Abstract
Background
The risk of colorectal cancer (CRC) among subjects with a positive faecal immunochemical test (FIT) who do not undergo a colonoscopy is unknown. We estimated whether non-compliance with colonoscopy after a positive FIT is associated with increased CRC incidence and mortality.
Methods
The FIT-based CRC screening programme in the Veneto region (Italy) invited persons aged 50 to 69 years with a positive FIT (>20 µg Hb/g faeces) for diagnostic colonoscopy at an endoscopic referral centre. In this retrospective cohort study, we compared the 10-year cumulative CRC incidence and mortality among FIT positives who completed a diagnostic colonoscopy within the programme (compliers) and those who did not (non-compliers), using the Kaplan-Meier estimator and Cox-Aalen models.
Results
Some 88 013 patients who were FIT positive complied with colonoscopy (males: 56.1%; aged 50–59 years: 49.1%) while 23 410 did not (males: 54.6%; aged 50–59 years: 44.9%).
The 10-year cumulative incidence of CRC was 44.7 per 1000 (95% CI, 43.1 to 46.3) among colonoscopy compliers and 54.3 per 1000 (95% CI, 49.9 to 58.7) in non-compliers, while the cumulative mortality for CRC was 6.8 per 1000 (95% CI, 5.9 to 7.6) and 16.0 per 1000 (95% CI, 13.1 to 18.9), respectively. The risk of dying of CRC among non-compliers was 103% higher than among compliers (adjusted HR, 2.03; 95% CI, 1.68 to 2.44).
Conclusion
The excess risk of CRC death among those not completing colonoscopy after a positive faecal occult blood test should prompt screening programmes to adopt effective interventions to increase compliance in this high-risk population.
Keywords: cancer prevention, colonoscopy, colorectal cancer screening, colorectal cancer
Significance of this study.
What is already known on this subject?
Subjects with a positive faecal immunochemical test (FIT) have a high prevalence of colorectal cancer (CRC) and advanced adenomas at colonoscopy.
Compliance with colonoscopy among FIT positives is suboptimal.
CRC incidence and mortality among FIT positives who do not comply with colonoscopy is not known.
What are the new findings?
The 10-year cumulative incidence of CRC was 44.7 per 1000 among colonoscopy compliers and 54.3 per 1000 in non-compliers.
The 10-year cumulative mortality from CRC was 6.8 per 1000 and 16.0 per 1000, respectively.
The risk of dying of CRC among non-compliers was 103% higher than among compliers (adjusted HR, 2.03; 95% CI, 1.68 to 2.44).
How might it impact on clinical practice in the foreseeable future?
It is critical that as many patients as possible complete a diagnostic colonoscopy after a positive FIT.
Failure to complete a colonoscopy after a positive FIT markedly increases the risk of dying of CRC.
Additional interventions are needed to engage these patients and decrease their burden of CRC.
Introduction
The faecal immunochemical test (FIT), one of several tests available for colorectal cancer (CRC) screening,1 is currently used in many countries.1–5 FIT screening is a two-step process, whereby a follow-up colonoscopy is recommended for participants with a positive FIT.1 5 6 Different methods of increasing colonoscopy completion have been proposed, with moderate evidence supporting the use of patient navigators and provider reminders.7 Other active outreach approaches include actively calling all patients with a positive FIT and maintaining a registry of patients requiring follow-up. However, many screening programmes struggle to ensure timely colonoscopy completion among those with a positive FIT, with rates as low as 50%.8 9 A meta-analysis found that compliance with a diagnostic evaluation was 80% in real-world FIT-based screening programmes.10
In the Veneto region (north-east Italy), subjects with a positive FIT are contacted via phone call and follow-up colonoscopy is offered free of charge. With this active approach, the programme has maintained rates of colonoscopy adherence of 80% at just 3 months after a positive test.11
Among participants with a positive FIT, the yield of clinically significant lesions at colonoscopy is high. In our experience, the positive predictive value of FIT for advanced neoplasia was 32.4% (4.6% for CRC and 27.8% for advanced adenoma).12 Previous studies have shown that delays in colonoscopy completion longer than 9 months are associated with a greater risk of CRC and more advanced disease at the time of colonoscopy.13–16 However, only one study has analysed the association between colonoscopy completion and burden of CRC disease, demonstrating an association between not undergoing colonoscopy and a significant increase in CRC incidence and mortality.17
The objective of this study was to quantify differences in CRC-specific cumulative incidence and mortality in the cohort of FIT positives who did not comply with an invitation to complete a diagnostic colonoscopy within the screening programme (‘non-compliers’), as compared with FIT positives who complied with colonoscopy (‘compliers’).
Methods
Setting
This study was carried out in the Veneto region, where a CRC screening programme using FIT has been operating since 2002. Its target population includes residents aged 50–69 years, who are invited to complete a FIT every 2 years. Subjects with a positive FIT (>20 µg Hb/g faeces) are contacted by phone by trained operators to undergo a precolonoscopy intake meeting. The phone call is standardised and information is provided about FIT positivity, the corresponding risk of disease, the recommendation for an endoscopic assessment and practical issues (precolonoscopy meeting, delivery of bowel preparation, etc). During the precolonoscopy meeting, colonoscopy is organised at an endoscopic referral centre during dedicated time slots. The programme does not exclude subjects with a positive FIT who are symptomatic, nor those who recontact the programme after initially refusing colonoscopy because they subsequently developed symptoms or changed their mind. Both the FIT test and colonoscopy are free of charge.
Barriers to colonoscopy outside the screening programme are minimal, as the Regional Health System guarantees a colonoscopy appointment within 30 days for patients with symptoms, with a copay of about 100€ by the patient. Furthermore, citizens may perform a colonoscopy in a private clinic, at a cost ranging between 150€ and 500€. Private colonoscopy may be covered by private insurance without a co-pay; however, private insurance is uncommon.
All screening data collected were recorded using dedicated software, and are available as individual records.
Cohort creation
We first identified the cohort of subjects who performed a FIT within the screening programme from 1 January 2004 to 30 September 2017. For each screening episode, we recorded the result of the FIT, whether the subject was excluded from colonoscopy, whether a colonoscopy was performed, and the outcome of the colonoscopy (invasive cancer, advanced adenoma, non-advanced adenoma, negative). Subjects already in follow-up because of a history of adenoma, CRC or IBD, who were affected by serious disease or disability, who had had a recent colonoscopy, who moved to other regions, who were deceased, and those who could not give informed consent for colonoscopy were excluded from the study. For subjects with more than one positive FIT during the study period, only the first test was considered.
Primary exposure
We defined all subjects who had a colonoscopy within the screening programme as ‘compliers’, the vast majority (99.93%) of whom had their colonoscopy within 12 months of the positive FIT. We considered all other subjects as ‘non-compliers’, irrespective of whether or not they had a follow-up colonoscopy outside of the screening programme because of the positive FIT or because of subsequent symptoms.
Outcomes
All subjects were linked using a regional, individual identification code with the database of the regional Tumor Registry (available up to 31 December 2015) and the regional database of Pathology Records (available up to 31 December 2018), to identify those who had been diagnosed with a CRC. Stage at diagnosis was collected through consultation of pathology and clinical records for CRCs diagnosed from 2013 to 2018, as cancer stage was available only for a minority of cases diagnosed before 2013. Cases were classified according to the tumour, node, metastases (TNM) seventh edition.18
The vital status of all subjects was assessed through record linkage with the population file of residents, as available from the regional Healthcare System, and with the regional Mortality Registry (available up to 31 December 2018) to identify those who died of CRC.
For follow-up analysis of incidence, each patient was followed from the date of the positive FIT to the date of CRC diagnosis, emigration or last available follow-up (ie, 31 December 2018), whichever came first. For follow-up analysis of mortality, patients were followed up to the date of death, emigration or last available follow-up.
The following indicators were computed for both compliers and non-compliers:
Cumulative incidence of CRC.
Cumulative mortality from CRC. An incidence-based mortality approach was used, that is, only deaths due to a CRC diagnosed after the date of the positive FIT were considered;
Cumulative mortality from all causes.
Statistical analysis
Descriptive statistics were used to summarise the main characteristics of the two study cohorts (colonoscopy compliers and non-compliers).
The cumulative incidence and mortality at 10 years of follow-up were computed using the Kaplan-Meyer estimator.
A Cox model, adjusted for gender, age and screening round (first, subsequent), was initially fit to estimate the effect of colonoscopy compliance on CRC incidence and mortality. In the model testing CRC incidence, the proportional hazard assumption was not met by colonoscopy compliance. A Cox-Aalen model19 was then applied, including gender, age and screening round in the multiplicative part of the model, and time-varying colonoscopy compliance in the additive part of the model. An extended-Cox model with piecewise-constant, time-varying coefficients estimated the HR of CRC incidence for compliers versus non-compliers.20
Given the long follow-up period, a significant number of deaths from other causes could bias the estimates of cumulative mortality from CRC, since the occurrence of death from other causes would preclude the possibility of dying for CRC. To overcome this limitation, we estimated cumulative mortality from CRC in a competing events framework using the Fine-Gray regression models,21 considering CRC death as an event of interest, and deaths from other causes as competing risks.
Since the timing of CRC incidence was different between the study cohorts (mostly <1 year after FIT for compliers versus >1 year after for non-compliers), we performed a sensitivity analysis including only subjects with a positive FIT prior to 31 December 2013 who had a potential follow-up time of 5 years or more.
Statistical tests were two-sided, with statistical significance set at 0.05. Statistical analyses were performed using SAS, V.9.4 statistical package (SAS Institute, Cary, North Carolina, USA) and the R-software environment.
Ethics
Italian legislation identifies Cancer Registries as collectors of personal data for surveillance purposes without explicit individual consent. We did not require approval from a research ethics committee as this study was a descriptive analysis of individual data without any direct or indirect intervention on patients.22
Results
During the study period, 1 127 093 subjects underwent one or more FIT within the regional screening programme and 113 008 had ≥1 positive test (online supplemental figure S1).
gutjnl-2020-322192supp001.pdf (278.3KB, pdf)
A total of 1585 subjects who were FIT positive were excluded: 643 were in follow-up because of a personal or family history of adenoma, CRC or IBD (40.6%); 407 had a recent colonoscopy (25.7%); 158 were deceased (10%); 108 were affected by serious disease or disability (6.8%); 49 could not give informed consent (3.1%); 41 left the region (2.6%); and 179 were excluded for other reasons (11.3%). Of the 111 423 remaining subjects, 88 013 complied with colonoscopy within the screening programme (79%), while 23 410 did not (21%).
Table 1 shows the main characteristics of the two study groups. The proportion of males was 56.1% (95% CI, 55.8 to 56.4) among compliers and 54.6% (95% CI, 53.9 to 55.2) among non-compliers; subjects aged 50–59 years were 49.1% (95% CI, 48.8 to 49.5) and 44.9% (95% CI, 44.3 to 45.6), respectively. At follow-up, 3549 CRC were diagnosed in the cohort of compliers (4.03%). Of these, 2717 (76.6%) were located in the colon and 793 (22.4%) in the rectum. Among non-compliers, 882 CRC were diagnosed overall (3.77%), 659 of which were colon cancers (74.7%) and 204 were rectum cancers (23.1%).
Table 1.
Demographic characteristics of the study population, numbers of CRCs diagnosed at follow-up and numbers of deaths, according to compliance with colonoscopy within the screening programme
| Compliers | Non-compliers | |||
| Number | % | Number | % | |
| Total | 88 013 | 100 | 23 410 | 100 |
| Gender | ||||
| Male | 49 416 | 56.1 | 12 770 | 54.6 |
| Female | 38 597 | 43.9 | 10 640 | 45.4 |
| Age (years) | ||||
| 50–59 | 43 227 | 49.1 | 10 510 | 44.9 |
| 60–69 | 44 786 | 50.9 | 12 900 | 55.1 |
| Screening round | ||||
| First | 31 549 | 35.9 | 9535 | 40.7 |
| Subsequent | 56 464 | 64.1 | 13 875 | 59.3 |
| Diagnosis of CRC | ||||
| Total | 3549 | 4.03 | 882 | 3.77 |
| Colon | 2717 | 3.09 | 659 | 2.82 |
| Rectum | 793 | 0.9 | 204 | 0.87 |
| Not available | 39 | 0.04 | 19 | 0.08 |
| Timing of the diagnosis of CRC | ||||
| Within 1 year of the FIT | 3197 | 90.1 | 555 | 62.9 |
| Between 1 and 10 years after FIT | 352 | 9.9 | 327 | 37.1 |
| Deaths | ||||
| Total | 3494 | 3.97 | 1995 | 8.52 |
| CRC | 336 | 0.38 | 169 | 0.72 |
| Other cancers | 1741 | 1.98 | 922 | 3.94 |
| Other causes | 1417 | 1.61 | 904 | 3.86 |
CRCs, colorectal cancers; FIT, faecal immunochemical test.
The proportion of CRC diagnosed <1 year after the positive FIT was significantly higher among compliers (90.1%) than among non-compliers (62.9%, (p<0.0001).
Stage at diagnosis was available for 1499/1711 CRC diagnosed from 2013 onward among compliers (87.6%) and for 361/425 among non-compliers (84.9%, table 2). Overall, 46.4% CRC diagnosed in compliers were stage I and 22.6% were stage II; the corresponding figures for non-compliers were 48.8% and 23.8%, respectively (p=0.46). Most CRC detected <1 year after the positive FIT were in stage I or II for both compliers (stage I: 46.8%; stage II: 22.3%) and non-compliers (stage I: 57.0%; stage II: 20.3%, p=0.014). Conversely, 34.7% of cancers in compliers and 38.2% in non-compliers diagnosed >1 year after the positive FIT were stages III or IV (p=0.90).
Table 2.
Distribution by TNM stage at diagnosis of CRCs diagnosed within and after 1 year of the positive FIT, according to compliance with colonoscopy
| TNM stage | Compliers (n=1499) | Non-compliers (n=361) | P value* | ||
| Number | % | Number | % | ||
| All CRCs | |||||
| I | 695 | 46.4 | 176 | 48.8 | 0.46 |
| II | 339 | 22.6 | 86 | 23.8 | |
| III | 387 | 25.8 | 79 | 21.9 | |
| IV | 78 | 5.2 | 20 | 5.5 | |
| Total | 1499 | 100 | 361 | 100 | |
| CRCs diagnosed within 1 year of the positive FIT | |||||
| I | 678 | 46.8 | 143 | 57.0 | 0.014 |
| II | 324 | 22.3 | 51 | 20.3 | |
| III | 373 | 25.7 | 46 | 18.3 | |
| IV | 75 | 5.2 | 11 | 4.4 | |
| Total | 1450 | 100 | 251 | 100 | |
| CRCs diagnosed after 1 year of the positive FIT | |||||
| I | 17 | 34.7 | 33 | 30.0 | 0.90 |
| II | 15 | 30.6 | 35 | 31.8 | |
| III | 14 | 28.6 | 33 | 30.0 | |
| IV | 3 | 6.1 | 9 | 8.2 | |
| Total | 49 | 100 | 110 | 100 | |
*Comparison between cohorts through the χ2 test.
CRCs, colorectal cancers; FIT, faecal immunochemical test; TNM, tumour, node, metastases.
During follow-up, 3494 (3.97%) compliers died, 336 (0.38%) of whom died of CRC. The number non-compliers who died was 1995 (8.52%), 169 (0.72%) of whom died of CRC.
Figure 1 shows the cumulative incidence of CRC in the two cohorts during the study period. The median follow-up for incidence assessment was 4.9 years (IQR, 2.9–8.0) in compliers and 4.5 years in non-compliers (IQR, 2.6–7.1). The 10-year cumulative incidence was 44.7 per 1000 (95% CI, 43.1 to 46.3) in compliers and 54.3 per 1000 (95% CI, 49.9 to 58.7) in non-compliers. The shape of the two curves was different. In the cohort of compliers, incidence increased quickly in the first months after the FIT, with modest increases thereafter. The rise in cumulative incidence in non-compliers was lower in the first year and steeper later. The cumulative incidence of CRC in non-compliers exceeded that of compliers from year 6 of follow-up onward. The results of multivariable models are reported in table 3. The hazard of being diagnosed with a CRC was estimated separately up to, and beyond, the sixth month of follow-up, according to the slope of the Cox-Aalen’s hazard curve between the two cohorts (see details in online supplemental appendix 2). In the first 6 months of follow-up, CRC incidence in non-compliers was 44% lower than compliers’ (HR 0.56; 95% CI, 0.51 to 0.61). After 6 months the hazard among non-compliers was about three and a half times higher than among compliers (HR 3.68; 95% CI, 3.20 to 4.22). CRC incidence was higher for males, for 60–69 year olds and for subjects at the first screening round over the whole follow-up period.
Figure 1.
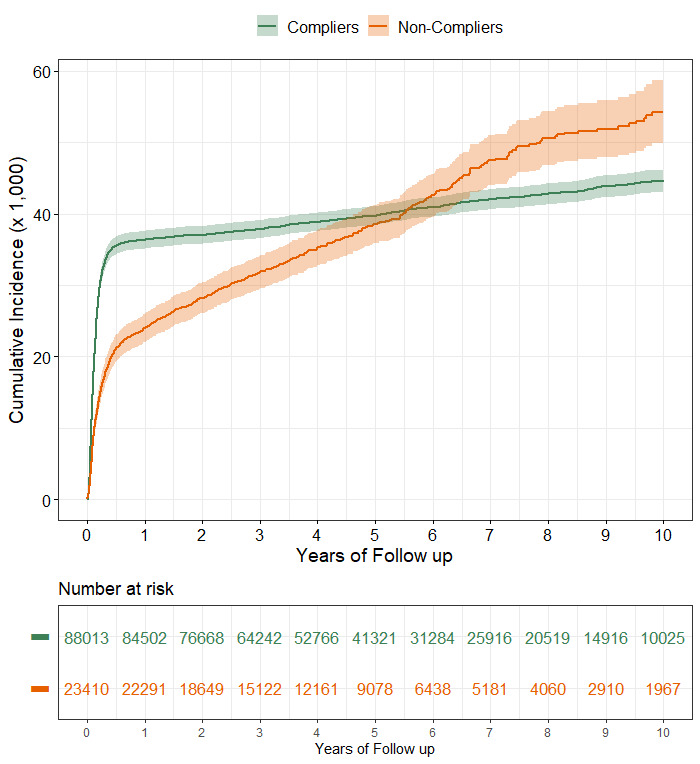
Cumulative incidence of CRC (per 1000) in a cohort of subjects with a positive FIT, according to compliance with follow-up colonoscopy. CRC, colorectal cancer; FIT, faecal immunochemical test.
Table 3.
Adjusted HR of CRC incidence and of CRC mortality in subjects with a positive FIT, with 95% CIs
| Adjusted* HR | 95% CI | |
| CRC incidence† | ||
| Gender | ||
| Male | 1.00 | – |
| Female | 0.84 | 0.79 to 0.89 |
| Age at time of FIT (years) | ||
| 50–59 | 1.00 | – |
| 60–69 | 1.7 | 1.59 to 1.80 |
| Screening round | ||
| First | 1.00 | – |
| Subsequent | 0.5 | 0.47 to 0.53 |
| Compliance with colonoscopy | ||
| Yes | 1.00 | – |
| No (up to month 6) | 0.56 | 0.51 to 0.61 |
| No (beyond month 6) | 3.68 | 3.20 to 4.22 |
| CRC mortality‡ | ||
| Gender | ||
| Male | 1.00 | – |
| Female | 0.68 | 0.56 to 0.81 |
| Age at time of FIT (years) | ||
| 50–59 | 1.00 | – |
| 60–69 | 1.88 | 1.56 to 2.26 |
| Screening round | ||
| First | 1.00 | – |
| Subsequent | 0.45 | 0.37 to 0.54 |
| Compliance with colonoscopy | ||
| Yes | 1.00 | – |
| No | 2.03 | 1.68 to 2.44 |
*Adjusted by all the variables reported in the table.
†According to Extended-Cox model.
‡According to Cox model.
CRC, colorectal cancer; FIT, faecal immunochemical test.
The median length of follow-up for mortality assessment was 4.9 years (IQR, 3.1–7.9) for compliers and 4.2 years (IQR, 2.5–6.7) for non-compliers. In compliers, the cumulative mortality due to CRC after 10 years of follow-up was 6.8 per 1000 (95% CI, 5.9 to 7.6, figure 2). The cumulative mortality due to CRC was greater for non-compliers during the whole study period and by the end of follow-up it was 16.0 per 1000 (95% CI, 13.1 to 18.9).
Figure 2.
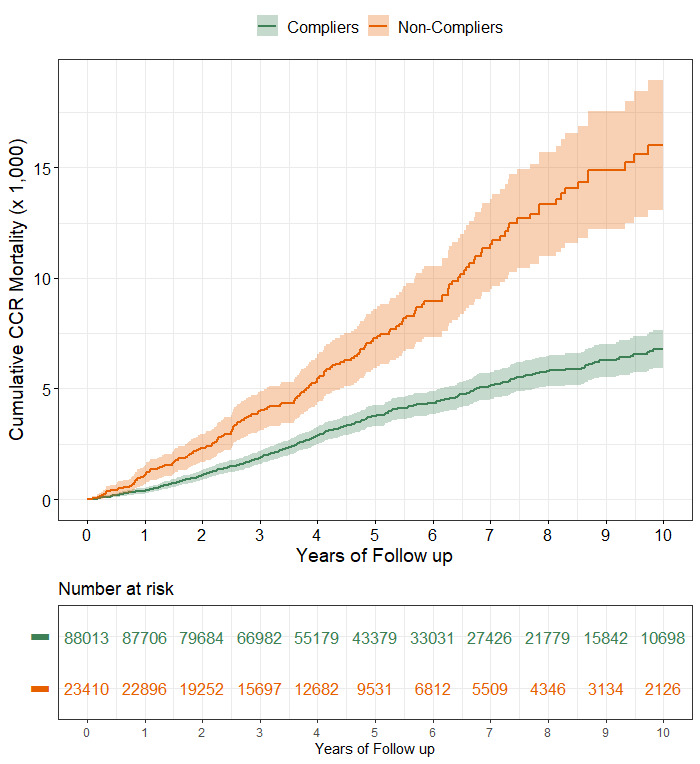
Cumulative CRC-specific mortality (per 1000) in a cohort of subjects with a positive FIT, according to compliance with follow-up colonoscopy. CRC, colorectal cancer; FIT, faecal immunochemical test.
The cumulative mortality from all causes among compliers was 83.4 per 1000 (95% CI, 80.2 to 86.6), while among non-compliers it was 164.2 per 1000 (95% CI, 155.6 to 172.6).
The risk of death from CRC was more than doubled among non-compliers (HR 2.03; 95% CI, 1.68 to 2.44). The competing risk analysis yielded similar results (HR 1.94; 95% CI, 1.61 to 2.33). The risk of death was higher for males, for 60–69 year olds and for subjects at the first screening round.
The sensitivity analysis performed on subjects with a potential follow-up time of 5 years or more involved 44 975 compliers and 10 475 non-compliers, who were diagnosed with respectively 2413 (5.4%) and 607 (5.8%) CRC. The cumulative incidence at 10 years was 55.8 per 1000 (95% CI, 53.6 to 58.0) for compliers and 66.5 per 1000 (95% CI, 61.0 to 72.0) for non-compliers. The HR of being diagnosed with CRC >6 months after the FIT among non-compliers was 3.86 (95% CI, 3.31 to 4.49). Details are reported in online supplemental appendix 3.
Discussion
This study compared CRC incidence and mortality between subjects with a positive FIT who were compliant and non-compliant with colonoscopy within the Veneto regional CRC screening programme. As expected, CRC incidence in compliers increased steeply in the first months after the FIT, due to CRC found during diagnostic colonoscopy. Over the subsequent 10 years, CRC incidence among compliers increased slowly, from a cumulative CRC incidence of 36.4 per 1000 after 1 year to 44.7 per 1000 after 10 years. This low incidence >1 year after the positive FIT is likely due to the early detection of cancers that would have become symptomatic and the removal of precancerous lesions during colonoscopy.23
The cumulative incidence of CRC in non-compliers also increased early after FIT, even if at a slower pace than in compliers. Cases diagnosed within 3 months of the FIT accounted for 40% of all cases and those diagnosed within 6, 9 and 12 months of the FIT accounted respectively for 57%, 60% and 63% of all cases. A steady increase in CRC incidence followed during the entire follow-up period, reaching 54 cases per 1000 10 years after the FIT, significantly higher than the 44 cases per 1000 observed among compliers. The counterintuitive lower absolute CRC incidence rate observed in non-compliers (3.77 vs 4.03 in compliers) was related to the shorter median duration of follow-up.
CRCs diagnosed early in non-compliers (ie, in the first months after the positive FIT) were likely among subjects who wanted a diagnostic colonoscopy, but preferred to have it outside the screening programme. The underlying reasons are not known; these subjects could have individual preferences for other endoscopy centres, or for a private setting. According to the regional database of outpatient services, approximately 5.4% of non-compliers underwent a follow-up colonoscopy in public endoscopy units not participating in the programme within 12 months of their positive FIT; however, the figure related to private facilities was not available. An increased risk of cancer and a worse distribution by stage have been reported for colonoscopies performed more than 7–9 months after a positive FIT.13–16 Most cases diagnosed <1 year after a FIT were early stage in both compliers and non-compliers.
While CRCs diagnosed in compliers >1 year after the FIT were postcolonoscopy (missed or interval) cancers, those in non-compliers were more likely to be among subjects who initially refused the diagnostic colonoscopy and then had their cancer detected on the appearance of symptoms. Compared with cases diagnosed within the first year, the stage of cases diagnosed after 1 year was much worse in both study cohorts, particularly in non-compliers. A delayed diagnosis was significantly more frequent in non-compliers (37% of the total >1 year after the positive FIT, compared with only 10% in compliers), which likely explains the excess CRC mortality, but not all-cause mortality, observed in this cohort.
CRC cumulative mortality was higher among non-compliers from the very beginning of the follow-up and the difference between the two cohorts progressively increased. Over the entire follow-up period, the cumulative mortality CRC in non-compliers was more than double that seen in compliers, after adjusting for available confounders.
Our results are in line with those by Lee and colleagues, who reported a 1.83-fold increased risk in detection of CRC and a 1.64-fold increased risk for CRC death among non-compliers with colonoscopy, after a follow-up of 8 years.15
This increase could be due to sick individuals in the cohort of non-compliers whose comorbidities prevented them from undergoing colonoscopy according to screening protocols. Furthermore, we observed a large difference in all-cause mortality between compliers and non-compliers. Previous studies have shown that those who are compliant with treatment recommendations have lower overall mortality that those who are non-compliant, even in randomised trials that should account for confounders such as socioeconomic status, education and other factors associated with negative outcomes.24 25 This effect may be especially important when studying CRC, as several lifestyle factors increase the risk of CRC (inactivity, alcohol, tobacco, etc). Therefore, the two study cohorts could be not completely comparable to each other, non-compliers being at higher risk of death from all causes. Compliance with colonoscopy would act as a selection mechanism to identify a population at high risk of premature death.
Though the proportion of deaths attributable to CRC was similar in compliers (9.6%) and non-compliers (8.5%), the higher overall mortality in non-compliers means that the absolute difference in CRC-specific mortality remains important. Because non-compliers are a group at high risk of death, and a high proportion of these deaths are attributable to CRC, they should be encouraged to have a procedure that may be lifesaving.
Our results show the importance of achieving the highest possible compliance with colonoscopy after a positive FIT. The critical role of actively calling the patients with a positive FIT to plan a diagnostic colonoscopy has been repeatedly reported in the literature.8 Without close monitoring, high proportions of patients with a positive FIT are lost to follow-up.26 27 There are multiple reasons why individuals may fail to get a colonoscopy after a positive FIT. A recent study from the Veterans Health Administration screening programme showed that the most common reason (in 35% of patients) was patient refusal,28 possibly related to colonoscopy preparation, invasiveness of the procedure and anticipated pain.29 Providers may also underestimate the likelihood of an advanced adenoma or cancer being the cause of the positive test, contributing to a lack of urgency.30
Subjects at subsequent screening rounds showed a significant reduction in cumulative incidence and mortality as compared with those at their first screening FIT. Comparing the quantitative results, and not just the qualitative positive–negative result, between subjects at the first versus subsequent rounds would facilitate the interpretation of this result, but quantitative data were not available in our study.
This study has some limitations. First, it cannot be excluded that non-compliers had an underlying risk of CRC different from compliers. However, for both the study cohorts, the post-test risk (ie, after a positive FIT) of CRC is so much higher than the pretest risk, that the relative contribution of any difference in the background risk is likely to be marginal.
Second, the favourable stage at diagnosis of CRC detected in the first year of the FIT in non-compliers suggests that some of them were asymptomatic, but chose to complete their colonoscopy outside the screening programme (ie, ‘non-programmatic compliers’). However, we do not know what proportion of non-compliers appropriately completed timely diagnostic colonoscopies outside of the programme, and were therefore non-programmatic compliers; if we assume non-programmatic compliers had a similar CRC incidence to programmatic compliers, we would expect the actual excess incidence of CRC among true non-compliers to be greater than that observed. Studies similar to ours from organised screening programmes have also lacked data about colonoscopy uptake among non-compliers.15
Third, because CRCs were diagnosed earlier in compliers (90% within 1 year) than in non-compliers (63%), the follow-up of non-compliers could be too short for undiagnosed cancers to surface. However, the results of the sensitivity analysis performed on subjects with a potential follow-up time of 5 years or more were similar to those observed on the whole study population.
Fourth, immortal time bias might affect our results because compliers could not be diagnosed with CRC or die before the date of their colonoscopy. A sensitivity analysis replacing the date of the FIT with the date of colonoscopy for compliers, thus excluding immortal time from analysis, showed minimal differences as compared with the main analysis (details in online supplemental appendix 4).
Fifth, stage at diagnosis was not available for CRCs diagnosed in the first years of the study. However, data from the Italian national surveys of CRC screening (which included the data from the Veneto region) showed a stable distribution of CRC stage in different years.11 31 Regarding non-compliers, it seems unlikely that stage at diagnosis of CRC diagnosed before 2013 could significantly differ from that collected from 2013 onward.
Finally, it has been shown that screen-detected CRCs have more favourable intrastage characteristics than non-screen-detected cancers, including a lower median number of positive lymph nodes for stage III–IV cancers.32 This difference likely gives an additional mortality advantage with screening, which could be concealed by the reported (traditional) distribution by stage.
Conclusions
The effectiveness of FIT-based screening depends on colonoscopy compliance after an initial positive result. We found that non-compliers with colonoscopy in an organised programme had more than double the rate of cumulative CRC mortality during 10 years of follow-up. Additional interventions are needed to engage these patients and decrease their burden of CRC.
Footnotes
Contributors: MZ was responsible for the study concept and design. MZ, JB and KS were involved in interpretation of data and drafting of the manuscript. JB, GC, SB, SR and EC were responsible for acquisition of data. JB and SG performed the statistical analysis and interpretation of data. MR helped in interpretation of data. All the authors made critical revision of the article for important intellectual content and gave final approval of the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available. Study data are not available.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The Italian legislation identifies Cancer Registries as collectors of personal data for surveillance purposes without explicit individual consent. The approval of a research ethics committee was not required, since this study is a descriptive analysis of individual data without any direct or indirect intervention on patients.
References
- 1. Lauby-Secretan B, Vilahur N, Bianchini F, et al. The IARC perspective on colorectal cancer screening. N Engl J Med 2018;378:1734–40. 10.1056/NEJMsr1714643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Basu P, Ponti A, Anttila A, et al. Status of implementation and organization of cancer screening in the European Union member States-Summary results from the second European screening report. Int J Cancer 2018;142:44–56. 10.1002/ijc.31043 [DOI] [PubMed] [Google Scholar]
- 3. Canadian Partnership Against Cancer . Colorectal cancer screening in Canada: monitoring & evaluation of quality indicators – results report, January 2013 – December 2014, 2017. [Google Scholar]
- 4. Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64:1637–49. 10.1136/gutjnl-2014-309086 [DOI] [PubMed] [Google Scholar]
- 5. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US preventive services Task force recommendation statement. JAMA 2016;315:2564–75. 10.1001/jama.2016.5989 [DOI] [PubMed] [Google Scholar]
- 6. European Colorectal Cancer Screening Guidelines Working Group, von Karsa L, Patnick J, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy 2013;45:51–9. 10.1055/s-0032-1325997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Selby K, Baumgartner C, Levin TR, et al. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med 2017;167:565–75. 10.7326/M17-1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chubak J, Garcia MP, Burnett-Hartman AN, et al. Time to colonoscopy after positive fecal blood test in four U.S. health care systems. Cancer Epidemiol Biomarkers Prev 2016;25:344–50. 10.1158/1055-9965.EPI-15-0470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal fit results in an integrated safety-net system. Am J Gastroenterol 2017;112:375–82. 10.1038/ajg.2016.555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gingold-Belfer R, Leibovitzh H, Boltin D, et al. The compliance rate for the second diagnostic evaluation after a positive fecal occult blood test: a systematic review and meta-analysis. United European Gastroenterol J 2019;7:424–48. 10.1177/2050640619828185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zorzi M, Da Re F, Mantellini P, et al. Screening for colorectal cancer in Italy: 2011-2012 survey. Epidemiol Prev 2015;39:93–107. [PubMed] [Google Scholar]
- 12. Zorzi M, Hassan C, Capodaglio G, et al. Long-Term performance of colorectal cancerscreening programmes based on the faecal immunochemical test. Gut 2018;67:2124–30. 10.1136/gutjnl-2017-314753 [DOI] [PubMed] [Google Scholar]
- 13. Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017;317:1631–41. 10.1001/jama.2017.3634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beshara A, Ahoroni M, Comanester D, et al. Association between time to colonoscopy after a positive guaiac fecal test result and risk of colorectal cancer and advanced stage disease at diagnosis. Int J Cancer 2020;146:1532-1540. 10.1002/ijc.32497 [DOI] [PubMed] [Google Scholar]
- 15. Lee Y-C, Fann JC-Y, Chiang T-H, et al. Time to Colonoscopy and Risk of Colorectal Cancer in Patients With Positive Results From Fecal Immunochemical Tests. Clin Gastroenterol Hepatol 2019;17:1332–40. 10.1016/j.cgh.2018.10.041 [DOI] [PubMed] [Google Scholar]
- 16. Zorzi M, Hassan C, Capodaglio G, et al. Colonoscopy later than 270 days in a fecal immunochemical test-based population screening program is associated with higher prevalence of colorectal cancer. Endoscopy 2020;52:871–6. 10.1055/a-1159-0644 [DOI] [PubMed] [Google Scholar]
- 17. Lee Y-C, Li-Sheng Chen S, Ming-Fang Yen A, et al. Association between colorectal cancer mortality and gradient fecal hemoglobin concentration in colonoscopy Noncompliers. J Natl Cancer Inst 2017;109:djw269. 10.1093/jnci/djw269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Edge SB, Byrd DR, Compton CC. Ajcc cancer staging manual. 7th edn. France: Springer, 2010. [Google Scholar]
- 19. Scheike TH, ZHANG MEI-JIE, Zhang MJ. An Additive-Multiplicative Cox-Aalen regression model. Scand J Stat 2002;29:75–88. 10.1111/1467-9469.00065 [DOI] [Google Scholar]
- 20. Therneau T, Grambsch P. Modeling survival data: extending the COX model. Springer, 2000. [Google Scholar]
- 21. Fine JP, Gray RJ. A proportional hazards model for the Subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 22. Decreto del Presidente del Consiglio dei Ministri, 3/3/2017, Identificazione dei sistemi di sorveglianza E dei registri di mortalit, di Tumori E di altre patologie, 17A03142, GU Serie Generale n.109 del 12/05/2017. Available: http://www.gazzettaufficiale.it/eli/id/2017/05/12/17A03142/sg [Accessed 12 Feb 2020].
- 23. Lee JK, Jensen CD, Levin TR, et al. Long-Term risk of colorectal cancer and related deaths after a colonoscopy with normal findings. JAMA Intern Med 2019;179:153–60. 10.1001/jamainternmed.2018.5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coronary Drug Project Research Group . Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med 1980;303:1038–41. 10.1056/NEJM198010303031804 [DOI] [PubMed] [Google Scholar]
- 25. Padula AM, Pressman AR, Vittinghoff E, et al. Placebo adherence and mortality in the heart and Estrogen/Progestin replacement study. Am J Med 2012;125:804–10. 10.1016/j.amjmed.2012.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carlson CM, Kirby KA, Casadei MA, et al. Lack of follow-up after fecal occult blood testing in older adults: inappropriate screening or failure to follow up? Arch Intern Med 2011;171:249–56. 10.1001/archinternmed.2010.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Partin MR, Burgess DJ, Burgess JF, et al. Organizational predictors of colonoscopy follow-up for positive fecal occult blood test results: an observational study. Cancer Epidemiol Biomarkers Prev 2015;24:422–34. 10.1158/1055-9965.EPI-14-1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. May FP, Yano EM, Provenzale D, et al. Barriers to follow-up Colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol 2019;17:469–76. 10.1016/j.cgh.2018.05.022 [DOI] [PubMed] [Google Scholar]
- 29. Partin MR, Gravely AA, Burgess JF, et al. Contribution of patient, physician, and environmental factors to demographic and health variation in colonoscopy follow-up for abnormal colorectal cancer screening test results. Cancer 2017;123:3502–12. 10.1002/cncr.30765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertels L, van der Heijden S, Hoogsteyns M, et al. Gps' perspectives on colorectal cancer screening and their potential influence on FIT-positive patients: an exploratory qualitative study from a Dutch context. BJGP Open 2019;3:bjgpopen18X101631. 10.3399/bjgpopen18X101631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zorzi M, Fedato C, Naldoni C, et al. Screening for colorectal cancer in Italy: 2007 survey. Epidemiol Prev 2009;33:57–74. [PubMed] [Google Scholar]
- 32. Zorzi M, Mangone L, Anghinoni E. Characteristics of the colorectal cancers diagnosed in the early 2000s in Italy. figures from the impact study on colorectal cancer screening. Epidemiol Prev 2015;39:108–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-322192supp001.pdf (278.3KB, pdf)
Data Availability Statement
No data are available. Study data are not available.


