Abstract
Objective:
HIV-infection increases the risk to progress to active-tuberculosis (TB). Detection of latent TB infection (LTBI) is needed to eventually propose preventive-therapy and reduce TB reservoir. QuantiFERON-TB Plus (QFT-Plus)-test identifies LTBI. Currently, only two studies on QFT-Plus accuracy in HIV-infected-population are available in high TB-endemic-countries. Therefore we aimed to evaluate the effect of HIV-infection on QFT-Plus accuracy to detect LTBI in a low TB-endemic-country.
Methods:
We enrolled 465 participants, among the 167 HIV-infected-persons: 32 with active-TB (HIV-TB), 45 remote-LTBI (HIV-LTBI) and 90 at low M. tuberculosis (Mtb)-infection risk. Among the 298 HIV-uninfected-persons: 170 with active-TB, 76 recent-LTBI, 34 remote-LTBI and 18 with low Mtb-infection risk.
Results:
QFT-Plus sensitivity was similar in TB regardless of HIV-status. CD4-count did not influence the distribution of IFN-γ values in HIV-TB and HIV-LTBI. Moreover HIV-LTBI and HIV-uninfected remote LTBI had a similar proportion of results in the uncertain range (IFNγ ≥0.2 ≤ 0.7 IU/ml) differently from those LTBI-persons reporting recent-exposure (p = 0.016). Cytometry results demonstrated that CD8-response was similar in HIV-infected- and -uninfected-persons whereas CD4-response was impaired in HIV-infected-persons (p = 0.011).
Conclusions:
HIV-infection does not affect QFT-Plus response in active-TB, whereas the time of exposure influences the proportion of uncertain-results in LTBI.
Keywords: Latent tuberculosis, HIV, IGRA, QuantiFERON-TB Gold Plus, M.tuberculosis
Introduction
It is estimated that one fourth of world population is infected with M. tuberculosis (Mtb) 1–5 and that only the 5–10% of Mtb-infected individuals will progress to tuberculosis (TB) disease during life time.2,5 In 2017, 1 million (9%) of the worldwide TB cases were related to HIV-infected persons leading to 300,000 deaths.5 HIV-infection is responsible of an increased risk of progression to active disease, representing one of the main cause for active-TB disease, even in the absence of a deep CD4 T-cell impairment and even if under antiretroviral therapy (ART).3,6,7 Latent TB infection (LTBI) should be investigated in each HIV-infected person and the preventive TB treatment should be eventually offered.5,8 T-cells from individuals that have been infected with Mtb will release interferon-gamma (IFN-γ) when stimulated with Mtb antigens and the majority of these Mtb specific T-cells represent a recall of the memory response.9–12 The Interferon-γ release assays (IGRAs) used to diagnose LTBI measure the immune reactivity to Mtb antigens. Recently, a new IGRA, the QuantiFERON-TB Gold Plus (QFT-Plus)13–20 has been proposed. Compared to the previous version, it includes an additional tube (TB2) containing peptides stimulating both CD4+ and CD8+ T-cells.15,20 Several studies on the CD8 Mtb-specific response have inspired the development of the QFT-Plus.12,21–26 Recently, it has been also demonstrated that TB1- and TB2- antigens induce IP-10, suggesting it as an additional LTBI biomarker.27,28 The literature comparing QFT-Plus with the QFT-gold in tube (QFT-GIT) reports a high agreement in LTBI detection.29–34 Only few studies in high TB endemic countries are available on the QFT-Plus accuracy in HIV-infected individuals.35,36 Therefore, aim of the present study is to evaluate the impact of HIV-infection on the accuracy of QFT-Plus in patients with active-TB and LTBI in a low TB endemic country. According to the manufacturer’s instructions, the QFT results interpretation is based on a cut-off of 0.35 IU/mL.20 Studies on the healthcare workers demonstrated that results falling near the cut-off have a high conversion or reversion rate.37–42 These results fall in a zone of uncertainty ranging between 0.20- and 0.70 IU/Ml.37,38,40,43 Recently it has been reported that LTBI patients with immune-mediated inflammatory diseases have a low IFN-γ response to QFT-P and a high proportion of results ranging in the grey zone. Moreover, a study conducted in a low TB endemic country such as Netherland, demonstrated an association between the results falling in the gray-zone range and relevant risk factors and/or evidence of Mtb infection.44 The same authors reported also a case of a pregnant woman, with a QFT result falling in the negative uncertainty range before starting a tumor necrosis gactor (TNF) inhibitor (infliximab) therapy that developed active TB after starting it. According to the authors, a borderline QFT result in individuals with higher risk to develop active TB, should be considered as a TB-specific response that justifies the TB preventive therapy.45 In a high TB incidence country the TB preventive therapy is not offered to all LTBI individuals, with the exception of persons living with HIV (PLWH). PLWH have a higher risk to develop active TB disease, therefore in this vulnerable population it is crucial to diagnose LTBI and offer TB preventive therapy in both high and low TB endemic country.8 For this reason, as secondary aim, we investigated the distribution of QFT-P results according to the uncertain range in the fragile population of PLWH. Finally, for the first time to our knowledge, we aimed to characterize by cytometry in PLWH the response to QFT-P assay to specifically measure the CD4+ and CD8+ T-cell response comparing it to individuals without HIV infection.
Methods
Population characteristics and study design
This study was approved by the Ethical Committee of L. Spallanzani National Institute of Infectious Diseases (INMI) in Rome, (approval number 72/2015) and Villa Marelli Institute, Niguarda Ca’ Granda Hospital in Milan (approval number 110–022019). Written informed consent was obtained. Research was performed following the STROBE-statement guidelines for observational studies.46,47 The study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).48 Patients were prospectively enrolled (April 2015/January 2019).
International guidelines for the management of HIV infection recommend to perform the screening for LTBI in those with recent HIV diagnosis infection.2,3,6 Therefore, we enrolled PLWH already in ART that performed the screening for TB after starting the antiviral therapy or ART-naïve undergoing LTBI screening as routine test after newly diagnosed with HIV infection.
Among the HIV-uninfected individuals, we enrolled individuals undergoing screening because contacts of active TB patients defined as “recent LTBI”, or we enrolled the individuals known to have had LTBI and never underwent to TB preventive therapy defined as “remote LTBI”.
PLWH with low risk of TB infection performed only QFT-plus as routine LTBI screening. For all the other individuals enrolled with or without HIV infection, we performed the QFT-Plus assay in our laboratory for research purposes and we used our generated results for the present study. Therefore, patients were prospectively enrolled either those included for research purposes, or for clinical routine purposes as the PLWH newly diagnosed or the HIV-uninfected recent contacts that underwent routine LTBI screening. Among them, we identified those with LTBI based on the positivity to QFT-plus.
Microbiological-confirmed TB cases were based on a positivity to at least one of these tests: Mtb culture (sputum, broncholavage, pleural fluid, abscesses); Mtb-specific RNA amplification (TRCReady M.TB, Tosoh, Japan) and/or Mtb -specific NAT (Home-made PCR (IS6110) GeneXpert, Cepheid; Genotype MTBDRPlus Hain Life-science); histo-pathological findings and presence of acid fast bacilli in tissues. Clinical TB diagnosis was based on clinical and radiologic criteria, including appropriate response to TB-specific therapy. TB patients were enrolled no later than the first week of TB-treatment. In the absence of clinical, microbiological and radiological signs of active-TB, LTBI definition was based on a positive QFT-Plus (Qiagen, Hilden, Germany) score. LTBI individuals were enrolled before starting preventive-therapy. Mtb-exposure was classified as recent for individuals reporting a contact with a smear positive active-TB patient no more than 3 months before. Individuals not reporting contacts with active-TB patients in the 3 years before the screening and with unknown TB contact, were defined as remotely exposed. As control we enrolled HIV-infected and–uninfected individuals with low risk of Mtb-exposure; LTBI was excluded on the basis of IGRA results and absence of clinical and radiological signs of active-TB. The low TB risk individuals were persons not reporting contact with an active TB patient. To note that in the study we have included 106 LTBI,14 18 healthy donors and 66 active-TB patients, already described in previous reports.14,17
QFT-Plus assay
QFT-Plus kits were donated by Qiagen and used according to manufacturer’s instructions. Levels of IFN-γ were quantified by ELISA and analyzed by a QFT-Plus Analysis Software.20
Intracellular staining assay
Intracellular staining was performed, concomitantly to QFT-Plus, in 13 HIV-TB, 12 HIV-LTBI, 12 TB, 5 remote LTBI and 5 recent LTBI. As reported,15 we isolated peripheral blood mononuclear cells (PBMC) and calculated frequencies of CD4 and CD8 T-cells producing IFN-γ, TNF-α and IL-2, acquiring at least 200,000 lymphocytes at FACS CANTO II (BD, Bioscences). Cytometry data were analyzed by FloJo software. Cytokine background found in the Nil tube was subtracted from each condition. We assigned a negative score when the background was higher than half of antigen-specific response. A frequency of cytokine producing T-cells of at least 0.03% was considered as positive response. Data analysis has been concomitantly performed in blind by EP and TC. Concordance of the analyses was 90% and agreement was achieved by discussion.
Statistical analysis
For quantitative measures median and interquartile ranges (IQRs) were reported and Kruskall-Wallis or Mann-Whitney U test were used for comparison across groups. Chi square or Fisher exact test was used for comparison among categorical variables. Logistic regression models were used to evaluate association among QFT-Plus results and diagnosis, odds ratio (OR) their confidence intervals (CI) and p-value were reported. In the HIV-positive subgroup, we used Spearman’s correlation to evaluate the relationship between antigen-specific response to TB1 and TB2 as well as between each antigen-specific response and CD4 cells/mm.3
Data analysis was performed using STATA (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) and Prism 6 (Graphpad Software 6.0, San Diego, USA).
Results
Population characteristics
We enrolled 465 participants: 167 HIV-infected and 298 HIV-uninfected. Among the HIV-infected individuals, 32 had active-TB (HIV-TB), 45 had LTBI (HIV-LTBI) and 90 had a low risk of Mtb infection (HIV-low TB risk). Among the HIV-uninfected individuals, 170 had active-TB disease (TB), 76 were recent LTBI (recent LTBI), 34 were remote LTBI (remote LTBI) and 18 were individuals with low risk of Mtb infection (low TB risk) (Table 1).
Table 1.
Demographic, epidemiological and clinical features of the enrolled patients.
| HIV-infected patients |
HIV-uninfected patients |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HIV-TB | HIV-LTBI | HIV-Low TB risk | TOTAL | P | TB | LTBI recent | LTBI remote | Low TB risk | TOTAL | P | |
|
| |||||||||||
| N | 32 | 45 | 90 | 167 | 170 | 76 | 34 | 18 | 298 | ||
| Sex male, N (%) | 24 (75.0) | 39 (86.7) | 74 (82.2) | 137 (82.0) | 0.448* | 100 (58.8) | 37 (48.7) | 14 (41.2) | 8 (44.4) | 159 (53.4) | #0.148 |
| Age, median (IQR) | 40 (32–51) | 36 (27–41) | 37 (29–49) | 37 (28–48) | 0.185§ | 37 (29–47) | 39(25–54) | 44 (32–56) | 39 (33–46) | 38 (28–49) | §0.461 |
| BCG-vaccinated, N (%) | 23 (71.9) | 35 (77.8) | 19 (21.1) | 77 (46.1) | <0.001* | 116 (68.2) | 38 (50.0) | 15 (44.1) | 1 (5.5) | 170 (57.0) | #<0.001 |
| TB diagnosis, N (%) | |||||||||||
| Microbiological confirmation | 25 (78) | - | - | - | - | 132 (78) | - | - | - | - | |
| Clinical diagnosis | 5(16) | - | - | - | - | 38 (22) | - | - | - | - | |
| ART, N (%) | 18 (56.2) | 28 (62.2) | 27 (30.0) | 73 (43.7) | <0.001* | - | - | - | - | - | |
| HIV RNA loglO copies/ml, median (IQR) | 4.91 | 1.84 | 4.65 | 4.47 | <0.001* | - | - | - | - | - | |
| (3.60–5.41) | (1.59–4.29) | (3.92–5.26) | (2.22–5.18) | ||||||||
| CD4 /mm3, Median (IQR) + | 124 (52–279) | 626 | 418 | 417 | <0.001* | - | - | - | - | - | |
| (418–860) | (253 −631) | (208–646) | |||||||||
| Origin, N (%) | <0.001* | *<0.001 | |||||||||
| West Europe | 9 (28.1) | 10 (22.2) | 71 (78.9) | 90 (53.9) | 55 (32.3) | 39 (51.3) | 19 (55.9) | 18 (100) | 131 (44.0) | ||
| East Europe | 7 (21.9) | 6 (13.3) | 7 (7.8) | 20 (12.0) | 65 (38.2) | 21 (27.6) | 8 (23.5) | 0 | 94 (31.5) | ||
| Asia | 1 (3.1) | 2 (4.4) | 0 | 3 (1.8) | 23 (13.5) | 8 (10.5) | 2 (5.9) | 0 | 33 (11.1) | ||
| Africa | 8 (25.0) | 7 (15.6) | 4 (4.4) | 19 (11.4) | 14 (8.4) | 5 (6.7) | 5 (14.7) | 0 | 24 (8.0) | ||
| South America | 7 (21.9) | 20 (44.4) | 8 (8.9) | 35 (21.0) | 13 (7.6) | 3 (3.9) | 0(0) | 0 | 16 (5.4) | ||
TB: tuberculosis; BCG: Bacillus Calmette et Guérin; N: number; IQR: interquartile range.
Kruskal Wallis.
Fisher test.
Chi Square test.
CD4 count is not available for 5 patients (1 HIV-TB, 3 HIV-LTBI, 1 HIV-Low TB risk).
Among the PLWH, the majority were from Western Europe (54%) and male (82%). We found significant differences regarding the Bacillus Calmette et Guerin (BCG) vaccination status (p < 0.001) and the origin (p < 0.001); to note that majority of HIV-TB and HIV-LTBI subjects came from high endemic TB countries where the vaccination is mandatory at the birth. We found significant differences also comparing the ART intake, the number of HIV-RNA copies and the CD4-count (Table 1, supplementary Table S1). Among the HIV-uninfected individuals, the majority were from Western Europe (44%), 53.4% were male; we observed significant differences regarding the BCG-vaccination and the origin (Table 1).
Sensitivity of the QFT-Plus assay in active-TB patients
Sensitivity of the QFT-Plus, which is a routine test for LTBI, was evaluated in active-TB as a surrogate of LTBI because it is not possible to isolate Mtb in LTBI individuals. Sensitivity for TB was 80% and it was not significantly different from that found in HIV-TB patients either including or not the indeterminate results (68.7% or 73%). As expected, we found a higher number of indeterminate results in the HIV-infected patients compared to HIV-uninfected (p = 0.024) (Table 2). Response to TB1 or TB2 did not significantly differ between HIV-TB and TB patients (p = 0.158 and p = 0.216 respectively). Performing a two-sample test to compare the proportion of the total TB1 response with the total TB2 response in each group, we did not observe significant differences (HIV-TB: p = 0.6; TB: p = 0.4; data no shown), therefore the tubes had a similar sensitivity both in HIV-infected and –uninfected TB patients.
Table 2.
QFT-Plus results in patients with active-TB with and without HIV-infection.
| QFT-Plus Results | HIV-TB (N = 32) N (%) | TB (N = 170) N (%) | TB vs HIV-TB OR (95% CI) | P | |
|---|---|---|---|---|---|
| Sensitivity of the test | TB1 or TB2 positive | 22 (68.7) | 136 (80.0) | 1.82 (0.79–4.20) | 0.161 |
| Indeterminate | 2 (6.2) | 0 (0) | NA | 0.024* | |
| Negative | 8 (25.0) | 34 (20.0) | 0.75 (0.31–1.81) | 0.524 | |
| Sensitivity of each test tube | Total TB1 | 20 (62.5) | 127 (74.7) | 1.77 (0.80–3.92) | 0.158 |
| Total TB2 | 22 (68.7) | 134 (78.8) | 1.69 (0.73–3.89) | 0.216 | |
| Type of response among QFT-Plus positive patients | TB1 and TB2 | 20 (90.9) | 125 (91.9) | 1.14 (0.23–5.51) | 0.874 |
| only TB | 0 (0) | 2 (1.5) | NA | 0.100* | |
| only TB2 | 2 (9.1) | 9 (6.6) | 0.71 (0.14–3.52) | 0.674 |
TB: tuberculosis; TB1: peptides of TB1 tube; TB2: peptides of TB2 tube; N: number; OR: Odds Ratio; CI: confidence Interval; NA: not available because one category has frequency = 0.
If OR was not available, we compared proportion using Fisher Test.
Stratifying the QFT-Plus results according to the ability to respond to both TB1 and TB2 (“TB1 and TB2”), only to TB1 (“only TB1”) or only to TB2 (“only TB2”) (Table 2), we found that the majority of patients simultaneously responded to both stimulations (HIV-TB 90.9, TB 91.9%), as reported in the HIV-uninfected individuals15,17 and we did not find significant differences between HIV-TB and TB.
HIV-infection does not have any impact on the distribution of results according to the “uncertain range” of QFT-Plus in active-TB patients
Positivity to the QFT-Plus assay is based on IFN-γ values ≥0.35 IU/mL.49 Several studies have highlighted the variability of the results falling close to the assay cut-off,37,50,51 identifying an “uncertain range” (0.2–0.7 IU/ml). To evaluate the distribution of the IFN-γ values in the “uncertain range”, we reported our results stratifying the results as: <0.2 IU/mL; ≥0.2 ≤ 0.34 IU/mL; ≥0.35≤0.7 IU/mL; >0.7 IU/mL (Table 3). Evaluating the QFT-Plus positive scores, we found that the majority of the positive responses were out of the “uncertain range” (>0.7 IU/mL) both in HIV-TB (TB1 81.8%; TB2 81.8%) and TB (TB1 83.1%; TB2 86.8%). Regrouping the data as “certain” and “uncertain” results, we found a similar number of “uncertain” and “certain” results in the HIV-infected individuals and in the HIV-uninfected individuals (Table 3). Similarly, performing the analysis of the data from active-TB patients scored negative to QFT-Plus, we found a higher number of results falling in the negative “certain range” in both HIV-infected and –uninfected groups (Table 3). Moreover, HIV-TB patients did not have a higher risk to have uncertain results vs certain results compared to active TB patients, as shown by the OR evaluation (Table 3).
Table 3.
Distribution of IFN-γ values in response to TB1 and TB2 of QFT-Plus assay in TB patients with and without HIV-infection according to the “uncertain zone range”.
| Subjects scored positive to QFT-Plus |
Subjects scored negative to QFT-Plus |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TB1- response |
TB2-response |
TB1- response |
TB2-response |
|||||||||
| Results in range IFN-γ IU/mL | HIV-TB TB (N = 22) N (%) | TB (N = 136) N (%) | p# | HIV-TB TB (N = 22) N (%) | TB (N = 136) N (%) | p# | HIV-TB TB (N = 8) N (%) | TB (N = 34) N (%) | p# | HIV-TB TB (N = 8) N (%) | TB (N = 34) N (%) | p# |
| Negative certain <0.2 | 0 (0) | 3 (2.2) | 1.000 | 0 (0) | 2 (1.5) | 1.000 | 7 (87.5) | 27 (79.4) | 1.000 | 6 (75.0) | 26 (76.5) | 1.000 |
| Negative uncertain 0.2–0.34 | 2 (9.1) | 6 (4.4) | 0.308 | 0 (0) | 0 (0) | NA | 1 (12.5) | 7 (20.6) | 2 (25.0) | 8 (23.5) | ||
| Positive uncertain 0.35–0.7 | 2 (9.1) | 14 (10.3) | 1.000 | 4 (18.2) | 16 (11.8) | 0.486 | - | - | - | - | ||
| Positive certain > 0.7 | 18 (81.8) | 113 (83.1) | 1.000 | 18 (81.8) | 118 (86.8) | 0.514 | - | - | - | - | ||
| Total certain <0.2 or > 0.7 | 18 (81.8) | 116 (85.3) | 0.748 | 18 (81.8) | 120(88.2) | 0.486 | - | - | - | - | ||
| Total uncertain 0.2–0.7 | 4 (18.2) | 20 (14.7) | 4 (18.2) | 16 (11.8) | - | - | - | - | ||||
| Risk of uncertain results vs certain for TB vs HIV-TB | OR (95% Cl) | P | OR (95% Cl) | P | OR (95% CI) | P | OR (95% CI) | P | ||||
| 0.77 (0.24–2.53) | 0.674 | 0.60 (0.18–2.00) | 0.405 | 1.81 (0.19–17.29) | 0.604 | 0.92 (0.15–5.50) | 0.930 | |||||
TB: tuberculosis; TB1: peptides of TB1 tube; TB2: peptides of TB2 tube; N: number; OR: Odds Ratio; CI: confidence Interval; NA: not available because one category has frequency = 0.
If OR was not available, we compared proportion using Fisher Test.
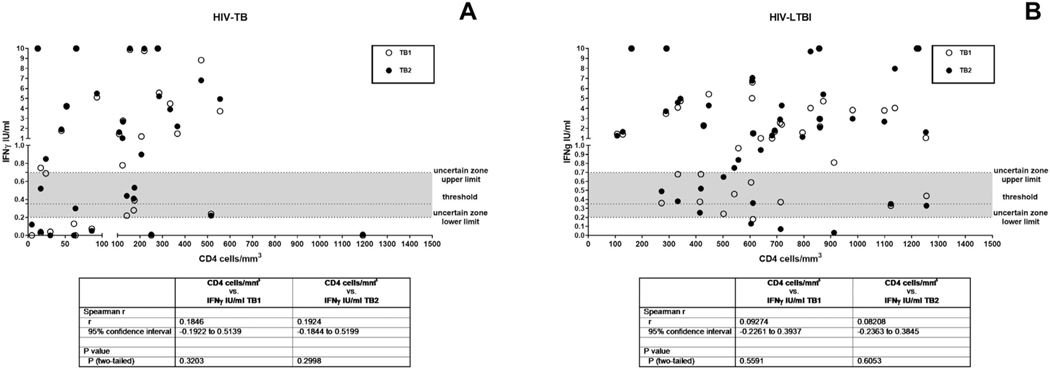
Based on previous studies demonstrating that CD4 counts have an impact on the IGRA response in HIV-infection,52–54 we analyzed the effect of immune suppression on QFT-Plus in HIV-TB. To verify the presence of a correlation between the IFNγ production expressed as International Unit (IU)/ml and the number of CD4 cells/mm3, we performed a Spearman correlation and we did not find any significant differences (Fig. 1A). Similarly, applying a univariable logistic regression, we did not find nor linear trend between the TB1- and TB2-response and CD4-count increment. (Supplementary Table S2), nor significant differences. Finally, we did not find any significant association neither between the TB1- or TB2-response and ART status (Supplementary Table S2).
Fig. 1. Correlation between the IFNγ production and the number of CD4 cells/mm.
3. Spearman correlation was performed. A) HIV-TB; B) HIV-LTBI. IFN: interferon; IU: international unit; white circles for TB1 stimulation; black circles for TB2 stimulation. CD4 count is not available for 4 individuals (1 HIV-TB and 3 HIV-LTBI).
QFT-Plus results in LTBI individuals
Evaluating the sensitivity to total TB1- and total TB2-stimulation, we found that both recent LTBI and remote LTBI individuals had a risk similar to HIV-LTBI to have a positive total TB1 or total TB2 response (Table 4). To understand the impact of HIV-infection on the QFT-Plus results in LTBI, we stratified the QFT-Plus results according to the ability of individuals to respond to both TB1 and TB2, only to TB1 or only to TB2 (Table 4). We found that recent LTBI individuals had a significant higher OR to have a TB1 and TB2 response compared to HIV-LTBI (OR: 4.5; p = 0.018), whereas no differences was observed in remote LTBI compared to HIV-LTBI.
Table 4.
QFT-Plus results in patients with LTBI with and without HIV-infection.
| QFT-Plus Results | HIV-LTBI (N = 45) N (%) |
LTBI recent (N = 76) |
LTBI remote (N = 34) |
|||||
|---|---|---|---|---|---|---|---|---|
| N (%) | vs HIV-LTBI OR (95% CI) | P | N (%) | vs HIV-LTBI OR (95% CI) | P | |||
| Sensitivity of each test tube | Total TB1 | 41 (91.1) | 75 (98.7) | 7.32 (0.79–67.65) | 0.079 | 30 (88.2) | 0.73 (0.16–3.16) | 0.676 |
| Total TB2 | 40 (88.9) | 73 (96.0) | 3.04 (0.69–13.39) | 0.141 | 33 (97.0) | 4.12 (0.45–37.08) | 0.206 | |
| Type of response among QFT-Plus | TB1 and TB2 | 36 (80.0) | 72 (94.7) | 4.5 (1.30–15.61) | 0.018 | 29 (85.3) | 1.45 (0.43–4.80) | 0.543 |
| only TB1 | 5 (11.1) | 3 (3.9) | 0.33 (0.07–1.15) | 0.141 | 1 (2.9) | 0.24 (0.03–2.18) | 0.206 | |
| only TB2 | 4 (8.9) | 1 (1.3) | 0.14 (0.01–1.26) | 0.079 | 4 (11.8) | 1.37 (0.32–5.91) | 0.676 | |
TB1: peptides of TB1 tube; TB2: peptides of TB2 tube; N: number; OR: Odds Ratio; CI: confidence Interval. LTBI subjects have been enrolled to be QFT-Plus positive, therefore it was not possible to calculate the sensitivity of the test.
Analyzing the distribution of the results according to the “uncertain range” (Table 5) we found that the majority of HIV-LTBI and remote LTBI individuals had values >0.7 IU/mL (HIV-LTBI: TB1-response 73.3% and TB2-respones 73.3%; remote LTBI: TB1-response 76.5% and TB2-response 88.2%). Differently, HIV-LTBI had a lower number of positive results >0.7 IU/mL compared to HIV-uninfected recent LTBI (TB1-response: HIV-LTBI: 73.3%, recent LTBI 93.4%, p = 0.005; TB2-response: HIV-LTBI 73.3%, recent LTBI 92.1%, p = 0.008). Regrouping the data as “certain” and “uncertain” results, we found a higher number of “uncertain” results in the HIV-infected individuals and a higher number of “certain” results in the HIV-uninfected individuals; however only comparing the recent LTBI with the HIV-LTBI individuals we observed significant differences (TB1 p = 0.010; TB2 p = 0.016). Consequently we found a lower significant risk to have uncertain results only for recent LTBI compared with HIV-LTBI (TB1: OR 0.22, p = 0.008, TB2: OR 0.22, p = 0.018).
Table 5.
Distribution of IFN-γ values in response to TB1 and TB2 of QFT-Plus assay in LTBI patients with and without HIV-infection according to the “uncertain zone range”.
| Results in range IFN-γ IU/mL | TB1-Positive resPonse |
TB2-positive response |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV-LTBI (N = 45) N (%) | LTBI Recent (N = 76) N (%) | HIV-LTBI vs LTBI recent#p | LTBI Remote (N = 34) N (%) | HIV-LTBI vs LTBI remote#p | HIV-LTBI (N = 45) N (%) | LTBI Recent (N = 76) N (%) | HIV-LTBI vs LTBI recent #p | LTBI Remote (N = 34) N (%) | HIV-LTBI vs LTBI remote #p | |
| Negative certain < 0.2 | 1 (2.2) | 0 (0) | 0.372 | 1 (2.9) | 0.100 | 3 (6.7) | 2 (2.6) | 0.359 | 1 (2.9) | 0.630 |
| Negative uncertain 0.2–0.34 | 3 (6.7) | 1 (1.3) | 0.144 | 3 (8.8) | 0.100 | 2 (4.4) | 1 (1.3) | 0.555 | 0 (0) | 0.503 |
| Positive uncertain 0.35–0.7 | 8 (17.8) | 4 (5.3) | 0.054 | 4 (11.8) | 0.540 | 7 (15.6) | 3 (3.9) | 0.038 | 3 (8.8) | 0.502 |
| Positive certain > 0.7 | 33 (73.3) | 71 (93.4) | 0.005 | 26 (76.5) | 0.799 | 33 (73.3) | 70 (92.1) | 0.008 | 30 (88.2) | 0.157 |
| Total certain <0.2 or > 0.7 | 34 (75.6) | 71 (93.4) | 0.010 | 27 (79.4) | 0.790 | 36 (80.0) | 72 (94.7) | 0.016 | 31 (91.2) | 0.216 |
| Total uncertain 0.2–0.7 | 11 (24.4) | 5 (6.6) | 7 (20.6) | 9 (20.0) | 4 (5.3) | 3 (8.8) | ||||
| Risk of uncertain results vs certain for LTBI vs HIV-LTBI | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| 0.22 | 0.008 | 1.24 | 0.686 | 0.22 | 0.018 | 0.39 | 0.181 | |||
| (0.07–0.68) | (0.42–3.65) | (0.06–0.77) | (0.09–1.56) | |||||||
TB: tuberculosis; TB1: peptides of TB1 tube; TB2: peptides of TB2 tube; N: number; OR: Odds Ratio; CI: confidence Interval.
Fisher test. LTBI subjects have been enrolled to be QFT-Plus positive.
Distribution of discordant QFT-Plus results
The proportion of discordant QFT-Plus results in HIV-infected and -uninfected population with active-TB and LTBI were not significantly different (Table 6). However, we observed that the HIV- LTBI had the highest proportion of discordant results, TB1 positive and TB2 negative or vice versa, (20%). Calculating the OR to have a discordant results, we found that HIV-uninfected recent LTBI had the lower probability to have discordant results compared to HIV- LTBI (OR 0.22, p = 0.018).
Table 6.
Distribution of discordant and concordant response to TB1 and TB2 stimulation according to the diagnosis among all subjects resulted QFT-Plus positive.
| Diagnosis | Concordant | Discordant | #p | OR for a discordant result among diagnosis |
|||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N | OR | 95% CI | p | ||
| HIV-LTBI remote | 36 (80.0) | 9 (20.0) | 45 | 0.081 | 1 | ||
| HIV-TB | 20 (90.9) | 2 (9.1) | 22 | 0.40 | 0.08–2.03 | 0.270 | |
| LTBI recent | 72 (97.7) | 4 (5.3) | 76 | 0.22 | 0.06–0.77 | 0.018 | |
| LTBI remote | 29 (85.3) | 5 (14.7) | 34 | 0.69 | 0.21–2.28 | 0.543 | |
| TB | 125 (91.9) | 11 (8.1) | 136 | 0.35 | 0.13–0.91 | 0.032 | |
| Total | 282 (90.1) | 31 (9.9) | 313 | 0.40 | 0.08–2.03 | 0.270 | |
LTBI: latent tuberculosis infection; TB: tuberculosis; TB1: peptides of TB1 tube; TB2: peptides of TB2 tube; N: number.
Fisher test; OR: Odds Ratio; CI: confidence interval.
Then, we analyzed the distribution of concordant and discordant results according to the “uncertain range”,37,50,51 (Table 7). We found that the majority or the half of discordant results fell in the “uncertain range” in all groups (HIV-TB: 100%, p = 0.043, TB: 81.8%, p < 0.001; HIV-LTBI: 88.9%, p < 0.001; recent LTBI: 50% p = 0.02; remote LTBI: 80% p = 0.007).
Table 7.
Distribution of discordant and concordant IFN-γ response to TB1 and TB2 stimulation according to the “uncertain” and “certain range”.
| HIV-TB* | TB | HIV-LTBI | LTBI recent | LTBI remote | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| TB1-TB2 results IFN-γ IU/mL | Concordant N(%) | Discordant N(%) | #p | Concordant N(%) | Discordant N(%) | #p | Concordant N(%) | Discordant N(%) | #p | Concordant N(%) | Discordant N(%) | #p | Concordant N(%) | Discordant N(%) | #p |
| §Certain <0.2 or >0.7 | 17 (85) | 0 (0) | 0.043 | 111 (88.8) | 2 (18.2) | <0.001 | 32 (88.9) | 1 (11.1) | < 0.001 | 69 (95.8) | 2 (50.0) | 0.020 | 25 (86.2) | 1 (20.0) | 0.007 |
| §§Uncertain 0.2–0.7 | 3 (15) | 2 (100) | 14 (11.2) | 9 (81.8) | 4 (11.1) | 8 (88.9) | 3 (4.2) | 2 (50.0) | 4 (13.8) | 4 (80.0) | |||||
| Total | 20 (100) | 2 (100) | 125 (100) | 11 (100) | 36 (100) | 9 (100) | 72 (100) | 4 (100) | 29 (100) | 5 (100) | |||||
LTBI:latent tuberculosis infection; TB: tuberculosis; N: number.
Fisher test.
if TB1 and TB2 results fall in the certain range.
if at least one result falls in the uncertain range.
indeterminate results have been excluded.
Time of Mtb exposure impacts the IFN-γ production in LTBI individuals
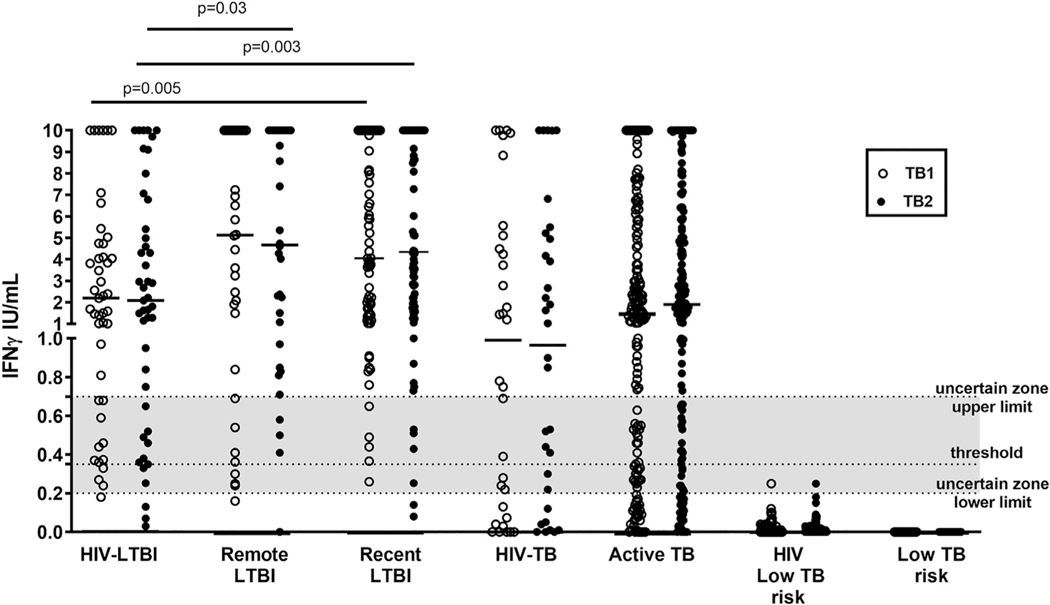
We evaluated the QFT-Plus results also by quantitative means (Fig. 2). We found that the HIV-LTBI individuals produce lower IFN-γ compared to LTBI, in response to TB1 (HIV-LTBI: median 2.2 IQR 0.7–4.7; remote LTBI: median 5.12, IQR 0.8025–10,recent LTBI: median 4.055, IQR 1.46–10 p = 0.005) and TB2 (HIV-LTBI: median 2.1 IQR 0.6–6.1; remote LTBI: median 4.675, IQR 0.94–10, p = 0.03; recent LTBI: median 4.345, IQR 1.757–10, p = 0.003). Differently in active-TB, HIV-infection did not affect the amount of IFN-γ response to TB1 (HIV-TB: median 1.0 IQR 0.09–4.9; TB: median 1.4 IQR 0.3–5; p = 0.3) or TB2 (HIV-TB: median 1.0 IQR 0.1–5.1; TB: median 1.9 IQR 0.5–5.9; p = 0.2).
Fig. 2. Quantitative IFN-γ response to stimulation with QFT-Plus antigen TB1 and TB2 in HIV-infected and –uninfected individuals with active-TB, LTBI and with low TB risk.
Horizontal lines indicate the median production. The data are presented as IU/mL. Footnotes: IFN: interferon; IU: international unit; white circles for TB1 stimulation; black circles for TB2 stimulation. The grey part of the graph represents the uncertain range of QFT-Plus results.
Comparing the IFN-γ level in response to TB1 and TB2 within the same group, we did not find significant differences. No response to QFT-Plus was found in the control groups with low risk of Mtb-exposure. Two HIV-infected individuals with a negative result falling in the uncertain zone (Fig. 2) were from Italy, therefore without TB-risk associated to the origin.
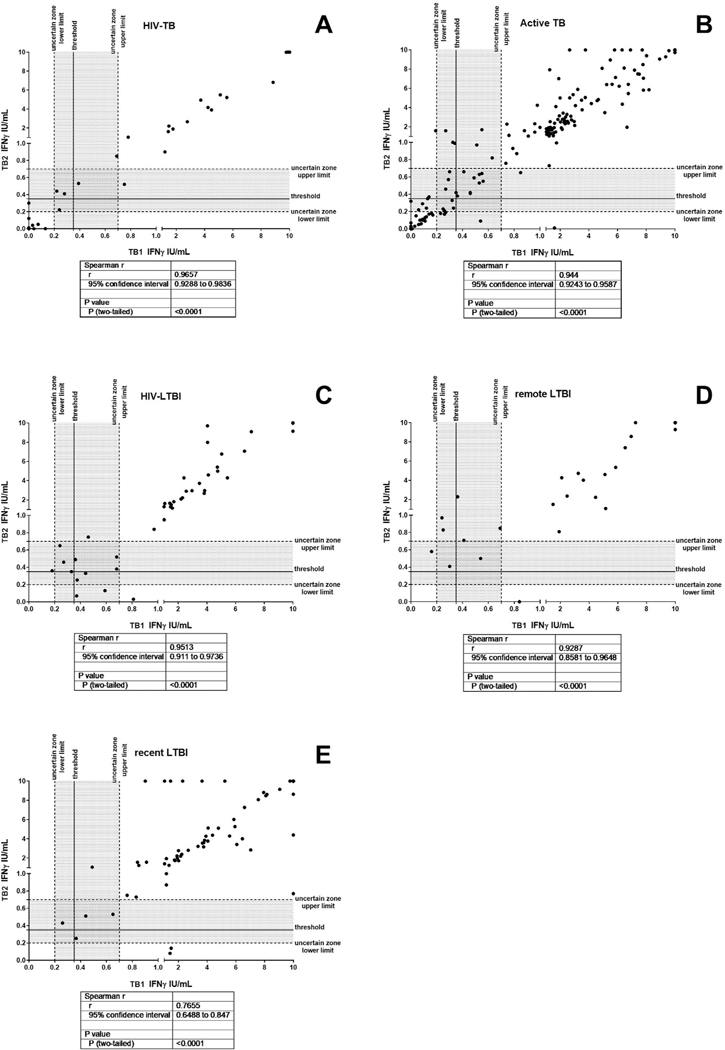
We also investigated the correlation between the amount of IFNγ produced in response to TB1 and/or TB2 stimulation (Fig. 3). Interestingly we found a positive and significant correlation in all groups between TB1 and TB2 IFNγ amount (HIV-TB r = 0.9657, p<0.0001; active TB: r = 0.944, p < 0.0001; HIV-LTBI: r = 0.9513, p<0.0001; remote LTBI: r = 0.9287, p < 0.0001, recent LTBI: r = 0.7655 p < 0.0001). To verify the presence of a correlation between the IFNγ production expressed as International Unit (IU)/ml and the number of CD4 cells/mm3, we performed a Spearman correlation and we did not find any significant differences (Fig. 1B).
Fig. 3.
Correlation between the IFNγ production in response to TB1 and TB2 stimulation, Spearman correlation was performed. A) HIV-TB; B) active TB; C) HIV-LTBI; D) remote LTBI; E) recent LTBI. Footnotes: IFN: interferon; IU: international unit; the grey part of the graph represents the uncertain range of QFT-Plus results.
Antigen-specific response within the CD4+ or CD8+ T-cells: proportion of QFT-Plus-responders by flow cytometry
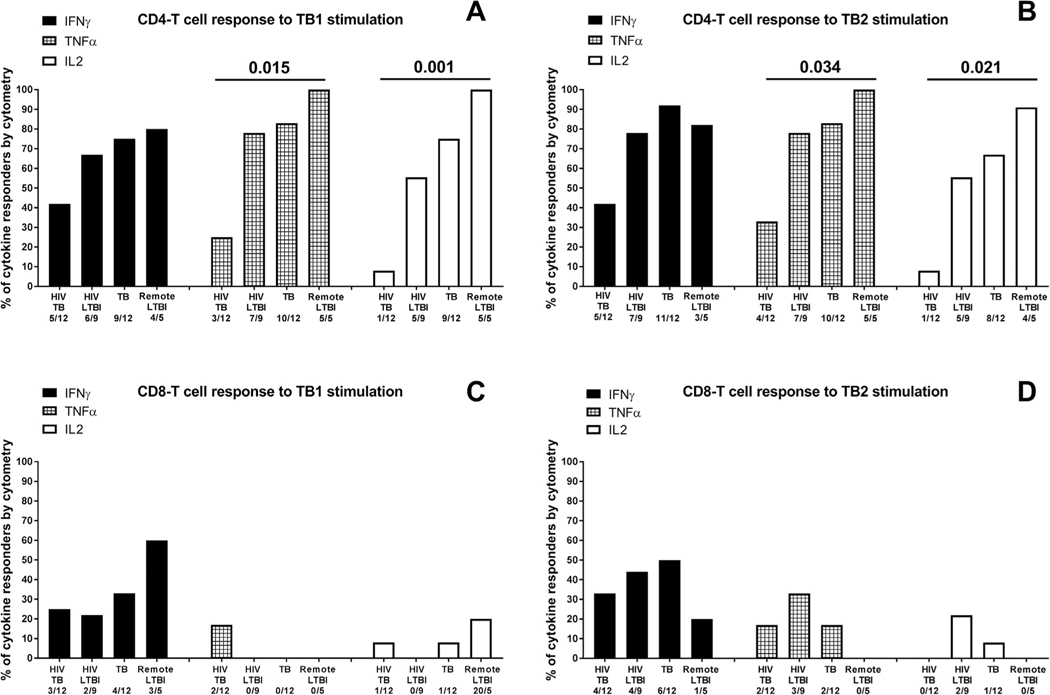
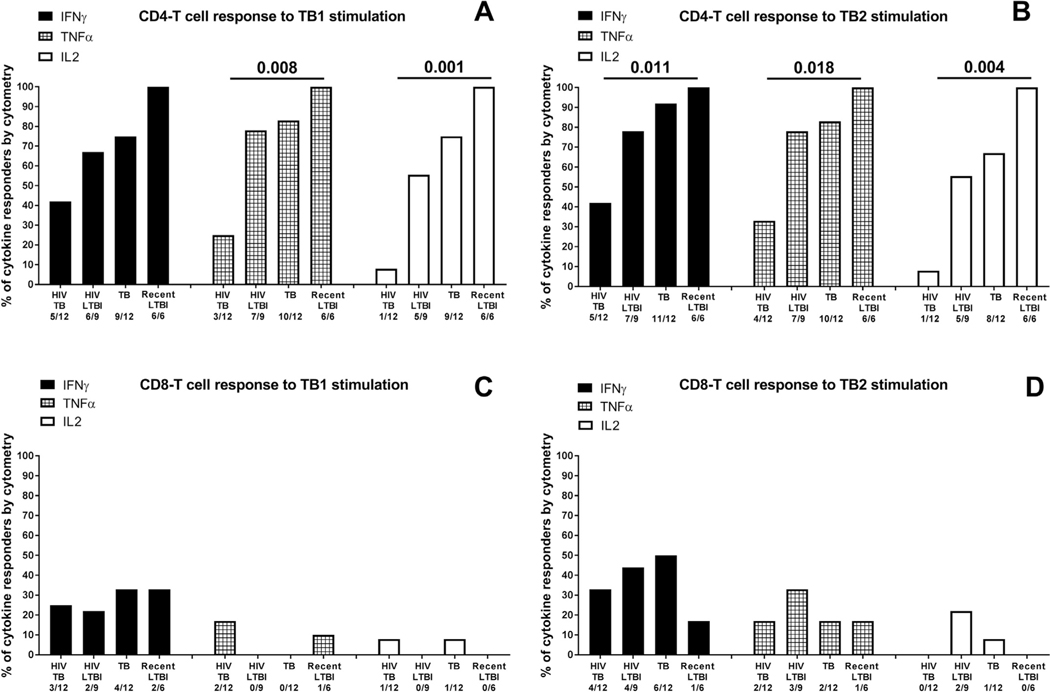
To characterize the antigen-specific response to TB1 or TB2 in a smaller cohort of patients, we evaluated by cytometry, the IFN-γ, TNF-α and IL-2 production in CD4+ and CD8+ T-cells (Figs. 4 and 5). We performed the analysis comparing the results of all HIV-infected individuals with the results of all HIV-uninfected individuals. Regarding the CD4+ T-cells, we found a higher proportion of IFN-γ, TNF-α and IL-2 responders to TB1 and TB2 in HIV-uninfected individuals compared to HIV-infected (comparison including remote LTBI TB1: TNFα p = 0.015, IL2 p = 0.001; TB2: TNFα p = 0.034, IL2 p = 0.021 (Fig. 4A,B); comparison including recent LTBI TB1: TNFα p = 0.008, IL2 p = 0.001 TB2: IFNγ p = 0.011, TNFα p = 0.018, IL2 p = 0.004 (Fig. 5A–B). Differently, the analysis of CD8+ T-cells response did not show any significant difference among the groups (Fig. 4C–D; 5C–D).
Fig. 4. Proportion of responders to TB1 and TB2 stimulation of the QFT-Plus test evaluated by flow cytometry.
Responders were defined based on IFN-γ or TNF-α or IL-2 production by CD4+ and CD8+ T-cells. A) CD4 T-cell response and CD8+ T-cell response to TB1 and TB2 stimulation considering only the remote LTBI in the in the HIV- uninfected LTBI group.The “x axis” reports the number of responders over total, for each group and for each cytokine. The statistical analysis has been performed comparing all the HIV-infected individuals with all the HIV-uninfected individuals using the Fisher exact test. The data are presented as proportion of responders for each cytokine. Footnotes: IFN: interferon; TNF: tumor necrosis factor; IL: interleukin.
Fig. 5. Proportion of responders to TB1 and TB2 stimulation of the QFT-Plus test evaluated by flow cytometry.
Responders were defined based on IFN-γ or TNF-α or IL-2 production by CD4+ and CD8+ T-cells. CD4 T-cell response and CD8+ T-cell response to TB1 and TB2 stimulation considering only the recent LTBI in the HIV-uninfected LTBI group The “x axis” reports the number of responders over total, for each group and for each cytokine. The statistical analysis has been performed comparing all the HIV-infected individuals with all the HIV-uninfected individuals using the Fisher exact test. The data are presented as proportion of responders for each cytokine. Footnotes: IFN: interferon; TNF: tumor necrosis factor; IL: interleukin.
Discussion
We evaluated the accuracy of QFT-Plus in HIV-infected and -uninfected individuals at different TB-stages in a low TB endemic country. We found that HIV-infection does not have an impact on the detection of positive results to QFT-Plus in active-TB. Evaluating the quantitative values, among the remote LTBI, we found similar results falling in the uncertain range independently of HIV infection. Conversely, in the HIV-uninfected recent LTBI individuals we found a significant lower number of results falling in the uncertain range. In this study the CD4-count did not influence the IFNγ (IU/ml) release. However, the cytometry results demonstrated that the CD4-response to QFT-Plus was impaired in HIV-infected- persons whereas the CD8-response was similar in HIV-infected-and -uninfected-persons. Collectively, these results suggest that the observed similar accuracy of QFT-Plus in HIV-infected and uninfected individuals is due to the CD8 compensation for the impaired CD4-response related to HIV infection.
The analysis of the QFT-Plus results demonstrated a similar sensitivity of the assay in HIV-TB and TB patients and a comparable ability to respond concomitantly to both TB1 and TB2, as shown in HIV-infected individuals in Zambia35 and in uninfected active-TB patients in Italy.17 Moreover, the “only TB2” and “only TB1” response was observed with a similar frequency both in HIV-infected and HIV-uninfected TB patients and this was confirmed by cytometry. A recent meta-analysis on QFT-Plus highlighted a higher sensitivity of TB2-stimulation compared to TB1, in HIV-uninfected active TB patients.34 In our study, we observed a higher but not significant sensitivity of TB2-stimulation compared to TB1, in active TB patients independently of HIV-infection. Moreover, the sensibility to TB2-stimulation was similar in HIV-infected and –uninfected active-TB patients. Based on this results, in the population studied, HIV-infection does not influence the TB1 or TB2 response to QFT-Plus in active-TB.
As previously reported,35 HIV-infection did not influence the magnitude of the response to TB1- and TB2- stimulation in HIV-TB patients. Although active-TB patients had a low CD4 T-count, the high Mtb-load associated with TB-disease induced a strong immune response similar to that found in the HIV-uninfected patients. Differently, HIV-infection affects the IFN-γ production in individuals with LTBI, as demonstrated in Ethiopia in HIV-infected pregnant women.36 In Zambia, it has been demonstrated a higher sensitivity of QFT-Plus compared to QFT-GT in HIV-TB adults.35 Interestingly, the authors reported also a higher, but not significant, sensitivity of the QFT-Plus compared to QFT-GIT, in individuals with ≤100 CD4+ T-cells/mm3. In our study the CD4 T-cell counts did not affect neither the number of QFT-Plus responders nor the amount of the IFNγ produced in the HIV-TB population. In line with previous results,35 we observed that the intake or not of ART did not affect the number of QFT-Plus responders.
Several reports discussed about the “true negative and positive values” of QFT-Plus.37,50,51 A study based on serial QFT evaluations,50 suggests that values less than 0.2 IU/mL, should be considered as “true negative values”, whereas if at least one value is within the “uncertain range” (0.2–07 IU/mL) the conversions are uncertain and the results are partially explained by technical assay variability. In our study the majority of TB patients, both HIV-infected and –uninfected, displayed positive results out of the “uncertain range”, as expected.17 The analysis in LTBI patients demonstrated that the determining factor for the distribution of IFNγ values out or in the uncertain range, is the time of exposure to Mtb. Indeed, the QFT-Plus results from the HIV-LTBI individuals, all classified as with a remote exposure, did not significantly differ in terms of number of “certain and uncertain” when compared to the results from remote LTBI HIV-uninfected. To note that the majority of the QFT-Plus results from HIV-LTBI, HIV-TB and remote LTBI HIV-uninfected individuals were “discordant results” falling inside the uncertain range; differently in recent LTBI only 50% of discordant results were within the uncertain range, indicating that recent TB contacts are associated with “certain results”. On the other side, considering that the majority of the HIV-LTBI individuals enrolled had, as main factor for TB risk, an origin from high TB endemic countries, it is unlikely that the discordant results were false positive scores.
We observed that HIV-LTBI and HIV-uninfected remote LTBI had a similar QFT-Plus response to TB1 or TB2, similar distribution of uncertain and discordant results, a positive correlation between IFNγ in response to TB1 and TB2 stimulation, and that the CD4 count did not influence the IFNγ production of HIV-LTBI. Collectively these data, although limited, may suggest that the HIV infection does not impact the LTBI screening performed by QFT-Plus. Moreover it may be important to investigate about the time of Mtb exposure as influential factor for the quantitative QFT-Plus response. Indeed, based on the clinical practice in low TB-endemic countries, it is reasonable to assume that the PLWH undergoing the routine LTBI screening have likely had an Mtb remote exposure rather than a recent one.
We characterized by cytometry the involvement of CD4+ and CD8+ T-cells in the QFT-Plus response and demonstrated that the HIV-infection affected the CD4+ T-cell response; differently the CD8+T-cell response was similar in HIV-infected and HIV-uninfected individuals. These data are particularly important to show that the role of TB2 stimulation in the assay was not impaired in PLWH. Probably, the CD8-specific response compensates for the CD4-response impairment related to HIV-infection, determining a similar sensitivity of QFT-Plus in HIV-infected and uninfected individuals.
In conclusion, we assessed the impact of HIV-infection on the accuracy of QFT-Plus in patients with active-TB and LTBI in a low TB endemic country. We showed a similar sensitivity in active TB in HIV-infected and -uninfected population, and that CD4 count did not influence the distribution of IFN-γ values in HIV-TB and HIV-LTBI patients. In those with remote LTBI, HIV infection did not have any impact on the quantitative QFT-Plus results falling in the “uncertain range”. Finally, the cytometry results demonstrated that HIV infection reduced the CD4+ T-cells response but did not impact the CD8+ T-cells response which likely compensates for the CD4-response impairment related to HIV-infection. Further studies are needed to confirm these results worldwide.
Supplementary Material
Acknowledgments
The authors are grateful to all the patients, nurses and physicians who helped to perform this study. Delia Goletti is professor of General Pathology at Saint Camillus International University of Health and Medical Sciences in Rome.
Funding
This work was supported by the Italian Ministry of Health “Ricerca Corrente”, the European Union (643381-TBVAC2020-H2020-PHC-2014-2015) and National Institutes of Health of USA (NIH 1R21AI127133-01). The funders had no role in the decision to publish the study, in analyzing the data or drafting the manuscript. The Qiagen company did not give any input into the interpretation of the data and the study was financed exclusively from institutional funds.
Footnotes
Declaration of Competing Interest
DG received consultant fees for public speaking in international meetings by Qiagen and Diasorin.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.02.009.
References
- 1.Cohen A, Mathiasen VD, Schon T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J 2019;54 2019. Print 2019 Sep. doi: 10.1183/13993003.00655. [DOI] [PubMed] [Google Scholar]
- 2.Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016;13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girardi E, Sabin CA, d’Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 2005;41:1772–82. [DOI] [PubMed] [Google Scholar]
- 4.Sotgiu G, Goletti D, Matteelli A. Global tuberculosis prevention: should we start from the beginning? Eur Respir J 2019:54 2019. Print 2019 Sep.. doi: 10.1183/13993003.01394. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Global tuberculosis report 2019:https://www.who.int/tb/publications/global_report/en/
- 6.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS 2009;23:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiberi S, Carvalho AC, Sulis G, Vaghela D, Rendon A, Mello FC, et al. The cursed duet today: tuberculosis and HIV-coinfection. Presse Med 2017;46:e23–39. [DOI] [PubMed] [Google Scholar]
- 8.Goletti D, Navarra A, Petruccioli E, Cimaglia C, Compagno M, Cuzzi G, et al. Latent tuberculosis infection screening in persons newly-diagnosed with hiv infection in italy: a multicentre study promoted by the Italian society of infectious and tropical diseases. Int J Infect Dis 2019. [DOI] [PubMed] [Google Scholar]
- 9.Petruccioli E, Petrone L, Vanini V, Sampaolesi A, Gualano G, Girardi E, et al. IFNgamma/TNFalpha specific-cells and effector memory phenotype associate with active tuberculosis. J Infect 2013;66:475–86. [DOI] [PubMed] [Google Scholar]
- 10.Goletti D, Carrara S, Vincenti D, Saltini C, Rizzi EB, Schinina V, et al. Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: a pilot study. Clin Microbiol Infect 2006;12:544–50. [DOI] [PubMed] [Google Scholar]
- 11.Butera O, Chiacchio T, Carrara S, Casetti R, Vanini V, Meraviglia S, et al. New tools for detecting latent tuberculosis infection: evaluation of RD1-specific long-term response. BMC Infect Dis 2009;9:182 2334–9-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiacchio T, Petruccioli E, Vanini V, Cuzzi G, Pinnetti C, Sampaolesi A, et al. Polyfunctional T-cells and effector memory phenotype are associated with active TB in HIV-infected patients. J Infect 2014;69:533–45. [DOI] [PubMed] [Google Scholar]
- 13.Cirillo DM, Barcellini L, Goletti D. Preliminary data on precision of quantifer-on-TB plus performance. Eur Respir J 2016;48:955–6. [DOI] [PubMed] [Google Scholar]
- 14.Chiacchio T, Petruccioli E, Vanini V, Cuzzi G, Massafra U, Baldi G, et al. Characterization of QuantiFERON-TB-plus results in latent tuberculosis infected patients with or without immune-mediated inflammatory diseases. J Infect 2019;79:15–23. [DOI] [PubMed] [Google Scholar]
- 15.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, et al. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB plus. J Infect 2016;73:588–97. [DOI] [PubMed] [Google Scholar]
- 16.Petruccioli E, Chiacchio T, Vanini V, Cuzzi G, Codecasa LR, Ferrarese M, et al. Effect of therapy on QuantiFERON-plus response in patients with active and latent tuberculosis infection. Sci Rep 2018;8:15626 018–33825-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petruccioli E, Vanini V, Chiacchio T, Cuzzi G, Cirillo DM, Palmieri F, et al. Analytical evaluation of QuantiFERON- Plus and QuantiFERON- Gold In-tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb) 2017;106:38–43. [DOI] [PubMed] [Google Scholar]
- 18.Barcellini L, Borroni E, Brown J, Brunetti E, Campisi D, Castellotti PF, et al. First evaluation of QuantiFERON-tb gold plus performance in contact screening. Eur Respir J 2016;48:1411–19. [DOI] [PubMed] [Google Scholar]
- 19.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, et al. First independent evaluation of QuantiFERON-tb plus performance. Eur Respir J 2016;47:1587–90. [DOI] [PubMed] [Google Scholar]
- 20.QuantiFERON®-TB Gold Plus, ELISA package insert, quiagen. http://www.quantiferon.com/irm/content/PI/QFT/PLUS/2PK-Elisa/UK.pdf.
- 21.Rozot V, Patrizia A, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, et al. Combined use of mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis 2015;60:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, et al. Mycobacterium tuberculosis-specific CD8+ t cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol 2013;43:1568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolova M, Markova R, Drenska R, Muhtarova M, Todorova Y, Dimitrov V, et al. Antigen-specific CD4- and CD8-positive signatures in different phases of mycobacterium tuberculosis infection. Diagn Microbiol Infect Dis 2013;75:277–81. [DOI] [PubMed] [Google Scholar]
- 24.Lancioni C, Nyendak M, Kiguli S, Zalwango S, Mori T, Mayanja-Kizza H, et al. CD8+ t cells provide an immunologic signature of tuberculosis in young children. Am J Respir Crit Care Med 2012;185:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O’rie T, et al. Functional capacity of mycobacterium tuberculosis-specific t cell responses in humans is associated with mycobacterial load. J Immunol 2011;187:2222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiacchio T, Petruccioli E, Vanini V, Cuzzi G, La Manna MP, Orlando V, et al. Impact of antiretroviral and tuberculosis therapies on CD4(+) and CD8(+) HIV/M. tuberculosis-specific T-cell in co-infected subjects. Immunol Lett 2018;198:33–43. [DOI] [PubMed] [Google Scholar]
- 27.Petrone L, Vanini V, Chiacchio T, Petruccioli E, Cuzzi G, Schinina V, et al. Evaluation of IP-10 in quantiferon-plus as biomarker for the diagnosis of latent tuberculosis infection. Tuberculosis (Edinb) 2018;111:147–53. [DOI] [PubMed] [Google Scholar]
- 28.Goletti D, Lindestam Arlehamn CS, Scriba TJ, Anthony R, Cirillo DM, Alonzi T, et al. Can we predict tuberculosis cure? What tools are available? Eur Respir J 2018;52 2018. Print 2018 Nov. doi: 10.1183/13993003.01089. [DOI] [PubMed] [Google Scholar]
- 29.Chien JY, Chiang HT, Lu MC, Ko WC, Yu CJ, Chen YH, et al. QuantiFERON-TB gold plus is a more sensitive screening tool than QuantiFERON-tb gold in-tube for latent tuberculosis infection among older adults in long-term care facilities. J Clin Microbiol 2018:56 18. Print 2018. Aug. doi: 10.1128/JCM.00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallegos Morales EN, Knierer J, Schablon A, Nienhaus A, Kersten JF. Prevalence of latent tuberculosis infection among foreign students in Lubeck, Germany tested with QuantiFERON-tb gold in-tube and QuantiFERON-tb gold plus. J Occup Med Toxicol 2017;12:12 017–0159-4. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pieterman ED, Liqui Lung FG, Verbon A, Bax HI, Ang CW, Berkhout J, et al. A multicentre verification study of the QuantiFERON((r))-tb gold plus assay. Tuberculosis (Edinb) 2018;108:136–42. [DOI] [PubMed] [Google Scholar]
- 32.Ryu MR, Park MS, Cho EH, Jung CW, Kim K, Kim SJ, et al. Comparative evaluation of QuantiFERON-tb gold in-tube and quantiferon-tb gold plus in diagnosis of latent tuberculosis infection in immunocompromised patients. J Clin Microbiol 2018:56 18. Print 2018. Nov. doi: 10.1128/JCM.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theel ES, Hilgart H, Breen-Lyles M, McCoy K, Flury R, Breeher LE, et al. Comparison of the QuantiFERON-tb gold plus and QuantiFERON-tb gold in-tube interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol 2018:56 18. Print 2018. Jul. doi: 10.1128/JCM.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sotgiu G, Saderi L, Petruccioli E, Aliberti S, Piana A, Petrone L, et al. QuantiFERON tb gold plus for the diagnosis of tuberculosis: a systematic review and meta-analysis. J Infect 2019. [DOI] [PubMed] [Google Scholar]
- 35.Telisinghe L, Amofa-Sekyi M, Maluzi K, Kaluba-Milimo D, Cheeba-Lengwe M, Chiwele K, et al. The sensitivity of the QuantiFERON((r))-tb gold plus assay in Zambian adults with active tuberculosis. Int J Tuberc Lung Dis 2017;21:690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konig Walles J, Tesfaye F, Jansson M, Tolera Balcha T, Winqvist N, Kefeni M, et al. Performance of QuantiFERON-tb gold plus for detection of latent tuberculosis infection in pregnant women living in a tuberculosis- and HIV-endemic setting. PLoS One 2018;13:e0193589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. Test variability of the QuantiFERON-tb gold in-tube assay in clinical practice. Am J Respir Crit Care Med 2013;187:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nienhaus A, Ringshausen FC, Costa JT, Schablon A, Tripodi D. IFN-gamma release assay versus tuberculin skin test for monitoring tb infection in healthcare workers. Expert Rev Anti Infect Ther 2013;11:37–48. [DOI] [PubMed] [Google Scholar]
- 39.Ringshausen FC, Nienhaus A, Schablon A, Schlosser S, Schultze-Werninghaus G, Rohde G. Predictors of persistently positive mycobacterium-tuberculosis-specific interferon-gamma responses in the serial testing of health care workers. BMC Infect Dis 2010;10:220 2334–10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schablon A, Nienhaus A, Ringshausen FC, Preisser AM, Peters C. Occupational screening for tuberculosis and the use of a borderline zone for interpretation of the igra in german healthcare workers. PLoS One 2014;9:e115322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slater ML, Welland G, Pai M, Parsonnet J, Banaei N. Challenges with QuantiFERON-tb gold assay for large-scale, routine screening of U.S. healthcare workers. Am J Respir Crit Care Med 2013;188:1005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moon HW, Gaur RL, Tien SS, Spangler M, Pai M, Banaei N. Evaluation of QuantiFERON-tb gold-plus in health care workers in a low-incidence setting. J Clin Microbiol 2017;55:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tagmouti S, Slater M, Benedetti A, Kik SV, Banaei N, Cattamanchi A, et al. Reproducibility of interferon gamma (IFN-gamma) release assays. Syst Rev Ann Am Thorac Soc 2014;11:1267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uzorka JW, Kroft LJM, Bakker JA, van Zwet EW, Huisman E, Knetsch-Prins C, et al. Proof of concept that most borderline QuantiFERONresults are true antigen-specific responses. Eur Respir J 2017:50 2017. Print 2017 Nov. doi: 10.1183/13993003.01630.. [DOI] [PubMed] [Google Scholar]
- 45.Uzorka JW, Delfos NM, Witte AMC, Scheper H, van Soolingen D, Arend SM. Tuberculosis after a borderline QuantiFERONresult during screening before infliximab. Eur Respir J 2018:52 2018. Print 2018 Aug.. doi: 10.1183/13993003.00913. [DOI] [PubMed] [Google Scholar]
- 46.Strobe Group University of Bern. Strobe Statement. https://www.strobestatement.org/index_php?id=strobe-single-news-view&tx_news_pi1%5Bnews%5D=146&tx_news_pi1%5Bcontroller%5D=News&tx_news_pi1%5Baction%5D=detail&cHash=1313eacdf5efcb43416881ec91b8a1e6 2014.
- 47.PLOS Medicine Editors Observational studies: getting clear about transparency. PLoS Med 2014;11:e1001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World medical association. WMA declaration of Helsinki – ethical principles for medical research involving human subjects 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ [DOI] [PubMed]
- 49.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K, et al. Updated guidelines for using interferon gamma release assays to detect mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 2010;59:1–25. [PubMed] [Google Scholar]
- 50.Nemes E, Rozot V, Geldenhuys H, Bilek N, Mabwe S, Abrahams D, et al. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of mycobacterium tuberculosis infection. Am J Respir Crit Care Med 2017;196:638–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pai M, Joshi R, Dogra S, Zwerling AA, Gajalakshmi D, Goswami K, et al. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int J Tuberc Lung Dis 2009;13:84–92. [PMC free article] [PubMed] [Google Scholar]
- 52.Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2011;56:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santin M, Munoz L, Rigau D. Interferon-gamma release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLoS One 2012;7:e32482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincenti D, Carrara S, Butera O, Bizzoni F, Casetti R, Girardi E, et al. Response to region of difference 1 (RD1) epitopes in human immunodeficiency virus (HIV)-infected individuals enrolled with suspected active tuberculosis: a pilot study. Clin Exp Immunol 2007;150:91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.