Abstract
Objective
Elucidate the core clinical and genetic characteristics and identify the phenotypic variation between different regions and genotypes of fatal familial insomnia (FFI).
Methods
A worldwide large sample of FFI patients from our case series and literature review diagnosed by genetic testing were collected. The prevalence of clinical symptoms and genetic profile were obtained, and then the phenotypic comparison between Asians versus non-Asians and 129Met/Met versus 129Met/Val were conducted.
Results
In total, 131 cases were identified. The age of onset was 47.51±12.53 (range 17–76) years, 106 patients died and disease duration was 13.20±9.04 (range 2–48) months. Insomnia (87.0%) and rapidly progressive dementia (RPD; 83.2%) occurred with the highest frequency. Hypertension (33.6%) was considered to be an objective indicator of autonomic dysfunction. Genotype frequency at codon 129 was Met/Met (84.7%) and Met/Val (15.3%), and allele frequency was Met (92.4%) and Val (7.6%).129 Met was a risk factor (OR: 3.728, 95% CI: 2.194 to 6.333, p=0.000) for FFI in the non-Asian population. Comparison of Asians and non-Asians revealed clinical symptoms and genetic background to show some differences (p<0.05). In the comparison of 129 polymorphisms, a longer disease duration was found in the 129 MV group, with alleviation of some clinical symptoms (p<0.05). After considering survival probability, significant differences in survival time between genotypes remained (p<0.0001).
Conclusions
Insomnia, RPD and hypertension are representative key clinical presentations of FFI. Phenotypic variations in genotypes and geographic regions were documented. Prion protein gene 129 Met was considered to be a risk factor for FFI in the non-Asian population, and 129 polymorphisms could modify survival duration.
Keywords: clinical neurology, genetics, prion
Introduction
Fatal familial insomnia (FFI) is a rare and intractable inherited prion-based disease reported first by Lugaresi et al. It is characterised by organic sleep-related symptoms, rapidly progressive dementia (RPD) and sympathetic symptoms.1–3 It has an autosomal-dominant pattern linked to a point mutation (D178N) of the prion protein (PRNP) gene, which cosegregates with the methionine polymorphism at codon 129 of the mutated allele.4 However, FFI has high clinical heterogeneity, which causes some ambiguity for early identification. Krasnianski et al proposed an applicable pathway that makes the FFI diagnosis more readily understandable.5 We had further divided FFI symptoms and signs into three clusters (sleep related, neuropsychiatric andprogressive sympathetic) to facilitate FFI recognition.2 However, the frequency and core clinical features of the disease spectrum have not been determined.2
Hundreds of FFI patients have been reported worldwide, distributed mainly in Europe and Asia, especially increasing numbers have been reported in China in recent years6 7. The phenotype has been reported to be similar in different areas but, as we observed in our case series compared with that of case series from Spain, the phenotype of FFI might have a divergent distribution in the three clusters of symptoms.8 9 One may speculate that a regional difference in the phenotype might exist between China even in Asia, Europe and the USA.10–15 In addition, phenotypic variability in different regions might be related to the genetic background. It was hypothesised that the phenotype is related to a polymorphism at codon 129 of PRNP in a small-sample study, and this hypothesis needs testing in the whole entity of FFI disease.4 16 17
In this study, we conducted an evidence-based quantitative clinical investigation to clarify the core clinical and genetic characteristics, regional and genotypic differences reflected in the phenotype based on a large cohort of FFI patients.
Methods
Written informed consent was obtained from all participants (or their guardians) before study initiation. Protocols were carried out in accordance with the relevant guidelines and regulations for the use of human subjects in research set by the Chinese government.
Study cohort
Eleven consecutive patients were identified by established criteria for the FFI diagnosis2 and confirmed by genetic analyses (PRNP: D178N) in the Department of Neurology of Xuanwu Hospital from 2012 to 2021.
Then, a systematic literature review was done in PubMed, Embase, Wanfang, Sinomed and China National Knowledge Infrastructure databases with no language restrictions from 1986 up to September 2020. The search terms we used were “Insomnia, Fatal Familial” OR “familial insomnia” OR “fatal insomnia” OR ((familial insomnia OR fatal insomnia) AND (prion OR PRNP OR scrapie prion protein OR PrPSc OR PrPc OR PrP gene)). Patients diagnosed definitively by genetic results (PRNP: D178N) with sufficient data were included in our study, and 120 patients were identified. Finally, 131 FFI patients were selected to pooled data from our case series and the literature review.
Data extraction
To extract detailed information (demographic data as well as the clinical symptoms, auxiliary examinations, and genetic results of FFI), an ‘investigation sheet’ was created according to an Expert Consensus on Clinical Diagnostic Criteria for FFI.2 The sheet covered the following information: sex; age of disease onset; disease duration; definitive familial history; three clusters of clinical symptoms (sleep related, neuropsychiatric, progressive sympathetic); results of seven auxiliary examinations (genetic analyses, MRI of the brain, electroencephalography (EEG); polysomnography (PSG), positron emission tomography (PET), single-photon emission CT (SPECT) and detection of 14-3-3 protein in cerebrospinal fluid (CSF). Selection of articles and data extraction were conducted independently by two authors (JZ and MC) working as a pair. Discrepancies during study selection, data extraction, and quality assessment were resolved by rechecking the source articles and further discussion with a third author (LYW) to reach a consensus.
Statistical analyses
Statistical analyses were performed using SPSS V.22 (IBM). Continuous data are represented as the mean±SD. Dichotomous data are given as a percentage. The Student’s t-test was used for continuous data. The χ2 test and Fisher’s exact test were employed for categorical data. Kaplan-Meier curves for survival probability were drawn, and the difference was assessed by a log-rank test. A binary logistic regression model was employed to evaluate the predictive effect of the 129 polymorphism on FFI cases compared with the pooled normal control data from Caucasians, Japanese and Han Chinese populations published in the literature.18–20
Results
Clinical and genetic features of FFI patients
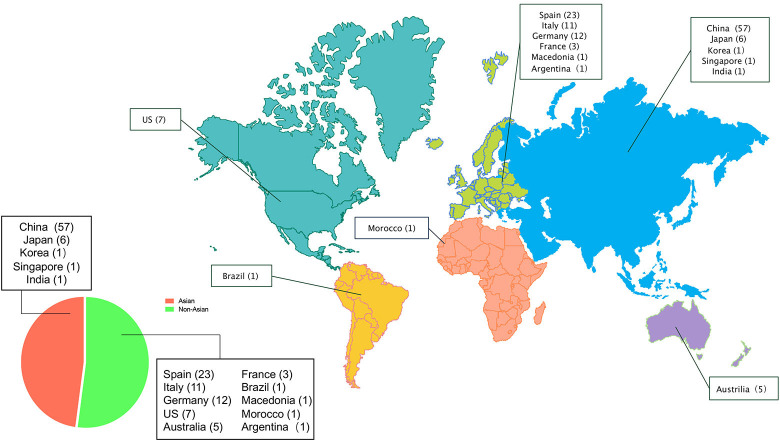
A total of 131 FFI patients (57 women, 72 men and 2 cases whose sex were not reported) were identified. The global regional distribution is shown in figure 1. The detailed demographic data of all FFI patients is summarised in table 1.
Figure 1.
Global regional distribution of FFI patients in Asia (n=66) and not in Asia (n=74). The numbers in parentheses represent the number of patients reported. FFI, fatal familial insomnia.
Table 1.
Demographic data, clinical symptoms and auxiliary examinations in all FFI patients
| Total (N=131) (n/%) | ||
| Demographics | Sex (F/M) | 57/72 |
| Age of onset (years) | 47.51±12.53 | |
| Disease duration (months) | 13.20±9.04 | |
| Definite familial history | 107 (81.7%) | |
| Clinical symptoms | Prevalence of sleep-related symptoms | 119 (90.8%) |
| Prevalence of neuropsychiatric symptoms | 124 (94.7%) | |
| Prevalence of progressive sympathetic symptoms | 95 (72.5%) | |
| Genetic analyses | D178N with 129 MM | 111 (84.7%) |
| D178N with 129 MV | 20 (15.3%) | |
| Brain MRI | Cerebral cortical atrophy | 26 (28.3%) |
| Hyperintense signals on DWI | 2 (2.2%) | |
| EEG | Diffusive excess of slow waves | 41 (40.6%) |
| Periodic spike discharges | 3 (3.0%) | |
| PSG | Reduced durations of REM | 47 (90.4%) |
| Sleep-related involuntary movements | 20 (38.5%) | |
| Sleep-related dyspnoea | 23 (44.2%) | |
| Laryngeal stridor | 10 (19.2%) | |
| PET | Hypometabolism in thalamus | 33 (78.6%) |
| SPECT | Induced blood flow in thalamus | 8 (80.0%) |
| CSF | Positive for 1433 protein | 20 (34.5%) |
For frequency calculation, the number of patients with abnormalities was the numerator. For calculation of the positive rate of clinical symptoms, the number of all patients was the denominator. When calculating the positive rate of auxiliary examination, the number of patients who completed each type of test was the denominator.
CSF, cerebrospinal fluid; DWI, diffusion-weighted imaging; EEG, electroencephalogram; F, female; FFI, fatal familial insomnia; M, male; PET, positron emission tomography; PSG, polysomnography; REM, rapid eye movement; SPECT, single-photon emission CT.
Based on the data of all FFI patients, the age of disease onset was 47.51±12.53 (range 17–76) years. A total of 106 patients were reported to have died. The disease duration was 13.20±9.04 (range 2–48) months. A definite familial history was documented in 107 (81.7%) patients. The prevalence of clusters A (sleep-related symptoms), B (neuropsychiatric symptoms) and C (progressive sympathetic symptoms) was 90.8%, 94.7% and 72.5%, respectively.
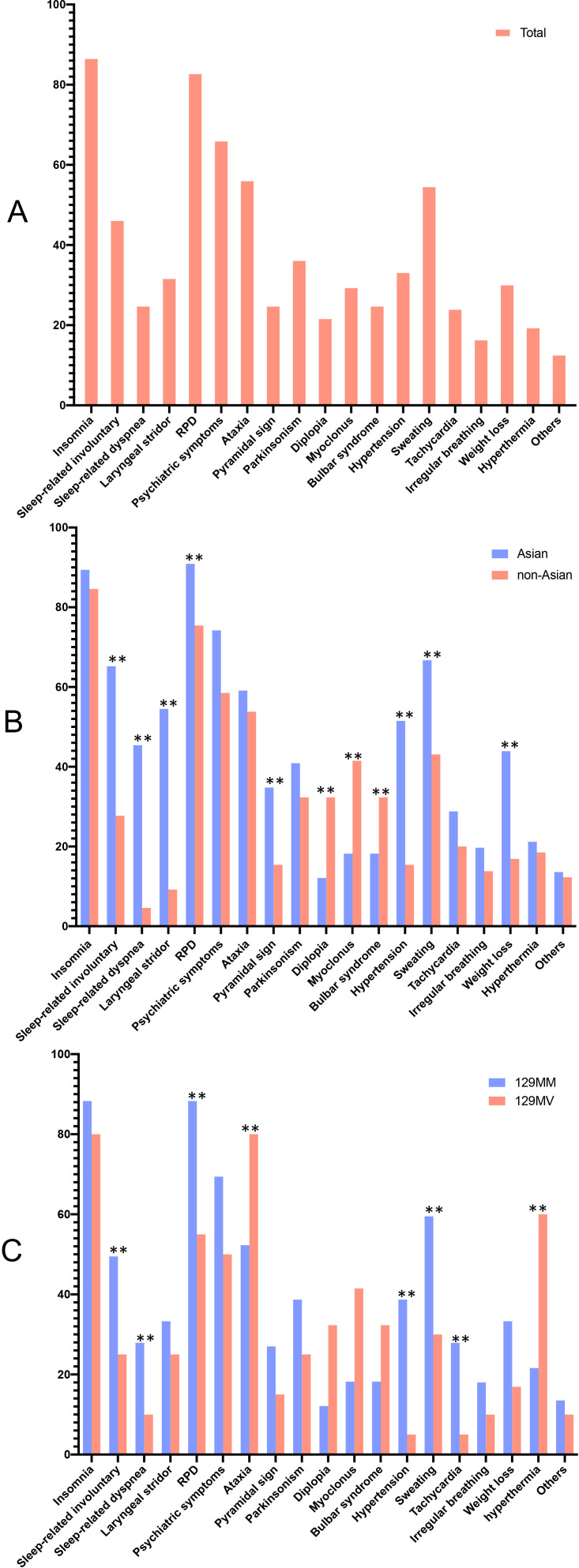
The prevalence of clinical symptoms was summarised to update the scheme of the FFI Expert Consensus (table 2). The prevalence of clinical features in all FFI patients is shown in figure 2A. Insomnia (87.0%) in cluster A and RPD (83.2%) in cluster B occurred with the highest frequency throughout the disease duration in all FFI patients. Hypertension (33.6%) in cluster C was considered to be an objective indicator of autonomic dysfunction. Detailed data on the auxiliary examination of all FFI patients is summarised in table 1. All patients underwent genetic testing, 92 (70.2%) had MRI of the brain, 101 (77.1%) underwent EEG, 52 (39.7%) completed PSG, 42 (32.1%) completed PET, 10 (7.6%) completed SPECT and 58 (44.3%) had CSF results. PSG was the most prevalent auxiliary examination, followed by SPECT and PET. Notably, at codon 129, the genotype frequency was 84.7% for Met/Met and 15.3% for Met/Val, and the allele frequency was 92.4% for Met and 7.6% for Val. Having Met at codon 129 was a risk factor (OR: 3.728, 95% CI: 2.194 to 6.333, p=0.000) for FFI in non-Asian people (table 3).
Table 2.
Clinical characteristics of all FFI patients
| Parameter | Common | Frequent | Less frequent | Rare |
| Cluster A (sleep-related symptoms) | ||||
| Insomnia | 87.0% | |||
| Sleep-related involuntary movements | 46.6% | |||
| Sleep-related dyspnoea | 25.2% | |||
| Laryngeal stridor | 32.1% | |||
| Cluster B (neuropsychiatric symptoms) | ||||
| Rapidly progressive dementia | 83.2% | |||
| Psychiatric symptoms | 66.4% | |||
| Ataxia | 56.5% | |||
| Pyramidal sign | 25.2% | |||
| Parkinsonism | 36.6% | |||
| Myoclonus | 29.8% | |||
| Bulbar syndrome | 25.2% | |||
| Diplopia | 22.1% | |||
| Cluster C (progressive sympathetic symptoms) | ||||
| Sweating | 55.0% | |||
| Hypertension | 33.6% | |||
| Weight loss | 30.5% | |||
| Tachycardia | 24.4% | |||
| Irregular breathing | 16.8% | |||
| Hyperthermia | 19.8% | |||
| Others* | 13.0% |
*Others in cluster C represent constipation, urinary retention and sexual dysfunction.
FFI, fatal familial insomnia.;
Figure 2.
Frequency of clinical symptoms and signs in total, Asian, non-Asian,129MM-genotype and 129MVgenotype-FFI patients (%). Others in cluster C represent constipation, urinary retention andsexual dysfunction. FFI, fatal familial insomnia; RPD, rapidly progressive dementia.* means significant difference
Table 3.
Logistic regression analysis for the predictive effect of 129 polymorphisms in FFI
| Allele | V* | M | OR (95% CI) | P value |
| Asian FFI (n=66) | 3 (2.3%) | 129 (97.7%) | 1 | |
| Asian controls† (n=279) | 18 (3.2%) | 540 (96.8%) | 1.433 (0.416 to 4.939) | 0.568 |
| Non-Asian FFI (n=65) | 17 (13.1%) | 113 (86.9%) | 1 | |
| Non-Asian controls‡ (n=398) | 286 (35.9%) | 510 (64.1%) | 3.728 (2.194 to 6.333) | 0.000 |
*Reference group.
†Pooled data for Japanese and Han Chinese.
‡Pooled data for Caucasians.
FFI, fatal familial insomnia.
Comparison between Asians and non-Asians
There was no significant difference in demographic data between Asians and non-Asians (table 4). The variation in clinical features in Asians and non-Asians is shown in figure 2B. Clinical symptoms, including sleep-related involuntary movements, dyspnoea and laryngeal stridor in cluster A, RPD and the pyramidal sign in cluster B and hypertension, sweating and weight loss in cluster C, carried a high prevalence, whereas diplopia and myoclonus had a lower prevalence in Asians than that in non-Asians (p<0.05). Comparison of detailed data regarding auxiliary examinations is summarised in table 4. Notably, the 129-polymorphism distribution was extremely different (129 MM genotype in Asians versus non-Asians: 95.5% versus 73.8%, p=0.001).
Table 4.
Comparisons between Asian FFI patients and non-Asians
| Asian (N=66) (n/%) | Non-Asian (N=65) (n/%) | P value | ||
| Demographic data | Sex (F/M) | 27/39 | 30/33 | 0.443 |
| Age of onset (years) | 46.79±12.60 | 48.25±12.50 | 0.507 | |
| Disease duration (months) | 11.82±6.40 | 14.74±11.15 | 0.108 | |
| Definite familial history | 51 (77.3%) | 56 (86.2%) | 0.189 | |
| Clinical symptoms | Prevalence of sleep-related symptoms | 64 (97.0%) | 55 (84.6%) | 0.014 |
| Prevalence of neuropsychiatric symptoms | 66 (100%) | 58 (89.2%) | 0.006* | |
| Prevalence of progressive sympathetic symptoms | 56 (84.8%) | 39 (60.0%) | 0.001* | |
| Genetic analyses | D178N with 129 MM | 63 (95.5%) | 48 (73.8%) | 0.001* |
| D178N with 129 MV | 3 (4.5%) | 17 (26.2%) | ||
| Brain MRI | Cerebral cortical atrophy | 21 (36.2%) | 5 (14.7%) | 0.027* |
| Hyperintense signals on DWI | 0 | 2 (5.9%) | 0.062 | |
| EEG | Diffusive excess of slow waves | 21 (36.8%) | 20 (45.5%) | 0.382 |
| Periodic spike discharges | 0 | 3 (6.8%) | 0.045* | |
| PSG | Reduced durations of REM | 25 (86.2%) | 22 (95.7%) | 0.251 |
| Sleep-related involuntary | 14 (48.3%) | 6 (26.1%) | 0.102 | |
| Sleep-related dyspnoea | 17 (58.6%) | 6 (26.1%) | 0.019* | |
| Laryngeal stridor | 9 (31.0%) | 1 (4.3%) | 0.015* | |
| PET | Hypometabolism in thalamus | 15 (71.4%) | 18 (85.7%) | 0.259 |
| SPECT | Induced blood flow in thalamus | 5 (100%) | 3 (71.4%) | 0.114 |
| CSF | Positive for 1433 protein | 15 (46.9%) | 5 (19.2%) | 0.028 |
For frequency calculation, the number of patients with abnormalities was the numerator. For calculation of the positive rate of clinical symptoms, the number of all patients was the denominator. When calculating the positive rate of auxiliary examination, the number of patients who completed each type of test was the denominator.
*Significant difference.
CSF, cerebrospinal fluid; DWI, diffusion-weighted imaging; EEG, electroencephalogram; F, female; FFI, fatal familial insomnia; M, male; PET, positron emission tomography; PSG, polysomnography; REM, rapid eye movement; SPECT, single-photon emission CT.
Comparison between 129MM and 129MV genotypes
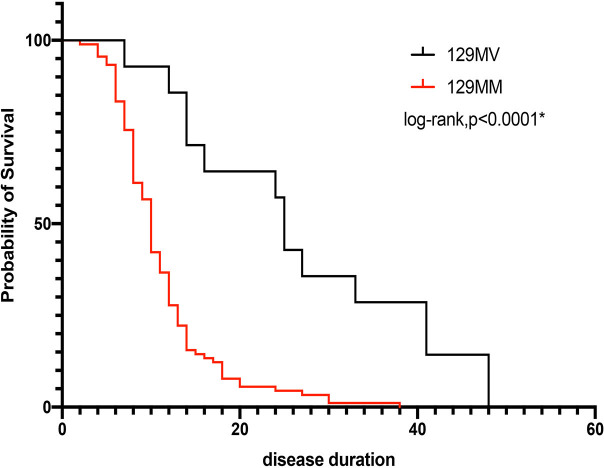
Notably, a significant difference was found in disease duration between people with the 129 MM genotype and those with the 129 MV genotype (11.13±5.92 versus 26.79±13.62, p=0.01) (table 5). Differences in clinical features between the two groups are shown in figure 2C. Sleep-related involuntary movements and dyspnoea in cluster A, RPD in cluster B and hypertension, sweating and tachycardia in cluster C carried a high prevalence, whereas ataxia in cluster B and hyperthermia in cluster C had a lower prevalence for those with a 129 MM genotype compared with those with a 129 MV genotype (p<0.05). Detailed data about auxiliary examinations is summarised in table 5. Periodic ‘spike discharges’ carried a higher prevalence in people with a 129 MV genotype. After considering the probability of survival, a significant difference remained between the 129 MM group and 129 MV group (p<0.0001) (figure 3).
Table 5.
Comparison between 129 MM and 129 MV genotypes in FFI patients
| 129 MM (N=111) (n/%) | 129 MV (N=20) (n/%) | P value | ||
| Demographics | Sex (F/M) | 51/58 | 6/134 | 0.096 |
| Age of onset (years) | 47.32±13.16 | 48.60±8.29 | 0.569 | |
| Disease duration (months) | 11.13±5.92 | 26.79±13.62 | 0.001* | |
| Definite familial history | 94 (84.7%) | 13 (65.0%) | 0.036* | |
| Clinical symptoms | Prevalence of sleep-related symptoms | 103 (92.8%) | 16 (80.0%) | 0.068 |
| Prevalence of neuropsychiatric symptoms | 106 (95.5%) | 18 (90.0%) | 0.314 | |
| Prevalence of progressive sympathetic symptoms | 86 (77.5%) | 9 (45.0%) | 0.003* | |
| Brain MRI | Cerebral cortical atrophy | 25 (30.5%) | 1 (10.0%) | 0.174 |
| Hyperintense signals on DWI | 2 (2.4%) | 0 | 0.618 | |
| EEG | Diffusive excess of slow waves | 37 (43.0%) | 4 (26.7%) | 0.234 |
| Periodic spike discharges | 0 | 3 (20.0%) | 0.000* | |
| PSG | Reduced durations of REM | 43 (89.6%) | 4 (100.0%) | 0.497 |
| Sleep-related involuntary movements | 20 (41.7%) | 0 | 0.100 | |
| Sleep related dyspnoea | 23 (47.9%) | 0 | 0.064 | |
| Laryngeal stridor | 10 (20.8%) | 0 | 0.163 | |
| PET | Hypometabolism in thalamus | 25 (78.1%) | 8 (80.0%) | 0.900 |
| SPECT | Induced blood flow in thalamus | 8 (88.9%) | 0 | 0.725 |
| CSF | Positive for 1433 protein | 18 (36.7%) | 2 (22.2%) | 0.400 |
For frequency calculation, the number of patients with abnormalities was the numerator. For calculation of the positive rate of clinical symptoms, the number of all patients was the denominator. When calculating the positive rate of auxiliary examination, the number of patients who completed each type of test was the denominator.
*Significant difference.
CSF, cerebrospinal fluid; DWI, diffusion-weighted imaging; EEG, electroencephalogram; F, female; FFI, fatal familial insomnia; M, male; PET, positron emission tomography; PSG, polysomnography; REM, rapid eye movement; SPECT, single-photon emission CT.
Figure 3.
Kaplan-Meier curve showing the difference in survival probability. Significant differences between genotypes (p<0.0001,* means significant difference) were documented.
Discussion
Our study, for the first time, characterised the core clinical manifestations and genetic features, and unveiled regional differences and genotype–phenotype correlations, of FFI based on reliable worldwide large-sample data. Our findings highlight the pronounced phenotypic heterogeneity of FFI and aid early recognition of this rare disease.
The core clinical characteristics of FFI were insomnia in cluster A and RPD in cluster B: they could be representative diagnostic items in future criteria to facilitate timely recognition of FFI. The key symptoms mainly associated with its pathology which is usually restricted to the thalamus, and cortex were also involved in cases who had a long duration of FFI.21 Insomnia in cluster A was intractable and resistant to sedatives with reduction of rapid eye movement sleep duration, and sometimes accompanied by involuntary movements and laryngeal stridor; moreover, poor sleep quality could lead to hyperinsomnia during daytime. Dementia in cluster B was associated with thalamus dysfunction involving various cognition domains and was accompanied frequently by hallucinations and illusions. The prevalence of cluster C was 72.5% in our research, which suggests that autonomic symptoms may not be rare. However, due to a lack of recognition of autonomic nervous systems by some neurologists, some symptoms may be neglected in some cases. To screen this cluster of symptoms more accurately, newly discovered hypertension co-occurring with the disease course is recommended as a typical symptom.
The role of PSG in the diagnostic workup of FFI may be underestimated. PSG had a high positive rate in all auxiliary examinations in our FFI patients, which is consistent with previous reports.5 22 Some unidentifiable sleep-related symptoms ignored by patients or their guardians (eg, laryngeal stridor, non-involuntary movements) can be documented sensitively through PSG. Therefore, PSG should be considered as an examination for a better understanding of sleep disturbance if FFI is suspected, which was also recommended in our expert consensus published in 2018.2
Neuronal loss and gliosis of thalamic nuclei are the main histopathological hallmarks of FFI, but thalamus impairment could be confirmed only in post-mortem examinations previously.3 23–27 SPECT and PET are promising in vivo examinations that could be employed to detect metabolism or perfusion abnormalities in the thalamus, which may contribute to the FFI diagnosis before genetic testing.28–30 Eighty percent of patients were found to have hypoperfusion on SPECT and 78.6% patients were found to have hypometabolism in the thalamus on PET, which indicates the high sensitivity of these modalities. Thus, timely use of SPECT or PET could be applied in well-resourced institutions.
We discovered significant phenotypic differences between Asians and non-Asians which may have been induced by a discrepancy in geographic areas, socioeconomic status and ethnicity. Notably, the genetic background was quite different between Asians and non-Asians: the 129 MM genotype was more common in Asians, which suggests that phenotype plasticity based on the geographic region may exist with nature–nurture interactions. In addition, the distribution of 129-polymorphisms in a healthy population has been reported to differ between Europeans and East Asians, and a similar tendency in FFI patients has been documented in our research.31 The 129 polymorphisms were crucial determinants in the prediction of at-risk populations. Met was demonstrated to be a risk factor in the non-Asian population in our research, indicating that non-Asian individuals with the 129M genotype are more susceptible to FFI.
Phenotypic variations had some relationships to the genotype in our research. Disease duration tended to be long in the 129 MV group, which may have been due to the alleviated clinical symptoms observed in this group, and is consistent with the hypothesis in some small-sample studies.32–34 129 polymorphisms upstream of PRNP may modulate production of PRNPs. D178N/M129 and D178N/V129 PRNPs differ in their folding and supramolecular assembly.35 36 Homozygotes could promote more severe conversion from a cellular PRNP into a pathogenic scrapie PRNP (PRNPSc) and aggravate PRNPSc accumulation, which would contribute to more predominant pathologic changes and lead to more severe symptoms, rapid progression and short lifespans than that for heterozygotes.37 Moreover, ataxia in cluster B was more common for people with the 129 MV genotype, as well as periodic spike discharges on EEG. These data indicate that 129 MV is more likely to mimic Gerstmann-Strussler-Scheinker syndrome or Creutzfeldt–Jakob disease profiles, which requires more careful differentiation in clinical practice.
Our study had some limitations. First, the sample size was restricted due to the extreme rarity of FFI. Second, the clinical data of FFI patients were collected retrospectively. Some data on symptoms were missing because there is a tendency to describe only ‘positive symptoms’ in the literature, as well as insufficient recognition of this rare disease in the early part of this century. Moreover, information regarding some auxiliary examinations was not sufficient and some may have been conducted at different timepoints. Third, some of the data of genotypes and alleles were from the literature but did not contain detailed information on age or sex, which may have led to some bias for calculation of risk factors.
Conclusions
We revealed the core clinical and genetic features of FFI as well as phenotype differences between genotypes and regions. Our findings could be useful for clinical practice and promote the accurate and early identification of this rare disease.
Footnotes
JZ, MC and ZT contributed equally.
Contributors: LYW designed and conceptualised the study. HY, JJL and LW provide the patients of study. JZ and MC collected and extracted the data from the literature. MC and ZCT analyzed and interpreted the data. MC and LYW drafted and revised the manuscript. JZ, MC, ZCT, KXX, YC, LL, YHW, JLM, HHY, Y-MYJ, ZYJ, T-XYX, DXW, XW, YZ, HY, JJL, LW and LYW approved the final draft.LYW is respnsible for the overall content as guarantor.
Funding: This work was supported by grants from the Ministry of Science and Technology of China (2019YFC0118600), National Natural Science Foundation of China (81971011) and Beijing Municipal Science and Technology Committee (D171100008217005, 7202060).
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request. The data in this research can be obtained by email to cmsddhr@sina.com.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol was approved by the Ethics Committee and Review Board of Xuanwu Hospital, Capital Medical University (Beijing, China).
References
- 1. Gallassi R, Morreale A, Montagna P, et al. Fatal familial insomnia: behavioral and cognitive features. Neurology 1996;46:935–9. 10.1212/WNL.46.4.935 [DOI] [PubMed] [Google Scholar]
- 2. Wu L-Y, Zhan S-Q, Huang Z-Y, et al. Expert consensus on clinical diagnostic criteria for fatal familial insomnia. Chin Med J 2018;131:1613–7. 10.4103/0366-6999.235115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lugaresi E, Medori R, Montagna P, et al. Fatal familial insomnia and dysautonomia with selective degeneration of thalamic nuclei. N Engl J Med 1986;315:997–1003. 10.1056/NEJM198610163151605 [DOI] [PubMed] [Google Scholar]
- 4. Goldfarb LG, Petersen RB, Tabaton M, et al. Fatal familial insomnia and familial creutzfeldt-jakob disease: disease phenotype determined by a DNA polymorphism. Science 1992;258:806–8. 10.1126/science.1439789 [DOI] [PubMed] [Google Scholar]
- 5. Krasnianski A, Sanchez Juan P, Ponto C, et al. A proposal of new diagnostic pathway for fatal familial insomnia. J Neurol Neurosurg Psychiatry 2014;85:654–9. 10.1136/jnnp-2013-305978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen C, Dong X-P. Epidemiological characteristics of human prion diseases. Infect Dis Poverty 2016;5:47. 10.1186/s40249-016-0143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montagna P. Fatal familial insomnia: a model disease in sleep physiopathology. Sleep Med Rev 2005;9:339–53. 10.1016/j.smrv.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 8. Zarranz JJ, et al. Phenotypic variability in familial prion diseases due to the D178N mutation. Journal of Neurology, Neurosurgery & Psychiatry 2005;76:1491–6. 10.1136/jnnp.2004.056606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prieto E, Domínguez-Prado I, Riverol M, et al. Metabolic patterns in prion diseases: an FDG PET voxel-based analysis. Eur J Nucl Med Mol Imaging 2015;42:1522–9. 10.1007/s00259-015-3090-x [DOI] [PubMed] [Google Scholar]
- 10. Krasnianski A, Bartl M, Sanchez Juan PJ, et al. Fatal familial insomnia: clinical features and early identification. Ann Neurol 2008;63:658–61. 10.1002/ana.21358 [DOI] [PubMed] [Google Scholar]
- 11. Liu L, Li C, Yang Q, et al. Clinical and neuroimaging features of a Chinese patient with fatal familial insomnia. Sleep Med 2017;32:280–1. 10.1016/j.sleep.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 12. Baldin E, Capellari S, Provini F, et al. A case of fatal familial insomnia in Africa. J Neurol 2009;256:1778–9. 10.1007/s00415-009-5205-4 [DOI] [PubMed] [Google Scholar]
- 13. Zarranz JJ, Digon A, Atarés B, et al. Phenotypic variability in familial prion diseases due to the D178N mutation. J Neurol Neurosurg Psychiatry 2005;76:1491–6. 10.1136/jnnp.2004.056606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabernero C, Polo JM, Sevillano MD, et al. Fatal familial insomnia: clinical, neuropathological, and genetic description of a Spanish family. J Neurol Neurosurg Psychiatry 2000;68:774–7. 10.1136/jnnp.68.6.774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi Q, Chen C, Gao C, et al. Clinical and familial characteristics of ten Chinese patients with fatal family insomnia. Biomed Environ Sci 2012;25:471–5. 10.3967/0895-3988.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 16. Montagna P, Cortelli P, Avoni P, et al. Clinical features of fatal familial insomnia: phenotypic variability in relation to a polymorphism at codon 129 of the prion protein gene. Brain Pathol 1998;8:515–20. 10.1111/j.1750-3639.1998.tb00172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bagyinszky E, Giau VV, Youn YC, Eva B, Van GV, Chul YY, et al. Characterization of mutations in prnp (prion) gene and their possible roles in neurodegenerative diseases. Neuropsychiatr Dis Treat 2018;14:2067–85. 10.2147/NDT.S165445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alperovitch A, Zerr I, Pocchiari M, et al. Codon 129 prion protein genotype and sporadic creutzfeldt-jakob disease. Lancet 1999;353:1673–4. 10.1016/S0140-6736(99)01342-2 [DOI] [PubMed] [Google Scholar]
- 19. Doh-ura K, Kitamoto T, Sakaki Y, et al. CJD discrepancy. Nature 1991;353:801–2. 10.1038/353801b0 [DOI] [PubMed] [Google Scholar]
- 20. Tsai MT, Su YC, Chen YH, et al. Lack of evidence to support the association of the human prion gene with schizophrenia. Mol Psychiatry 2001;6:74–8. 10.1038/sj.mp.4000790 [DOI] [PubMed] [Google Scholar]
- 21. Watts JC, Prusiner SB. Experimental models of inherited PrP prion diseases. Cold Spring Harb Perspect Med 2017;7:a027151. 10.1101/cshperspect.a027151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu L, Lu H, Wang X, et al. Clinical features and sleep analysis of Chinese patients with fatal familial insomnia. Sci Rep 2017;7:3625. 10.1038/s41598-017-03817-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montagna P. Fatal familial insomnia and the role of the thalamus in sleep regulation. Handb Clin Neurol 2011;99:981–96. 10.1016/B978-0-444-52007-4.00018-7 [DOI] [PubMed] [Google Scholar]
- 24. Cortelli P, Parchi P, Contin M, et al. Cardiovascular dysautonomia in fatal familial insomnia. Clin Auton Res 1991;1:15–21. 10.1007/BF01826053 [DOI] [PubMed] [Google Scholar]
- 25. Macchi G, Rossi G, Abbamondi AL, et al. Diffuse thalamic degeneration in fatal familial insomnia. a morphometric study. Brain Res 1997;771:154–8. 10.1016/S0006-8993(97)00902-5 [DOI] [PubMed] [Google Scholar]
- 26. Manetto V, Medori R, Cortelli P, et al. Fatal familial insomnia: clinical and pathologic study of five new cases. Neurology 1992;42:312–9. 10.1212/wnl.42.2.312 [DOI] [PubMed] [Google Scholar]
- 27. Lugaresi E. The thalamus and insomnia. Neurology 1992;42:28–33. [PubMed] [Google Scholar]
- 28. Cortelli P, Perani D, Montagna P, et al. Pre-symptomatic diagnosis in fatal familial insomnia: serial neurophysiological and 18FDG-PET studies. Brain 2006;129:668–75. 10.1093/brain/awl003 [DOI] [PubMed] [Google Scholar]
- 29. Montagna P, Gambetti P, Cortelli P, et al. Familial and sporadic fatal insomnia. Lancet Neurol 2003;2:167–76. 10.1016/S1474-4422(03)00323-5 [DOI] [PubMed] [Google Scholar]
- 30. Perani D, Cortelli P, Lucignani G, et al. [18F]FDG PET in fatal familial insomnia: the functional effects of thalamic lesions. Neurology 1993;43:2565–9. 10.1212/WNL.43.12.2565 [DOI] [PubMed] [Google Scholar]
- 31. Erginel-Unaltuna N, Peoc'h K, Komurcu E, et al. Distribution of the M129V polymorphism of the prion protein gene in a Turkish population suggests a high risk for Creutzfeldt-Jakob disease. Eur J Hum Genet 2001;9:965–8. 10.1038/sj.ejhg.5200754 [DOI] [PubMed] [Google Scholar]
- 32. Capellari S, Strammiello R, Saverioni D, et al. Genetic creutzfeldt-jakob disease and fatal familial insomnia: insights into phenotypic variability and disease pathogenesis. Acta Neuropathol 2011;121:21–37. 10.1007/s00401-010-0760-4 [DOI] [PubMed] [Google Scholar]
- 33. Gambetti P, Parchi P, Chen SG. Hereditary creutzfeldt-jakob disease and fatal familial insomnia. Clin Lab Med 2003;23:43–64. 10.1016/S0272-2712(02)00065-3 [DOI] [PubMed] [Google Scholar]
- 34. Cortelli P, Gambetti P, Montagna P, et al. Fatal familial insomnia: clinical features and molecular genetics. J Sleep Res 1999;8 Suppl 1:23–9. 10.1046/j.1365-2869.1999.00005.x [DOI] [PubMed] [Google Scholar]
- 35. Bouybayoune I, Mantovani S, Del Gallo F, et al. Transgenic fatal familial insomnia mice indicate prion infectivity-independent mechanisms of pathogenesis and phenotypic expression of disease. PLoS Pathog 2015;11:e1004796. 10.1371/journal.ppat.1004796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Apetri AC, Vanik DL, Surewicz WK. Polymorphism at residue 129 modulates the conformational conversion of the D178N variant of human prion protein 90-231. Biochemistry 2005;44:15880–8. 10.1021/bi051455+ [DOI] [PubMed] [Google Scholar]
- 37. Montagna P, Cortelli P, Avoni P, et al. Clinical features of fatal familial insomnia: phenotypic variability in relation to a polymorphism at codon 129 of the prion protein gene. Brain Pathol 1998;8:515–20. 10.1111/j.1750-3639.1998.tb00172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request. The data in this research can be obtained by email to cmsddhr@sina.com.