Abstract
The diversity and wide availability of trialkylamines render them ideal sources for rapid construction of complex amine architectures. Herein, we report that a nickel/photoredox dual catalysis strategy effects site-selective α-arylation of various trialkylamines. Our catalytic system shows exclusive N-Me selectivity with a wide range of trialkylamines under mild conditions, even in the context of late-stage arylation of pharmaceutical compounds bearing this common structural motif. Mechanistic studies indicate the unconventional behavior of Ni catalyst upon intercepting the α-amino radicals, in which only primary α-amino radical undergoes successful cross-coupling process.
Keywords: late-stage C–H functionalization, trialkylamine arylation, nickel/photoredox dual catalysis
Graphical Abstract
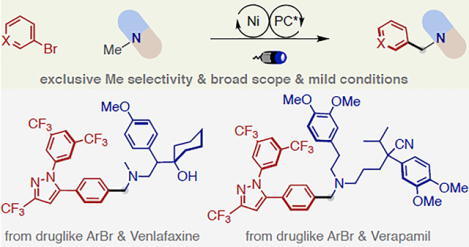
Trialkylamines are well-represented subunits in numerous alkaloid natural products, synthetic agrochemicals, clinical molecules and marketed pharmaceuticals (Figure 1A).1 The functionalization of C-H bonds alpha to N provides endless opportunities to fine-tune their physical properties as well as their biological activities and pharmacokinetics.2 The further development of direct late-stage α-C(sp3)–H functionalization of complex trialkylamines with improved structural modularity and functional diversity would enable novel synthetic disconnections and expedite discovery of lead compounds from existing medicinal chemistry libraries. To date, the late-stage α-alkylation of complex trialkylamines has experienced rapid growth (Figure 1B);3 however, the related arylation has remained underexplored with sporadic examples (Figure 1C, left).3e
Figure 1.
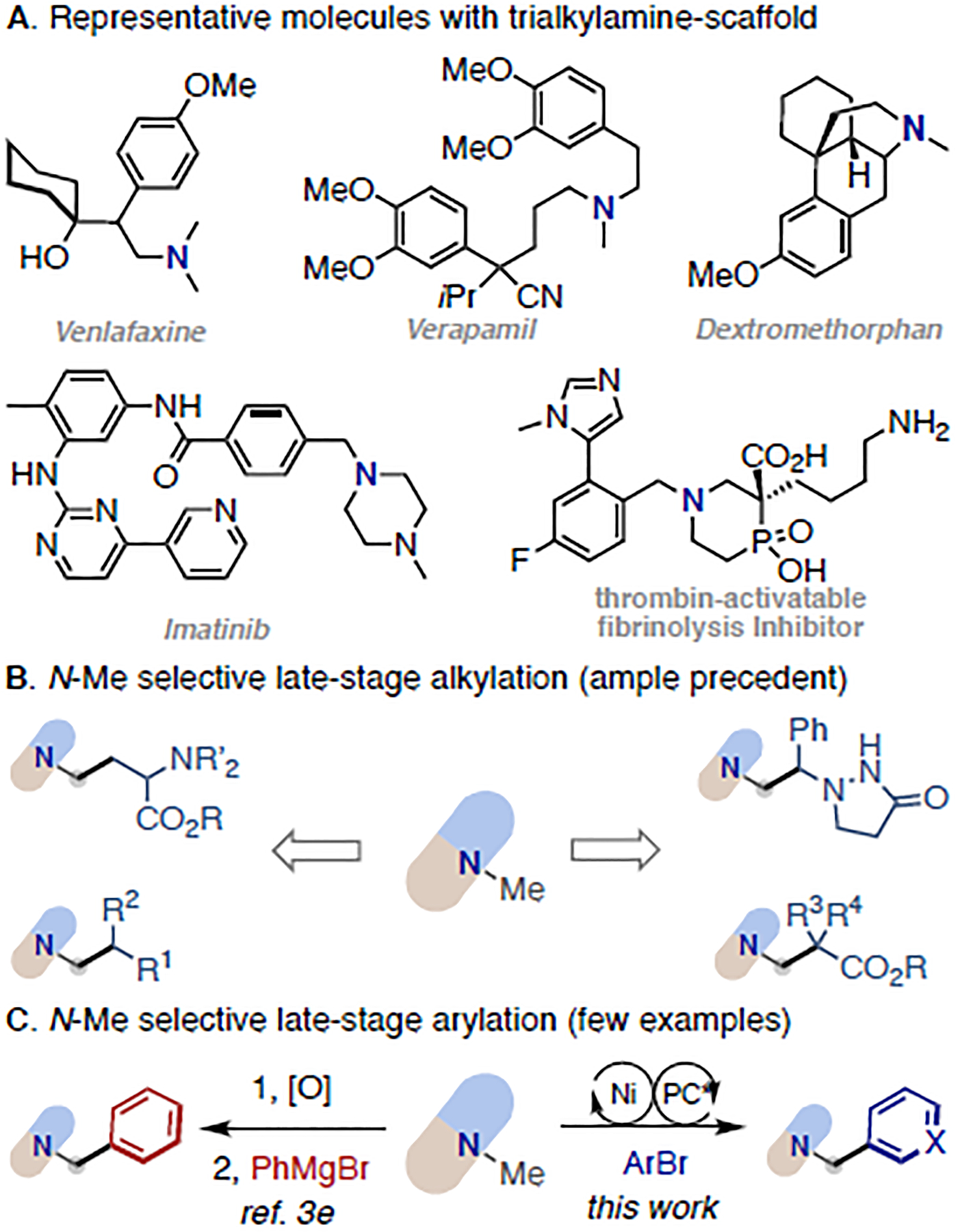
Prevalence and α-Functionalization of complex trialkylamines
Recently, transition-metal/photoredox dual catalysis has emerged as a transformative platform enabling a diverse set of formerly challenging chemical bond-forming events under mild conditions.4 Given that trialkylamines are commonly used as hydride, hydrogen or electron sources in a number of transition-metal/photoredox catalysed transformations,5 it would not be trivial to compete with the established reactivity for direct arylation of trialkylamines. However, Molander’s seminal work of desilylative arylation of α-silyl trialkylamines indicates otherwise,6 in which the silyl group is preinstalled from secondary amine to control the site-selective generation of α-amino radical.7 As part of our interest to derivatize amines,8 we questioned whether we could develop a general method for selective arylation of trialkylamines even in the context of late-stage functionalization with improved practicality and complexity (Figure 1C, right).
If accomplished, it would offer new opportunities to rapidly access novel benzyl dialkylamines — privileged moieties embedded within many bioactive molecules and lead compounds9 — with complementarity to classical alkylation or reductive amination strategies from secondary amines, where the complex benzaldehydes or benzyl electrophiles are often difficult to access.10
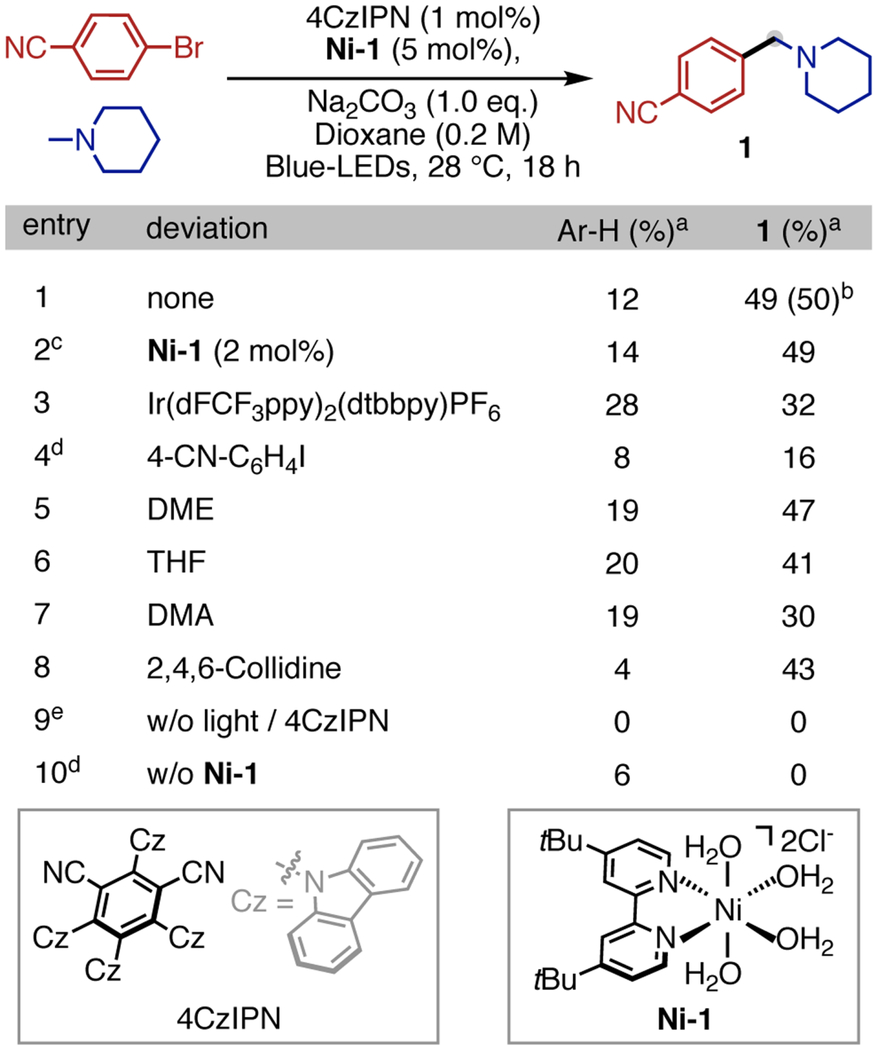
We started our investigation by conducting arylation of N-Me piperidine with 4-bromobenzonitrile under Ni/photoredox conditions.11 Gratifyingly, when reaction occurs, we observe exclusive N-Me arylation product 1. Further screening revealed the optimized conditions to be NiCl2(dtbbpy)(H2O)4 (Ni-1) (5 mol%), 4CzIPN (1 mol%) as organic photocatalyst,12,13 and Na2CO3 (1.0 equiv) as a base in Dioxane (0.2 M) under blue-LEDs irradiation (440 nm), affording 1 in 50% isolated yield (see SI for extensive screening details). Our system turns out to be highly efficient as 2 mol% of Ni-1 works equally well (entry 2). Switching to Ir-based photocatalyst leads to a decrease in yield (entry 3). While DME as solvent gives similar yield (entry 5), other solvents demonstrate lower chemoselectivity (entries 6 & 7). Using 4-iodobenzo-nitrile as aryl source (entry 4) shows low reactivity under our conditions. The use of an organic base, such as collidine, gives comparable results (entry 8). Control studies reveal the necessity of irradiation and both catalysts for arylation to occur (entries 9 & 10).
With the optimized conditions, we next studied the scope of our protocol. As shown in Table 2, the method displays exclusive N-Me selectivity. A variety of trialkylamines bearing various alkyl substituents underwent the desired arylation (1-9). Aryl bromides containing different functional groups and heterocycles (10-18, 21) are well accommodated. In addition, the ketone (11) and aldehyde (12) functionalities hold promise for further reductive amination events. Interestingly, arylation of sterically encumbered trialkylamines, often used as frustrated Lewis pair and hindered amine light stabilizers,14 is successful upon switching to a nickel/1,3-diketone-ligand combination (19-21) (see Table S4 for details), a system known for forging congested bonds.15
Table 2.
Scope of Trialkylamines and Aryl Bromides
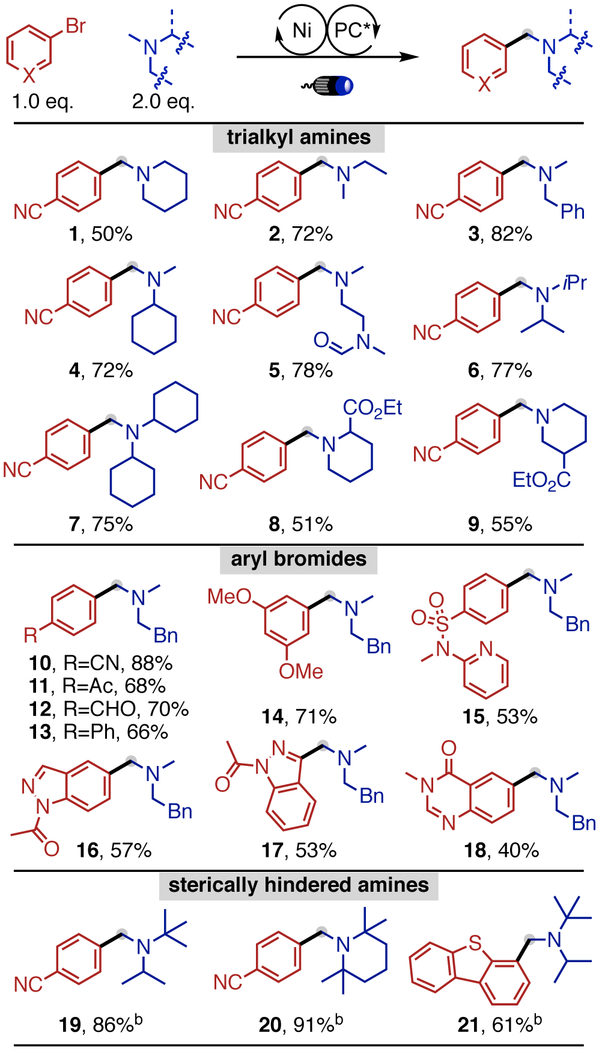
|
0.2 mmol scale; a Ni-1(5 mol%), 4CzIPN (1 mol%), Na2CO3 (1.0 eq.) in Dioxane (0.2 M) at 28 °C for 18 h; b NiCl2(H2O)6 (5 mol%), Dipivaloyl methane (10 mol%), 4CzIPN (1 mol%), Na2CO3 (1.0 equiv) in MeCN (0.2 M) at 28 °C for 24 h.
Encouraged by the broad generality of this approach, we anticipated that our protocol might not only streamline the synthesis of simple benzyl dialkylamines but might also be applicable to late-stage functionalization.16 To this end, we were pleased to find that a series of trialkylamine-containing pharmaceuticals and natural products could be coupled with complex aryl bromides. As evident by the results compiled in Table 3, a variety of valuable, yet not easily accessible druglike benzyl dialkylamines were rapidly synthesized with moderate to high yields (22-35), highlighting the potential impact of our protocol on generating complex amine architectures to accelerate lead compound discovery.
Table 3.
Late-stage N-Me Selective Arylation
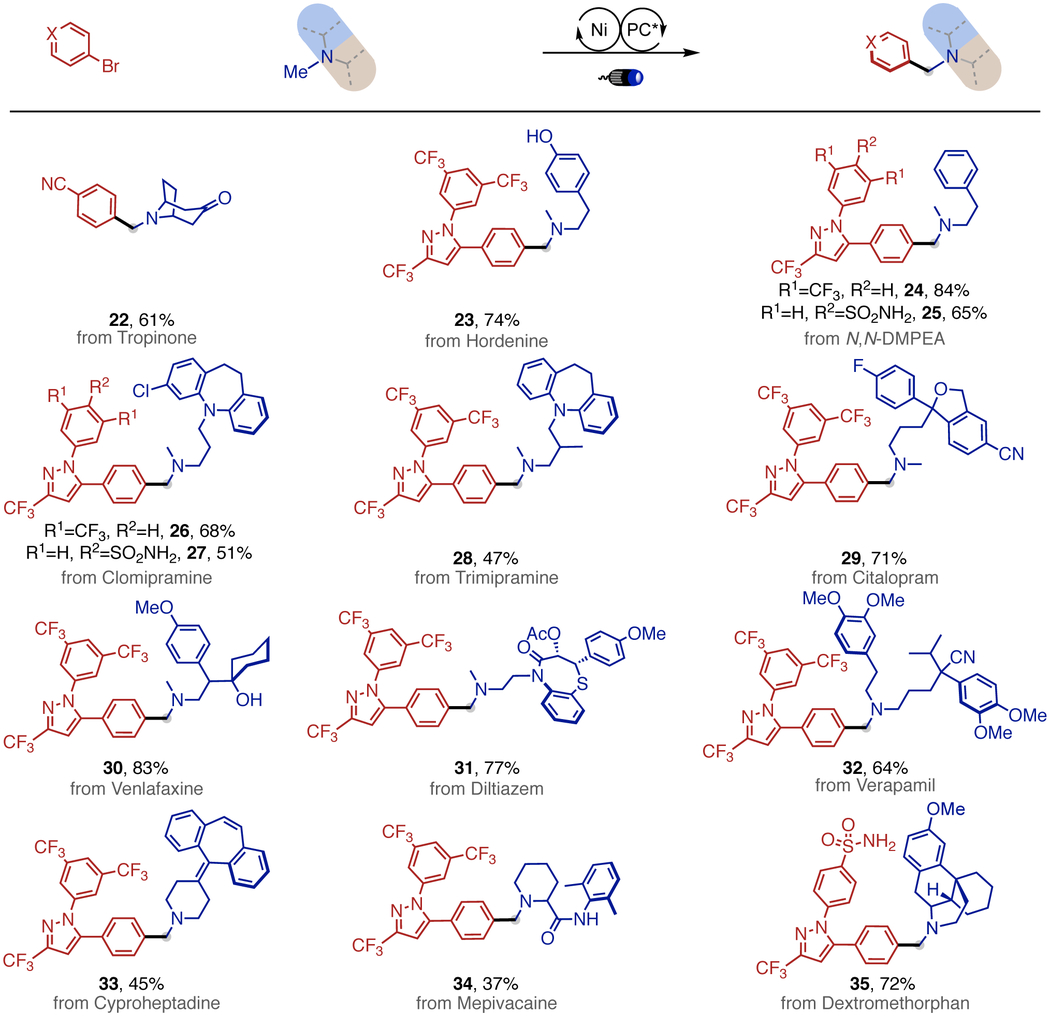
|
0.1 mmol scale; as Table 1 in Dioxane (0.1 M) for 24h.
In general, oxidation/deprotonation of trialkylamines furnishes the less hindered α-amino radical preferentially.7a,7b,8a However, this does not completely explain the exclusive N-Me selectivity observed in our photochemical arylation, especially for substrates that are known to generate a mixture of α-amino radicals with moderate ratios upon oxidation/deprotonation sequence (e.g. N-Me piperidine gives 2:1 selectivity for 1° over 2° under typical conditions8a,8c). We then turned our attention to understand the source of the high site-selectivity. A simple control experiment with triethylamine under standard conditions indicated the recalcitrance of a putative ArNiIILnBr (Ni-2) intermediate to intercept secondary α-amino radicals, as no desired arylation was observed. Instead, a considerable amount of hydrodebromination and homodimerization products from the aryl bromide were detected (Figure 2A). This helps to explain the parasitic by-products formed during the reaction screening of N-Me piperidine (Table 1). Further studies with α- and β-deutero- tributylamine as starting materials revealed that the H source of hydrodebromination partially comes from both α- and β-C(sp3)–H of tributylamine (Figure 2B), with the balance likely coming from the solvent (dioxane). When optimizing the arylation on sterically hindered trialkylamines, we found significant dimerization of the amine when the reaction was conducting in polar aprotic solvents (DMF or DMA) (Figure 2C). This likely stems from the amino radical attacking an iminium ion intermediate (Figure 2C).10b Indeed, adding H2O (50 equiv) to the standard arylation of N-Me piperidine completely shuts down the desired reactivity leading to exclusive generation of the hydrodebromination product, which presumably occurs via hydrolysis of the iminium ion intermediate (see Table S3 for H2O titration). On the basis of these results, along with the literature precedent on the coordination of Ni-complexes and iminium ions,10a,17 we tentatively propose that the arylation occurs through interception of α-amino radical by oxidative addition complex (Ni-2) to generate a NiIII intermediate (Ni-3),5g,6 which may undergo an off-cycle equilibrium between iminium ion and Ni-4 intermediate (Figure 2D). While Ni-3 may undergo reductive elimination to give rise to the desired product, the iminium ion with β-H instead results in the decomposition of Ni-3, leading to the hydrodebromination and homodimerization of the aryl bromide. These phenomena are consistent with the stability of (R3P)2Ni(0)/iminium ion complexation reported by Pierpont and Barefield.17a
Figure 2.
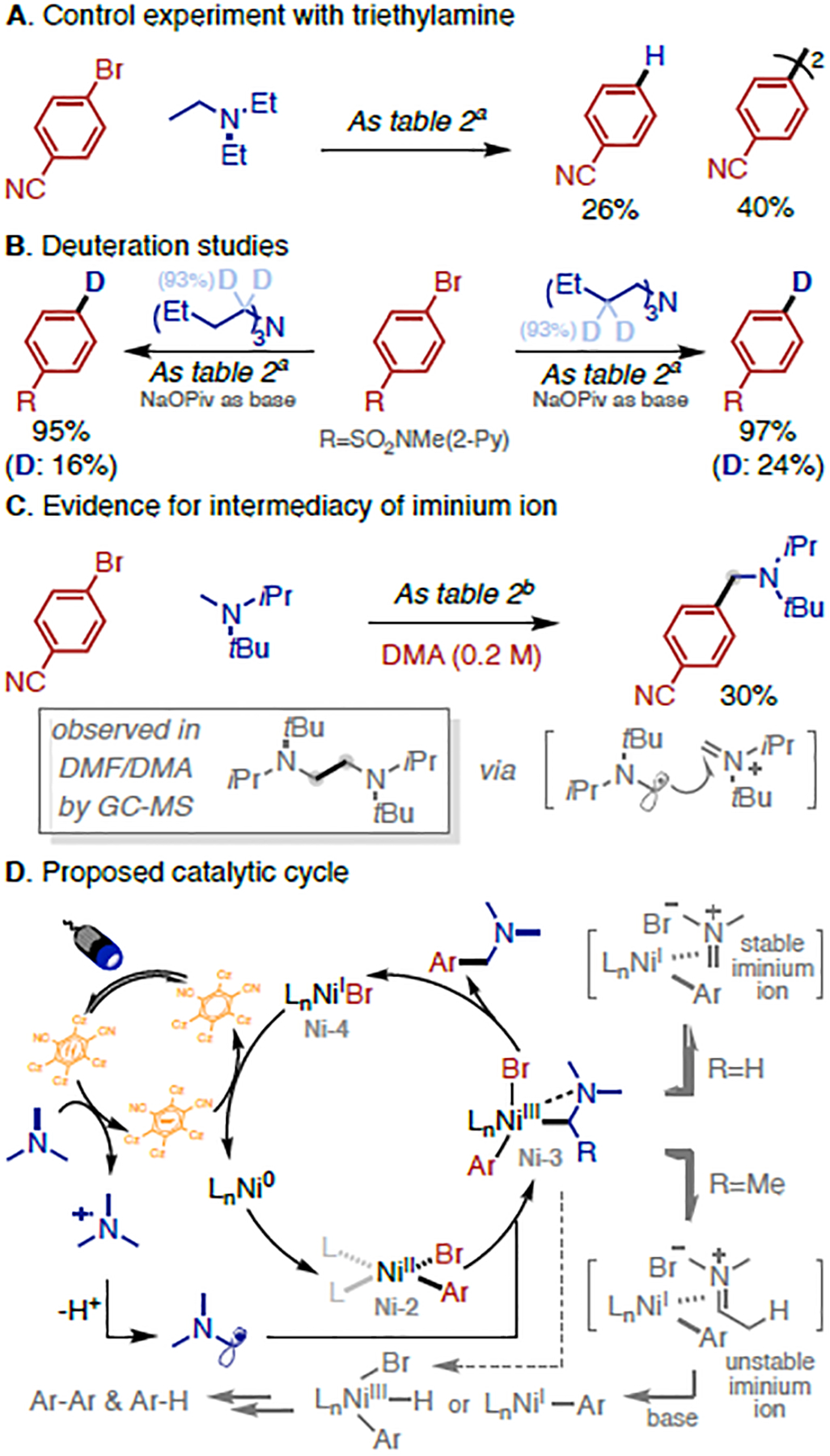
Mechanistic Studies.
Table 1.
Optimization and Control Studies
|
0.2 mmol scale; amine (2.0 equiv), homocoupling of ArBr accounts for mass balance. a GC-MS yield with ethyl benzoate as internal standard. b Isolated yield. c 24 h. d low conversion of ArBr. e no conversion of ArBr.
In summary, we have developed a late-stage arylation of trialkylamine-containing pharmaceuticals by employing Ni/photoredox dual catalytic platform under mild conditions. The reaction displays exclusive selectivity for N-Me C(sp3)-H bonds, not only streamlining the synthesis of benzyl dialkylamines, but also holding great promise to accelerate lead molecule discovery. Studies to enable arylation at higher α-substituted positions of trialkylamines are currently ongoing in our laboratory.
Supplementary Material
ACKNOWLEDGMENT
We thank NIGMS (GM125206) for support.
Footnotes
Reaction optimization, detailed experimental procedures and compound characterization. The Supporting Information is available free of charge on the ACS Publications website.
Funding Sources
The authors declare no competing financial interest.
REFERENCES
- (1).Blakemore DC; Castro L; Churcher I; Rees DC; Thomas AW; Wilson DM; Wood A Organic Synthesis Provides Opportunities to Transform Drug Discovery. Nature Chem. 2018, 10, 383–394. [DOI] [PubMed] [Google Scholar]
- (2).(a) Endoma-Arias MAA; Cox DP; Hudlicky T General Method of Synthesis for Naloxone, Naltrexone, Nalbuphone, and Nalbuphine by the Reaction of Grignard Reagents with an Oxazolidine Derived from Oxymorphone. Adv. Synth. Catal 2013, 355, 1869–1873; [Google Scholar]; (b) Roughley SD; Jordan AM The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem 2011, 54, 3451–3479. [DOI] [PubMed] [Google Scholar]
- (3).(a) He J; Hamann LG; Davies HML; Beckwith REJ Late-stage C–H Functionalization of Complex Alkaloids and Drug Molecules via Intermolecular Rhodium-Carbenoid Insertion. Nat. Commun 2015, 6, 5943; [DOI] [PubMed] [Google Scholar]; (b) Aycock RA; Pratt CJ; Jui NT Aminoalkyl Radicals as Powerful Intermediates for the Synthesis of Unnatural Amino Acids and Peptides. ACS Catal. 2018, 8, 9115–9119; [Google Scholar]; (c) Chan JZ; Chang Y; Wasa MB (C6F5)3-Catalyzed C–H Alkylation of N-Alkylamines Using Silicon Enolates without External Oxidant. Org. Lett 2019, 21, 984–988; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Matsuo BT; Correia JTM; Paixão MW Visible-Light-Mediated α-Amino Alkylation of Azomethine Imines: An Approach to N-(β-Aminoalkyl)Pyrazolidinones. Org. Lett 2020, 22, 7891–7896; [DOI] [PubMed] [Google Scholar]; (e) Barham JP; John MP; Murphy JA Contra-Thermodynamic Hydrogen Atom Abstraction in the Selective C–H Functionalization of Trialkylamine N-CH3 Groups. J. Am. Chem. Soc 2016, 138, 15482–15487; [DOI] [PubMed] [Google Scholar]; for a recent N-Me selective alkynylation, see:; (f) Chan JZ; Yesilcimen A; Cao M; Zhang Y; Zhang B; Wasa M Direct Conversion of N-Alkylamines to N-Propar-gylamines through C–H Activation Promoted by Lewis Acid/Organocopper Catalysis: Application to Late-Stage Functionalization of Bioactive Molecules. J. Am. Chem. Soc 2020, 142, 16493–16505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).For reviews on photoredox catalysis, see:; (a) Hopkinson MN; Sahoo B; Li J-L; Glorius F Dual Catalysis Sees the Light: Combining Photoredox with Organo-, Acid, and Transition-Metal Catalysis. Chem. Eur. J 2014, 20, 3874–3886; [DOI] [PubMed] [Google Scholar]; (b) Skubi KL; Blum TR; Yoon TP Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev 2016, 116, 10035–10074; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Levin MD; Kim S; Toste FD Photoredox Catalysis Unlocks Single-Electron Elementary Steps in Transition Metal Catalyzed Cross-Coupling. ACS Cent. Sci 2016, 2, 293–301; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Twilton J; Le C; Zhang P; Shaw MH; Evans RW; MacMillan DWC The Merger of Transition Metal and Photocatalysis. Nat. Rev. Chem 2017, 1, 0052; [Google Scholar]; (e) Marzo L; Pagire SK; Reiser O; König B Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed 2018, 57, 10034–10072; [DOI] [PubMed] [Google Scholar]; (f) Zidan M; Rohe S; McCallum T; Barriault L Recent Advances in Mono and Binuclear Gold Photoredox Catalysis. Catal. Sci. Technol 2018, 8, 6019–6028; [Google Scholar]; (g) Zhu C; Yue H; Chu L; Rueping M Recent Advances in Photoredox and Nickel Dual-Catalyzed Cascade Reactions: Pushing the Boundaries of Complexity. Chem. Sci 2020, 11, 4051–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).For the use of trialkylamines in metallophotoredox reactions, see:; (a) Revol G; McCallum T; Morin M; Gagosz F; Barriault L Photoredox Transformations with Dimeric Gold Complexes. Angew. Chem. Int. Ed 2013, 52, 13342–13345; [DOI] [PubMed] [Google Scholar]; (b) Shimomaki K; Murata K; Martin R; Iwasawa N Visible-Light-Driven Carboxylation of Aryl Halides by the Combined Use of Palladium and Photoredox Catalysts. J. Am. Chem. Soc 2017, 139, 9467–9470; [DOI] [PubMed] [Google Scholar]; (c) Murata K; Numasawa N; Shimomaki K; Takaya J; Iwasawa N Construction of a Visible Light-Driven Hydrocarboxylation Cycle of Alkenes by the Combined Use of Rh(I) and Photoredox Catalysts. Chem. Commun 2017, 53, 3098–3101; [DOI] [PubMed] [Google Scholar]; (d) Steiman TJ; Liu J; Mengiste A; Doyle AG Synthesis of β-Phenethylamines via Ni/Photoredox Cross-Electrophile Coupling of Aliphatic Aziridines and Aryl Iodides. J. Am. Chem. Soc 2020, 142, 7598–7605; [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Sun S; Duan Y; Mega RS; Somerville RJ; Martin R Site-Selective 1,2-Dicarbofunctionalization of Vinyl Boronates through Dual Catalysis. Angew. Chem. Int. Ed 2020, 59, 4370–4374; [DOI] [PubMed] [Google Scholar]; (f) Li J; Luo Y; Cheo HW; Lan Y; Wu J Photoredox-Catalysis-Modulated, Nickel-Catalyzed Divergent Difunctionalization of Ethylene. Chem 2019, 5, 192–203; [Google Scholar]; (g) Shaw MH; Shurtleff VW; Terrett JA; Cuthbertson JD; MacMillan DWC Native Functionality in Triple Catalytic Cross-Coupling: sp3 C–H Bonds as Latent Nucleophiles. Science 2016, 352, 1304–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Remeur C; Kelly CB; Patel NR; Molander GA Aminomethylation of Aryl Halides Using α-Silylamines Enabled by Ni/Photoredox Dual Catalysis. ACS Catal. 2017, 7, 6065–6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Lewis FD; Ho T-I Selectivity of Tertiary Amine Oxidations. J. Am. Chem. Soc 1980, 102, 1751–1752; [Google Scholar]; (b) Zhang X; Yeh S-R; Hong S; Freccero M; Albini A; Falvey DE; Mariano PS Dynamics of .alpha.-CH Deprotonation and .alpha.-Desilylation Reactions of Tertiary Amine Cation Radicals. J. Am. Chem. Soc 1994, 116, 4211–4220; [Google Scholar]; (c) Ruiz Espelt L; McPherson IS; Wiensch EM; Yoon TP Enantioselective Conjugate Additions of α-Amino Radicals via Cooperative Photoredox and Lewis Acid Catalysis. J. Am. Chem. Soc 2015, 137, 2452–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) Thullen SM; Rovis T A Mild Hydroaminoalkylation of Conjugated Dienes Using a Unified Cobalt and Photoredox Catalytic System. J. Am. Chem. Soc 2017, 139, 15504–15508; [DOI] [PubMed] [Google Scholar]; (b) Ashley MA; Yamauchi C; Chu JCK; Otsuka S; Yorimitsu H; Rovis T Photoredox-Catalyzed Site-Selective α-C(sp3)–H Alkylation of Primary Amine Derivatives. Angew. Chem. Int. Ed 2019, 58, 4002–4006; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shen Y; Funes-Ardoiz I; Schoenebeck F; Rovis T Site-selective α-C–H Functionalization of Trialkylamines via Reversible Hydrogen Atom Transfer Catalysis. ChemRxiv Preprint, April 16, 2021. DOI: 10.26434/chemrxiv.14442290.v1 (accessed 2021-04-19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Bolea I; Juárez-Jiménez J; de los Ríos C; Chioua M; Pouplana R; Luque FJ; Unzeta M; Marco-Contelles J; Samadi A Synthesis, Biological Evaluation, and Molecular Modeling of Donepezil and N -[(5-(Benzyloxy)-1-Methyl-1 H -Indol-2-Yl)Methyl]- N -Methylprop-2-Yn-1-Amine Hybrids as New Multipotent Cholinesterase/Monoamine Oxidase Inhibitors for the Treatment of Alzheimer’s Disease. J. Med. Chem 2011, 54, 8251–8270; [DOI] [PubMed] [Google Scholar]; (b) Mitchell D; Cole KP; Pollock PM; Coppert DM; Burkholder TP; Clayton JR Development and a Practical Synthesis of the JAK2 Inhibitor LY2784544. Org. Process Res. Dev 2012, 16, 70–81; [Google Scholar]; (c) Schaffner A-P; Sansilvestri-Morel P; Despaux N; Ruano E; Persigand T; Rupin A; Mennecier P; Vallez M-O; Raimbaud E; Desos P; Gloanec P Phosphinanes and Azaphosphinanes as Potent and Selective Inhibitors of Activated Thrombin-Activatable Fibrinolysis Inhibitor (TAFIa). J. Med. Chem 2021, 64, 3897–3910. [DOI] [PubMed] [Google Scholar]
- (10).For recent studies on benzyl dialkylamines synthesis, see:; (a) Heinz C; Lutz JP; Simmons EM; Miller MM; Ewing WR; Doyle AG Ni-Catalyzed Carbon–Carbon Bond-Forming Reductive Amination. J. Am. Chem. Soc 2018, 140, 2292–2300; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kumar R; Flodén NJ; White-hurst WG; Gaunt MJ A General Carbonyl Alkylative Amination for Tertiary Amine Synthesis. Nature 2020, 581, 415–420; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shi S; Qiu W; Miao P; Li R; Lin X; Sun Z Three-Component Radical Homo Mannich Reaction. Nat. Commun 2021, 12, 1006; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Liu RY; Buchwald SL CuH-Catalyzed Olefin Functionalization: From Hydroamination to Carbonyl Addition. Acc. Chem. Res 2020, 53, 1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For α-arylation of simple trialkylamines involving α-amino radical, see:; (a) Yoshikai N; Mieczkowski A; Matsumoto A; Ilies L; Nakamura E Iron-Catalyzed C–C Bond Formation at α-Position of Aliphatic amines via C–H Bond Activation through 1,5-Hydrogen Transfer. J. Am. Chem. Soc 2010, 132, 5568–5569; [DOI] [PubMed] [Google Scholar]; (b) Douglas JJ; Cole KP; Stephenson CR Photoredox Catalysis in a Complex Pharmaceutical Setting: Toward the Preparation of JAK2 Inhibitor LY2784544. J. Org. Chem 2014, 79, 11631–11643; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Singh A; Arora A; Weaver JD Photoredox-Mediated C–H Functionalization and Coupling of Tertiary Aliphatic Amines with 2-Chloroazoles. Org. Lett 2013, 15, 5390–5393; [DOI] [PubMed] [Google Scholar]; (d) Ikeda Y; Ueno R; Akai Y; Shirakawa E α-Arylation of Alkylamines with Sulfonylarenes through a Radical Chain Mechanism. Chem. Commun 2018, 54, 10417–10474; [DOI] [PubMed] [Google Scholar]; (e) Ide T; Barham JP; Fujita M; Kawato Y; Egami H; Hamashima Y Regio- and Chemoselective Csp3–H Arylation of Benzylamines by Single Electron Transfer/Hydrogen Atom Transfer Synergistic Catalysis. Chem. Sci 2018, 9, 8453–8460; [DOI] [PMC free article] [PubMed] [Google Scholar]; For α-arylation of tertiary anilines involving α-amino radical, see:; (f) Zuo Z; Ahneman DT; Chu L; Terrett JA, Doyle AG; MacMillan DWC Merging Photoredox With Nickel Catalysis: Coupling of α-Carboxyl sp3-Carbons with Aryl Halides. Science 2014, 345, 437–440; [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Prier CK; MacMillan DWC Amine α-Heteroarylation via Photoredox Catalysis: a Homolytic Aromatic Substitution Pathway. Chem. Sci 2014, 5, 4173–4178; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ahneman DT; Doyle AG C–H Funcationalization of Amines with Aryl Halides by Nickel-Photoredox Catalysis. Chem. Sci 2016, 7, 7002–7006; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Walker MM, Koronkiewicz B, Chen S, Houk KN, Mayer JM, Ellman JA Highly Diastereoselective Functionalization of Piperidines by Photoredox-Catalyzed α-Amino C–H Arylation and Epimerization. J. Am. Chem. Soc 2020, 142, 8194–8202; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Bergamaschi E; Weike C; Mayerhofer VJ; Funes-Ardoiz I; Teskey CJ Dual Photoredox/Cobaloxime Catalysis for Cross-Dehydrogenative α-Heterarylatio of Amines. Org. Lett 2021, 23, 5378–5382. [DOI] [PubMed] [Google Scholar]
- (12).For review on organic photoredox catalysis, see:; (a) Romero NA; Nicewicz DA Organic Photoredox Catalysis. Chem. Rev 2016, 116, 10075–10166; [DOI] [PubMed] [Google Scholar]; (b) Bryden MA; Zysman-Colman E Organic Thermally Activated Delayed Fluorescence (TADF) Compounds Used in Photocatalysis. Chem. Soc. Rev 2021, 50, 7587–7680. [DOI] [PubMed] [Google Scholar]
- (13).Luo J; Zhang J Donor–Acceptor Fluorophores for Visible-Light-Promoted Organic Synthesis: Photoredox/Ni Dual Catalytic C(sp3)–C(sp2) Cross-Coupling. ACS Catal. 2016, 6, 873–877. [Google Scholar]
- (14).(a) Sumerin V; Schulz F; Atsumi M; Wang C; Nieger M; Leskelä M; Repo T; Pyykkö P; Rieger B Molecular Tweezers for Hydrogen: Synthesis, Characterization, and Reactivity. J. Am. Chem. Soc 2008, 130, 14117–14119; [DOI] [PubMed] [Google Scholar]; (b) Yamashita H; Kawaguchi AW; Ohkatsu Y New Antagonism of Hindered Amine Light Stabilizers with Acidic Compounds Including Phenolic Antioxidants (Part 2) Formation Mechanism of Active Species of Peroxide Decomposition Reaction. J. Jpn. Petrol. Inst 2006, 49, 294–300; [Google Scholar]; (c) Hodgson JL; Coote ML Clarifying the Mechanism of the Denisov Cycle: How Do Hindered Amine Light Stabilizers Protect Polymer Coatings from Photo-Oxidative Degradation? Macromolecules 2010, 43, 4573–4583. [Google Scholar]
- (15).(a) Wang X; Wang S; Xue W; Gong H Nickel-Catalyzed Reductive Coupling of Aryl Bromides with Tertiary Alkyl Halides. J. Am. Chem. Soc 2015, 137, 11562–11565; [DOI] [PubMed] [Google Scholar]; (b) Primer DN; Molander GA Enabling the Cross-Coupling of Tertiary Organoboron Nucleophiles through Radical-Mediated Alkyl Transfer. J. Am. Chem. Soc 2017, 139, 9847–9850; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Green SA; Vásquez-Céspedes S; Shenvi RA Iron–Nickel Dual-Catalysis: A New Engine for Olefin Functionalization and the Formation of Quaternary Centers. J. Am. Chem. Soc 2018, 140, 11317–11324; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chen T; Zhang H; Mykhailiuk PK; Merchant RR; Smith CA; Qin T; Baran PS Quaternary Centers by Nickel-Catalyzed Cross-Coupling of Tertiary Carboxylic Acids and (Hetero)Aryl Zinc Reagents. Angew. Chem. Int. Ed 2019, 58, 2454–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Moir M; Danon JJ; Reekie TA; Kassiou M An Overview of Late-stage Functionalization in Today’s Drug Discovery. Expert Opinion on Drug Discovery 2019, 14, 1137–1149; [DOI] [PubMed] [Google Scholar]; (b) Börgel J; Ritter T Late-stage Functionalization. Chem 2020, 6, 1877–1887; [Google Scholar]; (c) Cannalire R; Pelliccia S; Sancineto L; Novellino E; Tron GC; Giustiniano M Visible Light Photocatalysis in the Late-stage Functionalization of Pharmaceutically Relevant Compounds. Chem. Soc. Rev 2021, 50, 766–897; [DOI] [PubMed] [Google Scholar]; (d) Guillemard L; Kaplaneris N; Ackermann L; Johansson MJ Late-stage C-H Functionalization Offers New Opportunities in Drug Discovery. Nat. Rev. Chem 2021, 5, 522–545. [DOI] [PubMed] [Google Scholar]
- (17).(a) Sepelak DJ; Pierpont CG; Barefield EK; Budz JT; Poffenberger CA Organometallic Chemistry of the Carbon-Nitrogen Double Bond. 1. Nickel Complexes Prepared from Iminium Cations and the x-Ray Structure of {[(C6H5)3P]Ni[CH2N(CH3)2]Cl}. J. Am. Chem. Soc 1976, 98, 6178–6185; [Google Scholar]; (b) Sylvester KT; Wu K; Doyle AG Mechanistic Investigation of the Nickel-Catalyzed Suzuki Reaction of N,O-Acetals: Evidence for Boronic Acid Assisted Oxidative Addition and an Iminium Activation Pathway. J. Am. Chem. Soc 2012, 134, 16967–16970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


