Vaccination against coronavirus disease 2019 (COVID-19) is presently the most promising intervention against severe acute respiratory syndrome coronavirus 2 infections. As the global vaccination program evolves, rare complications, such as COVID-19 vaccine-associated glomerular disease (CVAGD), have become clinically evident [1, 2]. We present two patients with CVAGD and their therapeutic management.
PATIENT 1
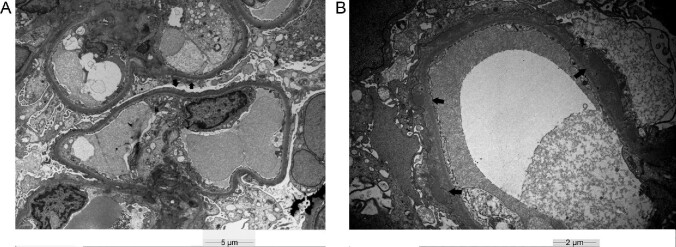
A 65-year-old Caucasian male had his Pfizer-BioNTech messenger RNA (mRNA) COVID-19 vaccination in April 2021. Ten days later he experienced a sudden onset of pedal edema associated with hypoalbuminemia (serum albumin 2.5 g/dL), proteinuria [protein: creatinine ratio (PCR) 9.7 g/g] and acute kidney injury [AKI, estimated glomerular filtration rate (eGFR) 52 mL/min/1.73 m2]. Serology for antineutrophil cytoplasmic antibodies (ANCA), anti-glomerular basement membrane (GBM) antibodies, free light chains (FLC), complement and routine viral studies were unremarkable. Kidney biopsy showed acute tubular injury with electron microscopy revealing extensive podocyte foot process effacement due to minimal change disease (MCD; Figure 1A). Therapy included maximally dosed perindopril and a 3-month follow-up. However, persisting proteinuria (PCR >10 g/g) warranted treatment with three methylprednisolone pulses (500 mg) followed by low-dose corticosteroids (16 mg/day tapered to 4 mg/day) and cyclosporine (target level 150 ng/mL). Complete remission was achieved within 10 days (eGFR 70 mL/min/m2, PCR 220 mg/g) that has been maintained even after corticosteroids cessation at 6 months.
FIGURE 1:
(A) Electron microscopic examination reveals diffuse podocytic foot process effacement (black arrows), supporting the diagnosis of MCD. Bar 5 µm. (B) Electron microscopic examination reveals subepithelial deposits with podocytic foot process effacement (black arrows), supporting the diagnosis of MN. Bar 2 µm.
PATIENT 2
A 68-year-old Caucasian male had his Pfizer-BioNTech mRNA COVID-19 vaccination in May 2021. Seven days later he presented with a sudden onset of lower extremities edema associated with AKI (eGFR 70 mL/min/1.73 m2 from 98 mL/min/1.73 m2), hypoalbuminemia (serum albumin 2.9 g/dL), glomerular hematuria and overt proteinuria (PCR 19 g/g). ANCA, anti-GBM, FLC, complement and routine viral studies were unremarkable. Kidney biopsy showed membranous nephropathy (MN; Figure 1B) with PLAR2-positive immunofluorescence. Treatment included maximally dosed irbesartan for 4 months. However, in September 2021, persisting proteinuria (PCR 14 g/g) and further deterioration of eGFR at 40 mL/min/1.73 m2 compelled immunosuppressive therapy with four weekly rituximab pulses (375 mg/m2). During the following 2 months, and while he remained under close follow-up, proteinuria decreased (PCR 6 g/g) and renal function improved (eGFR 69 mL/min/1.73 m2).
In both patients, immunosuppression was necessary to preserve renal function. These cases outline two important points. First, in rare instances COVID-19 vaccination seems to trigger glomerular disease. Second, due to the rarity of CVAGD, the treatment strategy is largely unknown [2, 3] but, as was shown in our cases, usual therapies for the corresponding glomerulopathies seem to be effective.
Glomerular involvement including cases of MCD and MN is documented after COVID-19 vaccination [2–4]. Concerning MCD, nephrotic syndrome occurs within days following COVID-19 vaccination, suggesting a vaccination-triggered immunological reaction. With steroid therapy, complete remission can be established [5].
In MN, the development of nephrotic syndrome has been observed weeks after the COVID-19 vaccination. Immune dysregulation toward certain podocyte antigens is possible [4]. A pitfall regarding the diagnosis of CVAGD may be the increased awareness postvaccination that may prompt medical attention for a preceding undiagnosed glomerular disease with subclinical manifestations [1].
The pleomorphic and unpredictable nature of CVAGD, further complicates its treatment strategy. Nevertheless, the use of already established therapeutic protocols may be both necessary and effective.
PATIENT CONSENT
We thank the patients for their informed consent regarding publication of their data.
Contributor Information
Anna Psyllaki, Department of Nephrology, University of Crete, Heraklion, Greece.
Ioanna Stavrakaki, Department of Nephrology, University of Crete, Heraklion, Greece.
Ariadni Androvitsanea, Department of Nephrology, Friedrich Alexander University Hospital, Erlangen, Germany.
Hariklia Gakiopoulou, Department of Renal Pathology, National Kapodistrian University of Athens, Athens, Greece.
Ioannis Petrakis, Department of Nephrology, University of Crete, Heraklion, Greece.
Kostas Stylianou, Department of Nephrology, University of Crete, Heraklion, Greece.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Li NL, Coates PT, Rovin BH. COVID-19 vaccination followed by activation of glomerular diseases: does association equal causation? Kidney Int 2021; 100: 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tan HZ, Tan RY, Choo JCJet al. . Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int 2021; 100: 469–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lebedev L, Sapojnikov M, Wechsler Aet al. . Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 2021; 78: 142–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aydin MF, Yildiz A, Oruc Aet al. . Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int 2021; 100: 464–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D'Agati VD, Kudose S, Bomback ASet al. . Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int 2021; 100: 461–463 [DOI] [PMC free article] [PubMed] [Google Scholar]