Abstract
Objective
Despite regular colonoscopy surveillance, colorectal cancers still occur in patients with Lynch syndrome. Thus, detection of all relevant precancerous lesions remains very important. The present study investigates Linked Colour imaging (LCI), an image-enhancing technique, as compared with high-definition white light endoscopy (HD-WLE) for the detection of polyps in this patient group.
Design
This prospective, randomised controlled trial was performed by 22 experienced endoscopists from eight centres in six countries. Consecutive Lynch syndrome patients ≥18 years undergoing surveillance colonoscopy were randomised (1:1) and stratified by centre for inspection with either LCI or HD-WLE. Primary outcome was the polyp detection rate (PDR).
Results
Between January 2018 and March 2020, 357 patients were randomised and 332 patients analysed (160 LCI, 172 HD-WLE; 6 excluded due to incomplete colonoscopies and 19 due to insufficient bowel cleanliness). No significant difference was observed in PDR with LCI (44.4%; 95% CI 36.5% to 52.4%) compared with HD-WLE (36.0%; 95% CI 28.9% to 43.7%) (p=0.12). Of the secondary outcome parameters, more adenomas were found on a patient (adenoma detection rate 36.3%; vs 25.6%; p=0.04) and a colonoscopy basis (mean adenomas per colonoscopy 0.65 vs 0.42; p=0.04). The median withdrawal time was not statistically different between LCI and HD-WLE (12 vs 11 min; p=0.16).
Conclusion
LCI did not improve the PDR compared with HD-WLE in patients with Lynch syndrome undergoing surveillance. The relevance of findings more adenomas by LCI has to be examined further.
Trial registration number
Keywords: colonic polyps, colonoscopy, inherited cancers, surveillance, imaging
Significance of this study.
What is already known on this subject?
Despite regular colonoscopic surveillance, interval colorectal cancers still occur relatively frequently in patients with Lynch syndrome.
One possible explanation could be that these cancers develop from missed polyps with a rapid adenoma-carcinoma sequence.
Linked Colour imaging (LCI), a push-button image-enhancing technique, has been demonstrated to increase the detection of polyps compared with white light endoscopy (WLE) in the average-risk population.
What are the new findings?
LCI did not improve the polyp detection rate compared with high-definition WLE in patients with Lynch syndrome undergoing surveillance, if performed by experienced endoscopists.
LCI resulted in an increase in adenoma detection without extending withdrawal time.
How might it impact on clinical practice in the foreseeable future?
When performed by experienced endoscopists, LCI is increasing adenoma detection and might therefore be of benefit for Lynch syndrome surveillance.
Introduction
Lynch syndrome is an autosomal dominant cancer predisposition syndrome. It is caused by a pathogenic gene variant in one of the DNA mismatch repair (MMR) protein genes (MLH1, MSH2, MSH6 or PMS2) or by deletions in the 3′ region of the EpCAM gene.1 Individuals with Lynch syndrome are at risk of early-onset colorectal cancer (CRC) and have a high cumulative lifetime risk of CRC that ranges from 15% to 70% by the age of 70.2–5 The prevalence of polyps in patients with Lynch syndrome does not seem to be higher than in the general population, but the microsatellite instable pathway to CRC seems to be accelerated in comparison with most sporadic CRC cases. Patients with Lynch syndrome have reported dwell times as low as 3–3.5 years compared with 10–15 years in sporadic CRCs.6 7 Therefore, it is apparent that regular colonoscopy surveillance, usually 2 yearly, is of utmost importance.5 8
It is well known that a significant number of adenomas are missed during colonoscopy. Back-to-back studies reveal an adenoma miss rate that ranges between 12% and 62% in the Lynch syndrome population.9 Among other reasons, these missed adenomas may progress rapidly to (interval) cancer in patients with Lynch syndrome.10 11 Recent publications report risks of CRC up to 46% in patients under surveillance illustrating the importance of optimising surveillance for patients with Lynch syndrome.4 11
Besides basic endoscopic quality skills, such as optimal withdrawal technique and position changes of the patient, advanced imaging techniques may also be able to reduce the number of missed polyps during colonoscopy.12 Advanced imaging techniques can be divided in two distinct forms: conventional chromoendoscopy (dye-based CE) and virtual chromoendoscopy (virtual CE). Virtual CE is easily activated by pushing a button on the endoscope and is based on a modification of the reflectance by either changing the incident wavelength or selection of wavelengths incident on the sensor. Based on the current evidence, the European Society of Gastrointestinal Endoscopy has recently recommended to make use of high-definition (HD) equipment in patients with Lynch syndrome, and suggests that advanced imaging techniques such as virtual CE may be of additional benefit.13
Linked Colour imaging (LCI) is a new advanced imaging technique (HDTV, 7000 System, Fujifilm Tokyo, Japan). This technique uses both preprocessing narrow band filter irradiation and postprocessing colour technology that separates acquired images into red, green and blue. The separated colours are reallocated and adjusted to enhance the colour contrast between polyps and the surrounding normal mucosa. Data on LCI for the detection of colorectal lesions are preliminary but promising.14–19
To this date, no study has assessed LCI for polyp detection in patients with Lynch syndrome. The aim of this study was to assess if LCI is superior to HD-white light endoscopy (WLE) for the detection of polyps during surveillance colonoscopy of patients with Lynch syndrome.
Patient and methods
Study design and setting
This prospective, randomised controlled trial compared polyp detection rates (PDRs) of LCI with HD-WLE in a cohort of patients with Lynch syndrome in eight centres in Belgium, Italy, the Netherlands, Poland, Spain and the United Kingdom. The study is reported in accordance with the Consolidated Standards of Reporting Trials statement for reporting randomised controlled trials.20
Patients
Consecutive eligible patients undergoing endoscopic surveillance for Lynch syndrome were approached for inclusion in this trial. Patients were considered eligible if they were aged 18 or older and had been diagnosed with a pathogenic gene variant in one of the MMR genes (MLH1, MSH2, MSH6, PMS2) or deletions in the 3′ region of the EpCAM gene. Exclusion criteria included surveillance colonoscopy within 1 year of the current examination, colonoscopy planned for the evaluation of symptoms, total proctocolectomy, known colonic neoplasia (referred patients), or a concurrent diagnosis of (serrated) polyposis syndrome or inflammatory bowel disease. Eligible patients were informed about the study aims, procedures and potential risks by the endoscopist or research nurse. After sufficient time to consider participation, written informed consent was obtained. Personal data were codified and registered in a secured online database (www.castoredc.nl, Castor Electronic Data Capture, Amsterdam, the Netherlands).
Endoscopists and endoscopy equipment
At each centre, participating endoscopists had extensive experience (>2000 colonoscopies), were familiar with virtual chromoendoscopy and performed already surveillance in patients with Lynch syndrome. At the start of the study, participating endoscopists had performed at least 10 procedures with the Fujifilm 7000 system. Both arms used the Fujifilm 7000 system (Fujifilm, Tokyo, Japan) which consists of four different wavelength light-emitted diodes as light sources, a processor and special scope series developed by Fujifilm (EC-760ZP and EC-760R, Fujifilm, Tokyo, Japan). HD monitor output was used for both arms placed at appropriate viewing distances at the discretion of the endoscopist.
Randomisation and allocation concealment
After signing informed consent, patients were stratified by centre and allocated randomly to undergo colonoscopy with either LCI or HD-WLE in a 1:1 ratio. Randomisation was performed by an independent computer-generated random numbers program (www.castoredc.nl, Castor Electronic Data Capture, Amsterdam, the Netherlands), with online access for all participating centres. Randomisation was performed prior to the start of the procedure by the endoscopist or research coordinator. Patients with a Boston Bowel Preparation Scale (BBPS)21 <6 or an incomplete colonoscopy were excluded from the study. Pathologists were blinded for the endoscopic technique as well as the optical diagnosis of the lesions detected during colonoscopy.
Endoscopic procedure and histopathology
All patients were prepared with osmotic laxatives according to the local hospital protocol. The procedures were performed under conscious sedation using intravenous midazolam and fentanyl or deep sedation with propofol at the discretion of the endoscopist and patient. Carbon dioxide insufflation was used for all procedures. The endoscope was advanced to the cecum with the endoscope set in the HD-WLE mode. Cecal intubation was confirmed by identification of the appendiceal orifice, ileocecal valve, intubation of the terminal ileum or the ileocolic anastomosis in patients with previous right hemicolectomy. On reaching the cecum, the quality of the bowel preparation was assessed using the BBPS.21 Butylscopolamine could be administered at the discretion of the endoscopist. When the patient was allocated to LCI, the imaging mode was switched to LCI for endoscopic inspection during withdrawal of the endoscope. All detected lesions were classified according to the Paris morphological classification.22 The size and the location of the lesion in the colon was noted. Endoscopists could use LCI, blue light imaging and HD-WLE to assess each lesion. Subsequently, the lesion was removed using standard polypectomy techniques. Per polyp, a unique histopathology container was used. The rectum was inspected in the retroflex position prior to extubation. Obvious hyperplastic lesions of 1–5 mm in the rectosigmoid could be left in situ at the discretion of the endoscopist. When the patient was allocated to HD-WLE, inspection on withdrawal was performed using HD-WLE in the same procedural steps as described earlier. Procedure time was defined as the time from endoscope insertion to extraction through the anus. The extubation time was defined as the time spent on inspection (withdrawal time), including time for cleaning of the bowel and the time spent on polypectomy (intervention time). All adverse events (AEs) related to the study intervention were recorded in the electronic case report form.
At each centre, an experienced gastrointestinal pathologist was designated to this study. Histological samples were collected in paraffin and processed using standard procedures. Histological findings were reported according to the Vienna classification of gastrointestinal neoplasia.23 As stated in the latest update of the WHO classification system, sessile serrated lesions were defined as serrated lesions with at least two irregular, dilated crypts, including dilatation of the base of the crypts that often have a boot, L-shape or inverted T-shape.24 Advanced adenoma was defined as an adenoma ≥10 mm, with villous morphology, or with high-grade dysplasia. Lesions located proximal to the splenic flexure were defined as proximal lesions. Hyperplastic polyps and sessile serrated lesions were grouped as serrated lesions.
Study outcomes and definitions
As all detected polyps are of clinical relevance in patients with Lynch syndrome, the primary outcome measure of the study was the PDR of HD-WLE and LCI. PDR was defined as the number of patients with at least one polyp detected. Diminutive hyperplastic lesions in the rectosigmoid, lesions not retrieved for pathology and histopathology outcomes as normal mucosa, lipomas, lymphoid aggregates, non-specific chronic inflammation inflammatory lesions were excluded for polyp detection comparisons. Hyperplastic polyps in the rectosigmoid were excluded for polyp comparisons as data have shown these polyps are harmless.25 Secondary outcome measures were the proportion of patients with at least one adenoma (adenoma detection rate (ADR)), the mean number of polyps, adenomas and serrated polyps per patient; median procedure and extubation times.
Sample size calculation
The required sample size was calculated by using a two-group χ2 test with 80% power and 0.05 two-sided alpha significance level. Based on the outcomes of the last two HD-WLE colonoscopies of 100 patients with Lynch syndrome at the Amsterdam UMC, we assumed an average PDR of 25% with HD-WLE, after excluding 1–5 mm hyperplastic polyps in the rectosigmoid. We hypothesised that the PDR of 25% using HD-WLE would increase to 40% using LCI, again excluding 1–5 mm hyperplastic polyps in the rectosigmoid. With these parameters, 332 patients were required to detect a statically significant difference in the PDR. Considering a dropout rate of 5% (ie, poor bowel preparation or incomplete cecum intubation) the sample size required was 348 patients.
Statistical methods
Descriptive statistics are used to describe patient, colonoscopy and polyp characteristics. Variables are reported as mean in case of continuous and normally distributed variables, as median with a 25th and 75th percentile (P25–P75) in case of non-normally distributed continuous variables, and as a number with a percentage in case of count or categorical variables. Comparisons between continuous variables were performed by Student’s t-tests or Mann-Whitney U tests for normal and asymmetric distributions, respectively. Categorical variables were compared by χ2 tests. The analysis of the primary outcome, the PDR, was performed on a superiority basis, examining the difference between HD-WLE and LCI. The Pearson χ2 test was used for the analysis of polyp detection. The relative risk ratio (RR) was calculated for dichotomous outcomes and presents the detection rate in the LCI group relative to detection rate in HD-WLE group, along with its 95% CI. Secondary dichotomous outcomes were analysed on a per-patient basis in an equivalent way to the primary outcome. For the number of lesions per patient (eg, polyps), the data were assumed to follow the negative binomial distribution, as it did not fit the Poisson distribution well due to overdispersion (ie, the variance was much greater than the mean). A negative binomial regression was used to compare between groups. Incidence rate ratios (IRRs) were calculated for continuous outcomes and present the number of lesions per patient in LCI group relative to the number of lesions per patient in the HD-WLE group, along with its 95% CI. A sensitivity analysis for the polyp detection study outcomes was performed to adjust for stratification per centre. For lesion detection rates, this was performed using a generalised linear model assuming a binomial distribution and a log link function. For the number of lesions, negative binomial regression was used again. In some cases, outcome data could not be obtained because the polyp was lost for histopathology or the histopathology result was missing. Missing outcome data were not replaced and excluded from the analysis. All outcomes were analysed on an intention-to-treat basis, which included all randomised patients except those with no data available (ie, incomplete colonoscopy or inadequate bowel preparation). Analyses were performed in statistical software R (V.3.6.1) using the reshape2, lme4 (glm) and MASS (glm.nb) packages. P values less than 0.05 were considered statistically significant.
Patient and public involvement and role of the funding source
The participating sites have been periodically monitored by the research team in collaboration with a senior Clinical Research Associate of the Clinical Research Unit of the Amsterdam UMC. Patients or the public were not involved in the design, conduct, or reporting, or dissemination plans of our research. Fuijifilm Europe GmbH provided research equipment on loan for this study and an unrestricted research grant that supported a research fellow to help execute the study. The sponsor had no role in the trial design, execution, data analysis, interpretation, decision to submit the paper or manuscript preparation. All authors had access to all study data and reviewed and approved the final manuscript.
Results
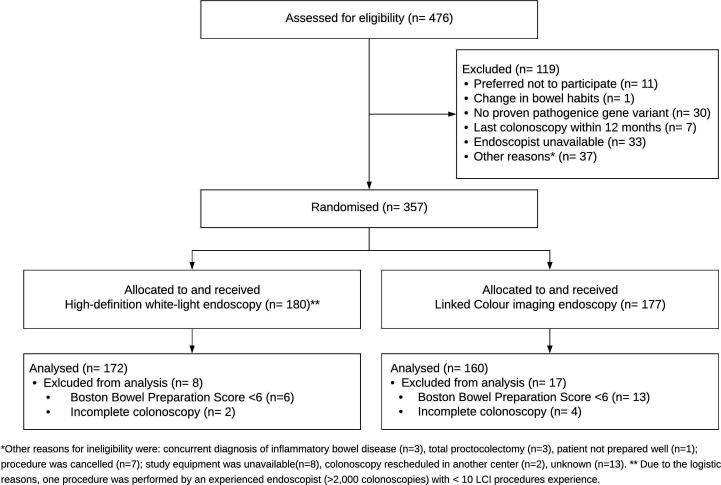
Between January 2018 and March 2020, 476 patients with Lynch syndrome were assessed for eligibility of which 357 patients were randomised to undergo inspection with either LCI or HD-WLE. Figure 1 shows the flowchart. Nineteen patients were excluded because of poor bowel preparation (BBPS <6) and six patients because of incomplete colonoscopy. After excluding these patients, 332 patients were included; 160 in the LCI group and 172 in the HD-WLE group. A total of 22 endoscopists from eight centres participated in the study. The number of colonoscopies per centre ranged from 15 to 81. No AEs related to the study intervention were reported.
Figure 1.
Consolidated Standards of Reporting Trials patient flowchart. LCI, linked colour imaging.
Baseline characteristics for patients who completed the trial were similar (table 1). The mean age of all participants was 48.4 years (SD 14.1), 141 (42%) were men and 72 (22%) had a personal history of CRC. In both groups the most common mutation type was MSH2, followed by MSH6 and MLH1 and PMS2; only two EpCAM mutation carriers were included. The median time since the previous surveillance colonoscopy was 17 months (13–24). First-time colonoscopies were performed in 42 (13%) of the patients. Of the 290 patients who had already undergone one or more colonoscopies before inclusion, 72 (22%) had neoplasia detected during the previous colonoscopy. Characteristics of the study procedures are shown in table 2.
Table 1.
Baseline characteristics
| Linked colour imaging (N=160)* |
High-definition white light endoscopy (N=172)* | |
| Age in years | 49.6 (14.4) | 47.32 (13.8) |
| Male gender | 74 (46%) | 67 (39%) |
| Time since diagnosis in years | 5 (2–10) | 6 (2–9) |
| Type pathogenic gene variant | ||
| MLH1 | 37 (24%) | 39 (23%) |
| MSH2 | 63 (39%) | 66 (38%) |
| MSH6 | 35 (22%) | 43 (25%) |
| PMS2 | 24 (15%) | 23 (14%) |
| Epcam | 1 (0%) | 1 (0%) |
| History of colorectal cancer | 35 (22%) | 37 (22%) |
| Surveillance interval in months | 17 (12–25) | 18 (13–24) |
| Number of previous colonoscopies | ||
| 0 | 20 (13%) | 22 (13%) |
| 1 | 24 (15%) | 25 (15%) |
| 2+ | 116 (72%) | 125 (72%) |
| Neoplasia detected during previous colonoscopy | 36 (23%) | 36 (21%) |
Data are n (%), n, mean (SD) or median (P25–P75).
*The baseline characteristics for patients who completed the trial were not statically different.
Table 2.
Colonoscopy characteristics
| Linked Colour imaging (N=160) | High-definition white-light endoscopy (N=172) | P value (two-sided) | |
| Boston Bowel Preparation Scale | 9 (6–9) | 9 (7–9) | 0.82 |
| Gloucester Comfort Score | 1 (1–2) | 1 (1–2) | 0.83 |
| Sedation | 0.82 | ||
| None | 7 | 6 | |
| Midazolam or fentanyl, or both | 69 | 73 | |
| Propofol | 80 | 93 | |
| Butylscopolamine | 30 (19%) | 22 (13%) | 0.18 |
| Colonoscopies per centre | 1.00 | ||
| Centre 1 | 39 | 42 | |
| Centre 2 | 31 | 38 | |
| Centre 3 | 22 | 23 | |
| Centre 4 | 19 | 19 | |
| Centre 5 | 19 | 19 | |
| Centre 6 | 15 | 16 | |
| Centre 7 | 7 | 8 | |
| Centre 8 | 8 | 7 | |
| Procedure time in minutes | 23 (17–31) | 22 (16–28) | 0.44 |
| Caecal intubation time in minutes | 7 (5–10) | 7 (5–10) | 0.99 |
| Extubation time in minutes | 15 (10–21) | 13 (9–19) | 0.09 |
| Withdrawal time in minutes | 12 (9–16) | 11 (8–15) | 0.16 |
| Intervention time in minutes | 1 (0–5) | 0 (0–4) | 0.39 |
Data are n (%), n or median (P25–P75).
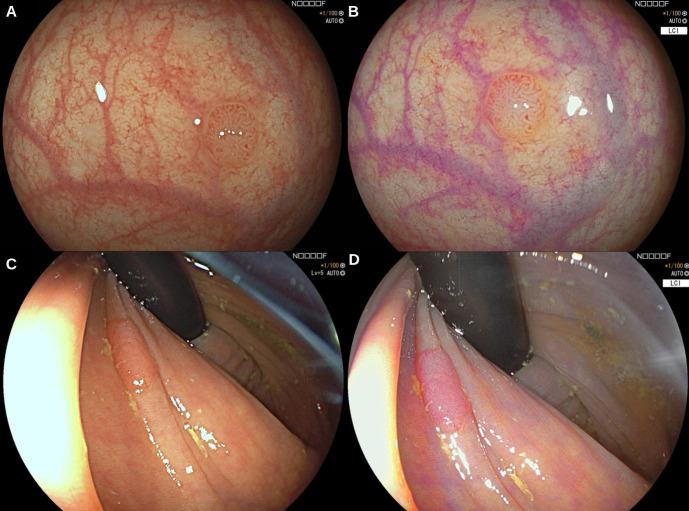
In total, 341 lesions were identified in the 332 patients (table 3). Of these lesions, 16 (5%) were not retrieved for histology and 33 (10%) were reported as normal mucosa or other non-neoplastic lesions (eg, inflammatory lesions). Of the 292 remaining lesions, 5 (2%) were carcinomas, 176 (54%) were adenomas, 28 (9%) were sessile serrated lesions, 79 (24%) were hyperplastic polyps and 4 (1%) were traditional serrated adenomas. After excluding 35 diminutive hyperplastic polyps in the rectosigmoid a remaining total of 257 histologically confirmed polyps were included for analysis. With HD-WLE five adenocarcinomas were detected, whereas with LCI no carcinomas were detected. All detected carcinomas were flat (Paris classification IIa or IIb) and located in the proximal colon. Sizes ranged from 6 to 35 mm. Patient characteristics of the patients with carcinomas found are shown in online supplemental file 1. Figure 2 shows images of a 4 mm and 10 mm flat elevated adenoma photographed with HD-WLE (A and C) and with LCI (B and D).
Table 3.
Characteristics of the detected lesions
| Linked Colour imaging (N=160) | High-definition white light endoscopy (N=172) | |
| All lesions | 190 | 151 |
| Size | ||
| ≤5 mm | 154 (81%) | 124 (82%) |
| 6–9 mm | 24 (13%) | 15 (10%) |
| ≥10 mm | 12 (6%) | 10 (7%) |
| Missing | 0 (0%) | 2 (1%) |
| Location | ||
| Caecum | 22 (12%) | 16 (11%) |
| Ascending* | 51 (27%) | 29 (19%) |
| Transverse | 41 (22%) | 32 (21%) |
| Descending† | 25 (13%) | 21 (14%) |
| Sigmoid | 35 (18%) | 32 (21%) |
| Rectum | 16 (8%) | 21 (14%) |
| Morphology, by Paris classification | ||
| Pedunculated (Ip) | 4 (2%) | 1 (0%) |
| Sub-pedunculated (Isp) | 0 (0%) | 0 (0%) |
| Sessile (Is) | 91 (48%) | 63 (42%) |
| Flat or flat elevated (IIa or IIb) | 90 (47%) | 81 (54%) |
| Depressed (IIc) | 0 (0%) | 0 (0%) |
| Missing | 5 (3%) | 6 (4%) |
| Histopathology | ||
| CRColorectal cancer | 0 (0%) | 5 (3%) |
| Adenoma | 104 (54%) | 72 (48%) |
| High-grade dysplasia | 3 | 2 |
| Villous features | 0 | 0 |
| Sessile serrated lesion | 15 (8%) | 13 (9%) |
| Dysplasia | 0 | 0 |
| Traditional serrated adenoma | 4 (2%) | 0 (0%) |
| Hyperplastic polyp | 40 (21%) | 39 (26%) |
| Normal mucosa | 15 (8%) | 12 (8%) |
| Other non-neoplastic | 5 (3%) | 1 (0%) |
| Not retrieved/not resected | 7 (4%) | 9 (6%) |
Data are n or n (%).
*Includes hepatic flexure.
†Includes splenic flexure.
CRC, colorectal cancer.
Figure 2.
Images of a 4 mm and 10 mm flat elevated adenoma with high-definition white light endoscopy (A and C) and corresponding Linked Colour imaging (B and D).
gutjnl-2020-323132supp001.pdf (53KB, pdf)
Primary outcome
Analysis for the primary and secondary outcomes are summarised in tables 4 and 5. The overall PDR was 40.1% (95% CI 34.7% to 45.6%). No significant difference was observed between the PDR with LCI (44.4%; 95% CI 36.5% to 52.4%) and HD-WLE (36.0%; 95% CI 28.9% to 43.7%) (RR 1.23, 95% CI 0.95 to 1.60, p=0.12).
Table 4.
Polyp detection rates
| Linked Colour imaging (N=160) | High-definition white light endoscopy (N=172) | Risk ratio (two-sided 95% CI) |
P value (two-sided) |
|
| Polyps† | 71 (44.4%) | 62 (36.0%) | 1.23 (0.95 to 1.60) | 0.12 |
| Adenomas | 58 (36.3%) | 44 (25.6%) | 1.42 (1.02 to 1.96) | 0.04* |
| Advanced adenomas | 7 (4.4%) | 8 (4.6%) | 0.94 (0.35 to 2.43) | 1.00‡ |
| Flat adenomas§ | 27 (16.9%) | 20 (11.6%) | 1.45 (0.85 to 2.48) | 0.18 |
| ≤5 mm adenomas | 52 (32.5%) | 38 (22.1%) | 1.47 (1.03 to 2.11) | 0.03* |
| >5 mm adenomas | 12 (7.5%) | 12 (7.0%) | 1.09 (0.50 to 2.32) | 0.85 |
| Proximal adenomas¶ | 45 (28.1%) | 32 (18.6%) | 1.51 (1.01 to 1.51) | 0.04† |
| Serrated polyps | 28 (17.5%) | 24 (14.0%) | 1.25 (0.76 to 2.07) | 0.38 |
| Sessile serrated lesions | 11 (6.9%) | 12 (7.0%) | 0.98 (0.45 to 2.17) | 0.97 |
| Proximal serrated polyps¶ | 18 (11.3%) | 17 (9.9%) | 1.14 (0.61 to 2.13) | 0.69 |
| Hyperplastic polyps | 17 (10.6%) | 15 (8.7%) | 1.21 (0.63 to 2.36) | 0.56 |
Data are n (%).
*p<0.05, **p<0.01.
†1–5 mm hyperplastic polyps in the rectosigmoid, normal mucosa, other non-neoplastic lesions and lesions not retrieved for pathology were excluded.
‡P value was calculated using Fisher’s exact test instead of χ2 test since the expected values in any cells of contingency table were below 10.
§Morphology 0-IIa, 0-IIb, or IIc according to Paris classification.
¶Proximal to splenic flexure.
Table 5.
Mean number of polyps per patient
| Linked Colour imaging (N=160) | High-definition white light endoscopy (N=172) | Incidence rate ratio (two-sided 95% CI) | P value (two-sided) |
|
| Polyps† | 0.94 (1.40) | 0.62 (1.14) | 1.51 (1.05 to 2.16) | 0.03* |
| Adenomas | 0.65 (1.11) | 0.42 (0.92) | 1.55 (1.02 to 2.35) | 0.04* |
| Advanced adenomas | 0.06 (0.28) | 0.05 (0.25) | 1.07 (0.37 to 3.15) | 0.89 |
| Flat adenomas‡ | 0.28 (0.75) | 0.19 (0.63) | 1.47 (0.76 to 2.84) | 0.26 |
| ≤5 mm adenomas | 0.54 (1.00) | 0.34 (0.76) | 1.58 (1.02 to 2.46) | 0.04* |
| >5 mm adenomas | 0.10 (0.40) | 0.07 (0.29) | 1.41 (0.60 to 3.30) | 0.43 |
| Proximal adenomas§ | 0.46 (0.92) | 0.26 (0.63) | 1.78 (1.10 to 2.88) | 0.02* |
| Serrated polyps | 0.29 (0.76) | 0.17 (0.49) | 1.64 (0.90 to 3.00) | 0.10 |
| Sessile serrated lesions | 0.09 (0.39) | 0.08 (0.29) | 1.24 (0.52 to 2.95) | 0.63 |
| Proximal serrated polyps§ | 0.17 (0.55) | 0.10 (0.33) | 1.61 (0.79 to 3.29) | 0.19 |
| Hyperplastic polyps | 0.17 (0.60) | 0.10 (0.34) | 1.71 (0.79 to 3.69) | 0.17 |
Data are mean (SD).
*p<0.05, **p<0.01.
†1–5 mm hyperplastic polyps in the rectosigmoid, normal mucosa or other non-neoplastic lesions (eg, inflammatory polyps) and lesions not retrieved for pathology were excluded.
‡Morphology 0-IIa, 0-IIb, or IIc according to Paris classification.
§Proximal to splenic flexure.
Secondary outcomes
The overall ADR with LCI (36.3%; 95% CI 28.8% to 44.2%) was significantly higher compared with HD-WLE (25.6%; 95% CI 19.2% to 32.9%) (RR=1.42, 95% CI 1.02 to 1.96, p=0.04). With LCI, a significantly higher proportion of patients had proximal adenomas (28.1% vs 18.6%; p=0.04) and ≤5 mm adenomas (32.5% vs 22.1%; p=0.03) compared with HD-WLE. For flat adenomas, adenomas >5 mm, serrated polyps, proximal serrated polyps, sessile serrated lesions and hyperplastic polyps no statistically significant differences were observed between groups. No significant difference in advanced adenoma detection rate and carcinoma detection rate was observed between the two groups (Fisher exact test). The mean number of polyps per patient was significantly higher with LCI compared with HD-WLE (0.94 (SD 1.40) vs 0.62 (SD 1.14); IRR 1.51, 95% CI 1.05 to 2.16, p=0.03). The mean number of adenomas detected per patient was also significantly higher for LCI compared with HD-WLE (0.65 (SD 1.11) vs 0.42 (SD 0.92); IRR 1.55, 95% CI 1.02 to 2.35, p=0.04). This difference was due to a significant increase in the mean number of proximal adenomas per patient (0.46 vs 0.26; p=0.02) and the mean number of diminutive adenomas per patient (0.54 vs 0.34; p=0.04) detected with LCI compared with HD-WLE. No differences in the mean number of serrated polyps, the mean number of flat adenomas, adenomas >5 mm, advanced adenomas, serrated polyps, proximal serrated polyps and the mean number of sessile serrated lesions per patient were detected between the two groups. In the subgroup of patients with a history of CRC surgery, no statistically significant differences in polyp/adenoma detection rate were observed between LCI and HD-WLE (online supplemental file 2).
gutjnl-2020-323132supp002.pdf (59KB, pdf)
Results from the sensitivity analyses to adjust for stratification per centre resulted in similar outcomes and are shown in online supplemental file 3. Online supplemental file 4 shows the wide variation in the PDRs and ADRs of the individual centres. In line with the overall increase in ADR with LCI compared with HD-WLE, LCI increased the adenoma detection rate in six of the eight individual centres.
gutjnl-2020-323132supp003.pdf (86.3KB, pdf)
gutjnl-2020-323132supp004.pdf (17.3KB, pdf)
The median procedure and withdrawal times were not significantly different between LCI and HD-WLE; 23 min (17–31) versus 22 min (16–28) (p=0.44) and 12 min (P25–P75; 9–16) versus 11 min (8–15) (p=0.16), respectively (table 2).
Discussion
This is the first international multicentre randomised controlled trial comparing LCI to HD-WLE for polyp detection in Lynch surveillance by experienced endoscopists. Although LCI did not significantly improve the total PDR, use of LCI resulted in an increase of the polyp detection overall by finding more polyps per patient. This increase was reflected by a clinically relevant increase in the ADR and the mean number of adenomas detected per patient, especially those that are proximally located or diminutive. In doing so, LCI did not extend the procedural or withdrawal time compared with HD-WLE. Hence, LCI is a non-invasive way of improving adenoma detection without any additional cost.
In this study, we observed no significant difference in the total PDR, which might be due to our sample size calculations, which was based on the assumption that the proportion of patients with Lynch syndrome with at least one polyp detected would be around 25% using HD-WLE, increasing to 40% when using LCI. In the current study, the actual proportion of patients with at least one polyp detected using HD-WLE was higher than expected: 36.0%. The reason for the unexpected high PDR with HD-WLE may be that all procedures were performed by dedicated endoscopists, while the sample size expectation was based on the last two colonoscopies of 100 patients with Lynch syndrome at the Amsterdam UMC, outside of a study environment. In the latter situation, part of the colonoscopies was performed by less experienced endoscopists. Furthermore, the Hawthorne effect may have occurred; endoscopists might put more effort in examining the colon than usual when they are aware that they are being monitored.26
Although no significance difference in the total PDR was observed, the present study did show that LCI detected more polyps per patient, reflected by a significance difference in mean number of polyps per patient. Many of these polyps were adenomas and this explains why the adenoma detection rate is significantly different and PDR is not. LCI detected a 10% higher proportion of patients with at least one adenoma compared with HD-WLE (36.3% vs 25.6%), a difference that was statistically significant (p=0.04). This means that 10 LCI procedures are required to detect one additional patient with at least one adenoma that would not be detected with HD-WLE. Moreover, the mean number of adenomas per colonoscopy did significantly differ between the LCI group and the HD-WLE group (0.65 vs 0.42, p=0.04). This increase in adenoma detection might be clinically relevant because we know that in the general population an increase in adenoma detection leads to a reduction in CRC incidence and mortality.27 28 In patients with Lynch syndrome, with a 7.9% 10 year cumulative incidence of PCCRC, this association was not directly proven.29 However, it was shown that a high quality colonoscopy is associated with an increased adenoma detection.29–31 Besides, an inverse association between colonoscopy quality and the risk of post colonoscopy CRC (PCCRC) in the subsequent colonoscopy was found.29–31 29 Therefore, since LCI is a non-invasive way to improve adenoma detection without additional costs, these technique might be clinically relevant for these high-risk patients.
This is in line with the results of a recent meta-analysis in the average-risk population.18 This meta-analysis showed that LCI increased the PDR and ADRcompared with WLE (61.4% vs 51.7%; RR 1.16; 95% CI 1.09 to 1.25; p<0.001 and 43.6% vs 33.5%; RR 1.26; 95% CI 1.14 to 1.39; p<0.001, respectively). The ADR and the mean number of adenomas per patient detected by HD-WLE in this study were 25.6% and 0.42, respectively, comparable to those reported in a large multicentre randomised controlled trial in patients with Lynch syndrome (28.1% and 0.52).32 Hence, also in this study the higher detection of adenomas with LCI seems not be attributed to a lower detection rate with HD-WLE.
The increased detection of adenomas with LCI was particularly reflected by a significant increase in detection of proximal and diminutive adenomas compared with HD-WLE. This suggests that an additional population of adenomas is being detected, of which their size and location makes them difficult to detect with HD-WLE alone. These additional diminutive and proximal adenomas are clinically relevant in Lynch syndrome, since the adenoma-carcinoma sequence is accelerated in patients with Lynch syndrome, and proximal adenomas might be more prone to rapid malignant transformation compared with distal adenomas.6 7 Though it is necessary to be cautious with drawing conclusions based on secondary outcomes, our consistent findings suggest that LCI seems of benefit to adenoma detection in patients with Lynch syndrome.
Compared with the average-risk population, some studies have reported a high proportion of adenomas with high-grade dysplasia and villous features in Lynch syndrome, particularly in the proximal colon.7 33 In our sample of 173 adenomas, only 5 (3%) contained high-grade dysplasia and none contained villous features. This may reflect the effectiveness of intensive modern endoscopic surveillance, as results similar to ours were seen in a recent detection study of Lynch syndrome surveillance.34 However, still five adenocarcinomas were detected. The five adenocarcinomas (2%) reported in our study were all flat (IIa or IIB) and proximally located. The surveillance intervals of these patients ranged from 12 to 14 months, suggesting that some of these lesions or its precursors might have been missed during a previous colonoscopy. Strikingly, all carcinomas were detected in the HD-WLE group for which we have no good explanation other than coincidence.
Apart from LCI, other advanced endoscopic imaging techniques have been assessed for polyp detection for Lynch syndrome surveillance. A meta-analysis of individual patient data of randomised controlled trials compared adenoma detection using dye-based CE and WLE in Lynch syndrome.35 In this study, dye-based CE did not increase ADR compared with HD-WLE (CE 30.8% vs HD-WLE 27.0%, p=0.42), but did increase extubation time. We can therefore conclude that the additional value of dye-based CE in adenoma detection is rather marginal, taking into account the non-significance in the meta-analysis. Three single-centre randomised cross-over studies were performed with virtual CE techniques (narrow band imaging, I-SCAN, autofluorescence imaging endoscopy), all documenting a benefit of virtual CE over WLE for adenoma and polyp detection.36–38 Our study clearly adds to the existing evidence that virtual CE helps detecting lesions in these high-risk patients. In addition, recent breakthroughs in artificial intelligence have shown potential to assist endoscopists with polyp detection in the near future.
This study is the first randomised trial showing that LCI is able to significantly increase adenoma detection compared with HD-WLE in patients with Lynch syndrome. Strengths of our study are that colonoscopies were of high quality, performed by 22 experienced endoscopists from eight international hospitals, and LCI and HD-WLE were consecutively performed in a random order. Data on polyp detection, procedure times and bowel preparation scores were prospectively recorded, ensuring accurate ldata collection of high-quality colonoscopies. We therefore believe that our results are reliable for daily clinical practice. However, potential limitations of this study must be acknowledged. Most importantly, the actual PDR with HD-WLE was higher than our initial assumption (36% vs 25%), resulting in a non-significant difference in our primary outcome (ie, type II error). With current knowledge, sample size calculations for future studies would include higher numbers of patients compared with our study. However, at the design stage of our study, HD-WLE technique was not yet thoroughly assessed for polyp detection in patients with Lynch syndrome. Another limitation of the study is that we did not standardise colonoscopy procedures between centres, such as use of sedatives, analgesics and antispasmodics. As this is a large randomised controlled trials and baseline characteristics were similar between the two techniques, we believe these factors did not confound the comparison. Furthermore, some endoscopists did more study colonoscopies than others, possibly introducing a learning curve for LCI during the study. To minimise this learning curve, participating endoscopists were required to have done at least 10 procedures with LCI before the start of the study. We have especially taken care that participating endoscopists had extensive experience (>2000 colonoscopies), were familiar with virtual chromoendoscopy and performed already surveillance in patients with Lynch syndrome. This potentially limits the generalisability of our findings. On the other hand, one can argue that only experienced (ie, high detectors) should perform endoscopic surveillance in high-risk CRC populations. Finally, since it is impossible to conceal the technique being used from endoscopists, there is an unavoidable observer bias as in every clinical study with an advanced imaging technique. The wide variation in baseline detection rates with WLE combined with the largest difference with LCI might suggests this type of bias. However, no difference in withdrawal time was observed between LCI and HD-WLE.
In summary, in this randomised controlled trial performed in an expert setting, LCI did not increase the proportion of patients with at least one polyp detected compared with HD-WLE in patients with Lynch syndrome. However, since LCI improved adenoma detection without additional withdrawal time or additional costs, it might still be valuable for surveillance of patients with Lynch syndrome.
Acknowledgments
We thank A Baker, C Camps, C Cohen, E Finati, H Beaumont, H Mues, H Willekens, J Dash, P Wilson, R Dierx, M Edmonds, M van der Ende-van Loon, R Moreira, S Arntz and V Roos for their invaluable help with patient recruitment and data collection. We thank Dr M van Leerdam and Professor C Cellier for their effort to set up the trial in their hospitals.
Footnotes
Twitter: @lisrs13, @frankbalaguer
Correction notice: This article has been corrected since it published Online First. The affiliations for Prof Repici have been updated.
Contributors: Conception and design: BBSLH, JLAV, YH, MGHvO, LK, PF, ED; data acquisition: all authors; data analysis and interpretation: all authors; writing of the manuscript: BBSLH, YH, ED; critical revision of the manuscript: all authors; statistical analyses: BBSLH, MGHvO; supervision: all authors.
Funding: FUJIFILM Europe GmbH.
Competing interests: AR has received loan equipment and a consultancy fee from Medtronic and Fujifilm. BAJB received speaking fees from Olympus, Tillotts Pharma AG and Ovesco Endoscopy. ED received equipment on loan from Olympus and Fujifilm, ED received a research grant from FujiFilm, a consulting fee for medical advice from Tillots, Olympus, Fujifilm, GI Supply and CPP-FAP and a speakers’ fee from Olympus, Roche and GI Supply. FB has endoscopic equipment on loan of Fujifilm, received an honorarium for consultancy from Sysmex, CPP-FAP speaking fees from Norgine, and an editorial fee from Elsevier. MFK has received speaking, teaching and consultancy fees from Olympus (2017 to present) and speaking and teaching fees, and a loan of equipment from Fujifilm (2019) and speaking fees from Medtronic (2019), Alfa Sigma (2017–2019) and Norgine (2018–2019). MP received a research grant from Fujifilm Spain and Casen Recordati, a loan of equipment from Fujifilm, received consultancy fee from Norgine, speaking fee from Norgine, Olympus, Casen Recordati, Janssen and an editorial fee from Thieme. RB has provided consultancy to and received research grants and speakings fees from Pentax (2008 to present) and Fujifilm (2015 to present); his department has received research grants and equipment from Pentax and Fujifilm (2015 to present). PB has received grant funding from Norgine, Fujifilm, Olympus, Pentax, Boston scientific. PF received research support from Boston Scientific research and a consulting fee from Olympus, Cook and Ethicon Endosurgery.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Human participants: the study was conducted according to the principles of the Declaration of Helsinki and the Medical Research Involving Human Subjects Act (WMO), and was approved by the Institutional Review Board of each participating centre (Research Ethics Committee References; the Netherlands: AMC 2016_34; Spain: HCB/2017/0114; Italy: ICH 1889; Poland: 75/2018/2019; Belgium: S60629; UK: 19/WS/0187).
References
- 1. Vasen HFA, Boland CR. Progress in genetic testing, classification, and identification of Lynch syndrome. JAMA 2005;293:2028–30. 10.1001/jama.293.16.2028 [DOI] [PubMed] [Google Scholar]
- 2. Ramsoekh D, Wagner A, van Leerdam ME, et al. Cancer risk in MLH1, MSH2 and MSH6 mutation carriers; different risk profiles may influence clinical management. Hered Cancer Clin Pract 2009;7:17. 10.1186/1897-4287-7-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Møller P, Seppälä T, Bernstein I, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut 2017;66:464–72. 10.1136/gutjnl-2015-309675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Møller P, Seppälä TT, Bernstein I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 2018;67:1306–16. 10.1136/gutjnl-2017-314057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000;118:829–34. 10.1016/S0016-5085(00)70168-5 [DOI] [PubMed] [Google Scholar]
- 6. Edelstein DL, Axilbund J, Baxter M, et al. Rapid development of colorectal neoplasia in patients with Lynch syndrome. Clin Gastroenterol Hepatol 2011;9:340–3. 10.1016/j.cgh.2010.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rijcken FEM, Hollema H, Kleibeuker JH. Proximal adenomas in hereditary non-polyposis colorectal cancer are prone to rapid malignant transformation. Gut 2002;50:382–6. 10.1136/gut.50.3.382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Jong AE, Hendriks YMC, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006;130:665–71. 10.1053/j.gastro.2005.11.032 [DOI] [PubMed] [Google Scholar]
- 9. Zhao S, Wang S, Pan P, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology 2019;156:1661–74. 10.1053/j.gastro.2019.01.260 [DOI] [PubMed] [Google Scholar]
- 10. Vasen HFA, Abdirahman M, Brohet R, et al. One to 2-year surveillance intervals reduce risk of colorectal cancer in families with Lynch syndrome. Gastroenterology 2010;138:2300–6. 10.1053/j.gastro.2010.02.053 [DOI] [PubMed] [Google Scholar]
- 11. Engel C, Vasen HF, Seppälä T, et al. No difference in colorectal cancer incidence or stage at detection by colonoscopy among 3 countries with different Lynch syndrome surveillance policies. Gastroenterology 2018;155:1400–9. 10.1053/j.gastro.2018.07.030 [DOI] [PubMed] [Google Scholar]
- 12. Kaminski MF, Thomas-Gibson S, Bugajski M, et al. Performance measures for lower gastrointestinal endoscopy: a European Society of gastrointestinal endoscopy (ESGE) quality improvement initiative. United European Gastroenterol J 2017;5:309–34. 10.1177/2050640617700014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bisschops R, East JE, Hassan C, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy 2019;51:1155–79. 10.1055/a-1031-7657 [DOI] [PubMed] [Google Scholar]
- 14. Fujimoto D, Muguruma N, Okamoto K, et al. Linked color imaging enhances endoscopic detection of sessile serrated adenoma/polyps. Endosc Int Open 2018;6:E322–34. 10.1055/s-0043-124469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Min M, Deng P, Zhang W, et al. Comparison of linked color imaging and white-light colonoscopy for detection of colorectal polyps: a multicenter, randomized, crossover trial. Gastrointest Endosc 2017;86:724–30. 10.1016/j.gie.2017.02.035 [DOI] [PubMed] [Google Scholar]
- 16. Oliveira Dos Santos CE, Malaman D, Pereira-Lima JC, et al. Impact of linked-color imaging on colorectal adenoma detection. Gastrointest Endosc 2019;90:826–34. 10.1016/j.gie.2019.06.045 [DOI] [PubMed] [Google Scholar]
- 17. Paggi S, Mogavero G, Amato A, et al. Linked color imaging reduces the miss rate of neoplastic lesions in the right colon: a randomized tandem colonoscopy study. Endoscopy 2018;50:396–402. 10.1055/a-0580-7405 [DOI] [PubMed] [Google Scholar]
- 18. Shinozaki S, Kobayashi Y, Hayashi Y, et al. Colon polyp detection using linked color imaging compared to white light imaging: systematic review and meta‐analysis. Dig Endosc 2020;32:874–81. 10.1111/den.13613 [DOI] [PubMed] [Google Scholar]
- 19. Paggi S, Radaelli F, Senore C, et al. Linked-color imaging versus white-light colonoscopy in an organized colorectal cancer screening program. Gastrointest Endosc 2020;92:723–30. 10.1016/j.gie.2020.05.044 [DOI] [PubMed] [Google Scholar]
- 20. Schulz KF, Altman DG, Moher D, et al. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston bowel preparation scale. Gastrointest Endosc 2010;72:686–92. 10.1016/j.gie.2010.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58:S3–43. 10.1016/S0016-5107(03)02159-X [DOI] [PubMed] [Google Scholar]
- 23. Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000;47:251–5. 10.1136/gut.47.2.251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snover D. Serrated polyps of the colon and rectum and serrated polyposis. who classification of tumours of the digestive system, 2010: 160–5. [Google Scholar]
- 25. Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107:1315–29. 10.1038/ajg.2012.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sewitch MJ, Carpentier S, Bessissow T. Adr improvement: the result of the intervention or the Hawthorne effect. Am J Gastroenterol 2013;108:1929. 10.1038/ajg.2013.309 [DOI] [PubMed] [Google Scholar]
- 27. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370:1298–306. 10.1056/NEJMoa1309086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaminski MF, Wieszczy P, Rupinski M, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology 2017;153:98–105. 10.1053/j.gastro.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 29. Sánchez A, Roos VH, Navarro M, et al. Quality of colonoscopy is associated with adenoma detection and Post-Colonoscopy colorectal cancer prevention in Lynch syndrome. Clin Gastroenterol Hepatol 2020;15. 10.1016/j.cgh.2020.11.002. [Epub ahead of print: 03 Nov 2020]. [DOI] [PubMed] [Google Scholar]
- 30. Haanstra JF, Vasen HFA, Sanduleanu S, et al. Quality colonoscopy and risk of interval cancer in Lynch syndrome. Int J Colorectal Dis 2013;28:1643–9. 10.1007/s00384-013-1745-2 [DOI] [PubMed] [Google Scholar]
- 31. Perrod G, Samaha E, Rahmi G, et al. Impact of an optimized colonoscopic screening program for patients with Lynch syndrome: 6-year results of a specialized French network. Therap Adv Gastroenterol 2018;11:1756284818775058. 10.1177/1756284818775058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rivero-Sánchez L, Arnau-Collell C, Herrero J, et al. White-Light endoscopy is adequate for Lynch syndrome surveillance in a randomized and Noninferiority study. Gastroenterology 2020;158:895–904. 10.1053/j.gastro.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 33. Rondagh EJA, Gulikers S, Gómez-García EB, et al. Nonpolypoid colorectal neoplasms: a challenge in endoscopic surveillance of patients with Lynch syndrome. Endoscopy 2013;45:257–64. 10.1055/s-0032-1326195 [DOI] [PubMed] [Google Scholar]
- 34. Haanstra JF, Dekker E, Cats A, et al. Effect of chromoendoscopy in the proximal colon on colorectal neoplasia detection in Lynch syndrome: a multicenter randomized controlled trial. Gastrointest Endosc 2019;90:624–32. 10.1016/j.gie.2019.04.227 [DOI] [PubMed] [Google Scholar]
- 35. Houwen BBSL, Mostafavi N, Vleugels JLA, et al. Dye-Based Chromoendoscopy in patients with Lynch syndrome: an individual patient data meta-analysis of randomized trials. Am J Gastroenterol 2021. 10.14309/ajg.0000000000001138. [Epub ahead of print: 11 Feb 2021]. [DOI] [PubMed] [Google Scholar]
- 36. Bisschops R, Tejpar S, Willekens H, et al. Virtual chromoendoscopy (I-SCAN) detects more polyps in patients with Lynch syndrome: a randomized controlled crossover trial. Endoscopy 2017;49:342–50. 10.1055/s-0042-121005 [DOI] [PubMed] [Google Scholar]
- 37. East JE, Suzuki N, Stavrinidis M, et al. Narrow band imaging for colonoscopic surveillance in hereditary non-polyposis colorectal cancer. Gut 2008;57:65–70. 10.1136/gut.2007.128926 [DOI] [PubMed] [Google Scholar]
- 38. Ramsoekh D, Haringsma J, Poley JW, et al. A back-to-back comparison of white light video endoscopy with autofluorescence endoscopy for adenoma detection in high-risk subjects. Gut 2010;59:785–93. 10.1136/gut.2008.151589 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2020-323132supp001.pdf (53KB, pdf)
gutjnl-2020-323132supp002.pdf (59KB, pdf)
gutjnl-2020-323132supp003.pdf (86.3KB, pdf)
gutjnl-2020-323132supp004.pdf (17.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.