ABSTRACT
Background
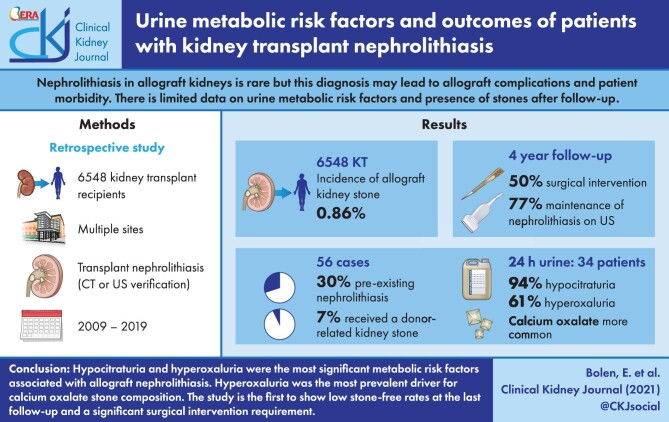
Nephrolithiasis in allograft kidneys is rare, but this diagnosis may lead to allograft complications and patient morbidity. Previous studies that have evaluated nephrolithiasis posttransplant have focused on surgical stone management, with limited data on urine metabolic risk factors and the presence of stones after follow-up.
Methods
We retrospectively evaluated kidney transplant recipients who were diagnosed with transplant nephrolithiasis between 2009 and 2019. Computed tomography and ultrasound imaging were used to confirm stone presence.
Results
The incidence of allograft kidney stone formation was 0.86% of 6548 kidney transplant recipients. Of the 56 cases identified, 17 (30%) had a pretransplant history of nephrolithiasis. Only four (7%) patients received a known kidney stone at the time of allograft implantation. Of the 56 cases, 34 had a 24-h supersaturation study. The urine supersaturation study showed 32 patients (94%) had a urine citrate of <450 mg excreted in 24 h (median 124.5 mg/24 h, reference range >500 mg/24 h), along with 22 patients (61%) having a urine oxalate excretion of ≥30 mg in 24 h (median 34.4 mg/24 h, reference range <30 mg/24 h). Calcium oxalate composition was most common (91% with >1 supersaturation for calcium oxalate crystals), with normal median urine calcium levels (median urine calcium 103.5 mg/24 h, reference range <200 mg/24 h). After a 4-year follow-up, 50% (n = 28) required surgical intervention and 43 (77%) patients continued to have evidence of transplant nephrolithiasis on imaging.
Conclusions
This is the largest study of transplant nephrolithiasis confirming that hypocitraturia and hyperoxaluria were the most significant urine metabolic risk factors associated with allograft nephrolithiasis and that hyperoxaluria was the most prevalent driver for calcium oxalate stone composition. Our study is first to show low stone-free rates at the last follow-up and a significant proportion requiring surgical intervention.
Keywords: hyperoxaluria, hypocitraturia, kidney stone, kidney transplantation, nephrolithiasis
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Renal transplant allograft nephrolithiasis has been repeatedly broached in the literature in small, mostly single-center trials, and reported rates range from 0.4 to 4.4% [1–5]. A large meta-analysis recently estimated the overall rate of nephrolithiasis in kidney transplant (KTx) recipients at 1%, though a significant proportion of stones contained in the analysis were found in the bladder and could not definitively be attributed to either the transplant or native kidney(s) [1]. While most studies have found the long-term risk to graft function to be low, particularly in the case of small and non-obstructive stones [6–10], there have been reports of acute allograft dysfunction and in some cases graft failure attributed to KTx nephrolithiasis [10–13].
Urine supersaturation tests are designed to measure key dietary and metabolic risk factors relevant to a patient's risk for nephrolithiasis and they provide complex ion supersaturation information, which has previously been shown to correlate with stone composition [14]. In addition, urine metabolic profiles have been used to provide more directed therapy for nephrolithiasis management [15].
A prior study that evaluated urine metabolic profile differences between 82 KTx patients and 82 matched healthy controls found that KTx recipients had more significant hypocitraturia and hyperoxaluria, both significant lithogenic risk factors [16]. However, this study was not limited to KTx recipients with a history of new-onset nephrolithiasis at the time of enrollment nor did it follow the patients longitudinally to assess the risk for eventual formation of kidney stones in either cohort. Additionally, previous large cohort studies analyzing new-onset nephrolithiasis in KTx recipients have focused on stone management, with limited data on risk factors and risk for recurrence after follow-up [17, 18].
As one of the largest KTx centers in the nation, we sought to add to the existing literature by evaluating our cohort of patients with transplant nephrolithiasis. Herein we report the largest study of KTx nephrolithiasis, with the aim of identifying risk factors, surgical intervention and the presence of stone disease at the last follow-up.
MATERIALS AND METHODS
After institutional review board (IRB) approval (19-011555), a retrospective chart review was conducted on all adult patients from 2009 to 2019 across multiple major enterprise sites using the electronic medical records and International Classification of Diseases (ICD) codes. ICD code for renal transplant with or without a simultaneous solid organ transplant and one of the following ICD codes were used: uric acid stones, ureterolithiasis, kidney stone, nephrolithiasis, calcium oxalate nephrolithiasis, urate nephrolithiasis, ureteral stone or calcium phosphate nephrolithiasis. The electronic medical records of the patients generated by ICD codes were then individually reviewed. For each patient on the list, computed tomography (CT) or ultrasound (US) imaging performed on or after the KTx date was reviewed to verify the presence of transplant nephrolithiasis. Donor-associated nephrolithiasis was determined based on the finding of a kidney stone in the transplanted kidney within 24–48 h after kidney transplantation. All KTx patients undergo kidney allograft US within that time frame after kidney transplantation. At our transplant center, protocol biopsies take place at 4 months, 1 year, 2 years and 5 years. Protocol biopsies are US guided and a US of the transplanted kidney is performed at the same time as the protocol biopsies, so all KTx patients receive at least one to two transplant allograft imagings within the first year posttransplant. The decision to pursue imaging with CT for some patients was based on the transplant team and was not standardized across the entire group. Exclusion criteria included patients who were <18 years old at the time of diagnosis and patients with stones that could not definitively be isolated to the transplant kidney or transplant ureter on imaging.
Multiple data points were collected, including information about the initial KTx, etiology of kidney failure, immediate post-transplant management, urine supersaturation studies (when available) and graft function and patient outcome at the last follow-up. The urine supersaturation panel was based on a 24-h urine evaluation of the following variables: volume, calcium, citrate, pH, uric acid, sodium, magnesium, potassium, phosphorus and supersaturation for calcium oxalate, calcium phosphate and uric acid crystals identified based on reference means using delta G (DG). This refers to the Gibbs energy of transfer, which is negative for undersaturated and positive for supersaturated solutions. When a patient had a history of multiple KTx, data from the transplant prior to the allograft nephrolithiasis incident were collected. When a patient had multiple supersaturation studies completed, data from the first study performed after the new stone event were collected. Fisher's exact test for count data and Kruskal–Wallis rank sum test were used to compare patients with urine metabolic assessment and patients without metabolic assessment.
RESULTS
A total of 553 charts underwent initial review, from which 56 cases of transplant nephrolithiasis that met all inclusion criteria were identified, as shown in Figure 1.
Figure 1:
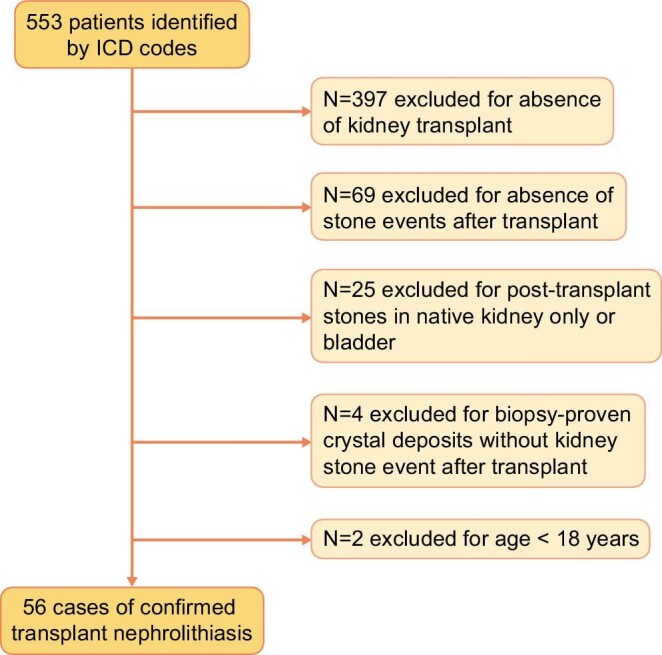
The number of patients with transplant nephrolithiasis (n = 56) included in the study after applying exclusion criteria from all patients identified based on ICD codes (n = 553).
There were 6548 unique KTxs between 2009 and 2019 across multiple sites for a transplant nephrolithiasis rate of 0.86%. The diagnosis of transplant nephrolithiasis was based on CT in 33 (59%) patients and US imaging for the remaining 23 (41%). The mean age at transplant was 56.5 (±12.1) years, 32 of 56 identified patients (57%) were male, 44 (79%) were White and 6 (11%) were Black. For most patients, this was their first KTx [46 (82%)]; 41 (76%) required dialysis before transplant and 17 (30%) had at least one stone event in their native kidney prior to transplant. The most common cause of initial kidney failure was glomerulonephritis [17 (30%)]. A complete set of pretransplant characteristics can be found in Table 1.
Table 1.
Pretransplant characteristics (N = 56)
| Characteristics | Values, n (%) |
|---|---|
| Female gender | 24 (43) |
| Race | |
| White | 44 (79) |
| Black | 6 (11) |
| Other/unknown | 6 (11) |
| Dialysis | 41 (73) |
| History of urolithiasis/nephrolithiasis in recipient | 17 (30) |
| Number of prior stone events (N = 17) | |
| 1 | 4 (24) |
| 2–4 | 4 (24) |
| ≥5 | 9 (53) |
| Stone composition | |
| Unknown | 13 (77) |
| Oxalate | 3 (18) |
| Uric acid | 1 (6) |
| Predisposing stone risk prior to transplant (N = 15) | |
| Polycystic kidney disease | 6 (40) |
| Hyperoxaluria | 2 (13) |
| Hyperuricemia/gout | 2 (13) |
| Multiple | 5 (33) |
| Cause of end-stage kidney disease | |
| Diabetes | 9 (16) |
| Hypertension | 3 (5) |
| Glomerulonephritis | 17 (30) |
| Polycystic kidney disease | 7 (13) |
| Stone | 1 (2) |
| Other | 14 (25) |
| Multiple | 5 (9) |
| Prior KTx | 10 (18) |
| Stone present in native kidney prior to transplant | 10 (18) |
A total of 31 patients (55%) received a deceased-donor organ and 25 (45%) received a KTx from a living donor. Eight (14%) had delayed graft function (six deceased-donor recipients and two living donor recipients). Mycophenolate mofetil (MMF) was part of the maintenance immunosuppression in 50 (89%) patients. At the time of diagnosis, 21 patients (38%) had at least two discrete stones in the allograft. The median size of the largest stone was 6 mm (range 0.1–35.0 mm) and the most common location for the largest stone was the inferior pole [20 (36%)]. Most stones were not analyzed for composition, but of those that were, six (11% of patients) had stones made completely of calcium oxalate, while five (9%) had stones of mixed composition. Posttransplant stone characteristics are shown in Table 2.
Table 2.
Transplant nephrolithiasis characteristics (N = 56)
| Characteristics | Values |
|---|---|
| Number of stones in KTx, n (%) | |
| 1 | 35 (63) |
| 2–4 | 21 (38) |
| Largest stone size (mm) | |
| Mean (SD) | 7.4 (7.1) |
| Median (range) | 6.0 (0.1–35.0) |
| Stone composition, n (%) | |
| Unknown | 44 (79) |
| Calcium oxalate | 6 (11) |
| Calcium phosphate | 1 (2) |
| Multiple | 5 (9) |
| Location of stone, n (%) | |
| Unknown | 8 (14) |
| Superior pole | 8 (14) |
| Inferior pole | 20 (36) |
| Renal pelvis | 6 (11) |
| Ureter | 9 (16) |
| Multiple | 5 (9) |
| Stone with associated obstruction, n (%) | 15 (27) |
| Time from transplant date to transplant nephrolithiasis identified on imaging (years) | |
| Mean (SD) | 3.9 (6.2) |
| Median (range) | 1.0 (0.0–32.0) |
SD, standard deviation.
A total of 34 patients (61%) had a 24-h urine supersaturation study completed at a median follow-up of 2 years after transplant. Patients who had a history of a stone event prior to transplant were more likely to have a 24-h urine metabolic assessment compared with those without [14/34 (41%) versus 3/22 (14%); P = 0.04]. Supersaturation was highest for calcium oxalate, as shown in Table 3. Other notable findings on the urine supersaturation studies included profound hypocitraturia [median 124.5 mg/24 h (range 20.0–763.0, reference range >500)], with 32 patients (94%) demonstrating a urine citrate of <450 mg excreted in 24 h. Urine oxalate was a median of 34.35 mg/24 h (range 9.8–136, reference range <30) with 22 patients (61%) having an excretion of ≥30 mg in 24 h and 10 patients (29%) having an excretion of >40 mg in 24 h. Urine magnesium was a median of 88.5 mg/24 h (range 22.0–240.0, reference range >30) with four patients (12%) excreting <30 mg in 24 h.
Table 3.
24-hour urine supersaturation results (N = 34)
| Tests | Values |
|---|---|
| Urine volume (mL) | |
| Mean (SD) | 2159.5 (797.2) |
| Median (range) | 2174.5 (745.0–3960.0) |
| Supersaturation calcium oxalate (DG) (reference mean 1.77 DG) | |
| Mean (SD) | 2.5 (1.9) |
| Median (range) | 1.9 (−0.4–9.0) |
| Supersaturation brushite/calcium phosphate (DG) (reference mean 0.21 DG) | |
| Mean (SD) | −1.2 (1.6) |
| Median (range) | −1.1 (−3.6–1.8) |
| Supersaturation hydroxyapatite (DG) (reference mean 3.96 DG) | |
| Mean (SD) | 1.8 (2.1) |
| Median (range) | 1.0 (−1.1–7.8) |
| Supersaturation uric acid (DG) (reference mean 1.04 DG) | |
| Mean (SD) | 0.1 (2.0) |
| Median (range) | 0.2 (−6.9–3.7) |
| Supersaturation sodium urate (DG) (reference mean 1.76 DG) | |
| Mean (SD) | −0.2 (1.2) |
| Median (range) | −0.1 (−2.7–2.0) |
| Urine sodium (mmol/24 h), n (%) | |
| Mean (SD) | 147.3 (84.0) |
| Median (range) | 125.5 (26.0–400.0) |
| Urine sodium (mmol/24 h) | |
| High (≥150) | 15 (44) |
| Acceptable (100–150) | 8 (24) |
| Ideal (<100) | 11 (32) |
| Urine calcium (mg/24 h) | |
| Mean (SD) | 149.1 (135.1) |
| Median (range) | 103.5 (26.0–592.0) |
| Urine calcium (mg/24 h), n (%) | |
| High (≥200) | 7 (21) |
| Normal (<200) | 27 (79) |
| Urine magnesium (mg/24 h) | |
| Mean (SD) | 98.5 (59.2) |
| Median (range) | 88.5 (22.0–240.0) |
| Urine magnesium (mg/24 h), n (%) | |
| Low (<30) | 4 (12) |
| Urine citrate (mg/24 h) | |
| Mean (SD) | 169.8 (167.3) |
| Median (range) | 124.5 (20.0–763.0) |
| Urine citrate (mg/24 h), n (%) | |
| Low (<450) | 32 (94) |
| Urine oxalate (mg/24 h) | |
| Mean (SD) | 40.7 (27.2) |
| Median (range) | 34.35 (9.8–136.0) |
| Urine oxalate (mg/24 h), n (%) | |
| High (≥30) | 22 (61) |
| Urine pH | |
| Mean (SD) | 6.0 (0.6) |
| Median (range) | 5.9 (5.1–7.7) |
SD, standard deviation.
In our cohort we identified four patients (7%) with donor-associated allograft nephrolithiasis at the time of transplant. All donor-derived transplant nephrolithiasis cases [4/34 (12%)] had a urine metabolic assessment. The baseline characteristics and the metabolic risk factors were similar for donor-derived cases compared with the rest of the cohort.
The median time from transplant to a new-onset stone event was 1 year (range 0–32 years). The cumulative stone event plot is shown in Figure 2. Twenty-four patients had a stone event up to 1 year after KTx (including the 4 patients with donor-associated transplant nephrolithiasis) and 32 patients had a stone event after 1 year. There was no significant difference in baseline characteristics between the two groups. For patients with 24-h urine supersaturation, urine volume was significantly lower among patients with a stone event >1 year after transplant compared with those with a stone event within the first year after transplant (median 1.8 versus 2.6 L; P = 0.02).
Figure 2:
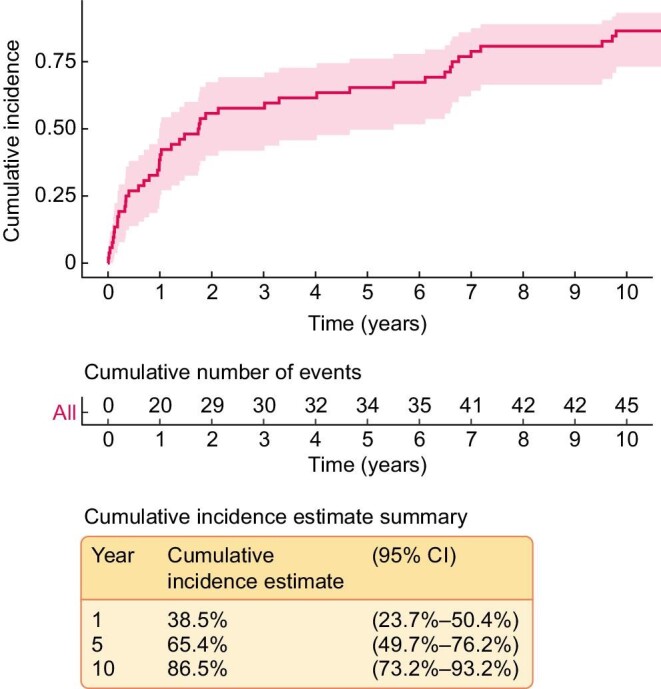
The cumulative incidence of stone events after KTx excluding the four cases of donor-associated nephrolithiasis.
Medical management included dietician referral in 18/56 patients (32%), potassium citrate supplementation in 14 (25%) and calcium citrate in 10 (18%). Most of the patients were on magnesium supplementation [34 (61%)], but supplementation was in the immediate posttransplant course rather than as a response to a stone event. None of the patients with urine oxalate ≥30 mg or patients with urine oxalate >40 mg in 24 h received a prescription for magnesium supplementation. A total of 45 patients (80%) were seen by the urology department, of whom 28 (50%) required surgical management: an initial nephrostomy tube in 10 (36%), ureteroscopy in 19 (68%) and percutaneous nephrolithotomy in 7 (25%). The decision to pursue surgical intervention was determined based on the size, location of the stone and any associated obstruction. Dietician referral for stone prevention was more common for patients who underwent urine metabolic assessment [16/34 (47%) versus 2/22 (9%); P = 0.003].
After a median follow-up of 4 years after the transplant nephrolithiasis diagnosis, 43/56 patients (77%) continued to have evidence of transplant nephrolithiasis on imaging. There was no difference in the rate of stone-free status on last imaging for patients with and without urine metabolic assessment [7/34 (19%) versus 6/22 (25%)]. The median creatinine was 1.4 mg/dL (range 0.5–4.3). A total of three patients (5%) had graft failure, defined as a glomerular filtration rate of <15 mL/min/1.73 m2 or being relisted for KTx; none of the graft failure events was directly attributed to stone-related complications. Two (4%) patients had died, with neither directly attributed to stone events.
DISCUSSION
This is the largest study of KTx nephrolithiasis examining clinical and urine metabolic risk factors and stone outcomes. We found that only a third of patients who developed nephrolithiasis in the allograft had a prior history of kidney stones and only 11% received a known kidney stone at the time of allograft implantation. Thus the majority of transplant nephrolithiasis cases developed due to lithogenic factors occurring in the post-transplant period. Moreover, we found that the stone resolution rate after urological intervention was low, with only 23% of patients stone free at the last follow-up. The rate of surgical intervention was significant at 50%.
The median time from KTx to nephrolithiasis diagnosis was 1 year, similar to prior studies. Other studies have shown that nephrolithiasis occurs during the first few months to 1 year after transplant, with the longest time from transplant to a stone event reported at 17 years [10, 19]. In another report, the majority of cases had a mean time to stone diagnosis of 8.5 years, with a range of up to 14 years later [20]. Transplant nephrolithiasis identified at the time of transplant reflects cases of donor-derived nephrolithiasis, which was present in ∼7% of our cohort.
Among those with known stone composition (n = 12), 100% calcium oxalate was most common. This correlated with urine metabolic crystal supersaturation results showing the mean supersaturation of calcium oxalate to be significantly higher than the reference range compared with other crystal supersaturation. These findings are consistent with the largest published meta-analysis showing 77% of transplant nephrolithiasis to be calcium-based [1]. Our results show that the median urine calcium was 103.5 mg/24 h and 79% of individuals had urine calcium <200 mg/24 h. However, the median urine oxalate was 34 mg, 61% had urine oxalate ≥30 mg/24 h and nine (27%) had urine oxalate ≥50 mg/24 h. Therefore hyperoxaluria appears to be the primary trigger for calcium oxalate nephrolithiasis in KTx recipients rather than hypercalciuria. In the cohort of patients with urine oxalate ≥50 mg/24 h, six had an identified enteric risk for hyperoxaluria, including one celiac, two chronic diarrhea, two pancreatic insufficiency and one gastric bypass. Only one of those patients had radiographic evidence of an absence of nephrolithiasis in the allograft at the last follow-up. Further studies of KTx recipients with enteric risk factors for hyperoxaluria are needed to better define the risk for transplant nephrolithiasis and to guide the monitoring program and treatment approach.
Several previous studies reported clinical and urine metabolic risk factors for transplant nephrolithiasis; however, they were limited to patients without nephrolithiasis or to a small number of cases. Previously identified clinical risk factors include secondary hyperparathyroidism, recurrent urinary tract infections and renal tubular acidosis [20–23]. Our findings on urine supersaturation were similar to previously published results, including hyperuricosuria, hypocitraturia, hyperoxaluria and low urine volume [21]. Hypocitraturia, however, appears to be the common denominator among all studies of KTx recipients with or without nephrolithiasis [16]. Our study results highlight that hypocitraturia is the most significant urine metabolic risk factor, followed by hyperoxaluria.
Hypocitraturia is a significant risk factor for calcium-based nephrolithiasis. Citrate and magnesium have antilithogenic properties and have been shown to decrease the formation and growth of calcium oxalate crystals. There are several postulated causes for hypocitraturia in KTx recipients. It is known that hypokalemia or metabolic acidosis can increase intracellular acidosis and increase proximal tubular reabsorption of citrate, contributing to low urine citrate excretion [24–26]. Metabolic acidosis is common in transplant recipients and has been attributed to calcineurin inhibitor therapy causing renal tubular acidification defects and contributing to increased intracellular acidosis. Diarrhea also plays a role in the development of non-anion gap acidosis in KTx recipients. In fact, patient-reported rates of diarrhea are as high as 53% in KTx recipients, with a cumulative incidence 3 years after KTx of 22.6% [27, 28]. The etiology of diarrhea after transplant is broad, but both antibiotic exposure and MMF immunosuppression are key risks. MMF has been reported to cause diarrhea and enteric malabsorption, and 89% of our patients were maintained on MMF therapy [29–31]. Enteric malabsorption is known to cause decreased urinary excretion of magnesium, citrate and calcium and increase urinary oxalate in a manner that directly correlates with the degree of malabsorption of fecal fat excretion [32]. Neither a food frequency questionnaire nor assessment of fecal fat was available for our case series, and further studies investigating the etiology of hyperoxaluria in KTx recipients are needed.
In our cohort, 61% of patients required magnesium supplementation and decreased urinary magnesium excretion was identified in 12% of patients. Magnesium chelates citrate in the tubular lumen and thus traps citrate in the urine and decreases its reabsorption, thus increasing total urine magnesium and citrate concentrations [32]. Decreased excretion of urinary magnesium therefore can contribute to hypocitraturia. Decreased urinary magnesium excretion as a risk factor for transplant nephrolithiasis has been previously reported [21]. Our findings did not confirm this, likely because the majority of the patients at our institution (61%) were prophylactically managed with magnesium supplementation for treatment of hypomagnesemia associated with KTx.
Recognizing proposed mechanisms for hypocitraturia in KTx recipients with transplant nephrolithiasis may help individualize medical management by addressing culprit causes. In our cohort, only 38% of patients with a urine supersaturation study received citrate supplementation and 32% of the entire cohort received dietician counseling for stone prevention directed at a high-fluid, low-sodium and low-oxalate diet.
While graft failure (5%) and mortality (4%) rates were low, consistent with previous reports, it should be emphasized that the morbidity of transplant nephrolithiasis was significant. The overall stone burden in our cohort was significant, as 38% of patients had two to four stones in the allograft, 27% of patients had an associated obstruction and 50% of patients required surgical intervention. Anatomic factors related to the transplant may have limited the ability for stone passage or removal in some cases. Taken together, these factors may have contributed to the overall low rate of stone clearance (23%) and low rates of spontaneous stone passage (25%) in our cohort.
Several limitations warrant discussion. The findings of this study are representative of a large multisite medical center and the results may not be generalizable to other centers due to varying transplant protocols and patient demographics. It should be recognized that there is no standardized serial imaging at our transplant center, therefore the onset of transplant nephrolithiasis cannot be fully predicted. Despite this, the median time to transplant nephrolithiasis is similar to that in the existing literature. Primary inclusion criteria relied on ICD coding to identify patients with transplant nephrolithiasis. Due to inherent limitations of ICD coding, patients with transplant nephrolithiasis may have been missed. However, the rate of transplant nephrolithiasis in our study at 0.86% is consistent with the largest study to date showing a rate of 1% [1]. A control group of KTx recipients with urine metabolic results without nephrolithiasis is lacking, and there was a relatively low rate of metabolic urine assessment within this cohort. Lastly, the study does not address the effect of treatment on repeat urine metabolic assessment.
We have shown in this study that transplant nephrolithiasis is associated with significant morbidity, including multiple stones, obstruction with up to 50% surgical intervention and <25% of patients stone free at the last follow-up. We confirmed that a calcium-based stone composition is the most prevalent and profound hypocitraturia is the most significant urine metabolic risk, followed by hyperoxaluria. The medical management approach is not standardized. Further studies are needed to improve nephrolithiasis risk stratification and individualization of medical management to reduce transplant nephrolithiasis morbidity in KTx recipients.
ACKNOWLEDGEMENTS
The IRB application for this study was reviewed by Jill Marie Gelner and was determined to be exempt from the requirement for IRB approval by the Mayo Clinic IRB.
Contributor Information
Erin Bolen, Mayo Clinic Alix School of Medicine, Scottsdale, AZ, USA.
Karen Stern, Department of Urology, Mayo Clinic, Mayo Clinic College of Medicine and Science, Scottsdale, AZ, USA.
Mitchell Humphreys, Department of Urology, Mayo Clinic, Mayo Clinic College of Medicine and Science, Scottsdale, AZ, USA.
Alexandra Brady, Department of Nephrology and Hypertension, Mayo Clinic, Scottsdale, AZ, USA.
Todd Leavitt, Department of Statistics, Mayo Clinic, Scottsdale, AZ, USA.
Nan Zhang, Department of Statistics, Mayo Clinic, Scottsdale, AZ, USA.
Mira Keddis, Department of Nephrology and Hypertension, Mayo Clinic, Scottsdale, AZ, USA.
CONFLICT OF INTEREST STATEMENT
All authors have reviewed and completed the disclosure statements and declare no conflicts of interest related to this work. This article is original and has not been published elsewhere, nor is it under consideration for publication at any other outlet.
DATA AVAILABLITY STATEMENT
The data underlying this article will be shared on reasonable request to the corresponding author.
REFERENCES
- 1. Cheungpasitporn W, Thongprayoon C, Mao MAet al. Incidence of kidney stones in kidney transplant recipients: a systematic review and meta-analysis. World J Transplant 2016; 6: 790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rezaee-Zavareh MS, Ajudani R, Ramezani Binabaj Met al. Kidney allograft stone after kidney transplantation and its association with graft survival. Int J Organ Transplant Med 2015; 6: 114–118 [PMC free article] [PubMed] [Google Scholar]
- 3. Stravodimos KG, Adamis S, Tyritzis Set al. Renal transplant lithiasis: analysis of our series and review of the literature. J Endourol 2012; 26: 38–44 [DOI] [PubMed] [Google Scholar]
- 4. Ferreira Cassini M, Cologna AJ, Ferreira Andrade Met al. Lithiasis in 1,313 kidney transplants: incidence, diagnosis, and management. Transplant Proc 2012; 44: 2373–2375 [DOI] [PubMed] [Google Scholar]
- 5. Kim H, Cheigh JS, Ham HW. Urinary stones following renal transplantation. Korean J Intern Med 2001; 16: 118–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sarier M, Duman I, Callioglu Met al. Outcomes of conservative management of asymptomatic live donor kidney stones. Urology 2018; 118: 43–46 [DOI] [PubMed] [Google Scholar]
- 7. Strang AM, Lockhart ME, Amling CLet al. Living renal donor allograft lithiasis: a review of stone related morbidity in donors and recipients. J Urol 2008; 179: 832–836 [DOI] [PubMed] [Google Scholar]
- 8. Martin G, Sundaram CP, Sharfuddin Aet al. Asymptomatic urolithiasis in living donor transplant kidneys: initial results. Urology 2007; 70: 2–5; discussion 5–6 [DOI] [PubMed] [Google Scholar]
- 9. Lancina Martín JA, García Buitrón JM, Díaz Bermúdez Jet al. [Urinary lithiasis in transplanted kidney]. Arch Esp Urol 1997; 50: 141–150 [PubMed] [Google Scholar]
- 10. Cho DK, Zackson DA, Cheigh Jet al. Urinary calculi in renal transplant recipients. Transplantation 1988; 45: 899–902 [DOI] [PubMed] [Google Scholar]
- 11. Lusenti T, Fiorini F, Barozzi L. Obstructive uropathy and acute renal failure due to ureteral calculus in renal graft: a case report. J Ultrasound 2009; 12: 128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qazi YA, Ali Y, Venuto RC. Donor calculi induced acute renal failure. Ren Fail 2003; 25: 315–322 [DOI] [PubMed] [Google Scholar]
- 13. Fabbian F, Catalano C, Rizzioli Eet al. Acute renal failure due to a calculus obstructing a transplanted kidney. Nephron 2002; 91: 742–743 [DOI] [PubMed] [Google Scholar]
- 14. Corder CJ, Rathi BM, Sharif Set al. 24-hour urine collection. In: StatPearls. Treasure Island, FL: StatPearls Publishing, 2021. http://www.ncbi.nlm.nih.gov/books/NBK482482/(4 April 2021, date last accessed) [PubMed] [Google Scholar]
- 15. Parvin M, Shakhssalim N, Basiri Aet al. The most important metabolic risk factors in recurrent urinary stone formers. Urol J 2011; 8: 99–106 [PubMed] [Google Scholar]
- 16. Dumoulin G, Hory B, Nguyen NUet al. Lack of increased urinary calcium-oxalate supersaturation in long-term kidney transplant recipients. Kidney Int 1997; 51: 804–810 [DOI] [PubMed] [Google Scholar]
- 17. Branchereau J, Timsit MO, Neuzillet Yet al. Management of renal transplant urolithiasis: a multicentre study by the French Urology Association Transplantation Committee. World J Urol 2018; 36: 105–109 [DOI] [PubMed] [Google Scholar]
- 18. Emiliani E, Subiela JD, Regis Fet al. Over 30-yr experience on the management of graft stones after renal transplantation. Eur Urol Focus 2018; 4: 169–174 [DOI] [PubMed] [Google Scholar]
- 19. Cardella JF, Hunter DW, Hulbert Jet al. Obstructed calycocystostomy site in association with a transplanted kidney: percutaneous management. Radiology 1985; 156: 67–68 [DOI] [PubMed] [Google Scholar]
- 20. Verrier C, Bessede T, Hajj Pet al. Decrease in and management of urolithiasis after kidney transplantation. J Urol 2012; 187: 1651–1655 [DOI] [PubMed] [Google Scholar]
- 21. Harper JM, Samuell CT, Hallson PCet al. Risk factors for calculus formation in patients with renal transplants. Br J Urol 1994; 74: 147–150 [DOI] [PubMed] [Google Scholar]
- 22. Stapenhorst L, Sassen R, Beck Bet al. Hypocitraturia as a risk factor for nephrocalcinosis after kidney transplantation. Pediatr Nephrol 2005; 20: 652–656 [DOI] [PubMed] [Google Scholar]
- 23. Mamarelis G, Vernadakis S, Moris Det al. Lithiasis of the renal allograft, a rare urological complication following renal transplantation: a single-center experience of 2,045 renal transplantations. Transplant Proc 2014; 46: 3203–3205 [DOI] [PubMed] [Google Scholar]
- 24. Hamm LL. Renal handling of citrate. Kidney Int 1990; 38: 728–735 [DOI] [PubMed] [Google Scholar]
- 25. Simpson DP. Citrate excretion: a window on renal metabolism. Am J Physiol 1983; 244: F223–F234 [DOI] [PubMed] [Google Scholar]
- 26. Hamm LL, Hering-Smith KS. Pathophysiology of hypocitraturic nephrolithiasis. Endocrinol Metab Clin North Am 2002; 31: 885–893, viii. [DOI] [PubMed] [Google Scholar]
- 27. Ekberg H, Kyllönen L, Madsen Set al. Clinicians underestimate gastrointestinal symptoms and overestimate quality of life in renal transplant recipients: a multinational survey of nephrologists. Transplantation 2007; 84: 1052–1054 [DOI] [PubMed] [Google Scholar]
- 28. Bunnapradist S, Neri L, Wong Wet al. Incidence and risk factors for diarrhea following kidney transplantation and association with graft loss and mortality. Am J Kidney Dis 2008; 51: 478–486 [DOI] [PubMed] [Google Scholar]
- 29. Bia MJ. Diarrhea in a long-term kidney–pancreas recipient. Clin J Am Soc Nephrol 2017; 12: 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ducloux D, Ottignon Y, Semhoun-Ducloux Set al. Mycophenolate mofetil-induced villous atrophy. Transplantation 1998; 66: 1115–1116 [DOI] [PubMed] [Google Scholar]
- 31. Jehangir A, Shaikh B, Hunt Jet al. Severe enteropathy from mycophenolate mofetil. ACG Case Rep J 2016; 3: 101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rudman D, Dedonis JL, Fountain MTet al. Hypocitraturia in patients with gastrointestinal malabsorption. N Engl J Med 1980; 303: 657–661 [DOI] [PubMed] [Google Scholar]