering the amount of calcium in the arteries of the heart and improvement in bone density and muscle cramping during hemodialysis. Patients receiving hemodialysis with calcium in their heart arteries were prescribed an increased magnesium concentration in the hemodialysis fluid for 6 months. The results showed that the increased magnesium concentration did not lower the amount of calcium in the heart arteries in patients who already had a great deal of calcium to begin with but might lower the amount of calcium in those with a small-to-medium amount of calcium. Increasing the concentration of magnesium in the hemodialysis fluid did not improve bone density but markedly improved muscle cramping during the treatment.
Editorial, 100410
Cardiovascular calcification and osteoporosis are highly prevalent in patients with end-stage kidney disease. Alterations of bone mineral metabolites, hormones, and the quality and quantity of calcification inhibitors potentiate the development and progression of cardiovascular calcification and bone loss.1 Decreased serum magnesium concentration stimulates oxidative stress and inflammation and impairs endothelial function, resulting in atherosclerosis.2,3 Magnesium is also an important constituent of bone minerals, and its depletion is associated with suppressed osteoblastic and osteoclastic activities, osteopenia, and parathyroid hormone (PTH) resistance.4 The relationship between low normal serum magnesium concentration and more severe vascular calcification and osteoporosis has been documented in patients with chronic kidney disease.5, 6, 7, 8, 9, 10 Increased magnesium abundance could reduce carotid intima-media thickness, slow the progression of vascular calcification in patients with chronic kidney disease stages 3-5, and increase bone mineral density (BMD).11, 12, 13, 14 An inhibitory effect of extracellular magnesium on vascular calcification has been confirmed in vitro.15, 16, 17 In current hemodialysis (HD) practice, dialysate magnesium concentration is kept constant at a relatively low level between 0.35 and 0.5 mmol/L (0.7-1 mEq/L or 0.85-1.2 mg/dL), and most patients experience further magnesium removal during HD.18 In 2 small observational studies, increased dialysis magnesium concentration did not result in any immediate harmful effects.19,20 On the other hand, a decrease in the frequency and severity of muscle cramps was reported by most patients.19 The present pilot quasi-experimental study was designed to examine the potential benefits of increased dialysis magnesium concentration on the progression of coronary artery calcification (CAC) and changes in BMD in patients receiving maintenance HD.
Methods
The MIRACAL (High Magnesium Dialysate Retard the Progression of Arterial Calcification) study (Thaiclinicaltrials.org registry number TCTR20180313006) is an investigator-initiated, single-blind, parallel-group, 1:1-allocation-ratio, quasi-experimental design study that examined the effect of high dialysis magnesium concentration on changes in CAC and BMD in patients receiving maintenance HD. The primary outcomes were changes in CAC score and BMD from baseline. The secondary outcomes included changes in laboratory data and the rate of progression of CAC. The study was conducted at 6 different dialysis centers that belonged to the same network of Ramathibodi Hospital and Bhumirajanagarindra Kidney Institute, Bangkok, Thailand, between April 2018 and June 2020. The study was approved by the Human Research Ethics Committee of Faculty of Medicine, Ramathibodi Hospital, Mahidol University (approval number MURA 2018/09) and was performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants.
Study Population
Patients receiving maintenance HD who were ≥18 years old, had been receiving HD for at least 3 months, and did not have any acute or chronic illnesses that could result in a life expectancy of <6 months were assessed for eligibility. Patients who agreed to participate were screened for CAC and BMD, and only patients with a CAC Agatston score of ≥300 were included. All patients who agreed to participate were under the direct care of principal investigators or other nephrologists from the same network. All patients underwent regular HD sessions at 1 of the 6 HD units within the same network and city. The patients received 4-hour, thrice-weekly HD using a high-flux dialyzer with a surface area of 1.8-2.1 m2. The dialysate calcium concentration was 2.5-3 mEq/L.
Sample Size Calculation
A sample size calculation was not performed because this was a pilot trial and the standardized effect size was not known.
Allocation and Intervention
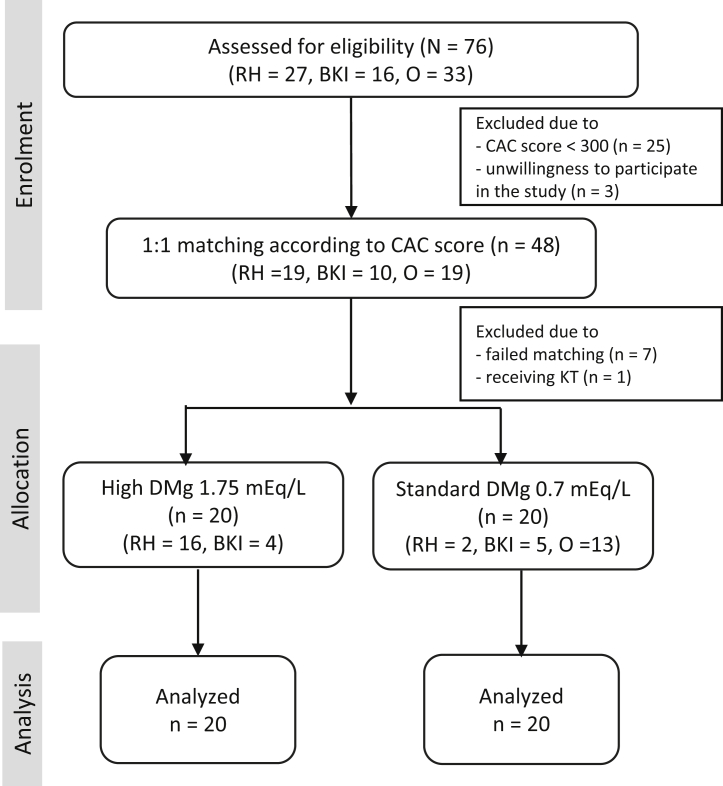
The flow chart for the study is shown in Fig 1. Seventy-six patients were screened for CAC and BMD, and 48 patients were eligible. To monitor safety in case of any adverse effects, the first 20 eligible patients who received dialysis at Ramathibodi Hospital and Bhumirajanagarindra Kidney Institute were assigned to receive a high dialysis magnesium concentration of 1.75 mEq/L. These patients were then matched in a 1:1 ratio according to the CAC score that fell within 20% of one another to the rest of the eligible patients. This control group continued to receive a standard dialysis magnesium concentration of 0.7 mEq/L. The concentration of 1.75 mEq/L was chosen because of the beneficial effect of a dialysis magnesium concentration of 2 mEq/L on calcification propensity reported previously.21 The intervention was continued for 26 weeks before the determination of follow-up CAC score and BMD. Dialysate electrolyte concentrations and medications related to mineral and bone disorders were kept unchanged during the study period.
Figure 1.
Study flow chart. Abbreviations: BKI, Bhumirajanagarindra Kidney Institute; CAC, coronary artery calcification; DMg, dialysate magnesium; KT, kidney transplantation; O, other HD units; RH, Ramathibodi Hospital.
Dialysate Preparation
All dialysis centers participating in the study used individual dialysate jugs to distribute dialysate to each patient. The standard dialysate was a mixture of 2 concentrated solutions (acid-mix A and base-mix B), which yielded final concentrations of 138 mEq/L of sodium, 2-3 mEq/L of potassium, 108.2 mEq/L of chloride, 2.5 mEq/L of calcium, 3 mEq/L of acetate, 0.7 mEq/L of magnesium, 32 mEq/L of bicarbonate, and 1 g/L of glucose. A solution containing a high dialysis magnesium concentration was prepared by adding 20.96 g of magnesium chloride powder to 5.5 L of a standard acid-concentrated solution, yielding the final magnesium and chloride concentrations of 1.75 and 108.7 mEq/L, respectively.
Safety Monitoring
During the first month of the study, all participants were asked or observed by HD nurses for signs and symptoms of hypermagnesemia, including headache, nausea, and flushing. Thereafter, the patients were asked to inform the nurses if any of these symptoms occurred. The symptom of muscle cramps was not specifically asked about but was part of the routine records documented by the HD nurses in the HD treatment record form. Intradialytic hypotension was characterized by a nadir systolic blood pressure of <90 mm Hg.22 In the high dialysate magnesium group, the serum and ionized magnesium, serum calcium, phosphate, and PTH levels were determined at baseline, after the first week, and every 8 weeks thereafter. To avoid magnesium toxicity, any patient who developed a serum magnesium concentration of >4 mg/dL, ionized magnesium concentration of >2.3 mEq/L, or arrhythmia underwent electrocardiography. Furthermore, the dialysis magnesium concentration was lowered to 1.5 mEq/L, and the serum and ionized magnesium concentrations were measured and electrocardiography was repeated.20,21
Measurements
All measurements were performed at the same laboratory and radiograph facilities. The baseline laboratory data were obtained before the HD session. The ionized magnesium concentrations were analyzed using the ion-selective electrode method. The serum calcium concentration was corrected based on the following equation: corrected calcium (mg/dL) = serum calcium (mg/dL) + [(40 − serum albumin (g/L))/10 × 0.8].
Coronary artery calcification was determined using cardiac computed tomography performed using a 320-slice computed tomography scanner (Aquilion ONE, Toshiba Medical Systems). The data were reconstructed at a 3-mm-axial slice thickness. The CAC score was determined using Smartscore software, version 4.0 (GE Healthcare), and calculated according to the method described previously by Agatston et al.23 The progression of CAC was defined as the difference between follow-up and baseline square root transformed Agatston score of ≥5 mm3 per year or volume score of ≥4 mm3 per year. These values were chosen to minimize interscan variability, maximize reproducibility, and distinguish rapid progressors from slow progressors and nonprogressors.24,25 Bone mineral density was determined using dual-energy x-ray absorptiometry (Hologic A, software 12.6.1). The results of the imaging studies were reviewed by the same experts from each field who were blinded to the study data.
Statistical Methods
Data are presented as mean ± standard deviation, median (interquartile range), or mean (95% confidence interval). To explore the treatment effect of the high dialysate magnesium concentration on outcomes over time, a mixed-effects regression analysis was performed with random intercept and maximum likelihood estimation. The t test, χ2 test, or Wilcoxon signed rank test was used to compare the difference between the 2 groups at the baseline. For subgroup analyses, the study participants were divided into 2 subgroups based on the median of the baseline Agatston and volume scores of the high dialysate magnesium group. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using Stata, version 16 (StataCorp LLC).
Results
All participants completed the study, and none of the patients missed an HD session. The baseline demographic and laboratory data are shown in Table 1. There were no significant differences in age, sex, body mass index, underlying diseases, smoking status, dialysis vintage, medications, baseline CAC scores, and BMD. Most of the baseline laboratory and dialysis adequacy data were comparable, except for lower serum albumin concentrations in the high dialysate magnesium group.
Table 1.
Baseline Demographic and Laboratory Data of All Patients
| Parameters | High Dialysate Mg (n = 20) | Standard Dialysate Mg (n = 20) | P |
|---|---|---|---|
| Male, n (%) | 13 (65%) | 11 (55%) | 0.52 |
| Age, y | 60.7 ± 10 | 63.2 ± 9.5 | 0.42 |
| Body mass index, kg/m2 | 25.1 ± 5.7 | 24.5 ± 4.7 | 0.71 |
| Diabetes mellitus, n (%) | 6 (30%) | 8 (40%) | 0.51 |
| Cardiovascular disease, n (%) | 3 (15%) | 2 (10%) | 0.63 |
| Smoker, n (%) | 4 (20%) | 5 (25%) | 0.71 |
| Dialysis vintage, mo | 80 (44-105) | 83 (38-128) | 0.8 |
| Phosphate binders | |||
| Calcium base, n (%) | 14 (70%) | 13 (65%) | 0.74 |
| Noncalcium base, n (%) | 5 (25%) | 6 (30%) | 0.72 |
| Calcimimetic, n (%) | 2 (10.5%) | 6 (30%) | 0.13 |
| Active vitamin D, n (%) | 13 (65%) | 9 (45%) | 0.2 |
| Warfarin, n (%) | 2 (10%) | 2 (10%) | >0.99 |
| Laboratory data | |||
| Hemoglobin, g/dL | 10.9 ± 1.3 | 10.9 ± 1.7 | 0.93 |
| Cholesterol, mg/dL | 165 ± 47 | 160 ± 36 | 0.72 |
| Albumin, g/L | 35.5 ± 3.2 | 37.8 ± 3.1 | 0.02 |
| Calcium, mg/dL | 9.34 ± 0.88 | 9.9 ± 1.22 | 0.1 |
| Phosphate, mg/dL | 4.76 ± 0.74 | 4.94 ± 1.18 | 0.57 |
| Intact PTH, pg/mL | 365 (158-660) | 412 (204-589) | 0.97 |
| Magnesium, mg/dL | 2.19 ± 0.35 | 2.14 ± 0.27 | 0.59 |
| C-reactive protein, mg/L | 1.07 (0.92-5.05) | 3.1 (0.63-8.37) | 0.38 |
| Kt/V | 1.83 ± 0.33 | 1.86 ± 0.34 | 0.74 |
| Coronary artery calcification | |||
| Agatston score | 1,672 (620-2,789) | 1,923 (825-2,937) | 0.64 |
| Volume score | 785 (280-1,241) | 720 (414-1,120) | 0.74 |
| Bone mineral density, g/cm2 | |||
| Total hip | 0.77 ± 0.15 | 0.7 ± 0.15 | 0.14 |
| Femoral neck | 0.65 ± 0.13 | 0.59 ± 0.15 | 0.21 |
| Lumbar spine | 0.87 ± 0.14 | 0.85 ± 0.17 | 0.68 |
Note: Data are presented as mean ± standard deviation or median (interquartile range).
Abbreviations: Mg, magnesium; PTH, parathyroid hormone.
Changes in CAC Score, BMD, and Laboratory Data
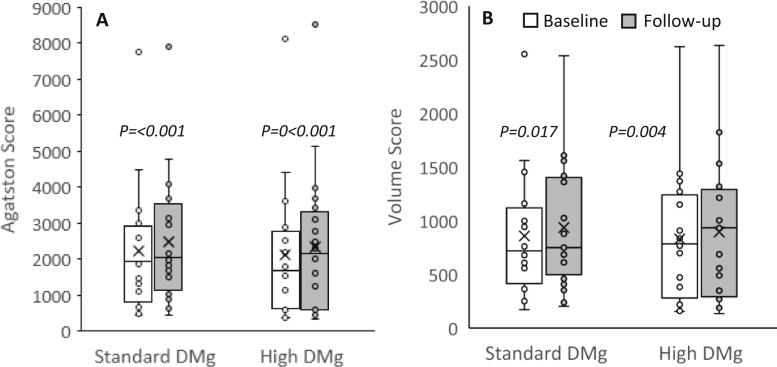
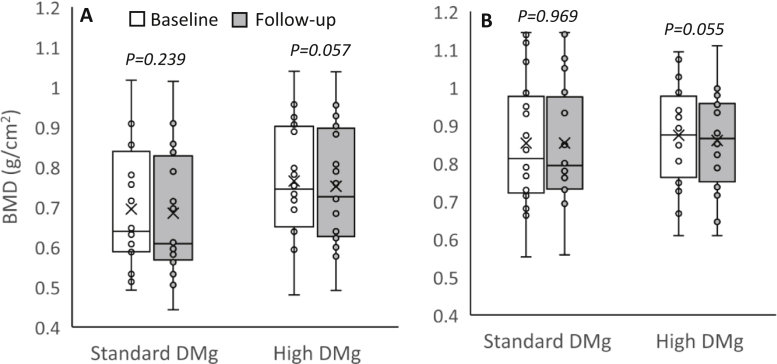
The results from the mixed-effects regression analysis are shown in Table 2. Figures 2 and 3 also illustrate the changes in the CAC score and BMD. A significant increase in the CAC score from the baseline was observed in both groups of patients at the end of the study. A tendency toward a decline in the BMD of the total hip, femoral neck, and lumbar spine from that at the baseline was observed in both the groups, but the difference did not reach statistical significance. Between-group comparisons also yielded negligible difference. The serum magnesium and ionized magnesium concentrations increased significantly in the high dialysate magnesium group. An insignificant increase in the serum calcium level in the standard dialysate magnesium group appeared to be more pronounced than that in the high dialysate magnesium group. There was no significant change in the serum phosphate level. A decline in the PTH level was observed in the high dialysate magnesium group, and an increase in the PTH level was observed in the standard dialysate magnesium group, but differences within and between the groups did not reach statistical significance. These results were largely unchanged after adjusting for possible confounders, including age, diabetes, serum albumin, dialysis vintage, and baseline CAC score.
Table 2.
Mixed-Effects Regression Analyses of Changes in Coronary Artery Calcification, Bone Mineral Density, and Laboratory Data
| Parameters | Time | Mean (95% Confidence Interval) |
Mean Change from Baseline within Group |
Mean Difference Between High and Standard Dialysate Magnesium Group | P | ||
|---|---|---|---|---|---|---|---|
| High DMg | Standard DMg | High DMg | Standard DMg | ||||
| CAC Agatston score | Baseline | 2,120 (1,256-2,985) | 2,220 (1,416-3,023) | 236 (105 to 366) | 268 (132 to 403) | −115 (−1,222 to 991) | 0.84 |
| 26 wk | 2,356 (1,424-3,288) | 2,487 (1,651-3,323) | |||||
| CAC volume score | Baseline | 830 (546-1,114) | 857 (590-1,125) | 65 (21 to 109) | 79 (14 to 144) | −34 (−396 to 327) | 0.85 |
| 26 wk | 895 (596-1,194) | 936 (662-1,211) | |||||
| Total hip BMD | Baseline | 0.77 (0.7-0.83) | 0.7 (0.62-0.77) | −0.01 (−0.03 to 0.00) | −0.01 (−0.03 to 0.01) | 0.07 (−0.02 to 0.16) | 0.14 |
| 26 wk | 0.75 (0.68-0.82) | 0.68 (0.61-0.76) | |||||
| Femoral neck BMD | Baseline | 0.65 (0.59-0.71) | 0.59 (0.52-0.66) | −0.01 (−0.01 to 0.00) | −0.01 (−0.02 to 0.01) | 0.06 (−0.03 to 0.14) | 0.17 |
| 26 wk | 0.64 (0.58-0.71) | 0.58 (0.52-0.65) | |||||
| Lumbar spine BMD | Baseline | 0.87 (0.81-0.94) | 0.85 (0.77-0.93) | −0.01 (−0.02 to 0.00) | 0.00 (−0.02 to 0.02) | 0.01 (−0.08 to 0.1) | 0.77 |
| 26 wk | 0.86 (0.8-0.92) | 0.85 (0.78-0.93) | |||||
| Magnesium | Baseline | 2.19 (2.03-2.36) | 2.14 (2.01-2.27) | 1.05 (0.92 to 1.18) | 0.07 (−0.05 to 0.18) | 0.55 (0.32 to 0.77) | <0.001 |
| 26 wk | 3.24 (3.05-3.43) | 2.21 (2.00-2.41) | |||||
| Ionized Mg | Baseline | 1.54 (1.40-1.68) | 1.34 (1.28-1.40) | 0.26 (0.10 to 0.42) | −0.02 (−0.07 to 0.04) | 0.34 (0.23 to 0.45) | <0.001 |
| 26 wk | 1.80 (1.67-1.93) | 1.33 (1.23-1.43) | |||||
| Calcium | Baseline | 9.34 (8.92-9.75) | 9.90 (9.33-10.47) | 0.21 (−0.19 to 0.61) | 0.43 (−0.13 to 1.00) | −0.67 (−1.23 to −0.12) | 0.02 |
| 26 wk | 9.55 (9.13-9.97) | 10.33 (9.73, 10.93) | |||||
| Phosphate | Baseline | 4.76 (4.41-5.11) | 4.94 (4.39-5.49) | 0.13 (−0.54 to 0.79) | 0.32 (−0.07 to 0.71) | −0.28 (−0.88 to 0.32) | 0.36 |
| 26 wk | 4.89 (4.25-5.52) | 5.26 (4.64-5.88) | |||||
| PTH | Baseline | 471 (296-646) | 532 (278-785) | −52 (−131 to 28) | 58 (−47 to 164) | −116 (−402 to 170) | 0.43 |
| 26 wk | 419 (273-565) | 590 (288-892) | |||||
Abbreviations: BMD, bone mineral density; CAC, coronary artery calcification; DMg, dialysate magnesium; Mg, magnesium; PTH, parathyroid hormone.
Figure 2.
A boxplot graph of coronary artery calcification score of all patients. (A) Agatston score. (B) Volume score. The boxplot graph represents median, interquartile range, 95% confidence interval, and outliers. The box represents interquartile range. The horizontal line and X mark within the box represent the median and mean, respectively. The whiskers represent 95% confidence interval. Abbreviation: DMg, dialysate magnesium.
Figure 3.
A boxplot graph of bone mineral density of all patients. (A) Total hip. (B) Lumbar spine. The boxplot graph represents median, interquartile range, and 95% confidence interval. The box represents interquartile range. The horizontal line and X mark within the box represent the median and mean, respectively. The whiskers represent 95% confidence interval. Abbreviation: DMg, dialysate magnesium.
Progression of CAC
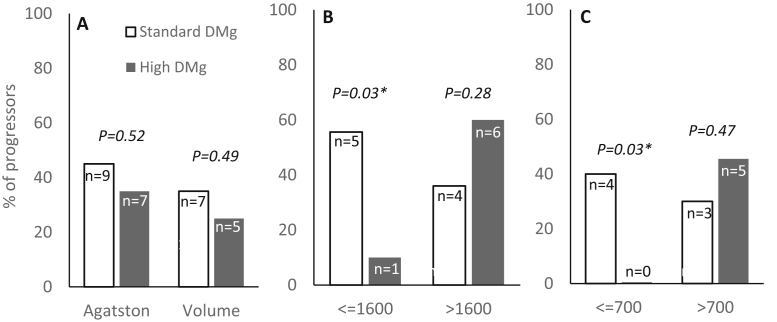
Figure 4 demonstrates the proportion of patients with significant progression of CAC. Progression, according to Agatston and volume score criteria, was observed in both groups of patients (Fig 4A). Because most of the study participants had prolonged dialysis vintage with a severe degree of CAC at baseline, the subgroup analyses using the median of the baseline Agatston and volume scores as cutoffs were attempted. Among patients with an Agatston score of ≤1,600 and a volume score of ≤700, the degree of CAC was stable in the high dialysate magnesium group but increased substantially in the standard dialysate magnesium group (Table 3). However, between-group comparisons did not yield meaningful differences. Further analyses of the rate of progression of CAC using both Agatston and volume score criteria revealed that the numbers of progressors were significantly fewer in the high dialysate magnesium group than in the standard dialysate magnesium group among patients with a lower baseline CAC score (Fig 4B and C).
Figure 4.
Proportion of patients with significant progression of coronary artery calcification. (A) All patients according to Agatston or volume score. (B) Subgroup according to Agatston score. (C) Subgroup according to volume score. Abbreviation: DMg, dialysate magnesium.
Table 3.
Mixed-Effects Regression Analyses on the Changes of CAC Score in Different Subgroups
| CAC Score | Mean Change from Baseline within Group |
Mean Difference between High and Standard DMg Group | P | |||
|---|---|---|---|---|---|---|
| High DMg | P | Standard DMg | P | |||
| Agatston | ||||||
| ≤1,600 | 87 (−58 to 233) | 0.24 | 228 (84 to 371) | <0.01 | −152 (−589 to 285) | 0.5 |
| >1,600 | 384 (211 to 557) | <0.01 | 300 (86 to 515) | 0.01 | 157 (−1,291 to 1,604) | 0.83 |
| Volume | ||||||
| ≤700 | 9 (−19 to 37) | 0.52 | 66 (24 to 108) | <0.01 | −138 (−300 to 24) | 0.09 |
| >700 | 111 (45 to 176) | <0.01 | 92 (−30 to 215) | 0.14 | −25 (−450 to 401) | 0.91 |
Note: Data are presented as mean (95% confidence interval).
Abbreviations: CAC, coronary artery calcification; DMg, dialysate magnesium.
Muscle Cramps and Safety Data
The symptom of muscle cramps and other side effects that occurred during the study period are shown in Table 4. At baseline, 11 (55%) patients in the high dialysate magnesium group and 8 (40%) patients in the standard dialysate magnesium group reported having muscle cramps during HD in the previous month (P = 0.34). At the end of the study, only 2 (10%) patients in the high dialysate magnesium group reported the symptom compared with 9 (45%) patients in the standard dialysate magnesium group (P = 0.01). The number of episodes of muscle cramps during the 26-week study period was also remarkably lower, with only 2 episodes in the high dialysate magnesium group compared with 285 episodes in the standard dialysate magnesium group. None of the patients reported headache, flushing, or nausea. The number of patients with intradialytic hypotension and the number of episodes of intradialytic hypotension were not different between the 2 groups. Most patients who were on the high dialysis magnesium concentration requested that the treatment be continued after study completion largely because of the alleviation of muscle cramps. Changes in the laboratory data in the high dialysate magnesium group are shown in Table 5. Predialysis serum and ionized magnesium concentrations reached a plateau by 8-16 weeks. The highest levels of serum and ionized magnesium were 3.8 mg/dL and 2.22 mEq/L, respectively. None of the patients required a reduction in the dialysis magnesium concentration. There were no significant changes in the serum calcium and phosphate levels. A significant decline in the PTH level from the baseline was observed only during the first 2 months of the study.
Table 4.
Muscle Cramps and Other Side Effects During the 26-Week Study Period
| Symptoms | Number of Patients (%) |
Number of Episodes |
||
|---|---|---|---|---|
| High DMg | Standard DMg | High DMg | Standard DMg | |
| Muscle cramps | 2 (10%) | 9 (45%) | 4 | 285 |
| Headache | 0 | 0 | 0 | 0 |
| Flushing | 0 | 0 | 0 | 0 |
| Nausea | 0 | 0 | 0 | 0 |
| IDH | 7 (35%) | 5 (25%) | 15 | 14 |
Abbreviations: DMg, dialysate magnesium; IDH, intradialytic hypotension.
Table 5.
Changes in Laboratory Data in the High Dialysate Magnesium Group
| Laboratory Data | Baseline | Week 1 | Week 9 | Week 17 | Week 25 |
|---|---|---|---|---|---|
| Magnesium, mg/dL | 2.19 ± 0.35a | 3.17 ± 0.41a | 3.23 ± 0.47a | 3.36 ± 0.58a | 3.24 ± 0.41a |
| Ionized Mg, mEq/L | 1.54 ± 0.27a | 1.86 ± 0.19a | 1.87 ± 0.19a | 1.75 ± 0.17a | 1.8 ± 0.24a |
| Calcium, mg/dL | 9.34 ± 0.88 | 9.4 ± 0.99 | 9.51 ± 1.03 | 9.29 ± 0.95 | 9.55 ± 0.89 |
| Phosphate, mg/dL | 4.76 ± 0.74 | 4.42 ± 1.04 | 4.88 ± 1.11 | 4.95 ± 1.12 | 4.89 ± 1.36 |
| PTH, pg/mL | 365 (158-660) | 328 (171-526)b | 312 (204-614)b | 362 (199-490) | 333 (210-604) |
Note: Data are presented as mean ± standard deviation or median (interquartile range).
Abbreviations: Mg, magnesium; PTH, parathyroid hormone.
P < 0.01 compared with baseline values.
P < 0.05 compared with baseline values.
Discussion
The high dialysis magnesium concentration administered for 26 weeks did not reduce the progression of CAC or improve BMD in patients receiving maintenance HD. In addition, the number of patients who progressed between the 2 groups was comparable. Patients were also categorized into 2 subgroups based on the median CAC score; there was a significant decline in the CAC score and number of patients who progressed in the lower CAC score subgroup who received the high dialysis magnesium concentration. There was no significant change in BMD in any of the subgroups. The number of patients who experienced muscle cramps and the number of episodes of muscle cramps during the study period were markedly lower in the group of patients who received the high dialysis magnesium concentration.
Vascular calcification is a cell-mediated process that occurs under appropriate conditions and tends to progress over time. Evidence has suggested that once the process starts, it is likely to be permanent and its regression is rare.26 Participants in the present study had prolonged dialysis vintage with severe calcification at the baseline; therefore, it is conceivable that any interventions in this later stage of calcification may be ineffective. Previous studies have confirmed the severity of CAC as an independent predictor of future progression, and interventions seemed to affect only those with less severe calcification.27, 28, 29 In the present study, the beneficial effect of the high dialysis magnesium concentration in slowing the progression of CAC was appreciable in the subgroup of patients with less severe calcification. However, the favorable effect of the high dialysis magnesium concentration on mild-to-moderate calcification cannot be confirmed in this pilot study that included only a small number of patients.
The effect of magnesium on bone in patients with chronic kidney disease has been largely unexplored. In the present study, the increased dialysis magnesium concentration administered for 6 months neither improved nor worsened BMD. In the general population, magnesium has been shown to protect against osteoporosis through inhibition of osteoclasts and stimulation of osteoblast activities and hydroxyapatite crystal formation.30 Mild hypermagnesemia has been shown to be associated with lower risk of hip fracture among patients receiving HD.9 Nevertheless, the lack of changes in bone mass due to the administration of the high dialysis magnesium concentration does not exclude alterations in bone strength and architecture. Further study is required to establish the effect of increased dialysis magnesium concentration on bone architecture.
Higher serum magnesium concentration and magnesium supplementation have been shown to be associated with lower PTH levels in patients with nondialysis-dependent chronic kidney disease and HD.11,21,31 In the present study, at the end of the study, a lower PTH level was observed in the high dialysate magnesium group and a higher PTH level was observed in the standard dialysate magnesium group, but differences within and between the groups did not reach statistical significance. However, a notable decline in the PTH level in the high dialysate magnesium group was documented during the first 2 months. This subtle change in the PTH level might be partly responsible for the less pronounced increase in the serum calcium level in the high dialysate magnesium group at the end of the study.
Muscle cramping is the second most common intradialytic symptom after hypotension. The discomfort produced by these intradialytic symptoms could be the cause of early cessation of HD session in up to 30% of patients.32,33 In the present study, the number of patients and episodes of muscle cramps during HD were remarkably lower among those who received the high dialysis magnesium concentration. In a small observational study, increasing the dialysis magnesium concentration from 0.5 to 1 mEq/L resulted in a lower frequency of and less severe muscle cramps.19 The pathophysiology of muscle cramps in patients receiving dialysis is poorly understood. A recent narrative review indicated a poor correlation of serum and body magnesium with magnesium stores. Despite most patients receiving HD having serum magnesium within the normal range, inadequate magnesium storage should not be overlooked and might be partly responsible for muscle cramps during HD.34 The favorable effect of increased dialysis magnesium concentration in alleviating muscle cramps is far reaching in improving the quality of life of patients receiving HD, and a larger clinical trial is warranted. Despite a significant increase in the serum magnesium concentration after the administration of the high dialysis magnesium concentration, the number of episodes of intradialytic hypotension was comparable between the 2 groups, indicating that the dialysis magnesium concentration of 1.75 mEq/L was well tolerated.
In the present study, the participants had prolonged dialysis vintage with a severe degree of vascular calcification at the baseline, and the findings might not apply to healthier patients with shorter dialysis vintage and less-established calcification. Because 1 cycle of bone turnover can take up to several months, the study duration of 6 months might not be long enough to determine the long-term effect of a high dialysis magnesium concentration on bone. It is also possible that a longer study duration may result in a more significant effect on vascular calcification. The high dialysis magnesium concentration was preferentially assigned to patients who received HD at 2 large dialysis centers where the investigators could conveniently monitor any adverse effects. Such a strategy might not be the best option for a study designed to establish causality. However, the primary outcomes of the present study were unlikely to be affected because CAC and BMD were determined at the same facilities and reviewed by the same experts for all participants. The patients assigned to the standard dialysate magnesium group were also under the care of the same group of nephrologists, who agreed to follow the protocol during the entire study period.
In summary, the high dialysis magnesium concentration administered for 26 weeks did not ameliorate the progression of CAC or improve BMD in patients with severe calcification receiving maintenance HD. The favorable effect of a high dialysis magnesium concentration may exist in subgroup of patients with mild-to-moderate CAC and will require further study.
Article Information
Authors’ Full Names and Academic Degrees
Praopilad Srisuwarn, MD, Sethanant Sethakarun, MD, Arkom Nongnuch, MD, Sutipong Jongjirasiri, MD, Chanika Sritara, MD, Pinkeaw Klyprayong, MS, and Sinee Disthabanchong, MD.
Authors’ Contributions
Research idea and study design: PS, AN, SD; data acquisition: PS, SS, AN, SJ, CS, PK; data analysis/interpretation; PS, SJ, CS, SD; statistical analysis: PS, SD; supervision or mentorship: AN, SD. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The study was funded by Faculty of Medicine, Ramathibodi Hospital, Mahidol University. The funders of this study had no role in the study design, data collection, data analysis, decision to publish, or preparation of the manuscript.
Financial Disclosure
Dr Srisuwarn received honoraria from Fresenius-Kabi, and Dr Disthabanchong received travel grants and honoraria from Amgen, Fresenius-Kabi, Kyowa Kirin, and Sanofi-Aventis and served as an advisory board member for Amgen and Sanofi-Aventis.
Peer Review
Received May 10, 2021. Evaluated by 2 external peer reviewers, with direct editorial input by the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form August 16, 2021.
Footnotes
Complete author and article information provided before references.
References
- 1.Disthabanchong S., Srisuwarn P. Mechanisms of vascular calcification in kidney disease. Adv Chronic Kidney Dis. 2019;26(6):417–426. doi: 10.1053/j.ackd.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Maier J.A. Low magnesium and atherosclerosis: an evidence-based link. Mol Aspects Med. 2003;24(1-3):137–146. doi: 10.1016/s0098-2997(02)00095-x. [DOI] [PubMed] [Google Scholar]
- 3.Tzanakis I., Virvidakis K., Tsomi A., et al. Intra- and extracellular magnesium levels and atheromatosis in haemodialysis patients. Magnes Res. 2004;17(2):102–108. [PubMed] [Google Scholar]
- 4.Rude R.K., Singer F.R., Gruber H.E. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr. 2009;28(2):131–141. doi: 10.1080/07315724.2009.10719764. [DOI] [PubMed] [Google Scholar]
- 5.Meema H.E., Oreopoulos D.G., Rapoport A. Serum magnesium level and arterial calcification in end-stage renal disease. Kidney Int. 1987;32(3):388–394. doi: 10.1038/ki.1987.222. [DOI] [PubMed] [Google Scholar]
- 6.Ishimura E., Okuno S., Kitatani K., et al. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin Nephrol. 2007;68(4):222–227. doi: 10.5414/cnp68222. [DOI] [PubMed] [Google Scholar]
- 7.Huang J.H., Cheng F.C., Wu H.C. Low magnesium exacerbates osteoporosis in chronic kidney disease patients with diabetes. Int J Endocrinol. 2015:380247. doi: 10.1155/2015/380247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi Y., Hamano T., Nakano C., et al. Association between density of coronary artery calcification and serum magnesium levels among patients with chronic kidney disease. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0163673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaguchi Y., Hamano T., Wada A., Hoshino J., Masakane I. Magnesium and risk of hip fracture among patients undergoing hemodialysis. J Am Soc Nephrol. 2018;29(3):991–999. doi: 10.1681/ASN.2017080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrè S., Li X., Adams-Huet B., et al. Association of serum magnesium with all-cause mortality in patients with and without chronic kidney disease in the Dallas Heart Study. Nephrol Dial Transplant. 2018;33(8):1389–1396. doi: 10.1093/ndt/gfx275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turgut F., Kanbay M., Metin M.R., Uz E., Akcay A., Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol. 2008;40(4):1075–1082. doi: 10.1007/s11255-008-9410-3. [DOI] [PubMed] [Google Scholar]
- 12.Tzanakis I.P., Stamataki E.E., Papadaki A.N., Giannakis N., Damianakis N.E., Oreopoulos D.G. Magnesium retards the progress of the arterial calcifications in hemodialysis patients: a pilot study. Int Urol Nephrol. 2014;46(11):2199–2205. doi: 10.1007/s11255-014-0751-9. [DOI] [PubMed] [Google Scholar]
- 13.Orchard T.S., Larson J.C., Alghothani N., et al. Magnesium intake, bone mineral density, and fractures: results from the Women's Health Initiative Observational Study. Am J Clin Nutr. 2014;99(4):926–933. doi: 10.3945/ajcn.113.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakaguchi Y., Hamano T., Obi Y., et al. A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol. 2019;30(6):1073–1085. doi: 10.1681/ASN.2018111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louvet L., Buchel J., Steppan S., Passlick-Deetjen J., Massy Z.A. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant. 2013;28(4):869–878. doi: 10.1093/ndt/gfs520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montes de Oca A., Guerrero F., Martinez-Moreno J.M., et al. Magnesium inhibits Wnt/beta-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ter Braake A.D., Tinnemans P.T., Shanahan C.M., Hoenderop J.G., De Baaij J.H. Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-20241-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leenders N.H., Van Ittersum F.J., Hoekstra T., Hoenderop J.G., Vervloet M.G. Routine hemodialysis induces a decline in plasma magnesium concentration in most patients: a prospective observational cohort study. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-28629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lynch P.G., Abate M., Suh H., Wadhwa N.K. Magnesium and muscle cramps in end stage renal disease patients on chronic hemodialysis. Adv Nephrol. 2014;2014:1–6. [Google Scholar]
- 20.Schmaderer C., Braunisch M.C., Suttmann Y., et al. Reduced mortality in maintenance haemodialysis patients on high versus low dialysate magnesium: a pilot study. Nutrients. 2017;9(9):926. doi: 10.3390/nu9090926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bressendorff I., Hansen D., Schou M., Pasch A., Brandi L. The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: a randomized, controlled clinical trial. Clin J Am Soc Nephrol. 2018;13(9):1373–1380. doi: 10.2215/CJN.13921217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flythe J.E., Xue H., Lynch K.E., Curhan G.C., Brunelli S.M. Association of mortality risk with various definitions of intradialytic hypotension. J Am Soc Nephrol. 2015;26(3):724–734. doi: 10.1681/ASN.2014020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 24.Callister T.Q., Cooil B., Raya S.P., Lippolis N.J., Russo D.J., Raggi P. Coronary artery disease: improved reproducibility of calcium scoring with an electron-beam CT volumetric method. Radiology. 1998;208(3):807–814. doi: 10.1148/radiology.208.3.9722864. [DOI] [PubMed] [Google Scholar]
- 25.Hokanson J.E., MacKenzie T., Kinney G., et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182(5):1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 26.Chen N.C., Hsu C.Y., Chen C.L. The strategy to prevent and regress the vascular calcification in dialysis patients. Biomed Res Int. 2017:9035193. doi: 10.1155/2017/9035193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chertow G.M., Burke S.K., Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 28.Adirekkiat S., Sumethkul V., Ingsathit A., et al. Sodium thiosulfate delays the progression of coronary artery calcification in haemodialysis patients. Nephrol Dial Transplant. 2010;25(6):1923–1929. doi: 10.1093/ndt/gfp755. [DOI] [PubMed] [Google Scholar]
- 29.Ok E., Asci G., Bayraktaroglu S., et al. Reduction of dialysate calcium level reduces progression of coronary artery calcification and improves low bone turnover in patients on hemodialysis. J Am Soc Nephrol. 2016;27(8):2475–2486. doi: 10.1681/ASN.2015030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welch A.A., Skinner J., Hickson M. Dietary magnesium may be protective for aging of bone and skeletal muscle in middle and younger older age men and women: cross-sectional findings from the UK biobank cohort. Nutrients. 2017;9(11):1189. doi: 10.3390/nu9111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohya M., Negi S., Sakaguchi T., et al. Significance of serum magnesium as an independent correlative factor on the parathyroid hormone level in uremic patients. J Clin Endocrinol Metab. 2014;99(10):3873–3878. doi: 10.1210/jc.2013-4396. [DOI] [PubMed] [Google Scholar]
- 32.Suprapti B., Nilamsari W.P., Alderman C. Medical problems in patients with chronic kidney disease undergoing hemodialysis and their therapy. J Basic Clin Physiol Pharmacol. 2019;30(6) doi: 10.1515/jbcpp-2019-0250. jbcpp-2019-0250. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez L., Brown D., Hu D., Chertow G.M., Vassalotti J.A., Prichard S. Intradialytic symptoms and recovery time in patients on thrice-weekly in-center hemodialysis: a cross-sectional online survey. Kidney Med. 2020;2(2):125–130. doi: 10.1016/j.xkme.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varghese A., Lacson E., Jr., Sontrop J.M., et al. A higher concentration of dialysate magnesium to reduce the frequency of muscle cramps: a narrative review. Can J Kidney Health Dis. 2020;7 doi: 10.1177/2054358120964078. 2054358120964078. [DOI] [PMC free article] [PubMed] [Google Scholar]